Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Stroke due to cardiac embolism is commonly of sudden onset, is more prone to hemorrhagic transformation, and is associated with higher mortality than other stroke subtypes.
The etiologic workup for stroke due to a cardiogenic source is crucial in determining appropriate secondary prevention.
Anticoagulation is the mainstay of therapy for patients with cardioembolic stroke secondary to atrial fibrillation, absent any contraindications.
Some cardioembolic sources, including infective endocarditis and some intracardiac tumors, present a contraindication to treatment with anticoagulation.
Cardiac embolism is a major mechanism of stroke in the United States, accounting for 20%–30% of all ischemic strokes. , Atrial fibrillation (AF) is the readily identifiable cause in up to 50% of cardioembolic strokes. AF affects 9% of persons aged 65 years and older. In addition, approximately 30% of ischemic strokes in the young (younger than 45 years) can be attributed to cardiac embolism. , Table 32.1 estimates the prevalence of various cardiac conditions in embolic ischemia and infarction. The economic toll of stroke in the United States amounts to more than $100 billion annually in both direct and indirect healthcare costs.
| Source | Percentage of All Cardiogenic Emboli |
|---|---|
| Nonvalvular atrial fibrillation | 45 |
| Acute myocardial infarction | 15 |
| Ventricular aneurysm | 10 |
| Rheumatic heart disease | 10 |
| Prosthetic cardiac valve | 10 |
| Other | 10 |
In comparison with other subtypes of stroke, the prognosis after a cardioembolic stroke is poor. There is up to a 6.5% risk of stroke recurrence within 7 days, and the in-hospital mortality rate is 27.3%. The 5-year mortality rate for cardioembolic stroke has been reported as high as 80%. ,
Cardioembolism as a cause of stroke can be inferred as the diagnosis and distinguished from other stroke subtypes on the basis of (1) presence of a cardiac source of cerebral embolism, (2) absence of a large-artery stenosis or occlusion in the vessel supplying the involved vascular territory, (3) a clinical syndrome or radiographic appearance inconsistent with a small-vessel (lacunar) stroke, (4) evidence of systemic embolism, (5) absence of unusual precipitants of stroke (e.g., vasculitis), and (6) absence of a complex atheroma of the aortic arch, defined as being larger than 4 mm in thickness, ulcerated, or containing a mobile component ( Fig. 32.1 , ![]() ).
).
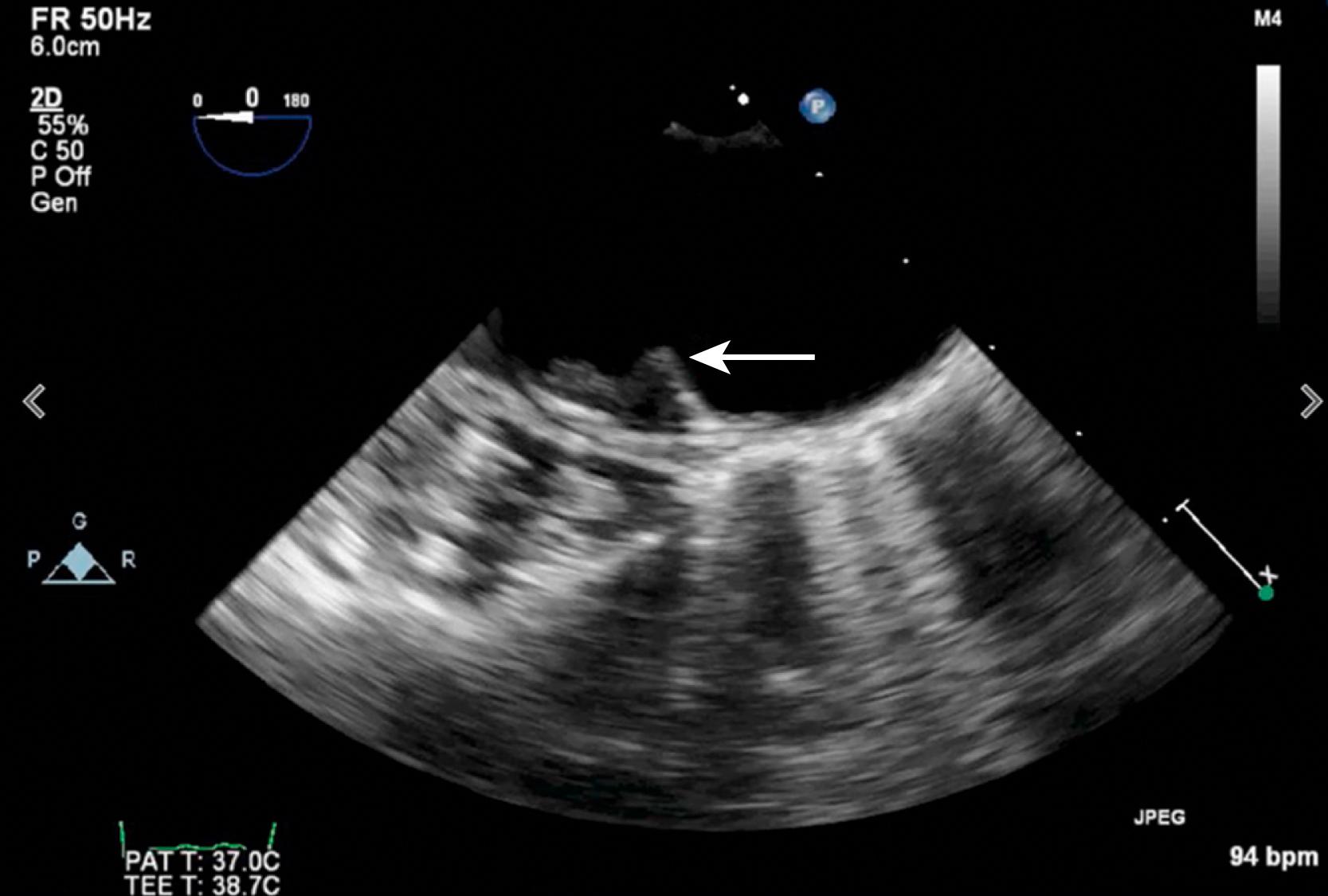
Clinical features of cardioembolic stroke are summarized in Box 32.1 . The onset of symptoms due to cardioembolism is often sudden, as observed in 25%–82% of possible cardioembolic strokes, but the rapidity of onset has low specificity, inasmuch as the onset is sudden in 66% of strokes from other mechanisms. , Sudden onset and loss of consciousness are also insensitive for determining that symptoms are due to embolism. , , In 1967, Fisher and Pearlman described “nonsudden” cerebral embolism with stuttering progression, which they attributed to vacillating flow around an embolus lodged in the intracranial circulation. Seizures related to acute stroke are more common in patients with cardiac embolism. There may be fluctuations in symptoms as the embolus lyses and fragments move downstream. In addition, early clinical improvement or recovery may be due either to the recruitment of collateral sources of blood flow or to distal migration of the embolus.
Neurologic history and findings
Sudden onset
Isolated focal deficit
Seizure at onset
Loss of consciousness at onset
Peak of deficit at onset
Involvement of more than one vascular territory
Evidence of systemic embolization
Multiple infarcts in more than one vascular territory
Deep and superficial infarctions
Hemorrhagic conversion
Absence of large-artery stenosis or occlusion in parent vessels
Rapid recanalization of intracranial vessels on transcranial Doppler ultrasonography
Repetitive stereotyped transient ischemic attacks (TIAs) are unusual in embolic stroke. Less than one-third of patients experience transient ischemic symptoms before the stroke. , , ,
The size of the emboli partially determines which vessels are affected. Small emboli can cause retinal ischemia. In a balloon catheter model of embolism, the majority of emboli were found in the middle cerebral artery or one of its branches; the next most commonly affected vessel was the basilar artery and its branches, and then the anterior cerebral artery. The size and composition of the embolus vary according to the underlying cardiac disorder ( Table 32.2 ). Valvular lesions may result in the embolization of calcified particles. Atrial myxomas can cause tumor emboli. In nonbacterial thrombotic endocarditis (NBTE), platelets are the main component of the embolic material, whereas emboli from left ventricular aneurysms contain mainly fibrinous material.
| Source | Type | Size |
|---|---|---|
| Atrial fibrillation | Fibrin | Large |
| Left ventricular thrombus | Fibrin | Large |
| Myxoma | Myxomatous | Small or large |
| Infective endocarditis | Septic debris | Small or large |
| Degenerative valvular disease | Calcium | Small |
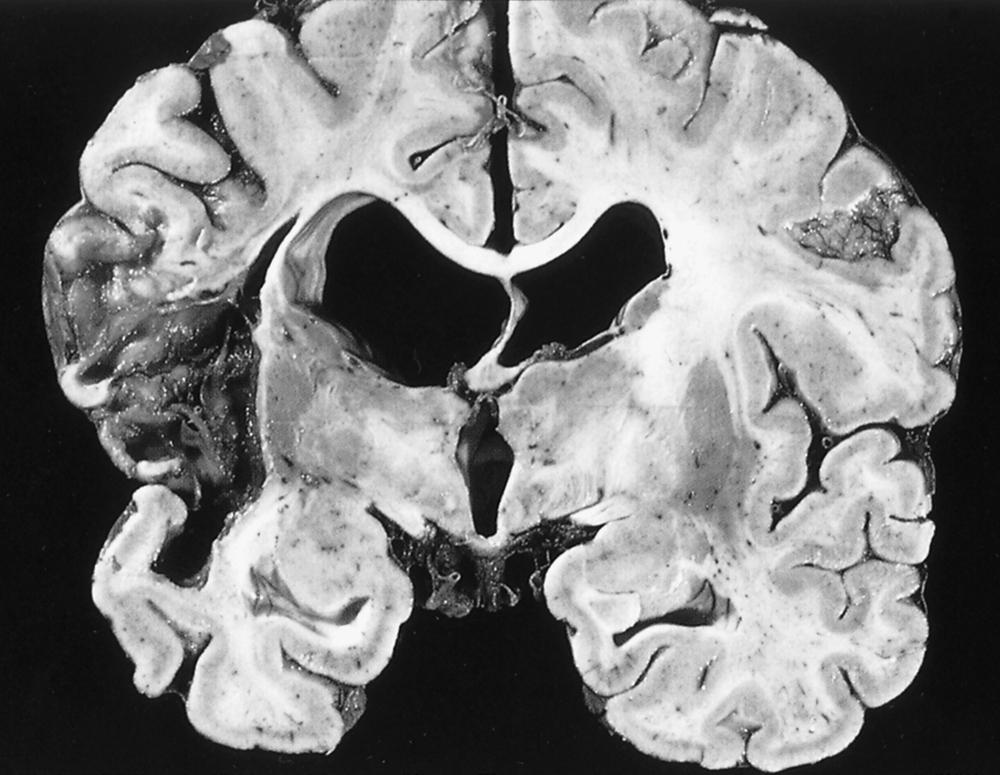
Patients at high risk for cardioembolism are more likely to have large infarcts that involve both deep and superficial structures ( Fig. 32.2 ) and are frequently visible on initial head computed tomography (CT). , , , Strokes caused by cardioembolism from AF commonly lead to a significant neurologic disability. A pattern of multiple infarctions involving multiple vascular territories is distinctive for cardioembolism. , Cerebral or cerebellar surface branch occlusion by an embolus may lead to focal infarctions causing specific syndromes of focal motor deficits, isolated aphasia, hemiataxia, or hemianopia. , , These syndromes can mimic small vessel occlusion or peripheral neuropathy, such as unilateral distal arm weakness due to infarction of the “hand knob” region of the precentral gyrus. Posterior cerebral artery territory infarcts are commonly caused by cardiac embolism. , Cardioembolism is also a foremost cause of large vessel occlusion, which may be amenable to mechanical thrombectomy. , Embolic strokes are believed to be more prone to hemorrhagic conversion, a complication detected on follow-up CT in approximately 20% of cardioembolic strokes. Hemorrhagic conversion occurs when there is spontaneous lysis of the thrombus with reperfusion into infarcted tissue.
Between 30% and 60% of patients with ischemic stroke have a possible source of cardiac embolism. , The detection of a potential cardiac source of embolism depends, to a large extent, on the thoroughness of the evaluation. For example, one study demonstrated that 15% of potential embolic sources were detected only after cardiac monitoring and two-dimensional (2D) transthoracic echocardiography (TTE). In addition, ascribing a stroke to a definite cardioembolic cause can be difficult because of a coexistent mechanism involving a potential noncardiac source of embolus (e.g., large-vessel atherosclerosis in approximately 30% of cases). , ,
A thorough cardiac assessment of patients with stroke is necessary for accurate diagnosis of the mechanism of cerebral ischemia and to help establish prognosis. A standard 12-lead electrocardiogram (ECG) can identify patients who are in sustained AF and can detect acute myocardial ischemia. A clinical history of angina pectoris or abnormal electrocardiography findings (e.g., anterior wall myocardial infarction [MI], left ventricular hypertrophy, or inverted T waves) has associated hazard ratios (HRs) of 1.6–3.2 for nonfatal MI or cardiac death in patients with TIA or minor stroke. Extended (>24 hours) cardiac monitoring is essential to detect paroxysmal AF, and the result is not always positive despite numerous such studies in the same patient. Long-term (>30 days) continuous cardiac monitoring after cryptogenic stroke with an event recorder or implanted cardiac monitor appears to detect AF more frequently than shorter intervals of monitoring. , Although greater duration (“burden”) of AF is associated with increased risk of stroke, the significance of short periods, minutes to hours, of new-onset paroxysmal AF detected after cryptogenic stroke, is uncertain.
TTE is an essential part of the embolic evaluation. M-mode and 2D echocardiography can define the cardiac chambers, valves, and left ventricular function. The use of agitated saline allows for detection of an intracardiac shunt, such as a patent foramen ovale (PFO). The proper detection of a shunt with this procedure requires an adequate Valsalva maneuver, with crossing of the bubbles from the right atrium to the left atrium seen upon release of the Valsalva maneuver after two to four cardiac cycles ( , ![]() ). A more delayed appearance of bubbles is suggestive of a pulmonary source of shunting (
). A more delayed appearance of bubbles is suggestive of a pulmonary source of shunting ( ![]() ), such as a pulmonary arteriovenous malformation (AVM). TTE may be insensitive for masses and thrombi in the left atrium and on the mitral valve, requiring further studies. , Selective use of contrast echocardiography, cardiac CT, or cardiac magnetic resonance imaging (MRI) are other noninvasive methods to detect a cardiac mass or thrombi.
), such as a pulmonary arteriovenous malformation (AVM). TTE may be insensitive for masses and thrombi in the left atrium and on the mitral valve, requiring further studies. , Selective use of contrast echocardiography, cardiac CT, or cardiac magnetic resonance imaging (MRI) are other noninvasive methods to detect a cardiac mass or thrombi.
Transesophageal echocardiography (TEE) is more sensitive than TTE in detecting abnormalities in the left atrium and appendage, atrial septum, mitral valve, and aortic arch. The cost-effectiveness of performing TEE in all patients with stroke remains controversial. , This evaluation is recommended for patients with a suspected embolic stroke in whom cardiac monitoring and TTE findings are unremarkable and in young patients without a clear other cause of stroke. It may be less sensitive for the detection of a PFO with agitated saline, because patients who are sedated for the procedure are less able to perform an adequate Valsalva maneuver. Although complication rates for TEE are very low, associated risks include esophageal injury, exposure to general anesthesia, hypertension or hypotension during the procedure, and aspiration.
Embolus detection by transcranial Doppler (TCD) ultrasonography can be used to identify microemboli due to a variety of potential cardiac sources (AF, infectious endocarditis, cardiomyopathy, aortic stenosis, mitral stenosis, and PFO). The frequency of high-intensity transient signals (HITSs) did not appear to vary according to echocardiographic diagnosis in one study, but another demonstrated a higher rate (33%) of microembolization in patients with high-risk sources of embolism (e.g., prosthetic valves) compared with patients with other, lower-risk cardiac conditions (e.g., AF [15%]). ,
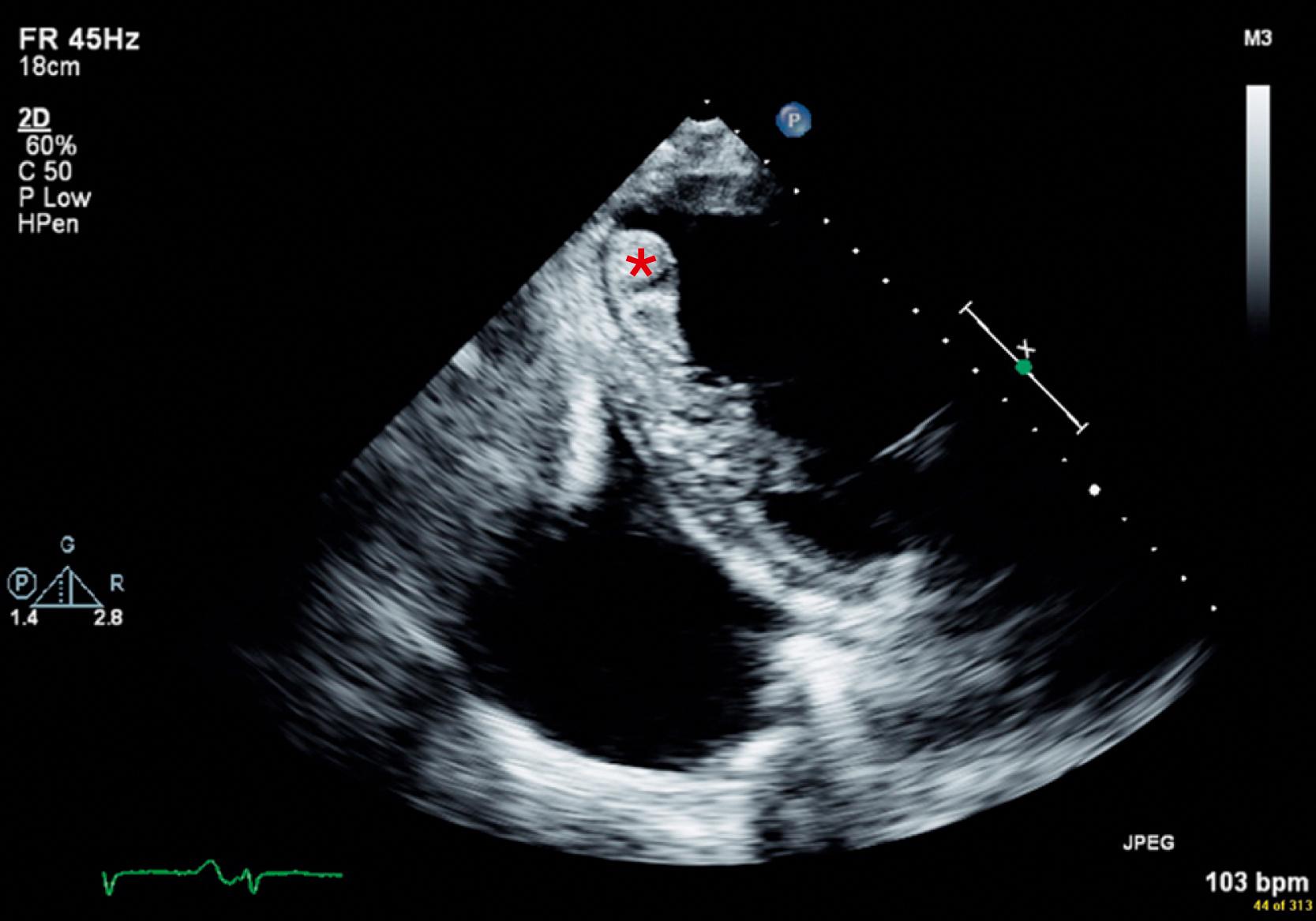
Box 32.2 summarizes potential cardiac conditions according to the strength of indication for anticoagulation for stroke prevention. Although the early period after stroke appears to be the highest risk period for recurrent embolization, it is also the period of greatest risk of hemorrhagic conversion. For example, the optimal timing of treatment with oral anticoagulants for nonvalvular AF after acute stroke is uncertain. However, in patients at higher risk for embolism, such as those with mechanical prosthetic valves or left ventricular thrombi ( Fig. 32.3 ), immediate anticoagulation should be considered.
Antithrombotic therapy considered the standard of practice
Left ventricular thrombus
Left atrial thrombus
Recent transmural anterior myocardial infarction
Rheumatic mitral or aortic valvular disease
Mechanical prosthetic heart valve
Atrial fibrillation (AF)
Antithrombotic therapy may be of value
Papillary fibroelastoma (where surgical resection is contraindicated)
Nonbacterial thrombotic endocarditis
Antithrombotic therapy contraindicated
Infective endocarditis
Atrial myxoma
Atrial cardiopathy (enlarged left atrium, left atrial fibrosis, atrial arrhythmias other than AF)
Atrial flutter
Bioprosthetic mitral heart valve
Chronic myocardial infarction or heart failure with low ejection fraction
Dilated cardiomyopathy
Left atrial spontaneous echo contrast (“smoke”) seen on transesophageal echocardiogram
Left ventricular aneurysm without thrombus
Mitral annular calcification
Mitral valve prolapse
Patent foramen ovale (particularly in young patients with cryptogenic stroke)
Patent foramen ovale with atrial septal aneurysm
Sick sinus syndrome
Valve strands
For long-term prevention of stroke, AF is the only condition for which anticoagulation has been conclusively shown to be superior to aspirin. Still, anticoagulation is often used in situations with the potential for recurrent embolization. In the Warfarin-Aspirin Recurrent Stroke Study (WARSS), warfarin was weakly (absolute risk reduction, 9%) superior to aspirin therapy in patients in whom an embolic stroke was suspected, but there was no evidence of a definite cardiac source; however, this difference was not statistically significant. Similarly, clinical trials have shown that direct oral anticoagulants are not superior to aspirin in preventing recurrent stroke in patients with embolic-appearing stroke of uncertain source. , A clinical trial comparing anticoagulation to aspirin treatment for prevention of stroke in patients with cryptogenic stroke, no AF, and atrial cardiopathy is underway.
The numerous structural and functional cardiac conditions associated with transient cerebral symptoms and infarction are described here in greater detail (see Table 32.1 ). Although cardiac testing can reveal a potential source, the clinician must determine whether the symptoms, physical findings, and results of neuroimaging support the causal relationship.
In patients with cardiomyopathy, the reported annual stroke rates (1.3%–3.5% per year) that were derived from cardiac trials likely underestimate the actual risk of stroke. , The risk of stroke is inversely related to the ejection fraction (EF), with as much as a 58% increase in thromboembolic events for every 10% decrease in EF, but not to the functional classification (New York Heart Association [NYHA] Functional Class). , There is a fourfold risk of stroke in patients who are 50–59 years old with heart failure. , The highest risk is in the first month after diagnosis. , The annual rate of stroke in patients with nonischemic dilated cardiomyopathy is approximately 1.5%–3.5%. , In patients with cardiomyopathy, AF is often a complicating factor that further raises the risk of stroke. Other factors include decreased apical flow and a possible intracardiac hypercoagulable state. Patients with heart failure have been found to have increased sympathetic activity, which may lead to increased platelet activation as well as increased levels of β-thyroid-binding globulin, D dimer, von Willebrand factor, fibrinopeptide A, thrombin-antithrombin complexes, β-thromboglobulin, and P-selectin; they also have impaired vascular endothelial function, which impairs the release of nitric oxide, further contributing to platelet adhesion. Takotsubo (stress) cardiomyopathy, caused in part by sympathetic activation and excess catecholamines, leads to left ventricular apical ballooning ( ![]() ), anterior wall dysfunction, and increased risk of stroke and TIA.
), anterior wall dysfunction, and increased risk of stroke and TIA.
In the Survival and Ventricular Enlargement (SAVE) study, patients with EF values of 29%–35% (mean 32%) had a stroke rate of 0.8% per year; the rate in patients with EF values of 28% or less (mean 23%) was 2.5% per year. Compared with aspirin, warfarin has been shown to significantly reduce the relative risk (RR) of stroke by approximately 40% in patients with heart failure, while also doubling the rate of major hemorrhage. The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial was a prospective, three-arm randomized trial comparing warfarin (international normalized ratio [INR] goal 2.0–3.0), aspirin (162 mg/day), and clopidogrel (75 mg/day) in patients with an EF of 35% or less and NYHA class II to IV. The study was stopped early at 1587 patients after poor enrollment rates and without showing a significant difference between the treatment arms, although there was a suggestion of a benefit for warfarin in reducing nonfatal stroke compared with aspirin (0.7% vs. 2.1%; P = 0.06), as a secondary end point. In the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial, patients with a reduced left ventricular EF (≤35%) without AF were randomized to receive warfarin adjusted to an INR of 2.0–3.5 versus aspirin 325 mg daily. The composite outcome of ischemic stroke, intracerebral hemorrhage or death from any cause was not different between the groups. Ischemic strokes were significantly reduced with the use of warfarin (0.72 vs 1.36 events per 100 patient-years, warfarin compared to aspirin), but the rate of major hemorrhage was significantly increased with warfarin (1.78 vs 0.87 events per 100 patient-years). Interestingly, the rates of intracerebral and intracranial hemorrhage did not differ between the groups.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here