Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The microarchitecture of cellular substructures involved in calcium signaling is highly organized in all forms of mammalian striated muscle, and the cardiac ventricular myocyte (VM) is no exception. The ultrastructural characteristics of the T-tubular system and sarcoplasmic reticulum (SR) play an important role in normal cardiac electrophysiology, and their degradation has dire consequences in a number of pathologic contexts. The following sections discuss several electrophysiologically important aspects of VM ultrastructure and place emphasis on describing structure-function relationships in the healthy and diseased myocardium. Because interaction within and between these structures often occurs at or below the limit of resolution for traditional live-imaging techniques, quantitative computational modeling has made an essential contribution to our understanding of how structure determines function. In addition, subcellular calcium signaling is a multiscale phenomenon with important dynamics that span the molecular and physiologic scales. Computational modeling has proven an important tool for integrating across these scales and across the broad range of methods used to define the structures involved and to measure their function. As such, we highlight ongoing computational efforts that make use of reconstructed subcellular geometries to describe the role of calcium in VM physiology, from the molecule to the micron.
The plasma membrane of ventricular myocytes, and most mammalian striated muscle, exhibits regular invaginations that project into the cell perpendicular to its surface ( Fig. 32.1 ). These structures align with the sarcomeric Z-disc and were originally termed transverse tubules (T-tubules) for their dominant orientation with respect to the long axis of the cell. In most species, however, a large number of longitudinal branches have also been observed, , which has caused different authors to describe the lattice architecture variously as the transverse-axial-tubular system (TATS), sarcolemmal tubule system, T-system, and sarcolemmal Z-rete. At a macroscopic level, the T-tubular lattice affects myocyte function through at least two important mechanisms. First, it expands VM surface area and thereby increases the density (per unit cell volume) of all sarcolemmal transport mechanisms. Second, it provides a platform for regionalization of specialized signaling structures. This chapter focuses on structures that are directly involved in cardiac Ca 2+ signaling, the best described of which are the calcium release unit (CRU; see Fig. 32.1 , right panel) and cardiac couplon.
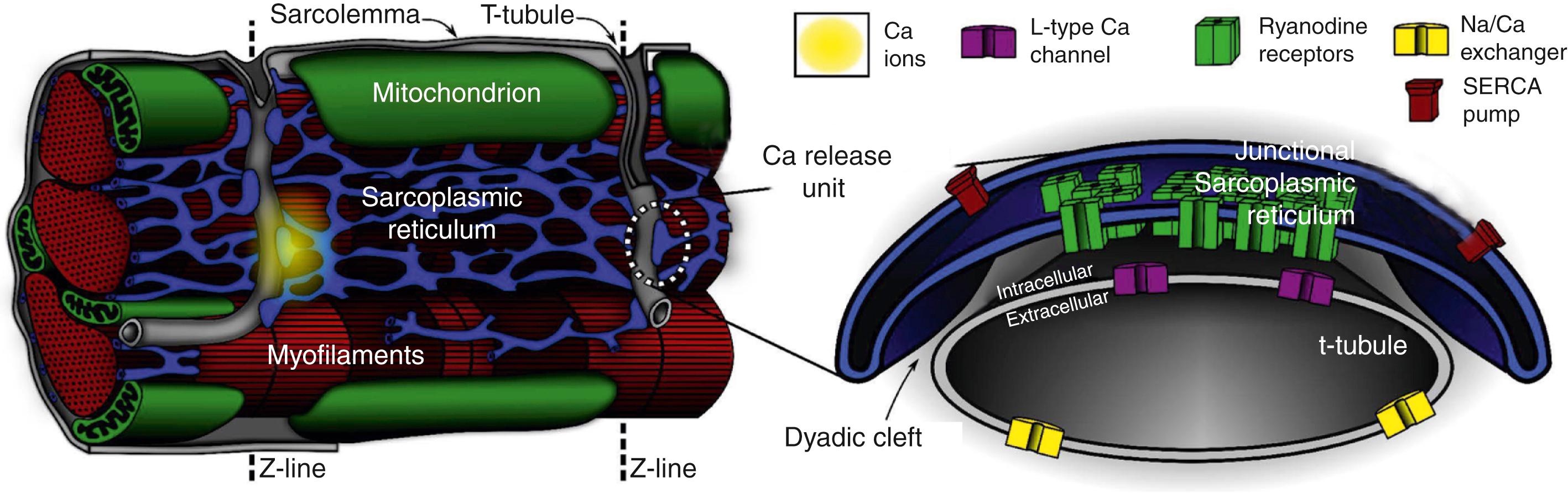
Initial impressions of the shape and structure of the CRU first appeared roughly half a century ago, well before the function of the couplon was understood or the term itself had been coined. At that time, transmission electron microscopy (EM) had shown junctions between the SR and T-tubular membranes, which in cardiac muscle were termed dyads for their characteristic two-component appearance in longitudinal tissue sections. A few years later, Constantin et al. identified these SR structures as the site of intracellular calcium storage, and Winegrad’s landmark study followed shortly after to show that they were also the site of intracellular Ca 2+ release. Fawcett and McNutt accordingly refined Porter and Palade’s well-known sketch of the cardiac sarcomere, and their rendition remains popular today (see Fig. 32.1 , left panel). In this schematic, Ca 2+ is released from specialized projections of the SR, which closely juxtapose the T-tubular membrane. These projections were also originally named for their anatomic appearance in transmission EM (i.e., junctional SR [jSR]; terminal cisternal SR) or characteristics of physical separation (i.e., heavy SR). Over the next several decades, classic studies were published by a number of groups to define Ca 2+ -induced Ca 2+ release (CICR) as the essential mechanism of excitation-contraction (EC) coupling occurring at cardiac dyads and responsible for activating contraction of the heart. , Key aspects of CICR are detailed in Chapter 2, Chapter 6 , but to review briefly, the voltage-dependent opening of local L-type Ca 2+ channels (LCC) permits influx of Ca 2+ , which binds to and activates nearby ryanodine receptors (RyR) in the jSR membrane. This results in the release, from the jSR, of the bulk of Ca 2+ that goes on to participate in contraction.
Over the last 4 decades, it has become clear that a number of important properties of cardiac EC coupling are critically dependent on the biophysical characteristics of the Ca 2+ -mediated interaction between the LCC and RyR. These processes and phenomena are discussed in detail later, but at this point it behooves us to introduce several terms that have been adopted to reflect fundamental structure-function relationships in the current paradigm of EC coupling, known as local control . First, the term CRU refers to a grouping of jSR terminals and RyR clusters, which together constitute a discrete functional ensemble. That is, the RyR clusters that make up a CRU are activated together in an all-or-nothing fashion but are otherwise functionally isolated from others by a sufficiently large diffusion distance. CRU structure is usually drawn schematically, as in Fig. 32.1 , which shows a single jSR terminal with a densely packed array of RyR. As will be seen, however, it is likely that real CRUs exhibit heterogeneous morphologies that incorporate multiple RyR clusters and probably also multiple functionally coupled jSR terminals. The term couplon was introduced to explicitly define the combination of an LCC cluster and associated CRU, which together are capable of functionally interacting to contribute to EC coupling. For this reason the couplon is, by definition, the elementary site of EC coupling in the heart (see Fig. 32.1 ) and, along with the CRU, is a feature of much further discussion in this chapter.
Much of our knowledge of cardiac ultrastructure has been informed by steadily improving approaches to both EM and light microscopy (LM). In combination with tomographic reconstruction algorithms, high-voltage EM (HVEM) has both enhanced resolution and improved penetration of thick-section preparations, allowing for the three-dimensional (3D) reconstruction of the T-tubule system and associated EC coupling structures. , Improvements in the acquisition and analysis of LM images have also now permitted imaging at or below the optical diffraction limit. This progress has brought the living T-tubule, , and even the detailed morphology of individual RyR clusters, , into view. Together, these techniques have been central to defining the structural characteristics of VM microdomains, describing protein localization in and around those domains, and permitting the geometrically detailed quantitative approaches described here.
The small space between the jSR and T-tubular membranes provides a confined volume (the so-called dyadic cleft, junctional cleft, or fuzzy space) in which large and rapid changes in local [Ca 2+ ] can be generated by the local transporters, particularly LCCs and RyRs. This constrained architecture is fundamental to high-fidelity coupling between those transporters and is maintained by the specialized anchoring protein, junctophilin, which tethers the jSR to the T-tubular membrane. In healthy myocytes, junctophilin keeps the distance between the jSR and the T-tubule to within 12 to 15 nm and appears to be interspersed among the RyR molecules within the dyadic surface of the jSR membrane.
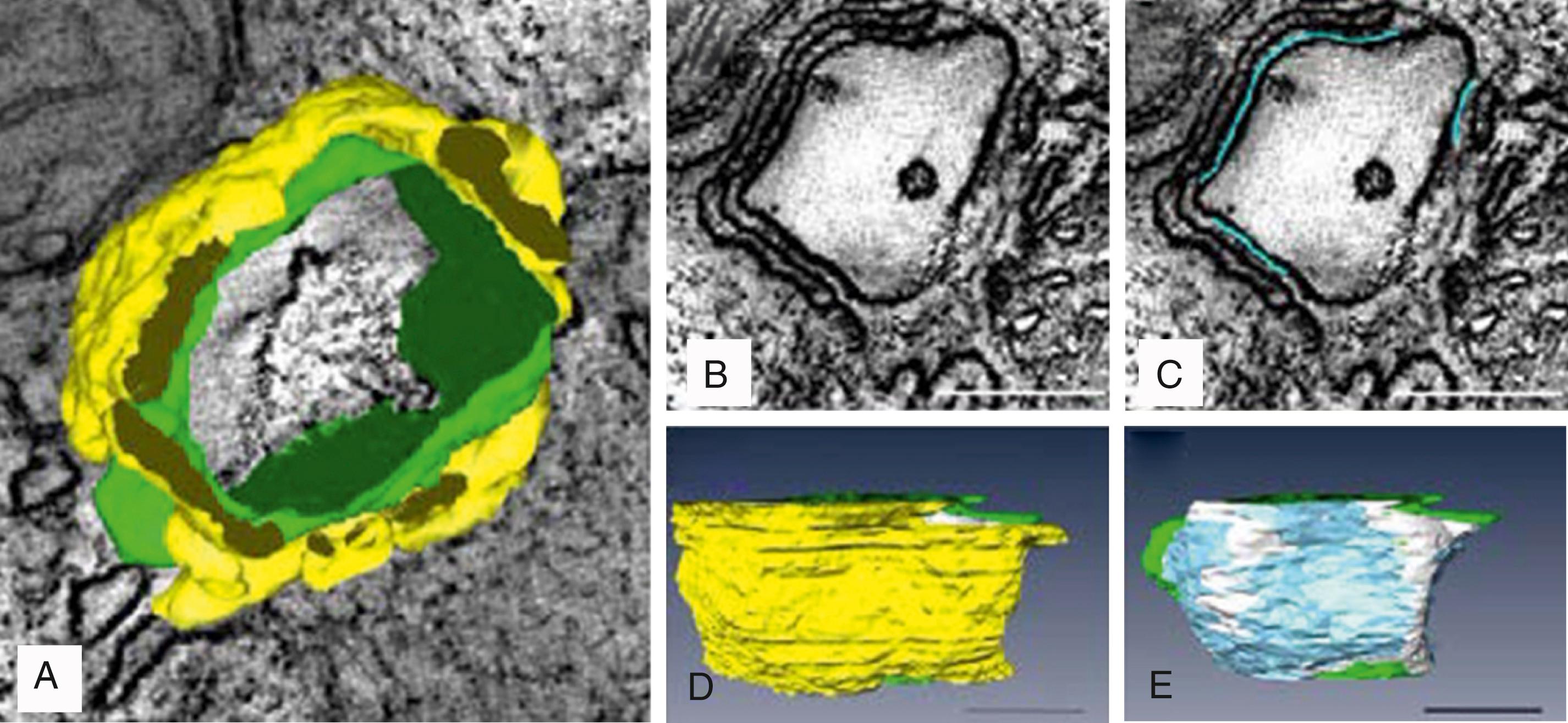
The exact number of LCCs and RyRs inside the couplon has been the topic of debate. Earlier studies by Franzini-Armstrong et al. used transmission EM to measure the cross-sectional diameter of individual CRU terminals and show that, in mammalian cardiac muscle, RyRs form dense clusters at individual CRU terminals. By assuming that the CRU terminals are circular and densely packed with receptors, these authors approximated the number of RyRs per couplon, estimating that 130 to 150 receptors are present in mouse ventricular couplons. Given that the RyR tetramer is around 30 nm on each side, this would require a dyad containing 100 RyRs to be 440 nm in diameter and 1.8 × 10 –12 μL in volume. Later studies have challenged these initial estimates. , , Using 3D EM tomography, Hayashi et al. reported that the size of each dyad is almost an order of magnitude smaller than previously reported (mean of 0.44 × 10 –12 μL), with a large fraction of tiny dyads (median 0.28 × 10 –12 μL). They also showed that the density of RyRs within each CRU was sparse. By segmenting the T-tubule (green), jSR (yellow), dyadic space (white), and RyR occupancy (blue), they observed spaces within the dyad that did not contain any RyRs ( Fig. 32.2E ). Interestingly, Hayashi et al. also found that 80% of all dyads have a neighboring dyad within 25 nm, which is dramatically closer than previously thought. Using super-resolution LM, Baddeley et al. found support for most of these surprising results. They similarly observed RyR clusters to be much smaller, more closely arranged, and irregularly shaped than had been reported or assumed in earlier work.
Meanwhile, work by Shen et al. used 3D super-resolution LM to image RyR clusters in rat cardiac myocytes. They observed that clusters internal to the cell are on average larger than those on the surface; however, both sets of clusters had on the order of 10 RyRs with an average of 13.1 RyRs per cluster for internal clusters and 10.1 RyRs per cluster for surface clusters. Jayasinghe et al. used a new DNA-PAINT microscopy method to achieve RyR imaging at around 10 nm resolution, enabling a fully quantitative analysis of RyR clusters; they reported an average of 8.81 RyRs per cluster with a mean nearest neighbor distance of 40.1 nm.
It is not completely clear why later studies have retrieved such different estimates of couplon size compared with those of Franzini-Armstrong et al., although it is at least clear that the early assumptions of a circular dyadic geometry and dense RyR packing is oversimplified and may explain this discrepancy. Based on agreement among more recent structural studies, and even earlier functional work, it is probably safe to conclude that the number of RyRs in the average CRU is on the order of tens rather than more than 100. Perhaps most importantly, it is now broadly appreciated that anchoring our understanding of cardiac EC coupling on the notion of an average dyadic structure is probably profoundly oversimplified.
Immunofluorescence (IF)-based colocalization studies have definitively shown that the LCCs present in VMs exhibit a punctate pattern of distribution and that around 90% of these puncta are coincident with RyR. , Thus the vast majority of LCCs are probably components of couplon structures. The number of LCCs within each couplon is less certain, as is the number of active LCCs required to trigger local Ca 2+ release during physiologic EC coupling. One range of estimates suggests that 17 to 53 LCCs are likely to be involved in couplon activation. Other studies, however, have observed higher coupling fidelities and therefore suggest that far fewer LCCs are required to trigger local SR Ca 2+ release. Part of the uncertainty here is because of a discrepancy in the estimates of RyR cluster size, which are required to extrapolate the LCC number in some studies. In addition, differences in the details of approach across studies, such as the potential ranges for LCC activation and partial pharmacologic inhibition of the LCC pool, have made it difficult to determine truly physiologically representative constraints for EC coupling. Localization of the myocardial Na + -Ca 2+ exchanger (NCX) is also worth mentioning at this point because its potential involvement in EC coupling is a long-debated topic that has recently been revisited. Although NCX does exhibit a punctate distribution in the T-tubules, precise colocalization of NCX with RyR occurs for only around 10% of the total NCX signal. , With this in mind, the latter authors also note that around 40% of NCX puncta reside within 150 nm of the nearest RyR cluster. Thus, although NCX is probably not selectively concentrated within the dyadic portion of the T-tubular membrane, it is likely to be nearby.
The protein exhibiting strongest colocalization with RyR is calsequestrin (CSQN). Ninety-five percent of all CSQN labeling is coincident with RyR, and only around 10% of RyR occur in the absence of CSQN. These observations are consistent with our understanding of CSQN function, which, through its ability to rapidly buffer Ca 2+ , acts to both limit the thermodynamic gradient that opposes SR Ca 2+ reuptake and provide a large local supply of Ca 2+ for RyR-mediated Ca 2+ release. It has also been shown to impose direct regulation of RyR gating. , Finally, localization of the sarco-endoplasmic reticulum Ca 2+ ATP-ase (SERCA2) is less clear than that of either RyR or CSQN. It is generally agreed that both the major (SERCA2a) and minor (SERCA2b) cardiac splice variants are present at the Z-disc and therefore in the vicinity of the jSR. Nevertheless, fluorescent labeling also appears to decorate the M-line SR, and no investigation to date has definitively demonstrated that SERCA2 exists within the same functional domain as RyR and CSQN. As described later, our quantitative approaches suggest that uncertainty in SERCA2 localization at this level may be functionally important, particularly with respect to Ca 2+ spark dynamics.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here