Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiac magnetic resonance (CMR) uses a strong magnet 1.5 to 3.0 Tesla (equivalent to 30,000–60,000 times the strength of the earth’s magnetic field), radiofrequency pulses, and gradient magnetic fields to obtain images of the heart. When placed in the bore of a magnet, positively charged protons, mainly from water, are aligned in the direction of the magnetic field creating a net magnetization. Radiofrequency pulses are used to tilt these protons away from their alignment, shifting them to a higher energy state. These protons then return to their equilibrium state through the process of relaxation and emit a signal. The relaxation consists of two components—T1 and T2 relaxation. Magnetic gradients are applied across the tissue of interest to localize these signals. The signals are then collected using a receiver coil and placed in a data space referred to as k-space, which is then used to create an image. CMR uses differences in relaxation properties between and among different tissues, fluids, and blood, and changes that occur due to pathological processes to create contrast in the image.
Similar to echocardiography, CMR allows the generation of images of the heart without exposure to ionizing radiation. Although the spatial resolution of CMR is comparable to echocardiography (~1 mm), the contrast-to-noise and signal-to-noise ratios are far superior ( Fig. 10.1 ). The latter allows easier delineation of borders between tissues and in particular between blood pool and myocardium. The contrast between blood pool and myocardium is generated using differences in signal properties of the different tissues without the use of contrast agents. CMR is also not limited by “acoustic windows” that may hinder echocardiography, and images can be obtained in any tomographic plane. Finally, CMR can provide information about tissue characteristics using differences in T1 and T2 signals, with the addition of contrast agents.
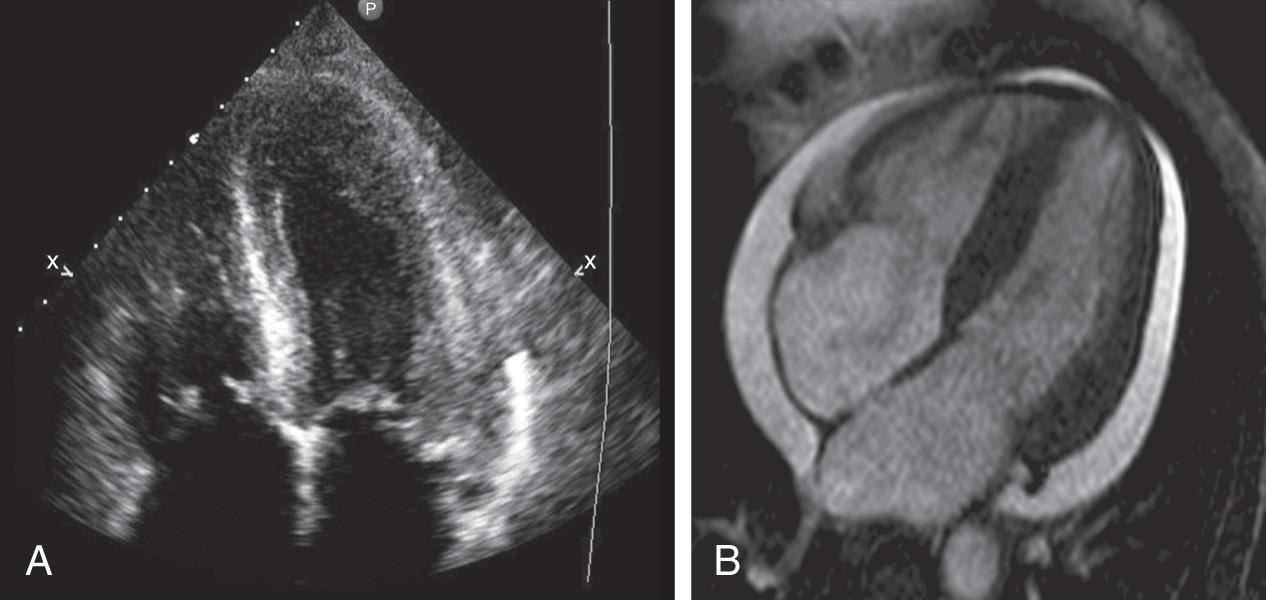
The major limitation of CMR is availability. Given the cost, the special construction necessary to host a CMR system, and the technical expertise and support necessary, CMR is not widely available at all centers. CMR is limited with respect to portability, unlike echocardiography where imaging can be performed at the patient’s bedside. There are also a set of contraindications ( Table 10.1 ) that limit the use of this technology in selected patient populations. Image acquisition can be challenging in patients with an irregular cardiac rhythm or difficulty breath-holding, though arrhythmia rejection and real-time techniques provide an opportunity to overcome these barriers ( Fig. 10.2 ). Finally since patients have to lie still in a long hollow tube for up to an hour during imaging, claustrophobia may be an important limiting factor.
|
|
|
|
|
|
|
|
|
|
|
|
|

Pulse sequences are orchestrated actions of turning on and off various coils, gradients, and radiofrequency pulses to produce a CMR image. In simple terms, the pulse sequences are based on either gradient echo or spin echo sequences. The most common sequences used are bright blood gradient echo sequences (where the blood pool is bright), dark blood spin echo sequences (where the blood pool is dark), steady-state free precession sequences (also a type of gradient echo sequence, and most commonly used for function or cine images), and inversion recovery sequences (e.g., late gadolinium enhancement [LGE] imaging used to assess myocardial scar).
Although echocardiography is usually the first-line imaging modality for questions of left ventricular (LV) function and assessment of valvular disease, cardiac MRI still has many important indications. The following questions and answers pertain to the appropriate use of cardiac MRI in the clinical setting as recommended in the multisociety multimodality appropriateness criteria from 2019. Appropriate uses of CMR are summarized in Table 10.2 .
|
|
|
|
|
|
|
|
|
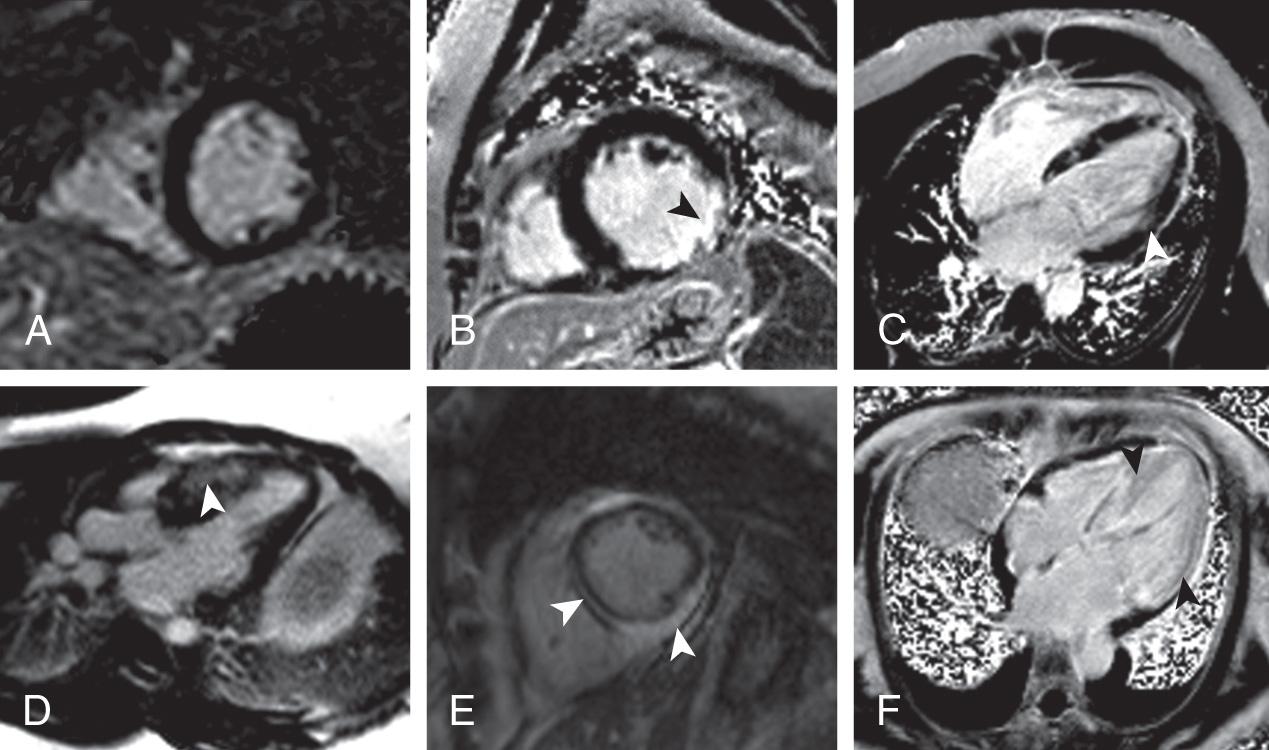
One of the unique aspects of CMR is the ability to identify myocardial scar/fibrosis. This is most commonly performed using LGE imaging (also known as delayed enhancement imaging). Gadolinium-based contrast agents (GBCAs) are administered intravenously, and then after waiting approximately 10 minutes and allowing time for the contrast to distribute into areas of scar/fibrosis, an inversion recovery gradient echo sequence is performed. This sequence nulls normal myocardium (makes it black), and anything that is bright within the myocardium is most likely myocardial scar/fibrosis ( Fig. 10.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here