Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The world of imaging continues to change thanks to advances in the field of breast imaging. Improvements in diagnosis, imaging, computer-assisted detection, surgical approach, therapy, and staging, to name a few, have contributed to the growth of this important field. As the body of knowledge for breast cancer diagnosis and treatment expands, it is increasingly important for the various specialties involved in the care of breast cancer patients to understand what is happening in related fields so that effective communication can occur. The types of imaging tests and biopsy techniques available for breast cancer diagnosis and staging have undergone many changes in recent years This chapter highlights the current diagnostic modalities and biopsy techniques available to the radiologist. Special attention is placed on the appropriate use of the various techniques and the imaging appearances of the various pathological entities that the pathologist will see. It is important for the pathologist to understand the strengths and limitations of imaging and how imaging can complement pathology.
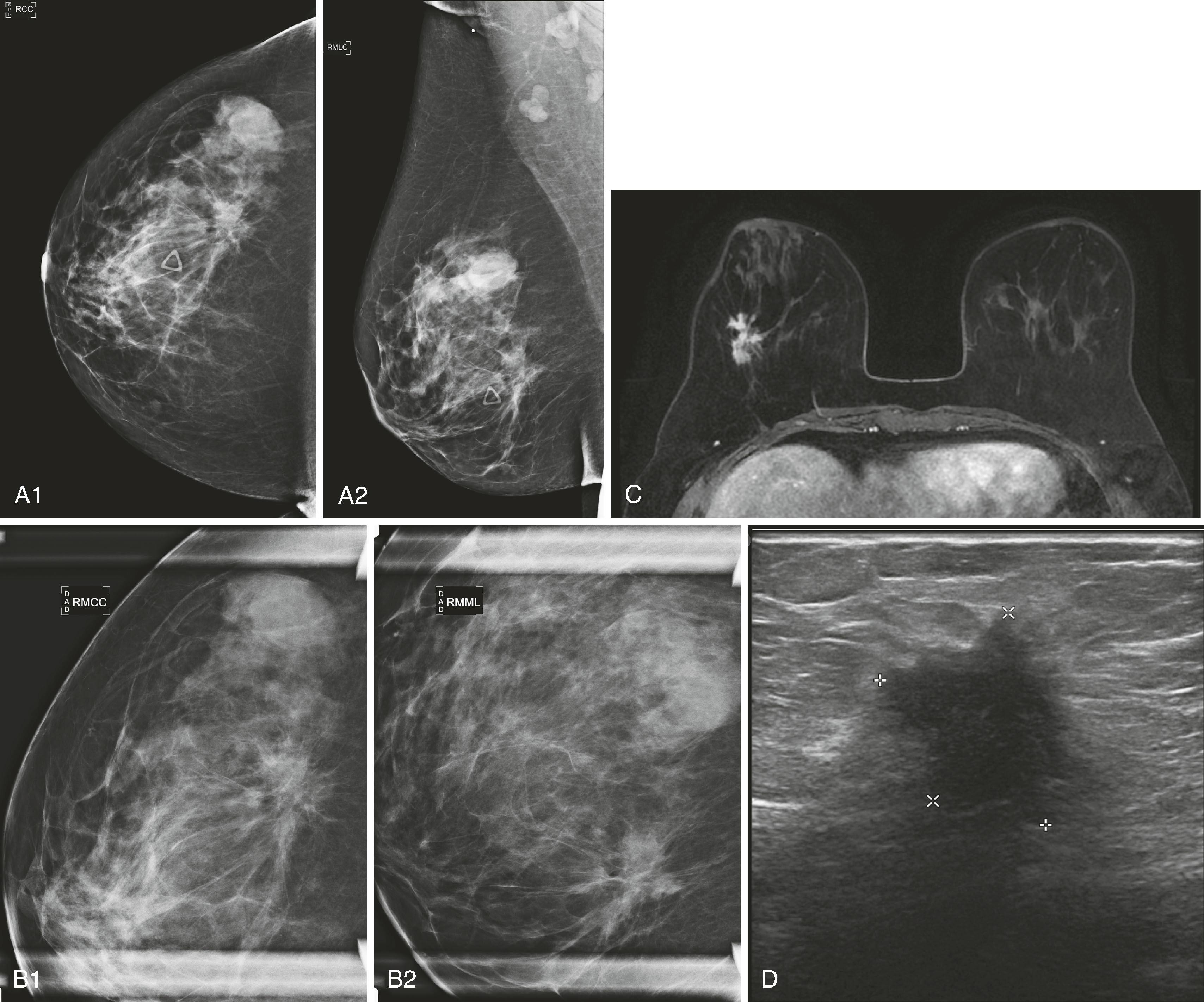
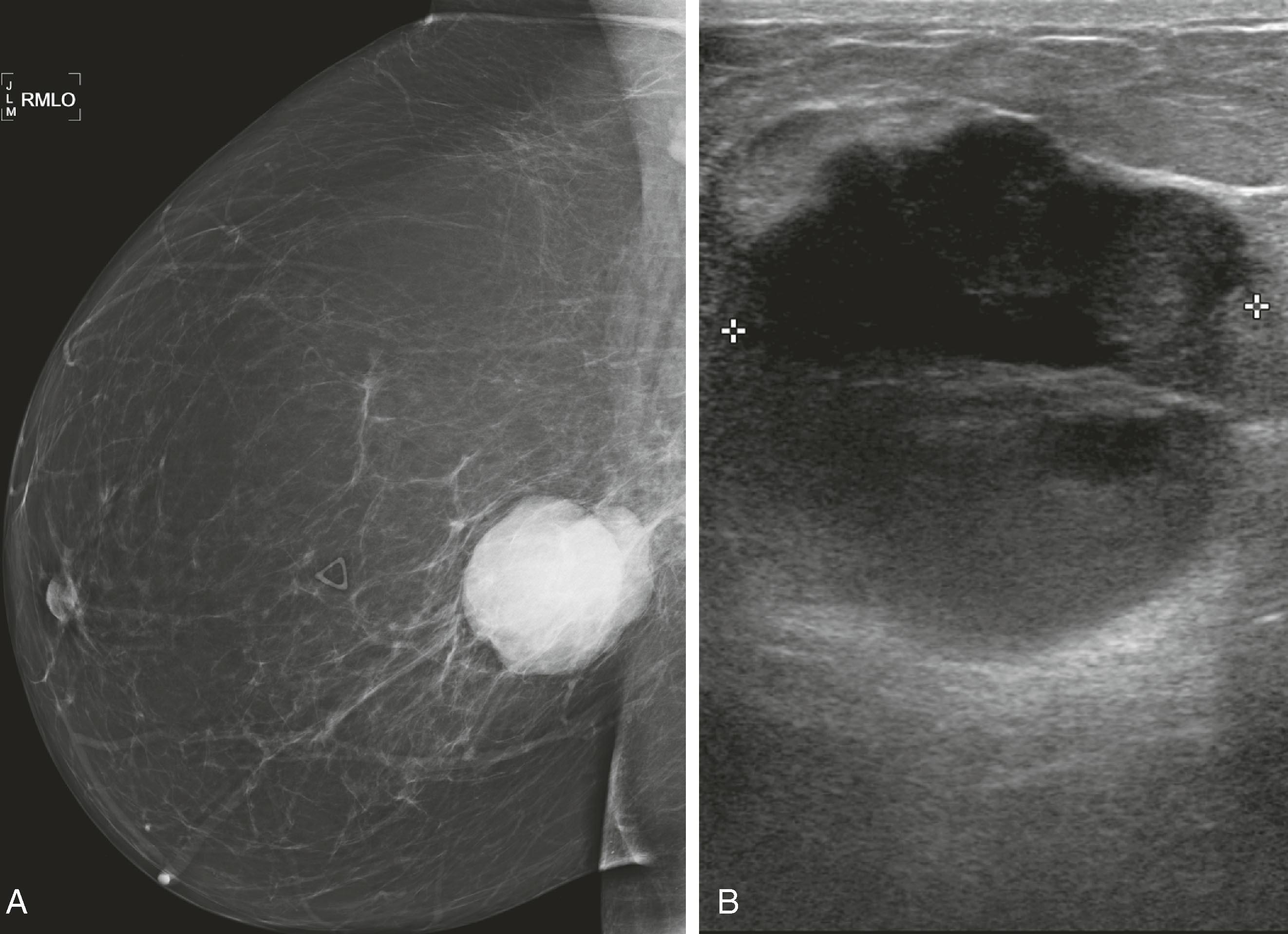
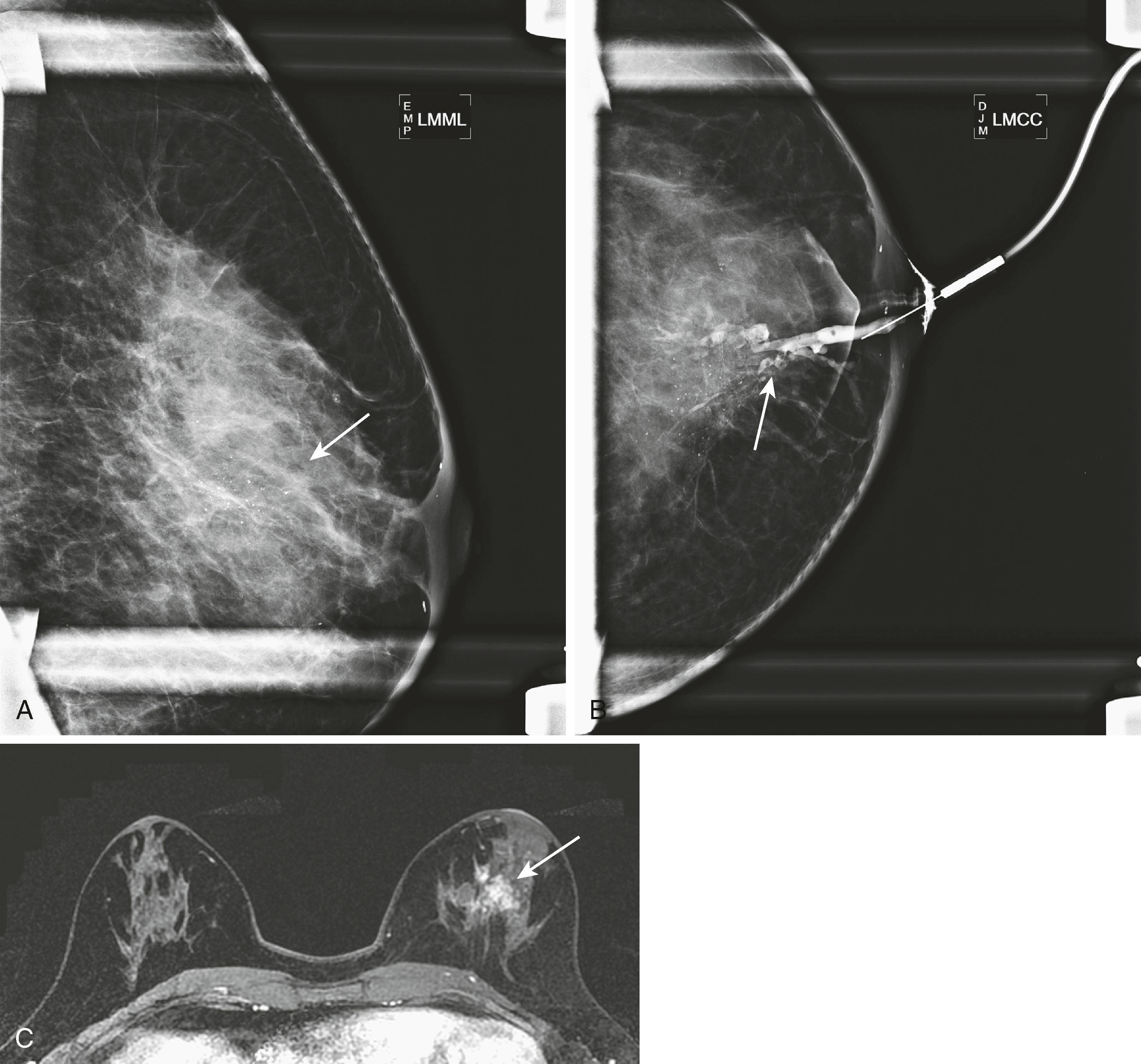
Mammography remains the most basic and important test in breast imaging. It has evolved from an analog technique to a digital one, but it is still typically the initial test that women will undergo in both the screening and diagnostic environments. There are ongoing controversies involving the age to initiate screening mammography, when to stop it, and at what intervals it should be used. Despite these controversies, all agree that some form of screening is important and improves survival by identifying cancer at an earlier and more treatable stage. Mammography is performed as both a screening test (asymptomatic women) and a diagnostic test (symptomatic women). When an abnormality is identified, it will be further evaluated by special mammographic techniques and other imaging modalities. Usually, the first additional modality to be used is ultrasound. Although there is new evidence to suggest that, in certain instances, ultrasound may be a valuable screening test, currently its primary use is to complement mammography. For example, when a woman has a palpable lesion that has no mammographic correlate, other tools become warranted. Fig. 8.1 shows a palpable abnormality in the right breast of a young woman. The mammogram is unimpressive; however, the ultrasound targeted to the area of interest demonstrates a densely shadowing lesion. Once a lesion is identified, the ultrasound can be used for guidance to perform a percutaneous biopsy. If a lesion is identified mammographically and is nonmass-like (e.g., calcifications), a biopsy could be performed with mammographic guidance (stereotactic biopsy). Fig. 8.2 shows new, indeterminate calcifications in a patient’s right breast. Special magnification views to further characterize the calcifications were performed, but they remained indeterminate by imaging criteria. Therefore, a mammography-guided stereotactic biopsy was performed. The diagnosis was ductal carcinoma in situ (DCIS), solid and cribriform, with moderate comedonecrosis.
Once a malignancy is defined by mammography and/or ultrasound, magnetic resonance imaging (MRI) may be performed to further define the extent of disease. In Fig. 8.1 , the MRI shows that the extent of the lobular cancer is considerably greater than expected by physical examination, mammography, and ultrasound. If the patient undergoes neoadjuvant chemotherapy, MRI is a way to follow tumor response.
On the day of surgery, wire localization or radioactive seed localization will be performed for nonpalpable lesions to assist the surgeon in finding and excising the tumor. If the lesion is an invasive cancer and there is no known axillary nodal disease, a sentinel lymph node injection will be performed so that the sentinel lymph node can be removed.
Depending on physical examination, the size of the tumor, the histology, and the appearance of the axilla, a metastatic workup will ensue before the anticipated surgery. This typically consists of computed tomography (CT) or positron emission tomography (PET)/CT of the chest, abdomen, and pelvis, and a bone scan if a plain CT is performed. If the patient is going to receive cardiotoxic chemotherapy, she will typically undergo a multigated angiogram (MUGA) scan to ensure that left ventricular function is normal.
Mammography, developed in the 1930s, has experienced progressive refinements in image quality and reduction in radiation dose because of improvements in the equipment generating X-rays and the receptors to display images, resulting in high-resolution and high-contrast radiographic images of the breast. Mammographic images are produced either digitally (digital mammography [DM]) and displayed on a high-resolution monitor, or directly onto special mammography film (film screen [FS] mammography).
For most women, the radiological workup begins with a mammogram. The American Cancer Society guidelines for evaluation of average-risk individuals include an annual mammogram beginning at age 40 years. Other groups advise beginning at age 45 or 50, annually or biennially, but annual mammography beginning at age 40 results in the greatest mortality reduction, allowing detection of tumors at smaller sizes and earlier stages. Mammography screening should begin earlier in patients who are BRCA mutation–positive or if there is a history of breast cancer in a first-degree relative younger than 50 years. Symptomatic women presenting with abnormalities, including a palpable lump, erythema, skin or nipple retraction, arm or breast swelling, or discoloration, will also begin with the standard mammographic views but will require additional mammographic views, ultrasound, or both.
To achieve a high-quality image, the skilled mammography technologist must include as much breast tissue as possible in the field of view and compress the breast firmly against the X-ray receptor plate, reducing the chance for motion blurring. The standard mammogram consists of a craniocaudal (CC) and a mediolateral oblique (MLO) view of each breast. The MLO view is used instead of a true lateral view to include the axillary tail of the breast and as much of the axilla as possible. The CC image on film is viewed rotated with the lateral side up and with the CC label on the lateral aspect of the breast ( Fig. 8.3 ).
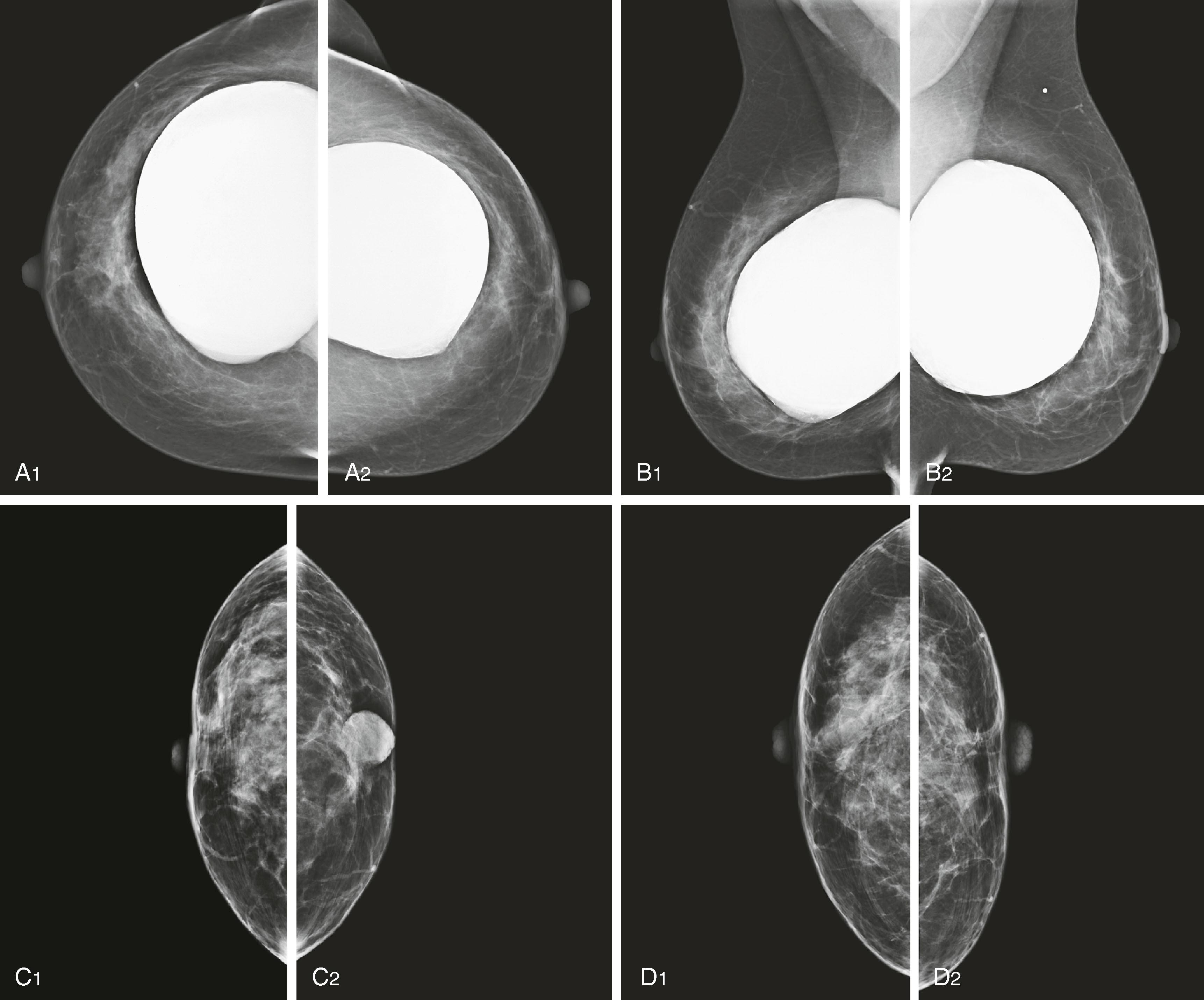
Owing to considerable variation in the mammographic appearance of women’s breasts, the detection of malignancy may be challenging, and comparison with prior mammograms is often critical to accurate interpretation of the current examination. The interpreting radiologist must meet experience and education requirements mandated by the federal government in accordance with the Mammography Quality and Standards Act (MQSA).
The density of breast tissue refers to the amount of glandular tissue relative to the amount of fat and has been classified into four groups: fatty, scattered, heterogeneously dense, and extremely dense. The more glandular the breast tissue is, the more likely a noncalcified lesion will be obscured by the dense tissue on one or both views. The topic of breast density is currently being addressed by legislation in multiple US states requiring that patients be notified of their breast density.
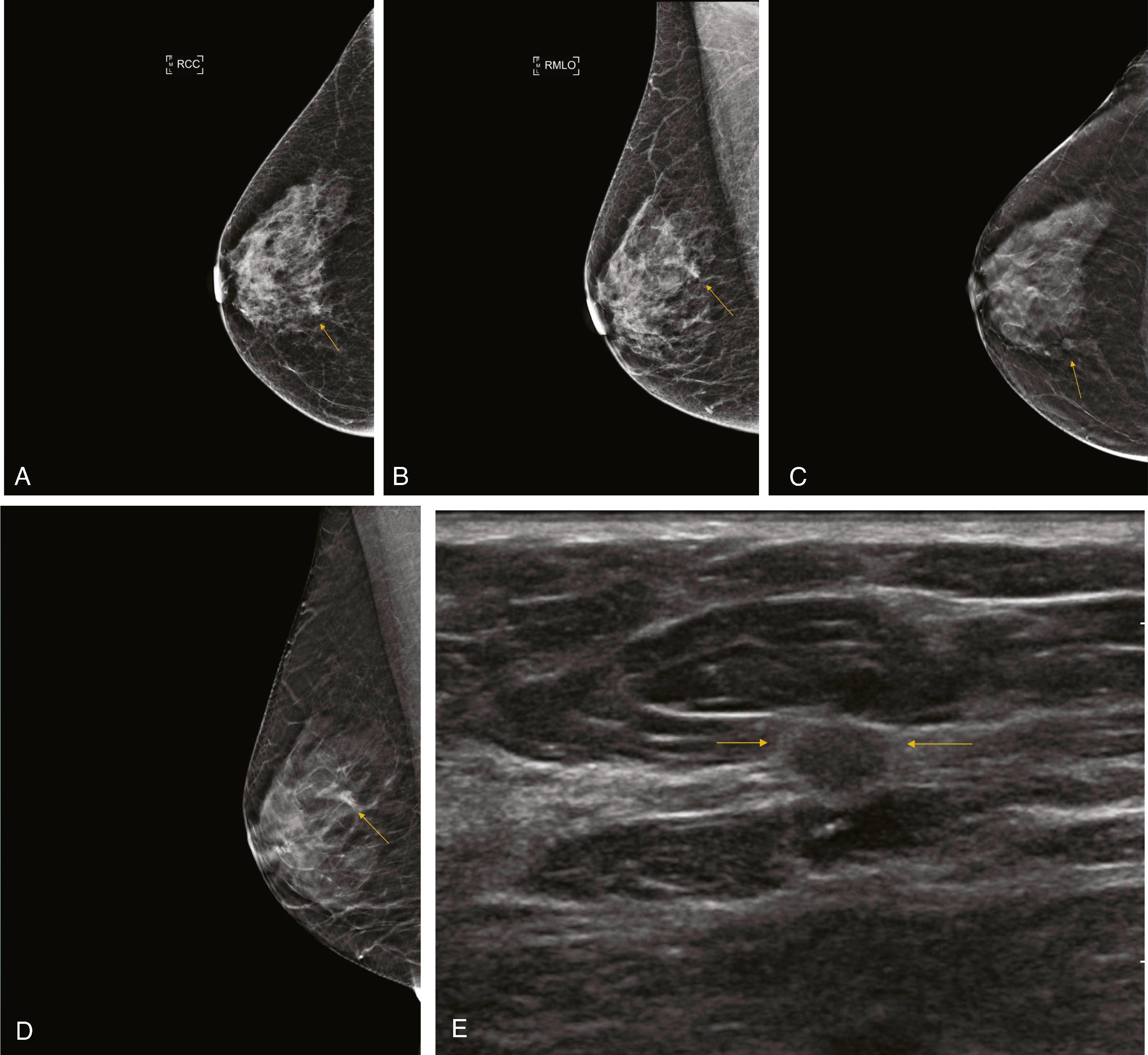
Similar to computer-aided Papanicolaou (Pap) smear analysis, computer-aided detection (CAD) has been used to aid radiologists in identifying abnormalities detected by mammography. The programs are designed to locate and highlight masses and calcifications with suspicious/indeterminate features. Some CAD programs attempt to assign a likelihood of malignancy by varying the size of the mark or providing a percentage depicting level of concern. The sensitivity and specificity of the CAD algorithm may be adjusted with the goal of assisting the radiologist in detecting malignancy without unnecessarily increasing the false positives ( Fig. 8.4 ).
![Fig. 8.4, ( A1 – A4 ) Diffuse bilateral benign secretory calcifications are present (arrow on linear calcification) [ A3 ]). ( B ) Computer-aided detection images have numerous marks, all denoting benign stable findings ( arrowheads , calcifications; asterisks , masses). Fig. 8.4, ( A1 – A4 ) Diffuse bilateral benign secretory calcifications are present (arrow on linear calcification) [ A3 ]). ( B ) Computer-aided detection images have numerous marks, all denoting benign stable findings ( arrowheads , calcifications; asterisks , masses).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/BreastImagingModalitiesforPathologists/1_3s20B9780323795227000084.jpg)
Facilities may perform either full-field (whole breast) digital mammography (FFDM), digital breast tomosynthesis (DBT), FFDM and DBT, or analog FS mammography. In the United States, almost all facilities have converted to digital mammography, and a large number have added DBT. The diagnostic accuracy of digital and FS imaging was studied in the Digital Mammographic Imaging Screening Trial (DMIST) conducted by the American College of Radiology Imaging Network (ACRIN). The DMIST study concluded that whereas both methods produce a mammogram of diagnostic quality, FFDM performed better for premenopausal and perimenopausal women younger than 50 years and for women of all ages with dense breast tissue.
A distinct advantage of FFDM systems is the speed at which the images are obtained and the ability of the radiologist to manipulate the images at the workstation. Digital mammography tends to shorten the length the examination, with a decrease in the number of additional images needed to complete the evaluation, but is more expensive than FS.
DBT, or 3D tomography, is essentially FFDM with the ability to examine the breast with thin (1-mm) sections. By removing overlapping structures, DBT helps to differentiate true lesions from summation densities and delineate the margins of lesions, which may help increase the specificity of mammography (e.g., the differentiation between benign and malignant lesions). In some studies, DBT has been shown to increase the sensitivity of mammography for finding additional breast cancers, and in others has been shown to decrease the recall rate in screening mammography by approximately 30% to 37% when used in combination with standard 2D mammography. The DBT machine is essentially a modified mammography unit with a moving X-ray tube; the digitally acquired data from several low-dose angled views is reconstructed to allow the imager to scroll through 1-mm slices of the breast, allowing superior visualization of subtle lesions and additional information about the location of a lesion. Recent software advances and subsequent US Food and Drug Administration (FDA) approval now allow the reconstruction of these data into a “synthetic 2D” mammographic image that can replace the standard 2D mammogram, reducing the radiation dose to the patient.
The mammographic abnormalities that may be seen as a manifestation of malignancy include microcalcifications, masses, architectural distortion, asymmetrical or developing densities, edema of the breast or skin, and axillary lymphadenopathy.
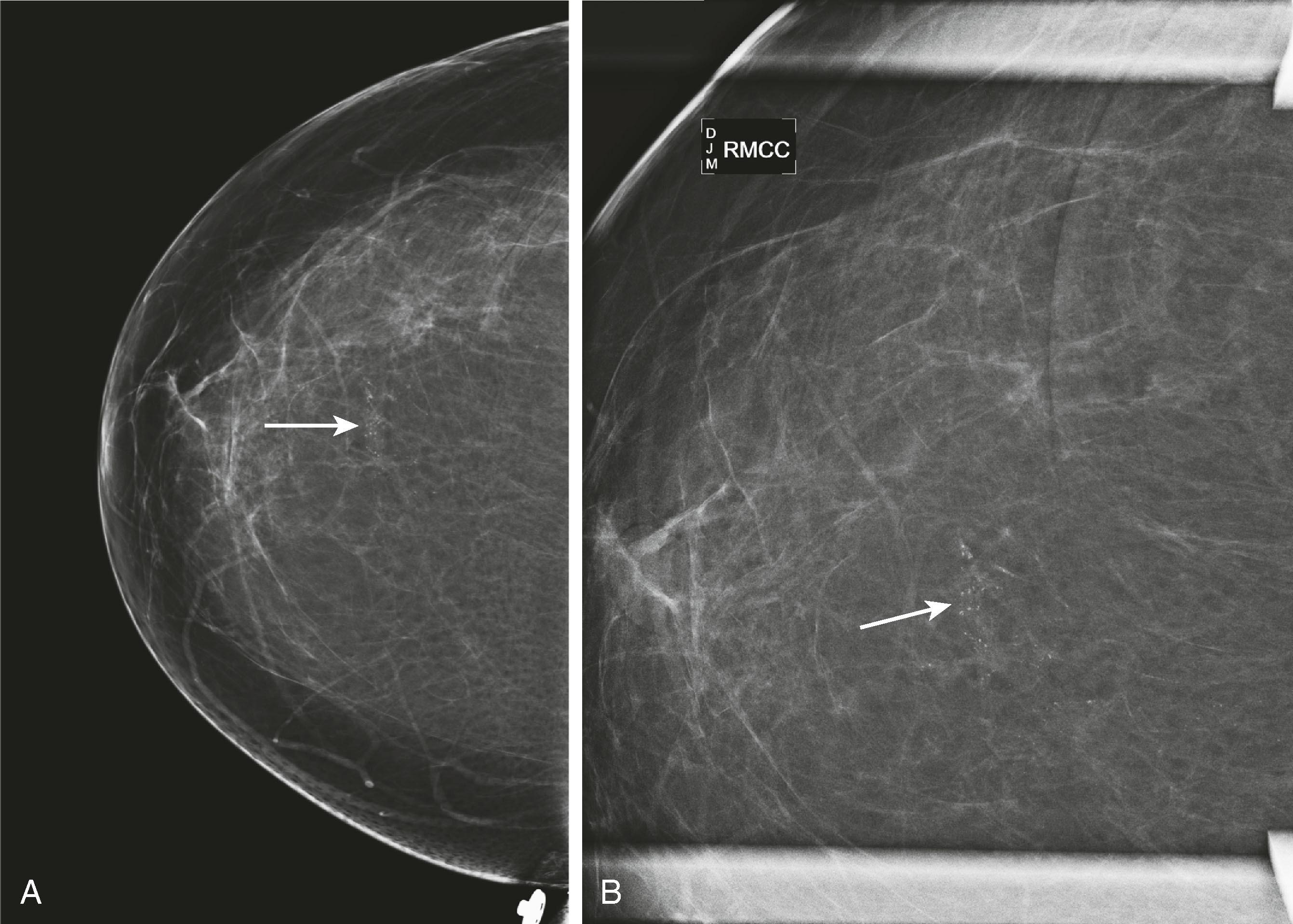
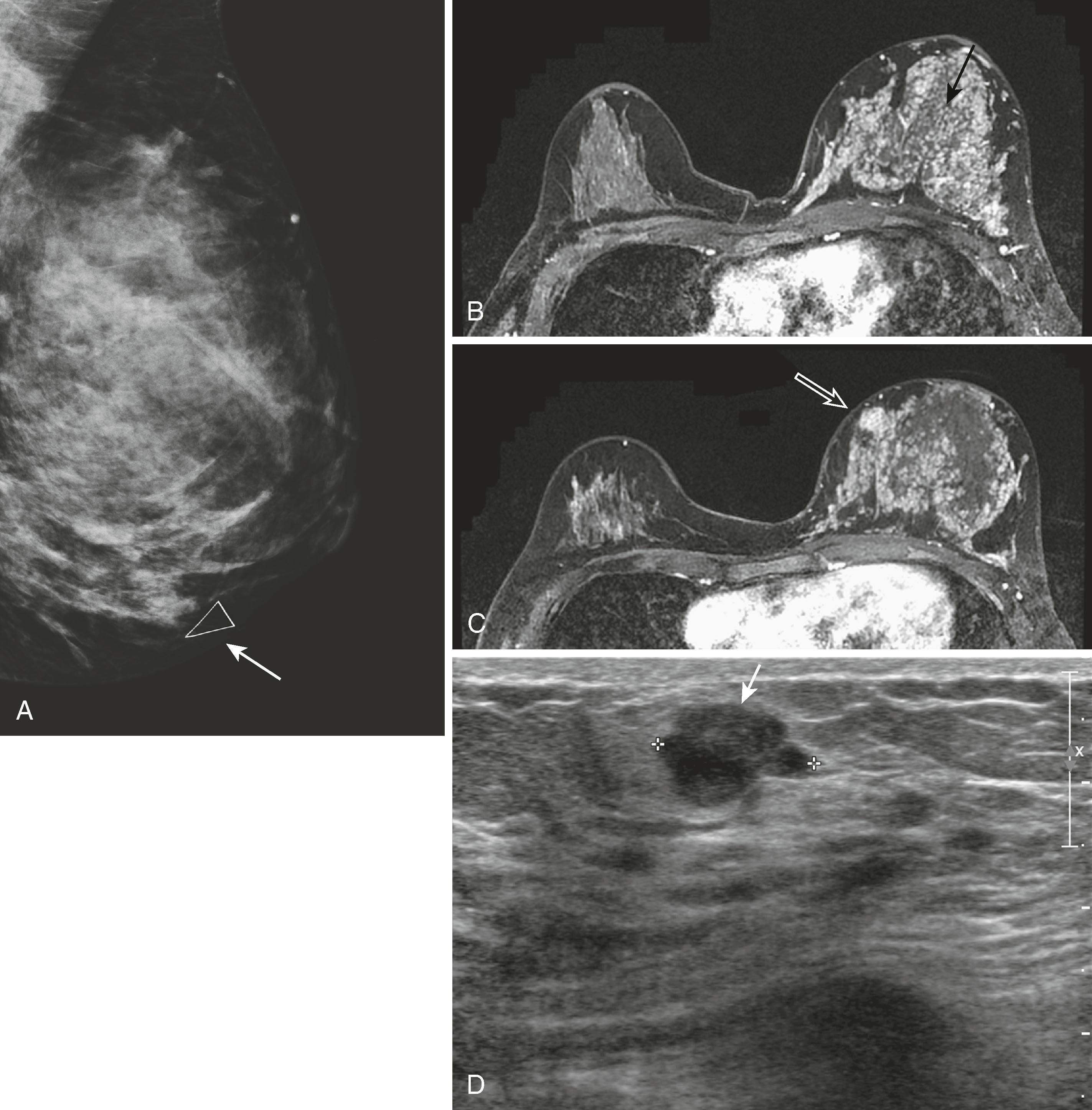
Microcalcifications can be associated with benign and malignant processes. Pleomorphism, linear shape and orientation, and clustered or segmental distribution of calcifications suggest malignancy, whereas a more diffuse distribution and rounded shape favor a benign etiology. Magnified views in the CC and ML (true lateral) projection are performed to more accurately assess their morphology. Certain features of calcifications such as central lucency or meniscal layering are classic for benign disease, but often the calcifications cannot be categorized as benign or malignant and are reported as “indeterminate.” Stereotactic biopsy is recommended for suspicious calcifications and usually for indeterminate calcifications, whereas 6-month follow-up mammography is advised for those thought to be probably benign ( Fig. 8.5 ).
Masses are evaluated mammographically with spot compression, spot-magnified views, or tomosynthesis to better visualize their margins. Smooth margins suggest a benign etiology, whereas irregular, microlobulated, ill-defined, or spiculated margins suggest malignancy. Large, coarse calcifications in a well-defined mass are typical features of a fibroadenoma ( Fig. 8.6 ).
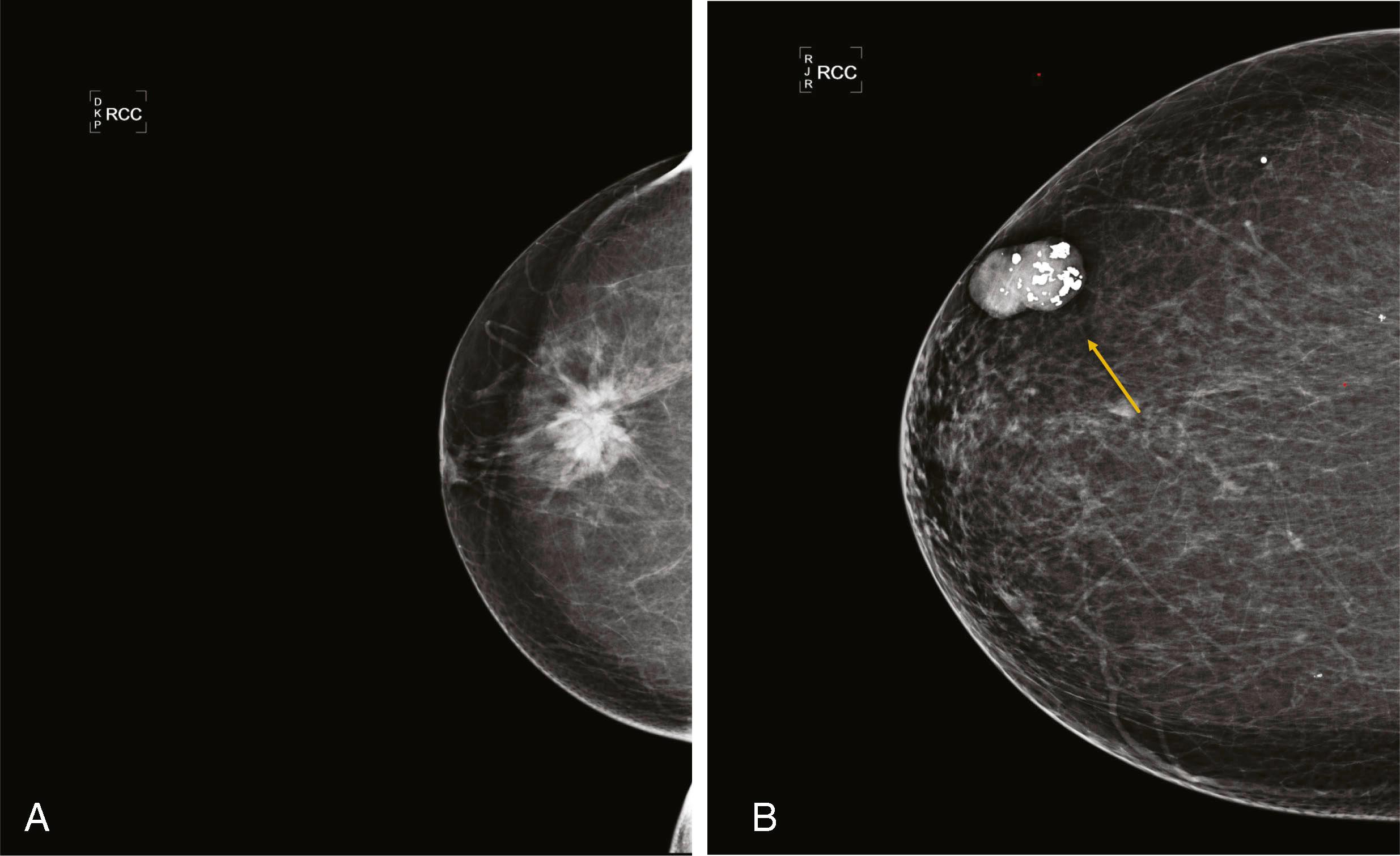
Carcinomas are rarely well defined but frequently are very dense for their size. Phyllodes tumors are well defined and often become very large.
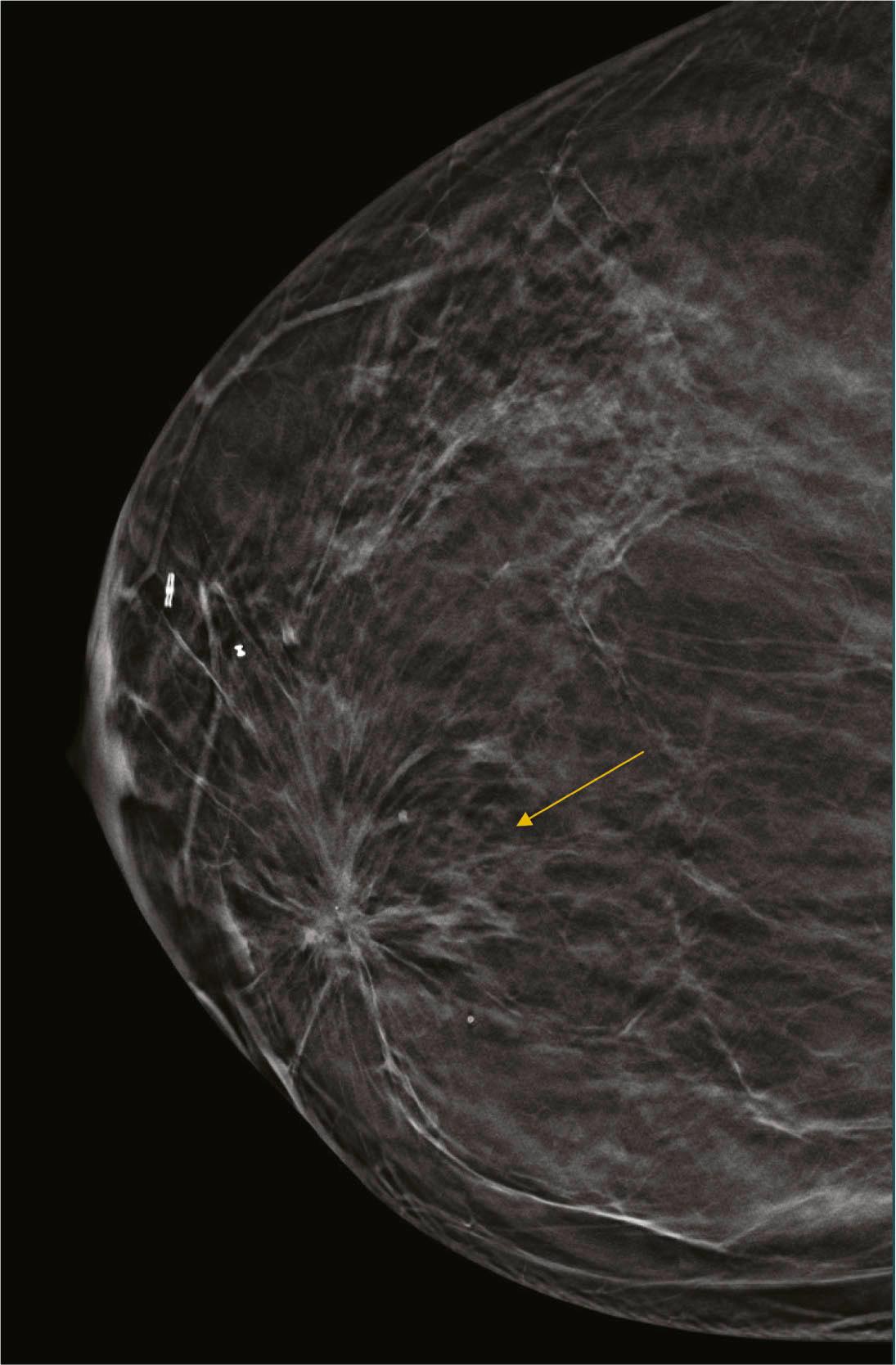
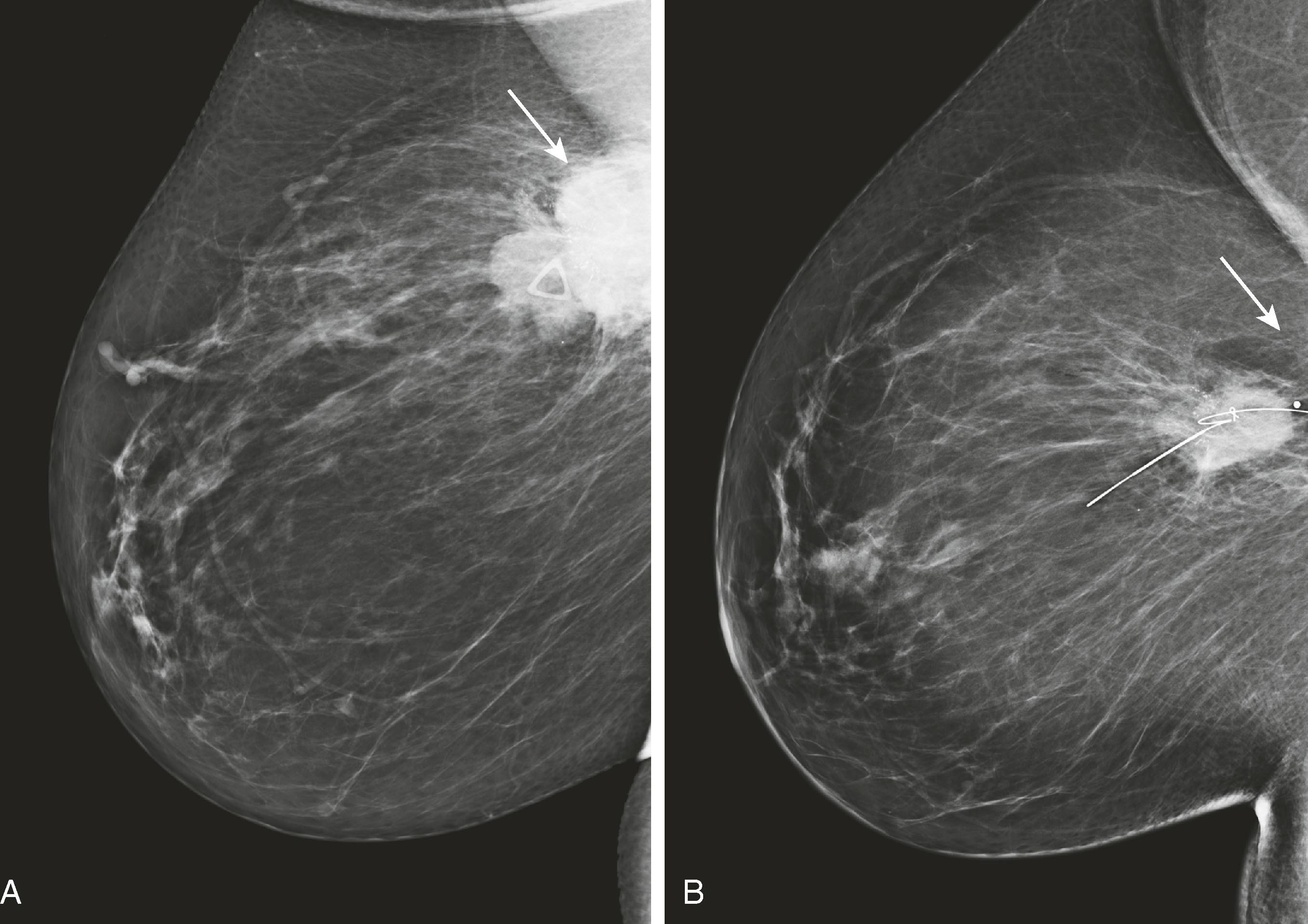
Architectural distortion is defined as disturbance of the normal architecture without an apparent mass. This appearance is most commonly the result of a surgical scar but can be attributable to a subtle invasive cancer, DCIS, radial scar, and other entities. Magnified views or tomosynthesis will better demonstrate the distortion and may reveal a subtle mass. Biopsy should be performed unless the area is shown to correspond to a surgical scar ( Fig. 8.7 ).
Asymmetrical densities , now called asymmetry and focal asymmetry , are sometimes attributable to malignancy but more often represent areas of glandular tissue. These are evaluated by comparison with old studies; spot compression views; angled or rolled views, often with tomosynthesis; and ultrasound. Persistence on multiple views and change from prior study suggest a true mass, which will be evaluated with ultrasound ( Figs. 8.8 and 8.9 ).
Edema of the breast and skin can be caused by radiation, mastitis, inflammatory carcinoma, or lymphatic obstruction. Lymphadenopathy may be visible on the mammogram if the axilla is visualized. Abnormally large, dense nodes may be reactive, metastatic, or attributable to lymphoma.
Establishing the size of a lesion as well as the multiplicity of lesions is important in planning definitive therapy. Other modalities such as ultrasound and MRI can be helpful in evaluating the true extent of disease once a diagnosis of malignancy is made.
Mammography is important not only in the diagnosis of breast cancer but also in the surveillance and assessment of neoadjuvant chemotherapy ( Fig. 8.10 ).
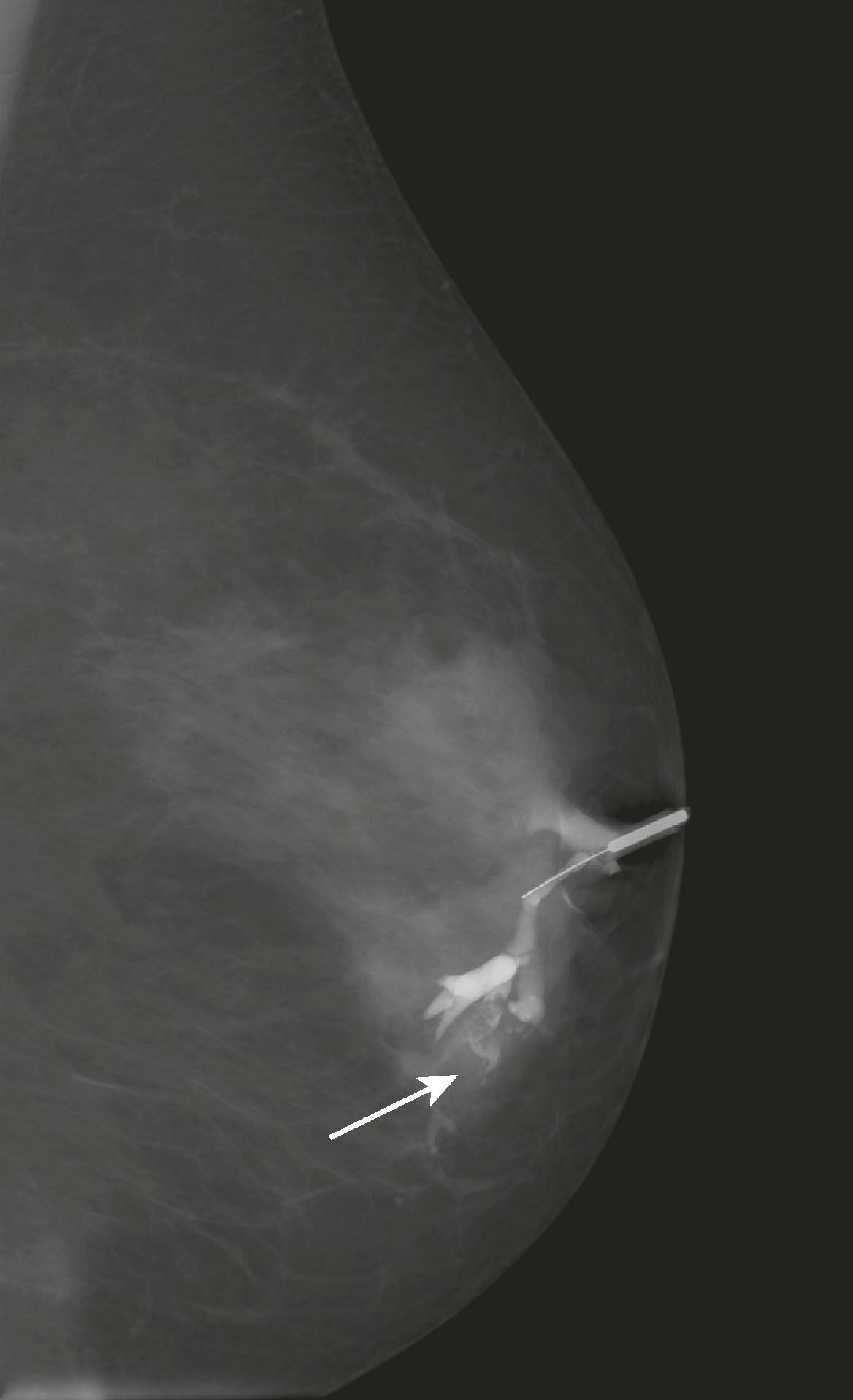
Ductography is performed to evaluate nipple discharge of clinical concern (i.e., bloody or clear to golden discharge from a single duct) to detect an intraductal pathology that is too subtle to be seen on mammography or ultrasound. A papilloma is often seen as a retroareolar lobulated filling defect, whereas DCIS may appear as a narrowed, irregularly marginated duct. Ductography is performed by cannulating the discharging duct with a 30-gauge sialogram cannula and injecting the duct with a small amount of contrast material. Mammogram views are performed with the cannula in place. The contrast opacifies the duct so that an intraluminal lesion will be visible ( Fig. 8.11 ). To identify and cannulate the proper duct, the radiologist must be able to express the discharge.
Screening for asymptomatic women, age of onset is controversial, 40 versus 50 years.
Diagnostic mammogram for lesions found on screening or for symptomatic patients.
CAD programs for assisted detection.
Digital (FFDM) performs better than analog (film based).
Calcifications classified as benign, indeterminate, or suspicious.
Breast ultrasound has become an important adjunct to mammography for the detection and diagnosis of breast cancer and for the evaluation of palpable breast masses. Ultrasound technology has advanced with improvements in image quality and resolution, expanding from its initial role in distinguishing cysts from solid masses to its current use in solid mass characterization and biopsy guidance. Ultrasound images are created by the reflection of sound waves emanating from the ultrasound transducer, from the interfaces in the tissue being insonated that return to the transducer and are converted into a grayscale image based on the distance of the reflected interfaces (echoes) from the transducer. The use of sound waves rather than X-rays allows this modality to be used freely in young patients and pregnant women.
In most patients older than 30 years, imaging will begin with mammography, and ultrasound will be used to evaluate a mass seen or suspected on the mammogram or to evaluate a palpable mass that cannot be visualized mammographically. In the case in which a well-defined mass is definitely present on the mammogram, sonography will usually enable the radiologist to determine whether or not it is fluid filled (a cyst or a seroma) or solid. In cases of uncertainty, ultrasound can be used to guide aspiration or biopsy of the lesion. If the lesion is solid, the shape, orientation, internal echogenicity, sound transmission features, vascularity, and margins of the lesion are evaluated to aid in determining the need for biopsy. The decision to biopsy versus follow with imaging surveillance will also be influenced by the risk status of the patient and the presence of other masses. If the abnormality on the mammogram cannot be seen on two orthogonal views, ultrasound will be used to help determine whether it is a true lesion or an area of glandular tissue appearing to be a lesion. If a true mass is present but hidden by glandular tissue on one or both orthogonal mammographic views, it can most often be found with sonography.
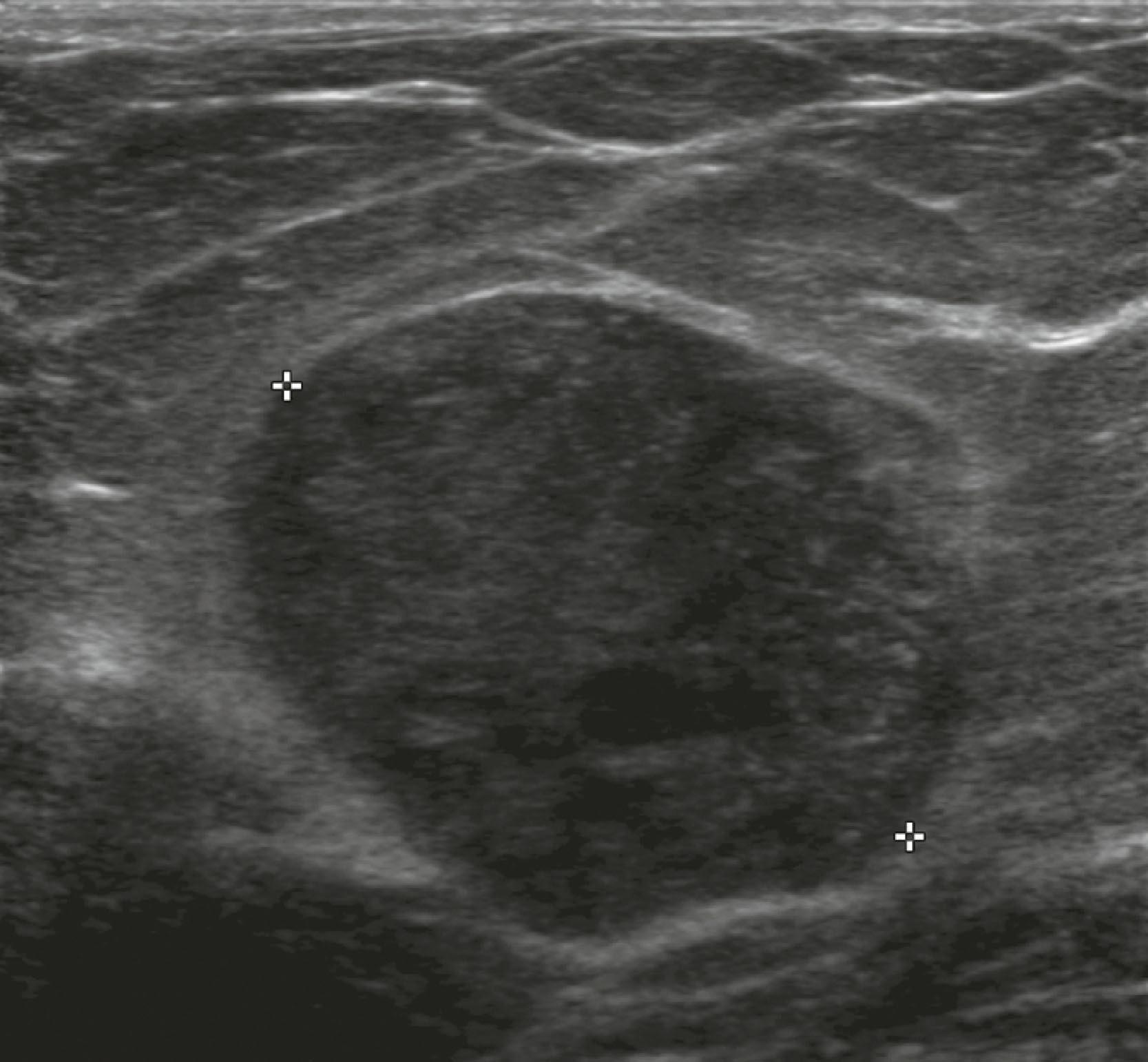
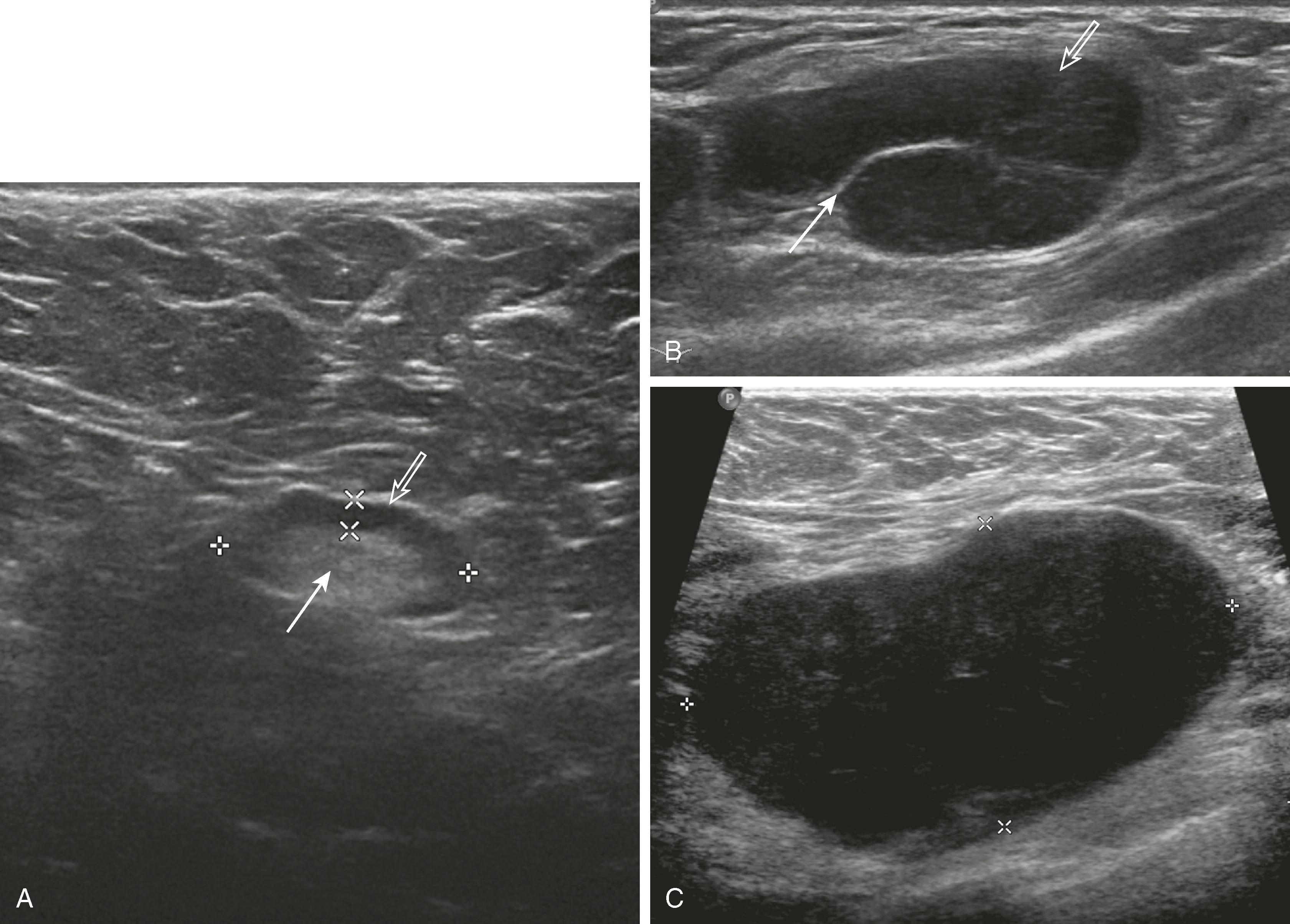
Sonographic features of simple cysts include well-defined margins, anechoic contents, and acoustic enhancement (increased sound transmission through the fluid compared with the surrounding tissue) ( Fig. 8.12 ). Simple cysts are rarely aspirated except for relief of patient symptoms, and the fluid obtained is often discarded rather than sent for cytological evaluation. However, some cysts contain internal echoes and are aspirated or undergo biopsy percutaneously to determine whether they are indeed complicated cysts or solid masses ( Fig. 8.13 ). The aspirate cytology of these complicated cysts does not always contain ductal epithelial cells, leading the pathologist to report the sample as suboptimal. A complex cyst (now referred to as a complex cystic and solid mass) is one that contains a mass or has complex internal architecture and will undergo biopsy of the noncystic portion, which may represent an intracystic papilloma or carcinoma ( Fig. 8.14 ).
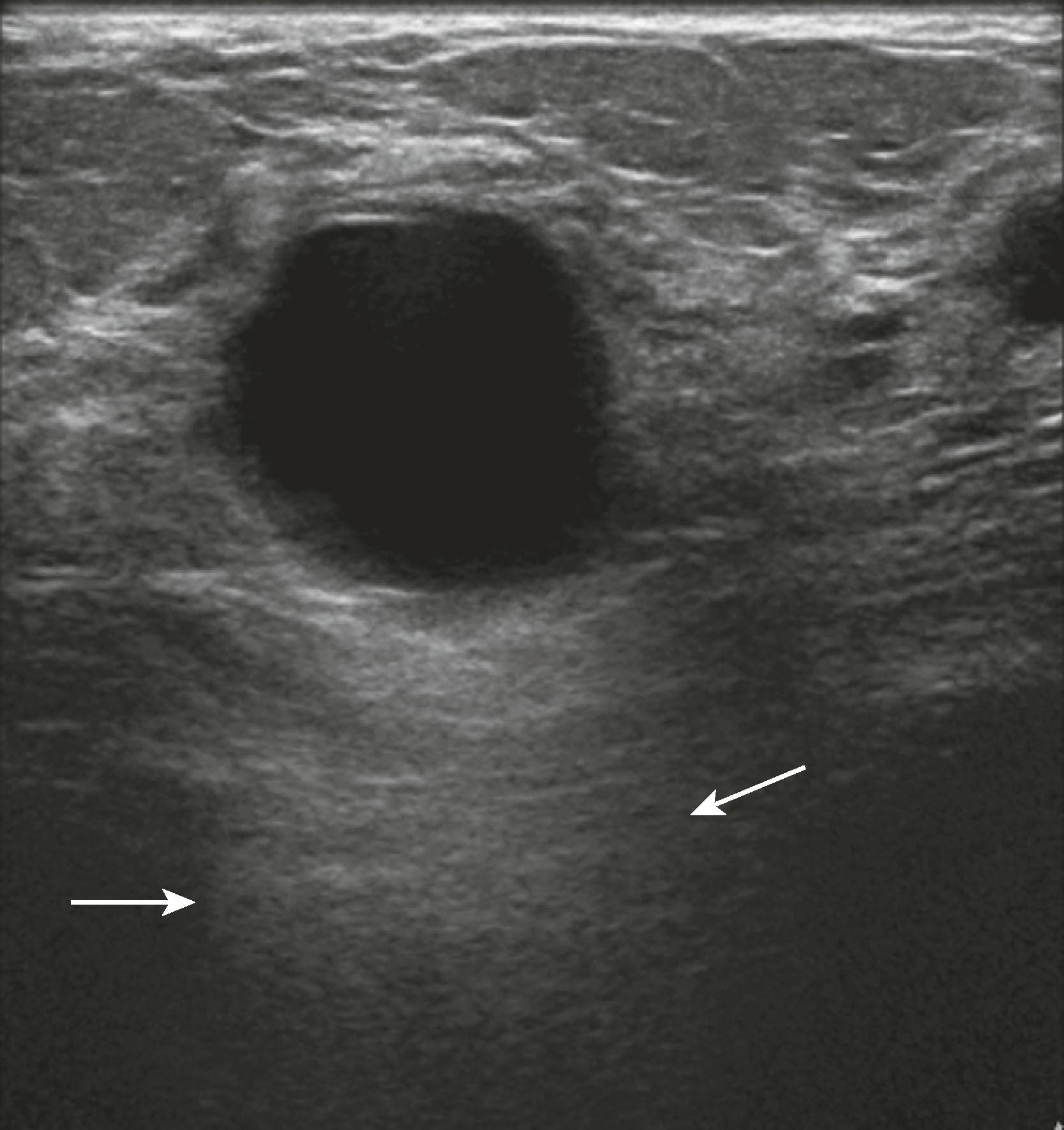
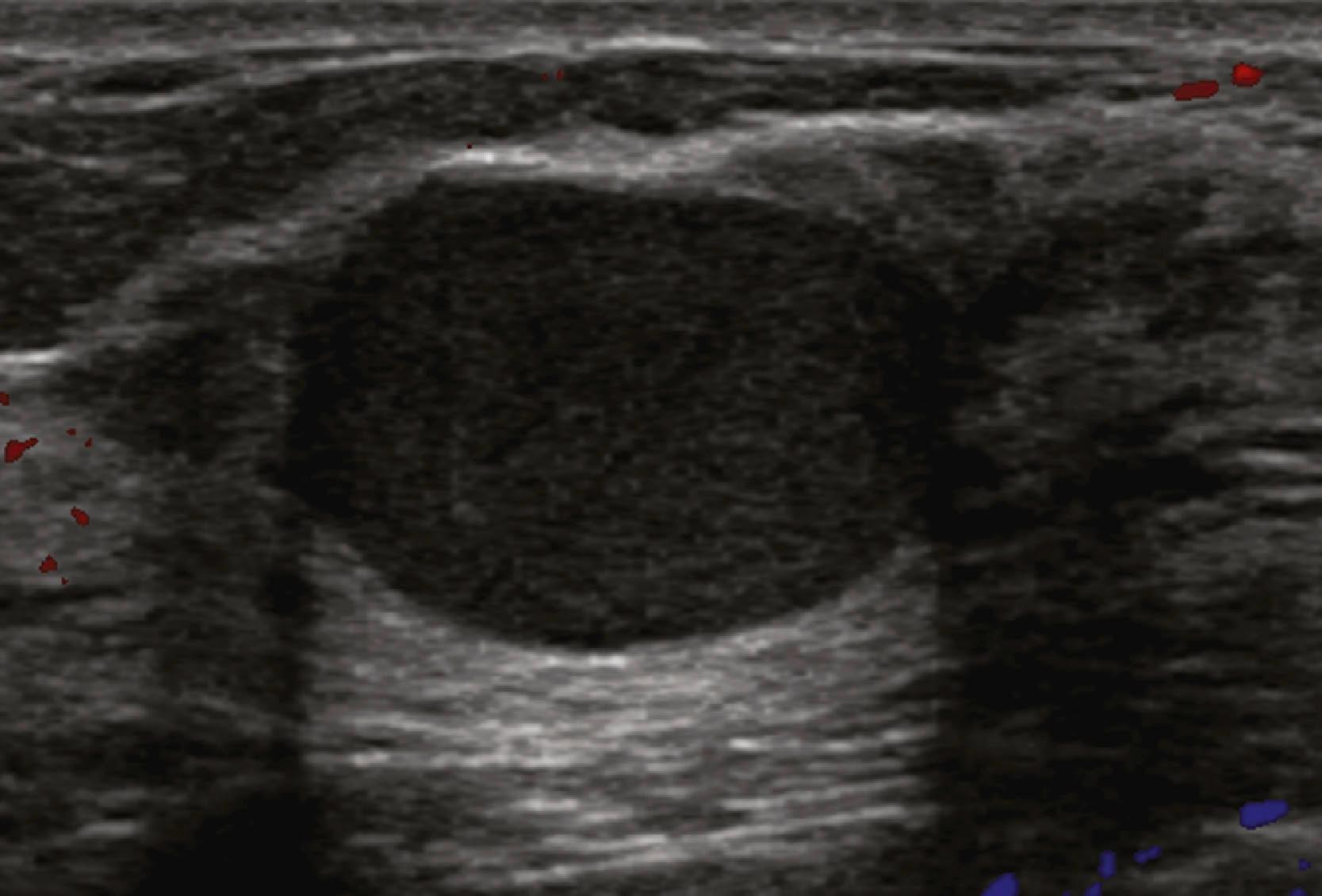
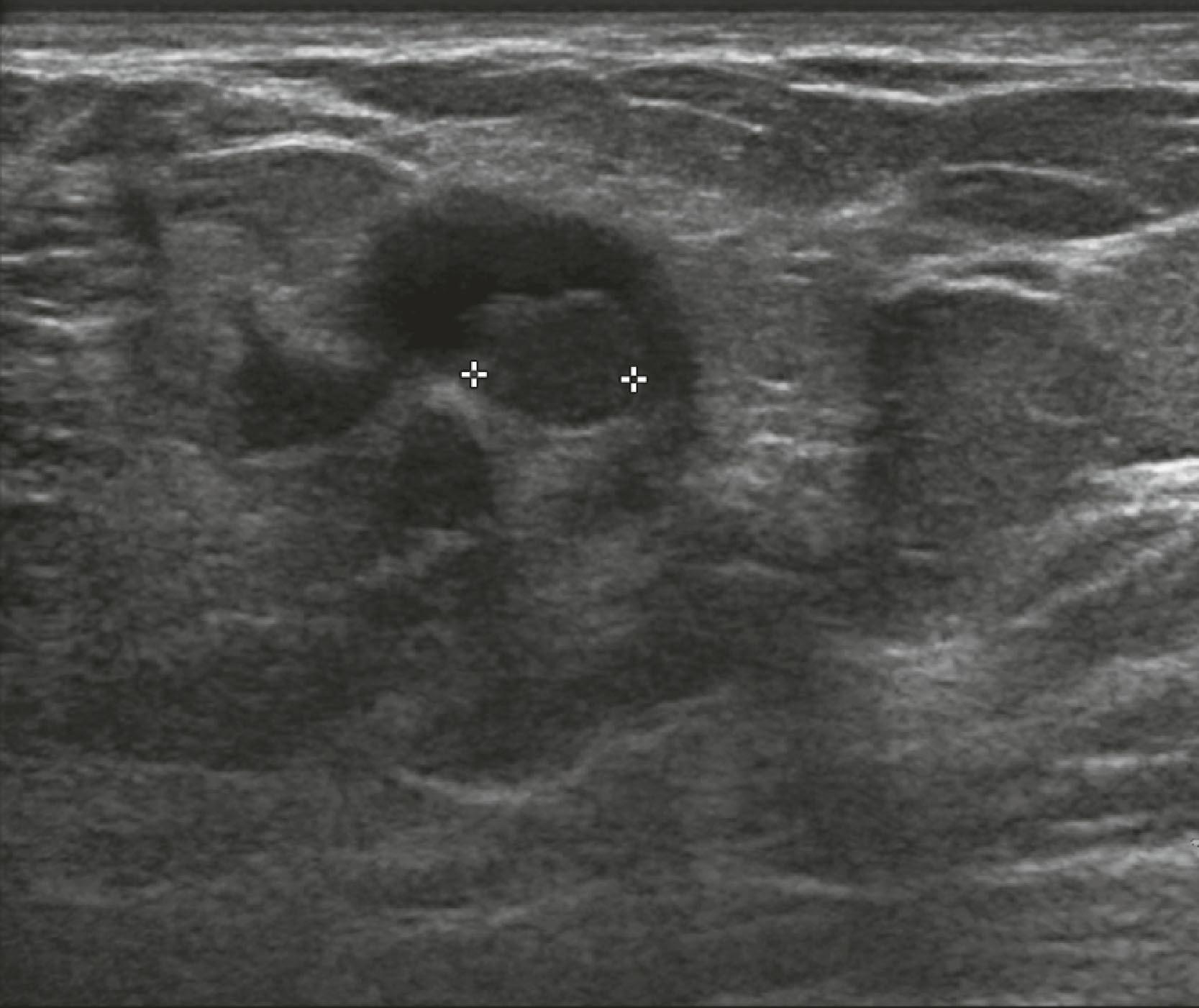
Sonographic features of benign masses, originally reported by Stavros and coworkers, include three or fewer gentle lobulations, orientation parallel to the chest wall, and a thin echogenic pseudocapsule, along with absence of any malignant features. Other features favoring a benign etiology are oval shape, circumscribed borders, and uniform hyperechogenicity ( Fig. 8.15 ). However, many benign-appearing masses undergo percutaneous or excisional biopsy because they are new or enlarging and cannot be unequivocally distinguished from well-circumscribed malignancies ( Fig. 8.16 ). A very large but well-defined mass is suspect for a phyllodes tumor ( Fig. 8.17 ).
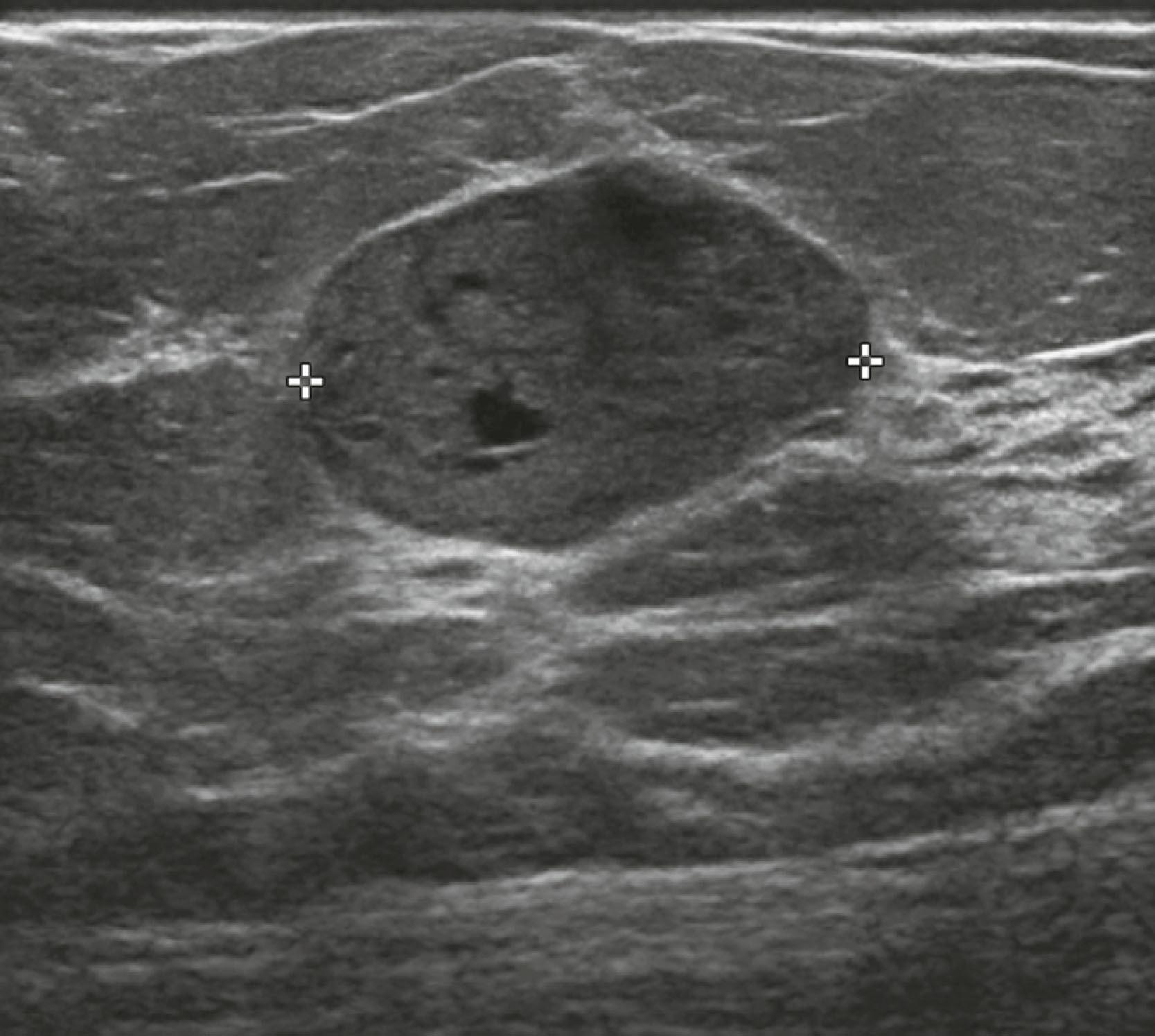
If a mass has suspicious features on mammography, ultrasound will be performed to further characterize it, to guide percutaneous needle biopsy, and to assess for additional disease in the breast and axilla. Sonographic features associated with invasive carcinoma are irregular shape, marked hypoechogenicity, ill-defined or angular margins, anteroposterior diameter greater than transverse diameter (taller than wide), and acoustic shadowing (greater attenuation of sound by the lesion compared with surrounding tissue, allowing less sound to pass through it) ( Fig. 8.18 ). The appearance of a surgical scar can mimic malignancy, so careful correlation with the mammogram, physical examination, and history is essential ( Fig. 8.19 ).
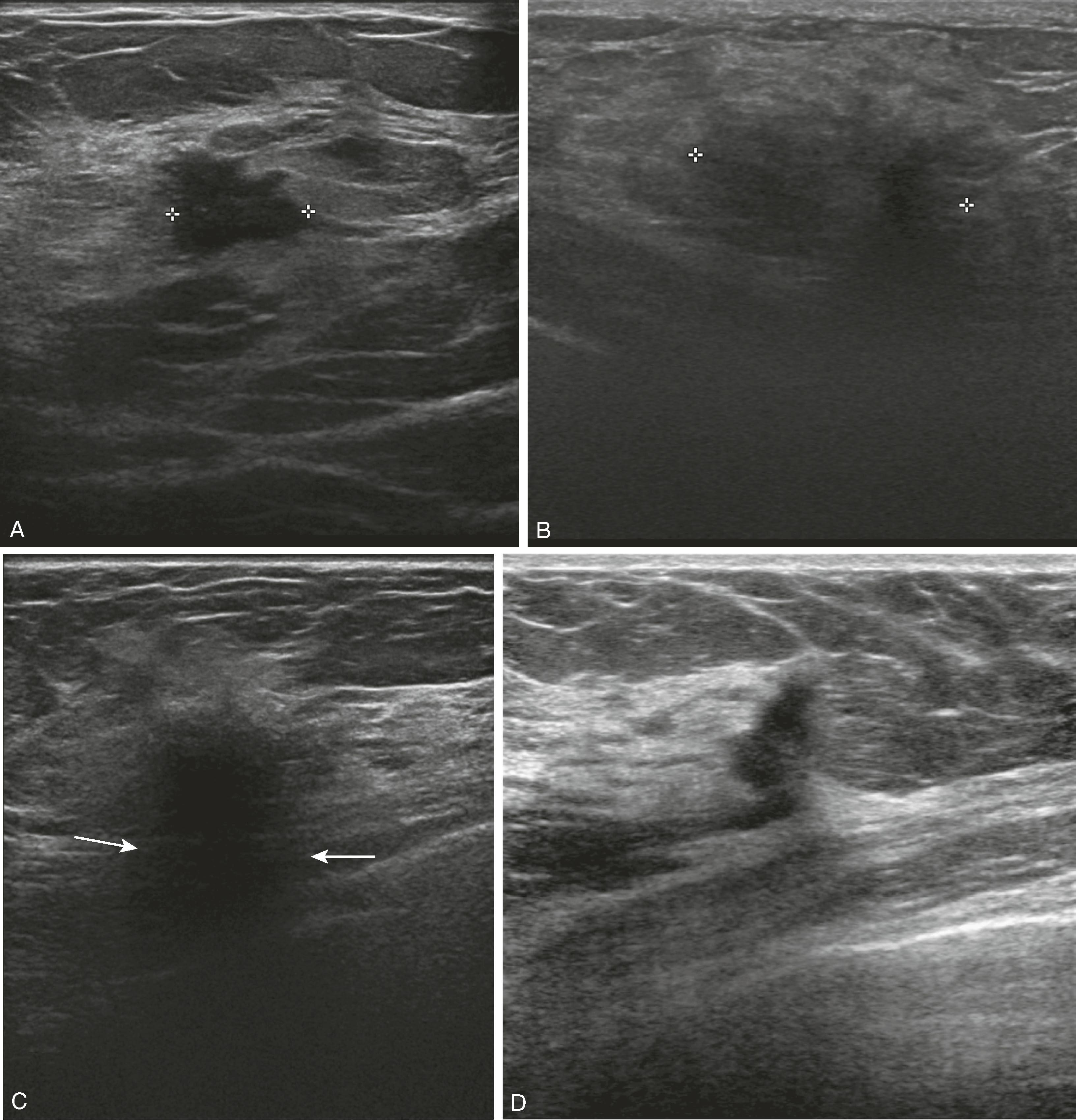
Ultrasound is ideal for evaluation of suspected breast infection because the patient’s breast is often too tender and swollen to tolerate the compression required for mammography. If an abscess ( Fig. 8.20 ) is identified, it can be drained with ultrasound guidance, and if there is any question of the area representing a necrotic tumor, its wall can be sampled with ultrasound guidance for pathological evaluation.
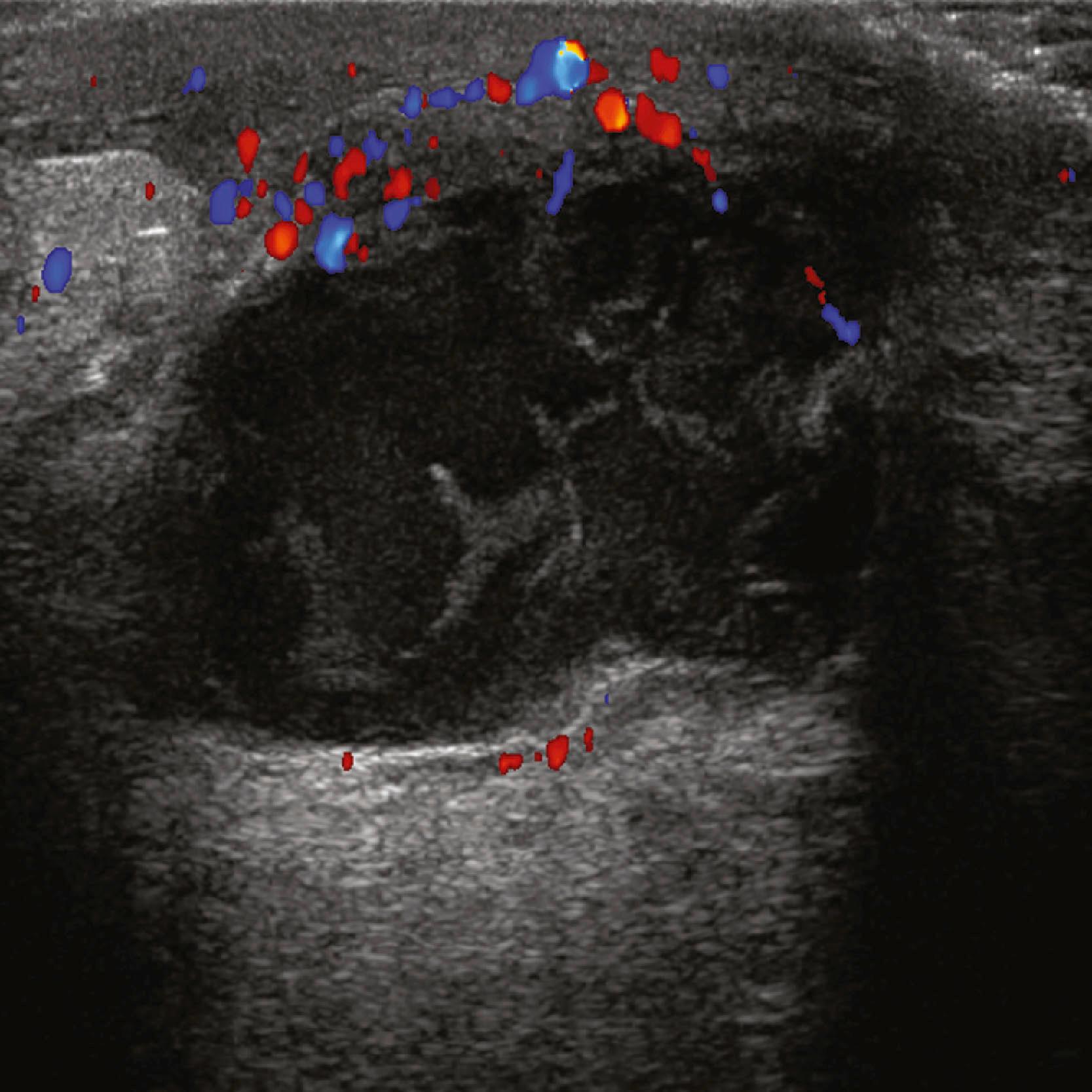
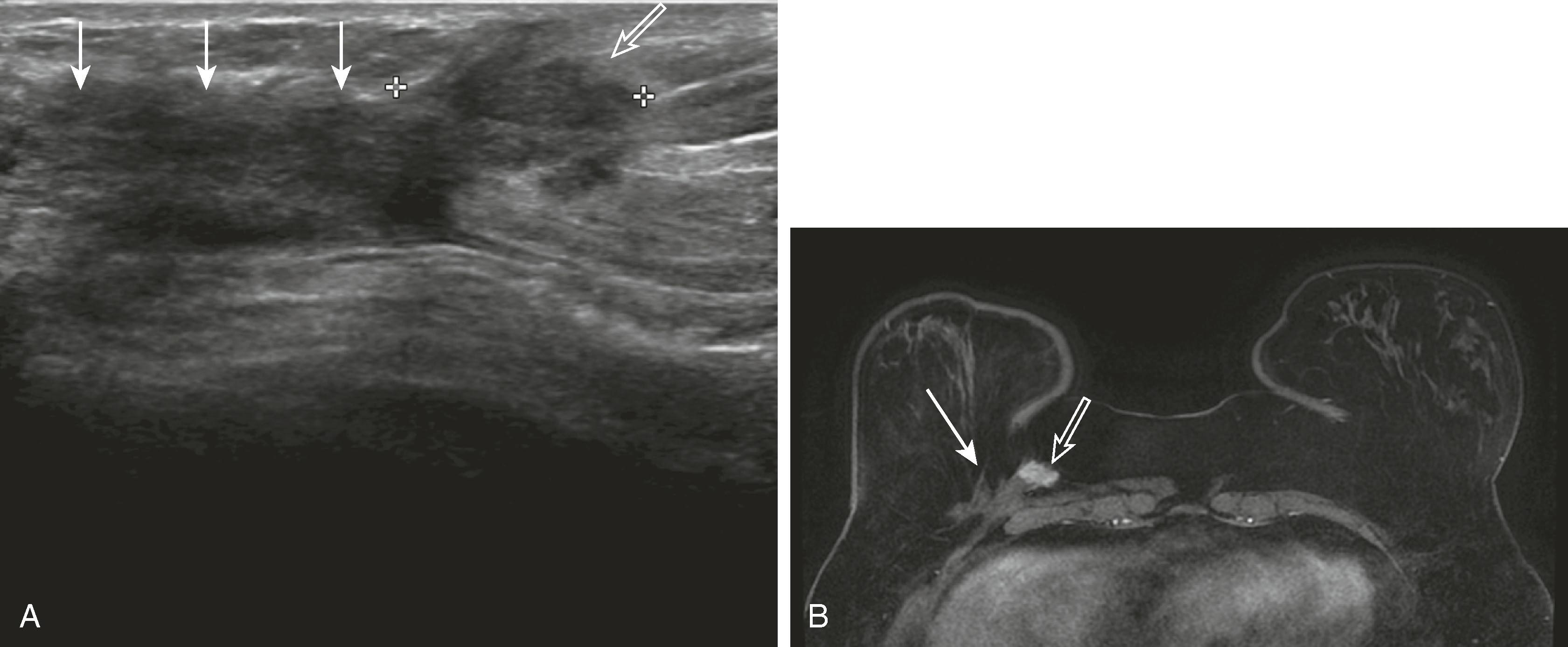
The axillary lymph nodes are well visualized sonographically in most patients, unless they are very deep in a large patient. Diffuse cortical thickening, rounded shape, loss or narrowing of the hilus, and eccentric bulging of the cortex of an axillary node are features that may be seen with reactive or metastatic lymphadenopathy ( Fig. 8.21 ). The presence of multiple bilateral abnormally cortically prominent axillary nodes suggests a chronic inflammatory process such as rheumatoid arthritis or a lymphoproliferative disorder.
Mammography screening has been shown to be an effective method for breast cancer detection, but it is less effective in patients with denser glandular tissue than in those with predominantly fatty tissue in the breast. Ultrasound is not hampered by dense tissue. Greater breast tissue density is associated with a higher risk of cancer. Screening ultrasound has been trialed in several studies. A large study of women with elevated risk for breast cancer, the ACRIN 6666 trial, demonstrated that, although additional cancers were detected, the positive predictive value (PPV) of screening ultrasound was significantly less than screening mammography, with many more benign lesions undergoing biopsy. Another criticism of sonography is that it is more operator dependent than mammography (i.e., the detection rate is more dependent on the skills of the sonographer). In addition, some lesions are very subtle and difficult to identify without a high degree of suspicion based on a mammogram or MRI finding. Screening ultrasound may supplement mammography in women with dense breasts, but it cannot replace mammography because sonography is poor at detecting calcifications, which are a common manifestation of malignancy, particularly DCIS.
Used to evaluate a mass seen or suspected on the mammogram or to evaluate a palpable mass that cannot be visualized mammographically.
Determines cystic versus solid, provides guidance for biopsy.
Features of benign masses include three or fewer gentle lobulations, orientation parallel to the chest wall, and a thin echogenic pseudocapsule.
Features associated with invasive carcinoma are irregular shape, marked hypoechogenicity, ill-defined or angular margins, anteroposterior diameter greater than transverse diameter, and acoustic shadowing.
Excellent for axillary evaluation.
May be more effective than mammography, but should not replace mammography, in patients with dense glandular tissue.
In the mid-1990s, breast MRI started to gain popularity as an ancillary breast imaging tool. It is a very valuable test because of its extremely high sensitivity (89% to 100%). Although the specificity is lower overall, it is similar to that of mammography and better than that of ultrasound. The PPV of MRI has been published in the range of 61% to 63%. More recently, publications report specificities between 81% and 97%. Therefore, although MRI rarely misses cancer, it can result in false positives, which have a significant cost in terms of subsequent imaging and biopsies, not to mention the emotional cost to the patient. Initially, MRI was used for local staging in patients with dense breast tissue and biopsy-proven cancer. More recently, the indications have expanded.
Currently, breast MRI has the following major indications:
Defining the extent of disease in patients with biopsy-proven cancers including the contralateral breast;
Answering questions in patients with equivocal conventional imaging or in patients with a palpable finding, pain, or nipple discharge, with negative mammography and ultrasonography;
High-risk screening for patients with detectable genetic mutations and family/personal history of breast cancer;
Assessing tumor response in patients undergoing neoadjuvant chemotherapy;
Search for occult breast cancer when patients present with carcinoma of unknown primary, especially when the initial presentation is with axillary adenopathy; and
Evaluation of tumor bed in patients with positive margins after surgical resection.
MRI detects breast disease by virtue of its ability, after the administration of a gadolinium-based contrast agent, to demonstrate neovascularity in tumors. Breast tumors, especially malignant ones, have more and larger vessels with higher permeability to contrast. In addition, there is an increase in the interstitial extravascular space of tumors. Typically, breast cancers enhance rapidly (within the first minute or so of contrast administration) followed by rapid washout over 10 to 15 minutes ( Fig. 8.22 ). This is the typical “kinetic signature” of cancer but, unfortunately, it is not absolute. There are a few cancers that demonstrate delayed enhancement with progressive accumulation of contrast and benign lesions, such as fibroadenomas, typically cellular, that enhance rapidly and wash out quickly ( Fig. 8.23 ). Therefore, when MRI-visible lesions are characterized, both the kinetic profile and the morphology must be considered. The morphological features that we have come to associate with breast cancer by most imaging techniques are indistinct borders, spiculation, and irregularity of shape.
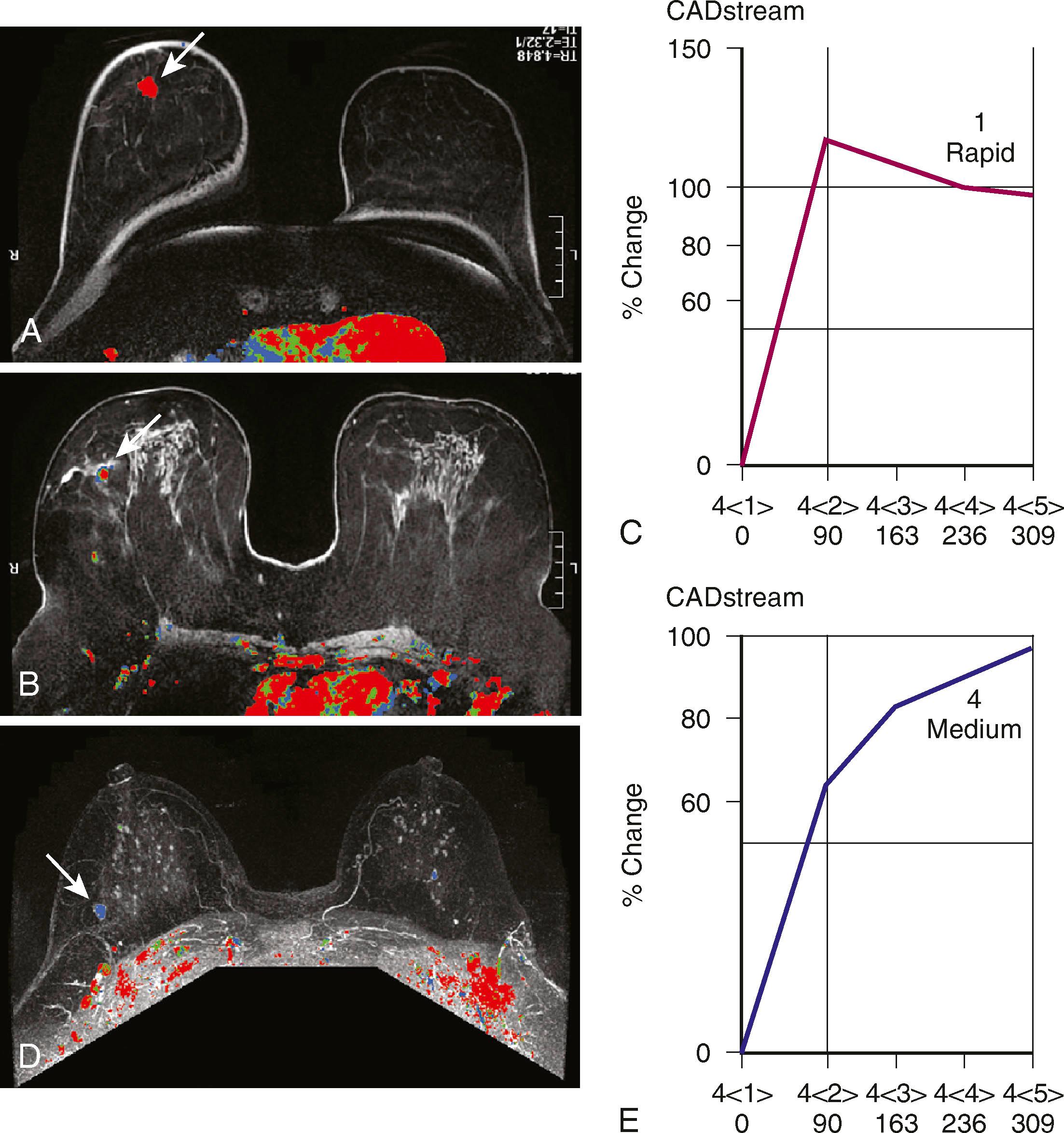
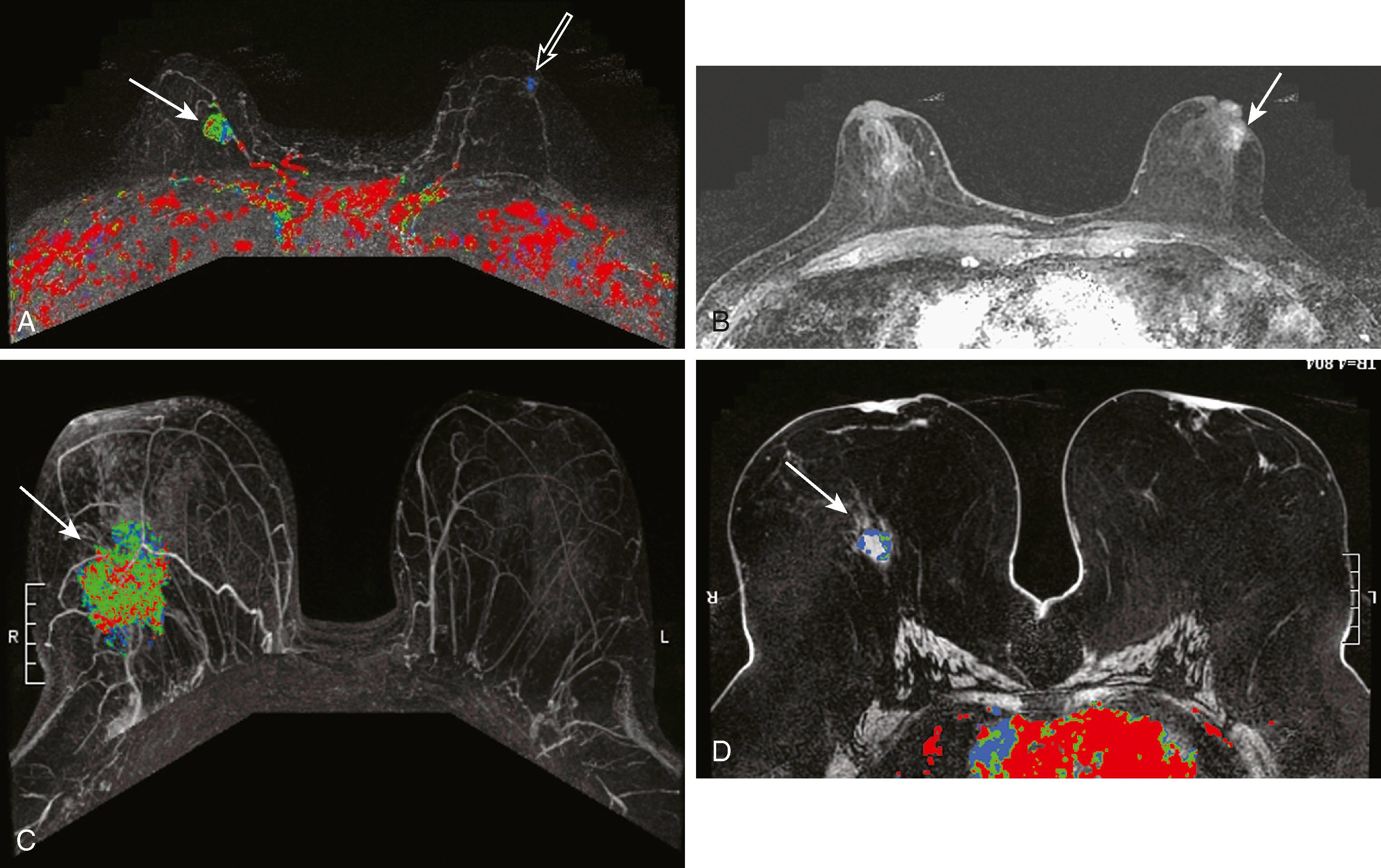
Protocols to image breast cancer can vary, but the basic technique of breast MRI is to scan both breasts rapidly over time before and after the administration of an intravenous gadolinium contrast agent. Scanning is usually performed in the axial plane, but in some institutions, the sagittal plane is preferred. Because these scans result in very large data sets (a typical study will commonly include well over 1,000 images), computer algorithms that simplify viewing are helpful. The software that displays the kinetic features of the breast is referred to as CAD software. CAD assigns a color map to areas of blood flow, which is color coded depending on the speed with which the contrast exits the lesion. To demonstrate this, the breasts must be scanned multiple times in rapid succession (called multiphasic imaging ) over a short period, typically 10 to 12 minutes. Newer protocols are evaluating the efficacy of shortening the exam time by limiting the number of post contrast series obtained. If the contrast exits the lesion quickly, it is called a washout pattern and is usually colored red. This is most typical of malignant lesions and benign or malignant lymph nodes. If the contrast stays at the same concentration throughout the scan, it is called a plateau pattern and is often colored green. This pattern is indeterminate for malignancy. If the contrast enters the lesion and the concentration of contrast continues to increase, it is characterized as a persistent pattern and is typically colored blue. This pattern is most commonly seen with benign lesions such as fibroadenomas and other fibrocystic conditions.
Post-processing software continues to help with detection and analysis of findings on MRI. For high-quality MRI of the breast to be performed, it is necessary to have hardware and software capable of scanning both breasts simultaneously and within the necessary time constraints of observing the inflow and egress of contrast material. In addition, the scanning protocol must suppress fat because much of the breast is composed of fatty tissue. Scanning parameters must have sufficient spatial resolution to depict the shape and margin so that accurate morphological characterization is possible. To do this, MRI units must have current software and a magnetic strength of at least 1.5 Tesla. Dedicated breast coils are critical.
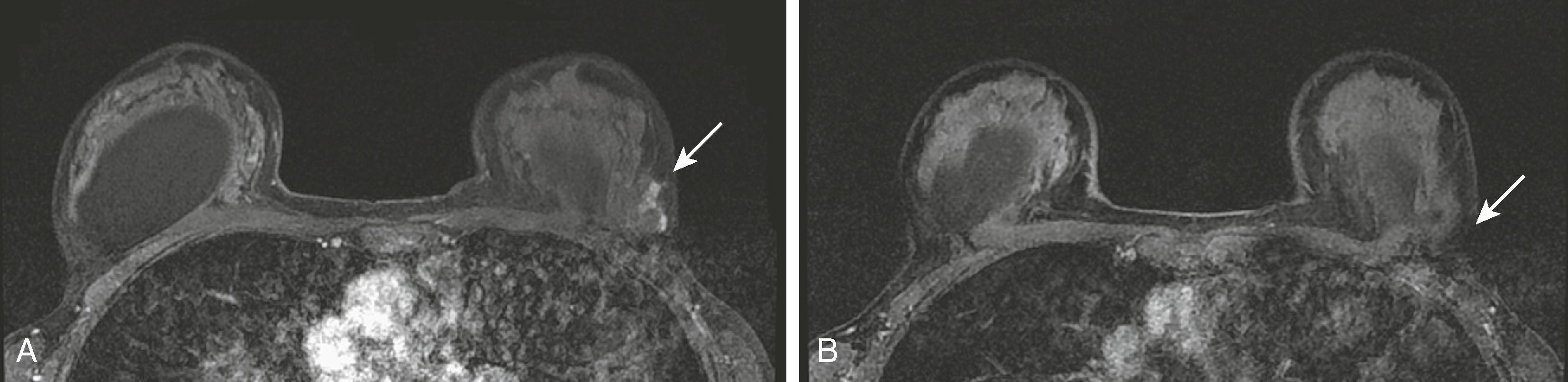
A confounding factor to be considered with MRI is the presence of physiological enhancement, a feature that also affects contrast-enhanced mammography (CEM) and molecular breast imaging (MBI). The enhancement of the breast is subject to physiological changes based on the hormonal status during the menstrual cycle or during pregnancy and lactation. At times, this type of enhancement can mimic cancer. Previously, it has been recommended that when MRI is completely elective, such as when it is performed for high-risk screening, it should be performed during the first week or so of the menstrual cycle. However, this practice is falling out of favor today. When a newly diagnosed breast cancer patient is scanned, it may not be possible to wait for the beginning of the cycle because treatment could be delayed. In addition, women who have had hysterectomy are problematic because the menstrual cycle is not apparent. Figs. 8.24 and 8.25 demonstrate these points in two different clinical scenarios.
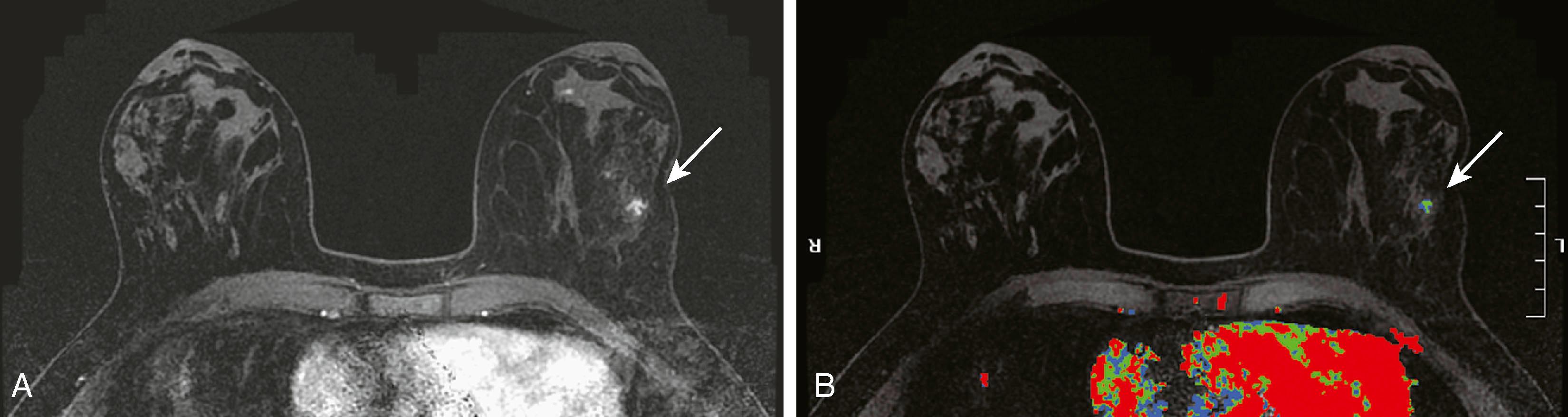
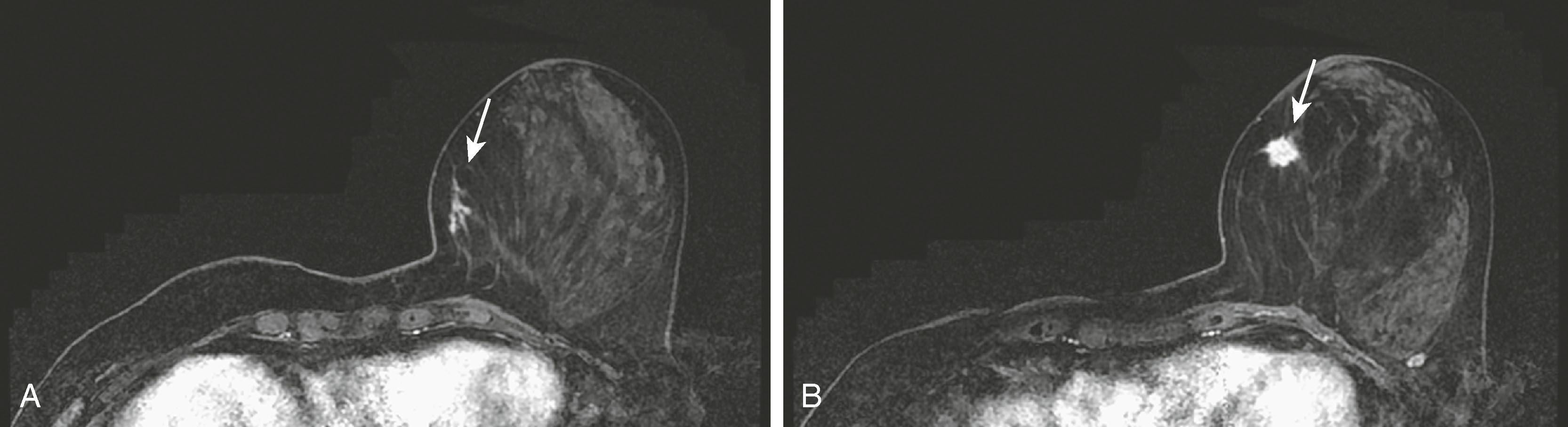
High-risk lesions such as atypical ductal hyperplasia (ADH) have a variable MRI appearance. The kinetic features are variable, ranging from washout to progressive. Contrast enhancement is thought to be the result of increased microvessel density and/or capillary permeability. The morphology is typically nonmass-like; however, sometimes these lesions can present as masses. When ADH is diagnosed by MRI-guided biopsy, recent literature suggests an upgrade rate of approximately 30% to malignancy. This is considerably higher than the upgrade rate of approximately 19% for stereotactic biopsy. According to Liberman and colleagues, this may be related to the fact that the women undergoing MRI are at a higher risk than those routinely undergoing mammography. Fig. 8.26 demonstrates one appearance of ADH. It is nonmass with a predominantly plateau pattern enhancement. Similar to high-risk lesions, the kinetic pattern of DCIS is variable. The typical morphological appearance of DCIS is described as nonmass clumped linear or segmental enhancement. Occasionally, an enhancing ductal branching pattern or filling defects within fluid-filled ducts can be identified ( Fig. 8.27 ). Similar to mammography and ultrasound, DCIS rarely may present as a mass on MRI. It is not currently possible to reliably predict whether there is a coexistent invasive component or the exact histological type of DCIS based on the morphology or kinetic characteristics. The sensitivity of MRI for detecting DCIS is lower than for invasive breast cancer, with reported sensitivities ranging from 77% to 96%. The specificity is considered to be similar to that of invasive cancer. MRI is better at depicting high-grade DCIS than low-grade disease. It is better at demonstrating high-grade DCIS without necrosis than mammography because DCIS is most frequently detected when the mammogram demonstrates calcifications. Unfortunately, it is well known that microcalcifications identified mammographically are often nonspecific. Because MRI does not detect DCIS by imaging the calcium, it is difficult at times to make a direct correlation between the mammogram and the MRI; therefore, at present, MRI and mammography are complementary tests. Fig. 8.28 shows malignant calcifications on a mammogram and the MRI demonstrates malignant morphology typical of DCIS.
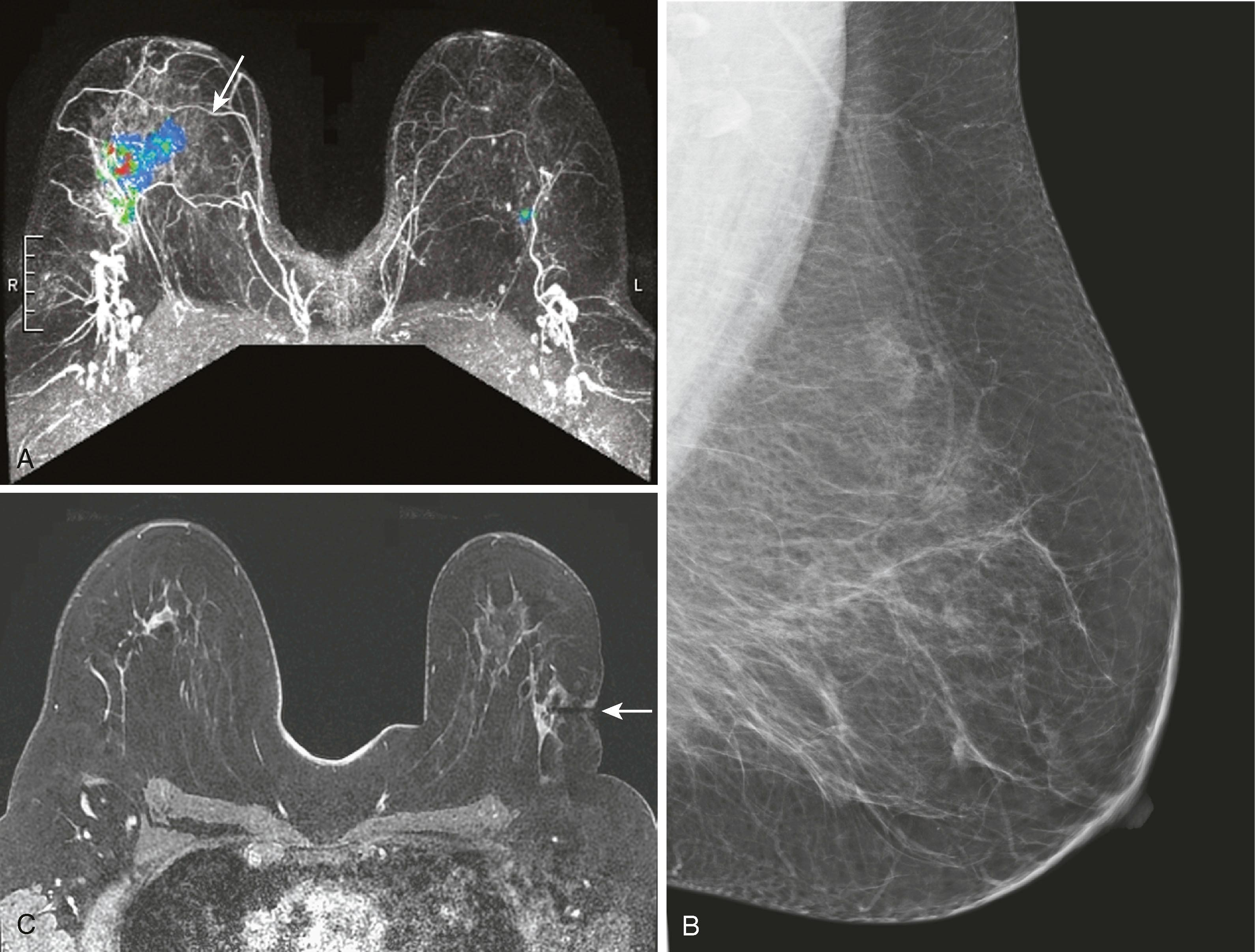
One of the most common uses of breast MRI is to evaluate for extent of disease in patients with biopsy-proven breast cancer. Liberman and associates, in a study across multiple series, reported that up to 48% of women with breast cancer will have unsuspected additional cancer that was not expected from mammography. This could be either multifocal or multicentric disease. MRI has been reported to depict additional tumors that necessitated wider excision in 27% to 34% of patients. Berg and coworkers point out that this closely mirrors the 25% to 36% rate of local recurrence in patients treated without chemotherapy or radiation therapy. MRI is relatively accurate at estimating tumor size but can overestimate and underestimate size on average by 15 mm in approximately 48% and 40% of findings, respectively. Because the size variation between MRI and pathological truth is relatively small, it is unlikely to have clinical implications. Lobular cancer is especially difficult to evaluate because it is well known that mammography, physical examination, and ultrasound tend to underestimate the extent and size of the tumors. Perhaps just as important, Lehman and colleagues reported that approximately 3% of women will have an unsuspected cancer in the contralateral breast ( Fig. 8.29 ).
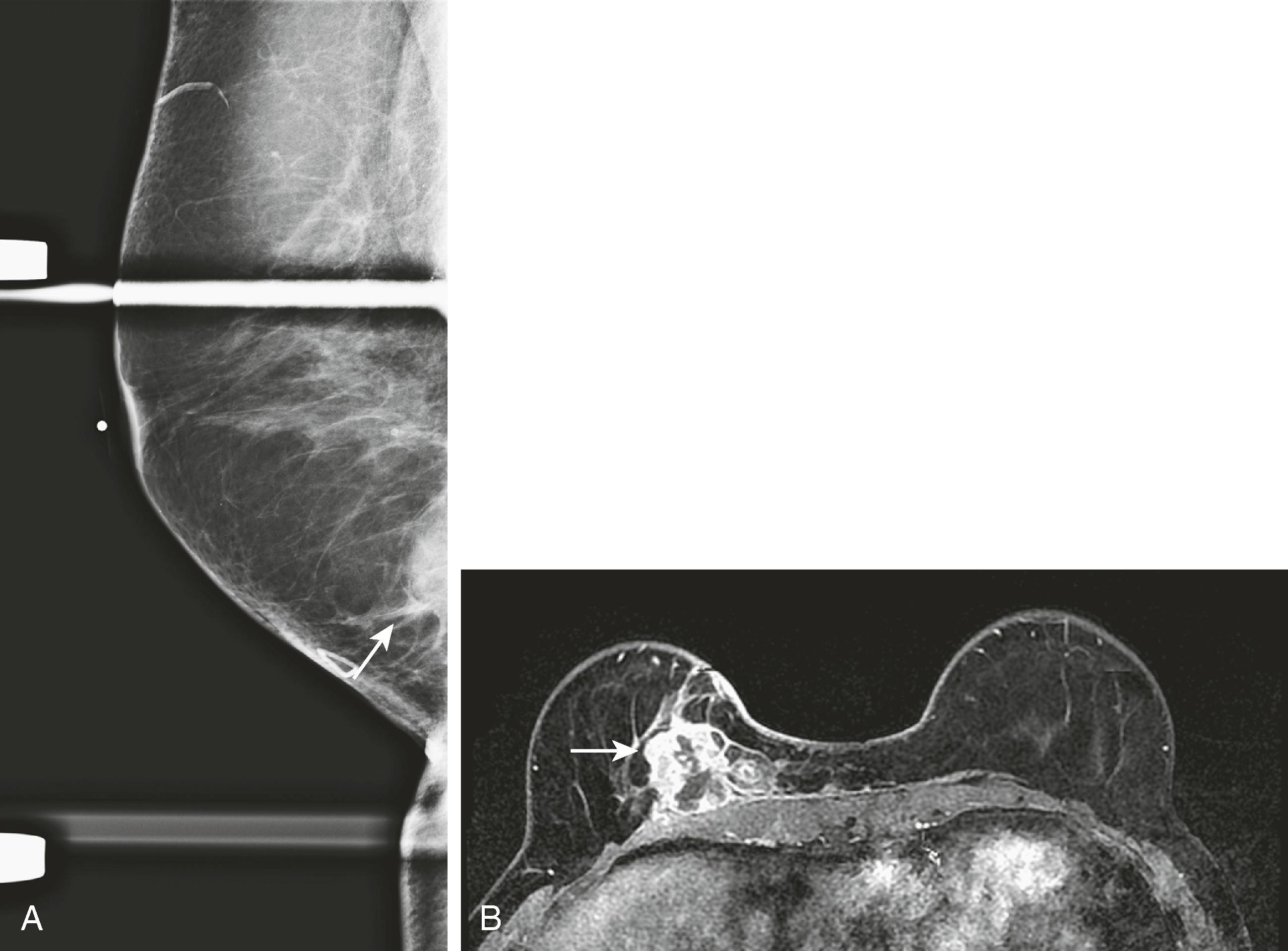
Despite the belief by most of the imaging community that breast MRI is a valuable staging tool, there are critics who believe that MRI may cause overestimation and overtreatment of breast cancer with no significant change in outcome. The Comparative Effectiveness of MRI in Breast Cancer (COMICE) trial, which compared randomly assigned preoperative breast cancer patients to MRI or no MRI, found a 19% reoperation rate in both groups. Others are concerned that, because of its high sensitivity in detecting additional disease, the mastectomy rate will increase. This increase in mastectomy rate was reported recently by Katipamula and associates at the Mayo Clinic.
Most still believe that breast MRI is a valuable test for preoperative surgical planning when breast conservation is being considered, and most importantly if pathology reveals lobular carcinoma. It appears to be most helpful in women with dense breast tissue in whom mammography is limited, but there are no generally accepted guidelines for which patients should be evaluated by MRI before surgery. Because of the high sensitivity and lower specificity of breast MRI, it is not infrequent that false-positive lesions are discovered. Fig. 8.30 demonstrates an instance in which MRI demonstrated more extensive tumor than expected.
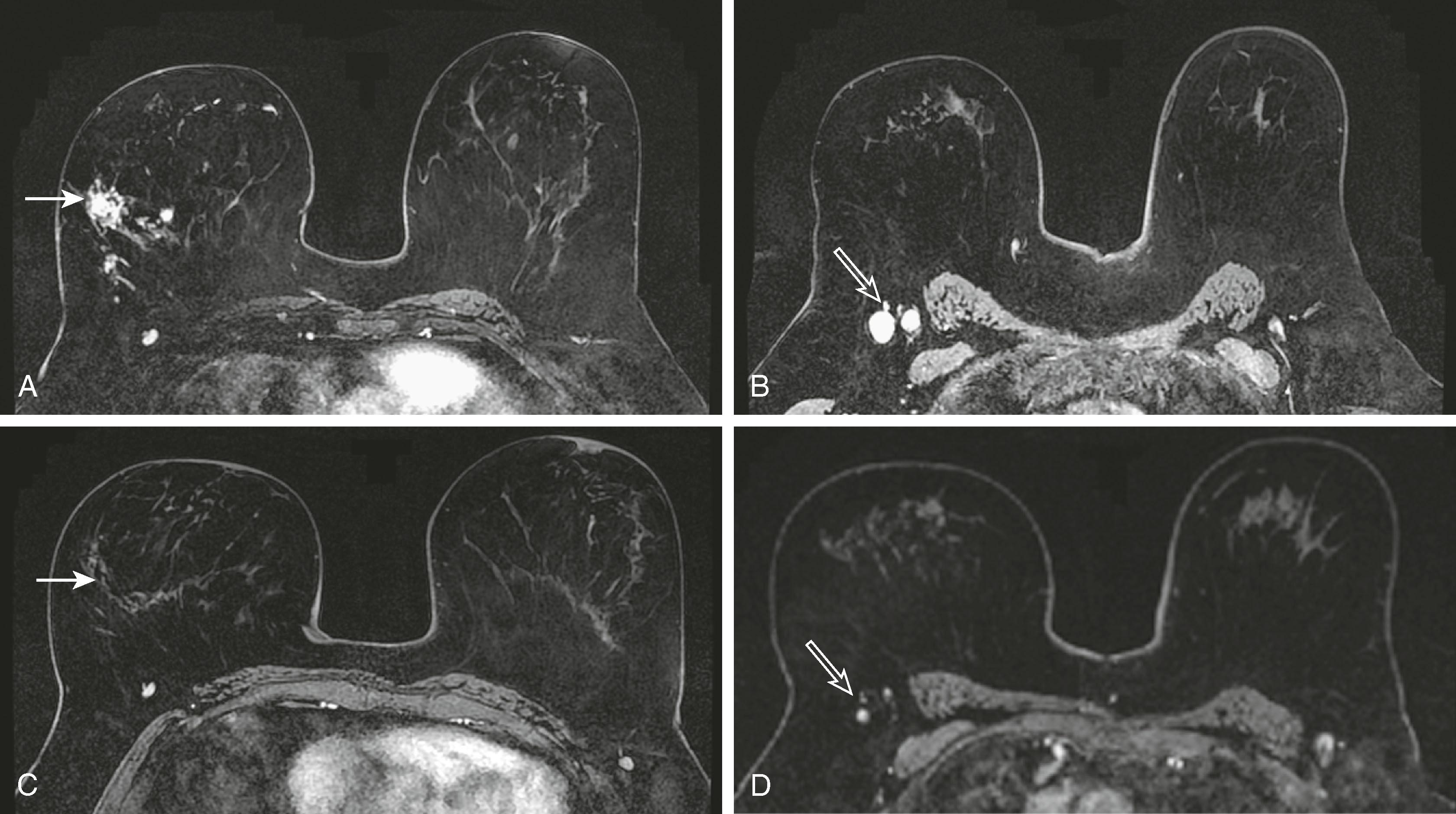
MRI is useful for following the response of tumors to neoadjuvant chemotherapy . Mammography, ultrasound, and physical examination are notoriously inaccurate in evaluating the response to chemotherapy. MRI and PET have proved to be superior in this regard. Kuhl reported that MRI findings have been shown to correlate better with tumor response than conventional imaging, with correlation coefficients ranging between 0.72 and 0.93 versus 0.30 and 0.52. One important caveat is that MRI, although very sensitive for detecting macroscopic invasive cancer, cannot exclude residual microscopic disease or low-grade DCIS. Although the MRI appears negative, there may be tumor remnants in 30% of patients. Interestingly, the underestimation is greater for patients who have a complete MRI response to chemotherapy than for patients who have a partial or no response. Fig. 8.31 demonstrates a complete MRI response to chemotherapy in a patient with lobular carcinoma. Fig. 8.32 demonstrates a patient with a partial response to chemotherapy. The tumor diminished in size with only several residual foci of tumor, and the kinetic pattern changed from plateau to progressive.
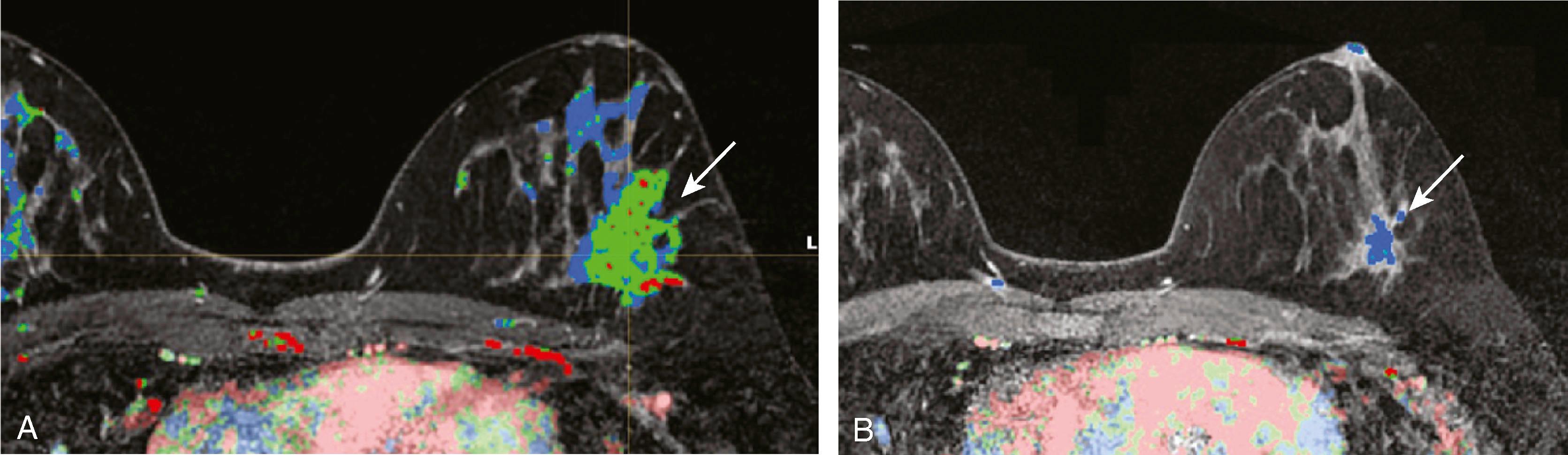
MRI for high-risk screening is now an accepted test for patients with high-risk profiles such as personal history of breast cancer and strong family history in premenopausal first-degree relative. One of the sentinel papers by Kriege and coworkers found that of 1,909 high-risk women screened with clinical breast examination, mammography, and MRI, the sensitivities were 17.9%, 33.3%, and 79.5%, respectively. The conclusion was that “MRI appears to be more sensitive than mammography in detecting tumors in women with an inherited susceptibility to breast cancer.” Initially published guidelines by the American Cancer Society helped to identify patients for whom screening breast MRI would be most effective. The published guidelines stated that women who have a greater than 20% lifetime risk for the development of breast cancer should undergo yearly MRI and mammography. The topic of breast cancer screening, especially for women at high risk, has been elaborated on by others, notably Monticciolo and colleagues, with recommendations from the Society of Breast Imaging and the American College of Radiology regarding the use of mammography, breast MRI, breast ultrasound, and other technologies. Figs. 8.33 and 8.34 demonstrate two screening patients with strong family histories of breast cancer; one is a false positive and the other a true positive.
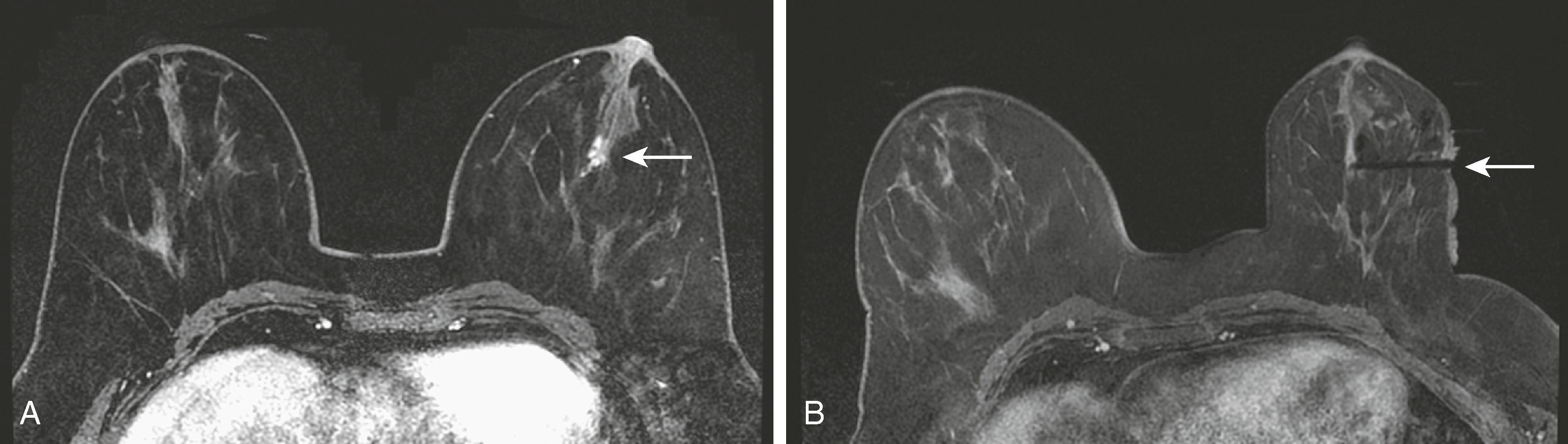
Breast MRI has become the gold-standard test to search for a breast primary when a patient presents with a carcinoma of unknown primary. It is valuable not only because of its high sensitivity in identifying the primary tumor but also because of its high negative predictive value. Liberman and associates noted in a small study group of 16 women that MRI identified the primary breast tumor in 13 out of 16 patients. MRI may be of greatest value when a patient presents with axillary lymphadenopathy and a negative mammogram. Fig. 8.35 demonstrates such a patient who presented with axillary lymphadenopathy and a negative mammogram.
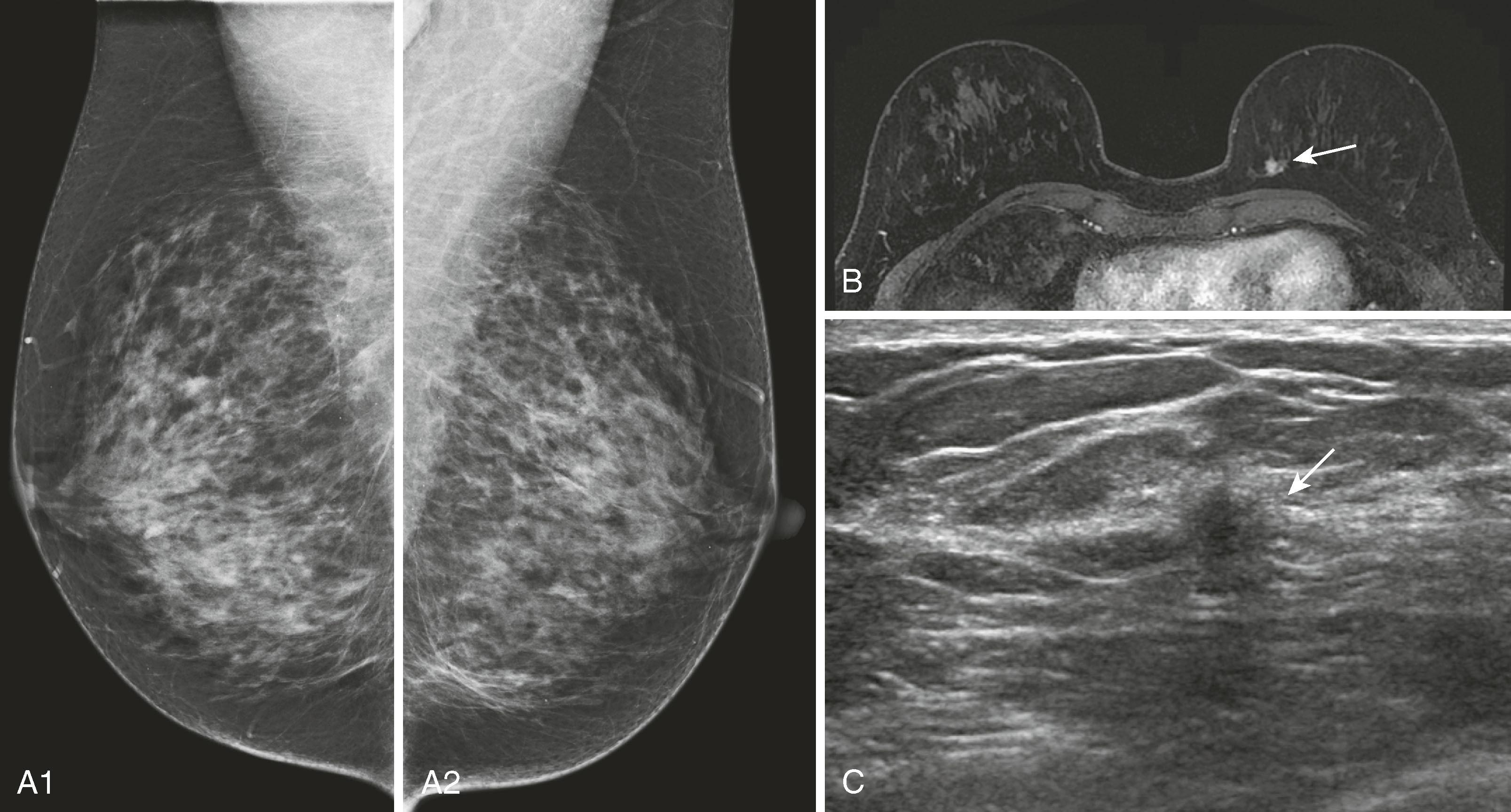
Not all lesions can be identified by second-look ultrasound, but if they can be identified, ultrasound-directed biopsy is preferable because of its ease and lower cost. DeMartini and coworkers reported that approximately 50% of lesions identified by MRI can be identified by ultrasound, and masses are easier to locate than nonmass-like lesions. Because of differences in breast position (MRI prone and ultrasound supine and decubitus), it is sometimes difficult to be certain that MRI and ultrasound are identifying the same lesion.
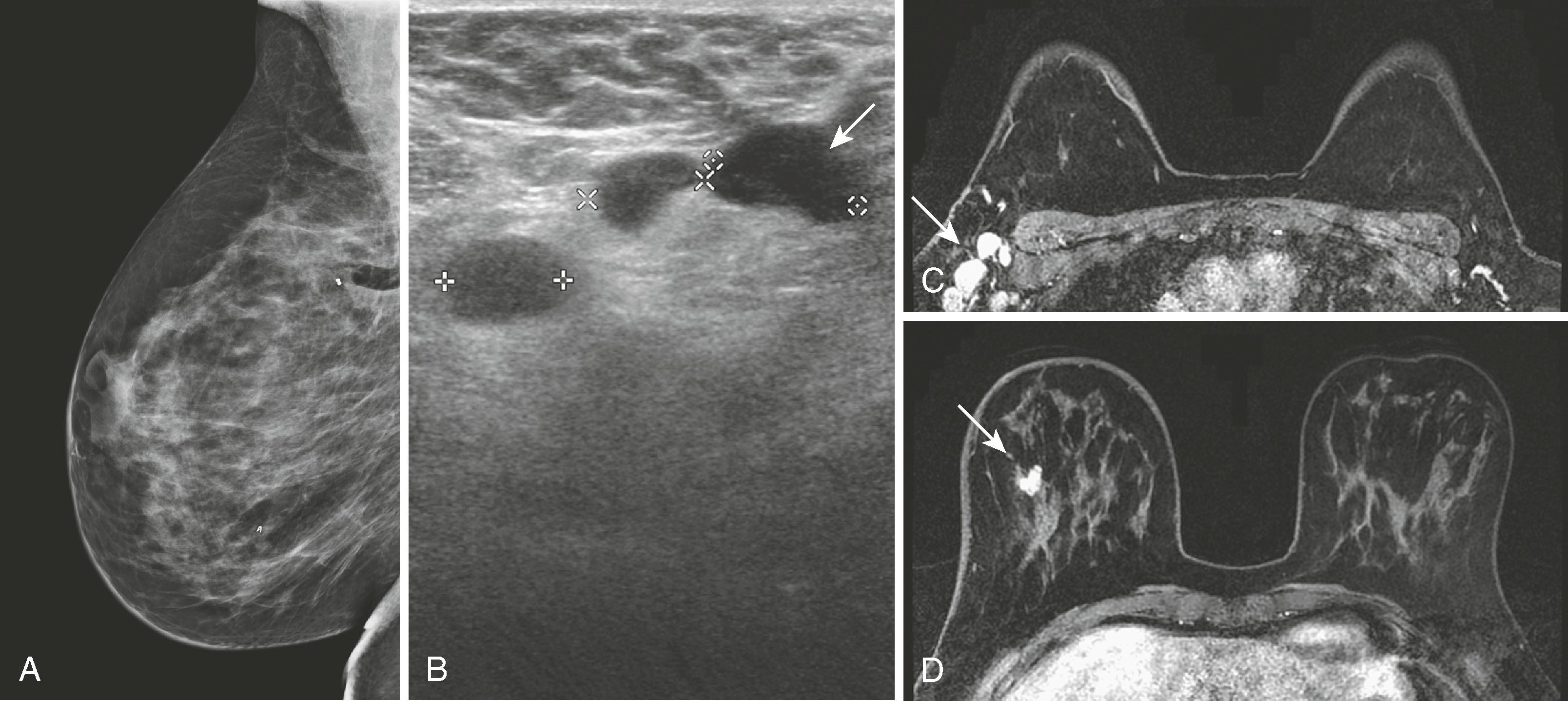
Approximately 32% to 63% of segmental mastectomies have positive margins, requiring either a re-excision or a mastectomy. Knowing the extent and distribution of residual disease within the breast can be helpful in making this decision. Lee and colleagues reported a sensitivity, specificity, and accuracy of 61.2%, 69.7%, and 64.6%, respectively, for showing the presence and extent of residual disease. The conclusion was that, although there is an overlap in the appearance of benign and malignant lesions in the postoperative breast, MRI can show additional lesions that, in their study, changed the original treatment plan in approximately 30% of patients. Therefore, although not necessary in every case with a positive margin, breast MRI can be very helpful for surgical planning in select cases. Fig. 8.36 shows an MRI performed on a patient with positive margins after segmental mastectomy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here