Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acknowledgments: The authors wish to recognize the work of previous authors, Drs. Robert Leffert, Vincent R. Hentz, Michelle James, and Peter Waters, for their contributions to Green’s Operative Hand Surgery and this chapter.
This chapter discusses the diagnosis, treatment, and long-term expected outcomes of infants and children with brachial plexus birth injuries (BPBIs). Because the type and severity of neural injuries are variable, treatment and outcome are not uniform for all infants. In those children with mild neurapraxic lesions, spontaneous recovery is to be expected over the first several months of life, with complete recovery evident by age 1 year. In contrast, children with severe avulsion injuries experience a lifetime of disability despite extensive therapy and surgical management. Although there have been many advances since Duchenne’s (1872) and Erb’s (1874) classic descriptions of infantile paralysis, many difficult challenges still remain. For instance, the treatment of severe injuries involving the entire brachial plexus with a combination of nerve ruptures and nerve avulsions remains insurmountable. Microsurgical reconstruction of the plexus in the first 3 to 9 months of life can improve the natural history. However, inability to directly repair nerve roots avulsions from the spinal cord and the resulting relative paucity of donor nerves for reconstruction results in persistent and permanent impairment.
Fortunately, the incidence of these multilevel avulsion and/or rupture injuries is relatively low (between 8% and 25%) for all brachial plexus birth palsies. , , In addition, secondary musculoskeletal deformities are common in children with incomplete recovery in the first 1 to 3 years of life. Although nonoperative and operative intervention, such as contracture releases, tendon transfers, and osteotomies, may improve outcomes, none can normalize limb function. The goal of this chapter is to explain the nature of the neurologic injury and its effect on the developing child. This knowledge will guide decision making regarding the initial neurologic injury and its secondary musculoskeletal manifestations. Issues and controversies with incomplete evidence for decision making will also be addressed.
Essential to any discussion of the natural history and treatment of a brachial plexus lesion is a thorough understanding of the anatomy. Therefore a brief review of the pertinent anatomy is described ( Fig. 40.1 ).
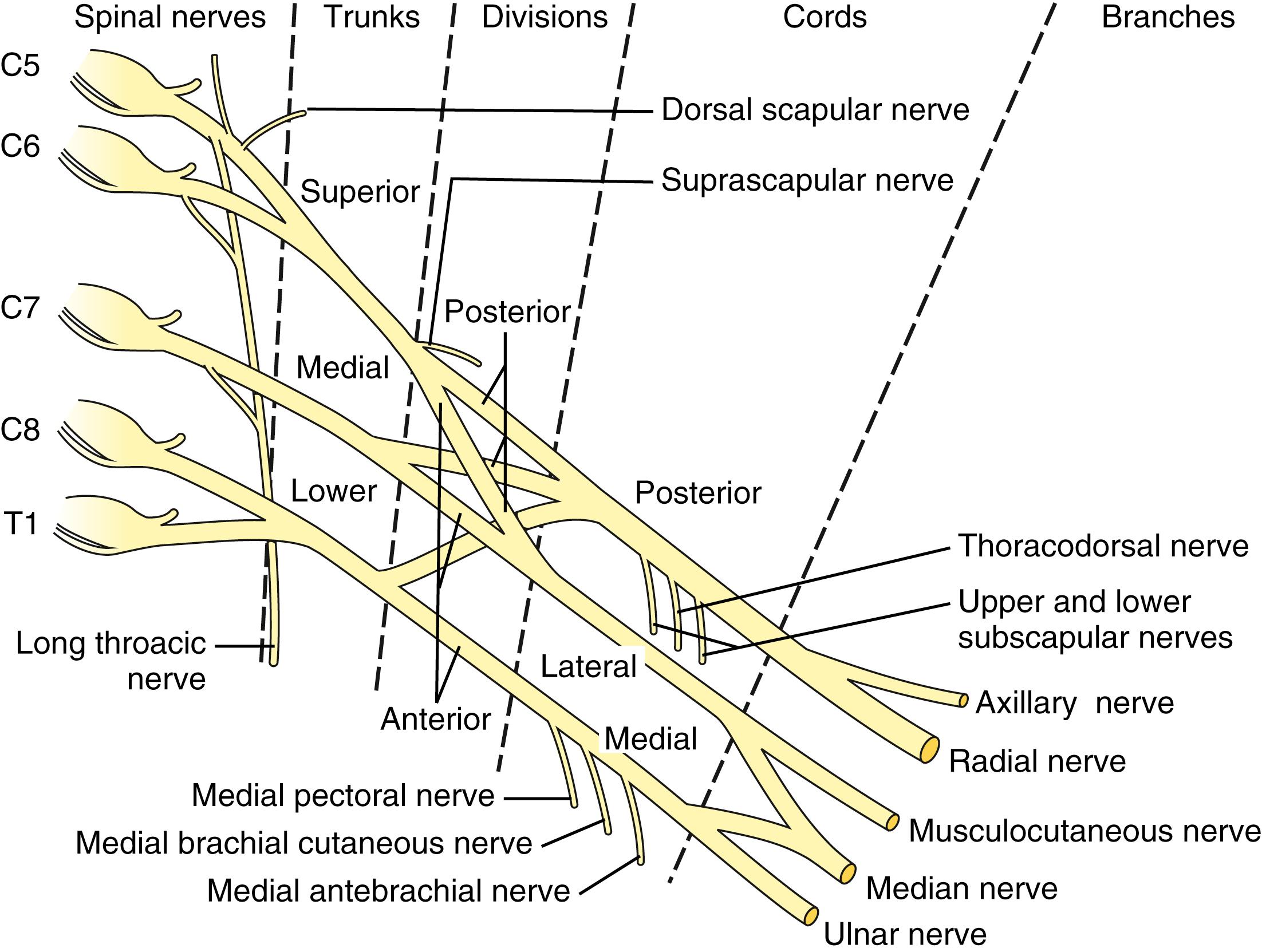
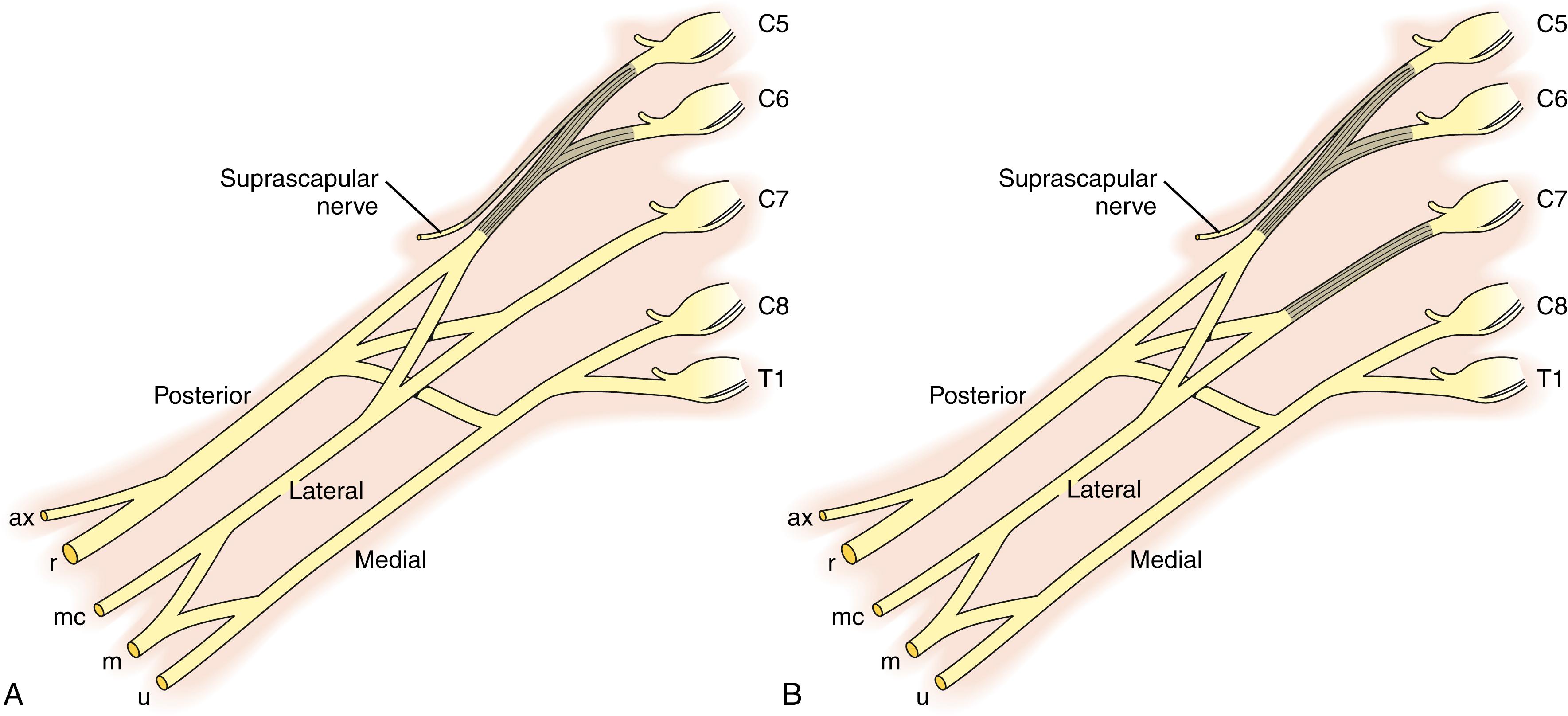
The brachial plexus most commonly receives contributions contiguously from the fifth cervical (C5) through the first thoracic (T1) ventral spinal nerve roots. Prefixed cords (22%) receive an additional contribution from the C4 nerve root, whereas the much less common postfixed cords (1%) receive a contribution from T2. The C5-6 nerve roots join to form the upper trunk, the C7 nerve root continues as the middle trunk, and the C8-T1 nerve roots combine to form the lower trunk. Each trunk bifurcates into anterior and posterior divisions. The posterior divisions of all three trunks make up the posterior cord. The anterior divisions of the upper and middle trunks form the lateral cord. Finally, the anterior division of the lower trunk forms the medial cord.
The major nerves of the upper extremity are terminal branches from the cords, with the ulnar nerve arising from the medial cord, the radial and axillary nerves from the posterior cord, the musculocutaneous nerve from the lateral cord, and the median nerve from branches of the medial and lateral cords. The other nerves to the upper extremity arise sequentially throughout the brachial plexus as illustrated in Fig. 40.1 . The dorsal scapular nerve arises from the C5 nerve root along with a branch to the phrenic nerve. The suprascapular nerve arises, often as a trifurcation along with the anterior and posterior divisions, from the upper trunk, and the long thoracic nerve arises from the C5-7 nerve roots. In addition, the lateral pectoral nerve originates from the lateral cord and the medial pectoral from the medial cord. Finally, the thoracodorsal nerve and the upper and lower subscapularis nerves arise from the posterior cord. The proximal-to-distal orientation of the brachial plexus is nerve roots, trunks, divisions, cords, and terminal branches. The divisions are located behind the clavicle. Every muscle in the upper limb is innervated by the brachial plexus, outside of a few muscles that stabilize the scapula (i.e., trapezius and partial levator scapulae).
Surgical exploration and reconstruction of the brachial plexus require a thorough understanding of its three-dimensional anatomy. As noted, the brachial plexus extends proximally from the C5 to the T1 nerve roots of the spinal cord and distally to the terminal branches in the upper brachium. In the neck, it is located between the anterior and middle scalene muscles. The plexus extends beneath the clavicle, superficial to the first rib, as it passes into the axilla. The plexus is divided into supraclavicular and infraclavicular portions. The brachial plexus is joined by the major blood vessels of the arm beneath the clavicle. As the plexus courses medial to the coracoid, the nerves surround the axillary artery. The relationship of the cords to the artery gives them their appropriate medial, lateral, and posterior designations. Complete exposure of the brachial plexus often requires retraction or osteotomy of the clavicle, release of the muscular insertions to the coracoid, and release of the clavicular origin of the pectoralis major muscle.
BPBI has an incidence of 0.38 to 1.56 per 1000 live births. The difference in incidence may depend on the type of obstetric care and the average birth weight of infants in different geographic regions. , In the United States, the incidence has been decreasing slowly over time, down from 1.6 per 1000 live births in 2000 to 0.9 per 1000 live births in 2012. Perinatal risk factors for brachial plexus injury include large size for gestational age (macrosomia), maternal diabetes, multiparous pregnancies, previous deliveries resulting in BPBI, , prolonged labor, breech delivery, , oxytocin administration, uterine tachysystole, shoulder dystocia, and assisted (vacuum or forceps) deliveries and difficult births. ,
Maternal body mass index is on the rise in the United States and other well-developed countries of the world and leading to increasing fetal size and a rising incidence of gestational diabetes. Both are risk factors for BPBI. , Unfortunately, shoulder dystocia is often an unanticipated and unpredictable obstetric emergency. Fetal distress may contribute to muscle hypotonia and provide less protection of the plexus from stretch or compression injury during delivery.
Mechanically, shoulder dystocia in vertex deliveries and difficult arm or head extraction in breech deliveries increase the risk for nerve injury. , The increased incidence of delivery by cesarean section may in part be due to an attempt to avoid dystocia and brachial plexus injuries. However, cesarean section does not guarantee prevention of a BPBI, although it decreases the incidence 100-fold. Further complicating the etiology are reports citing evidence of intrauterine onset of paralysis. , Thus, prevention of the injury remains elusive, underscoring the importance of optimal treatment of the sequelae.
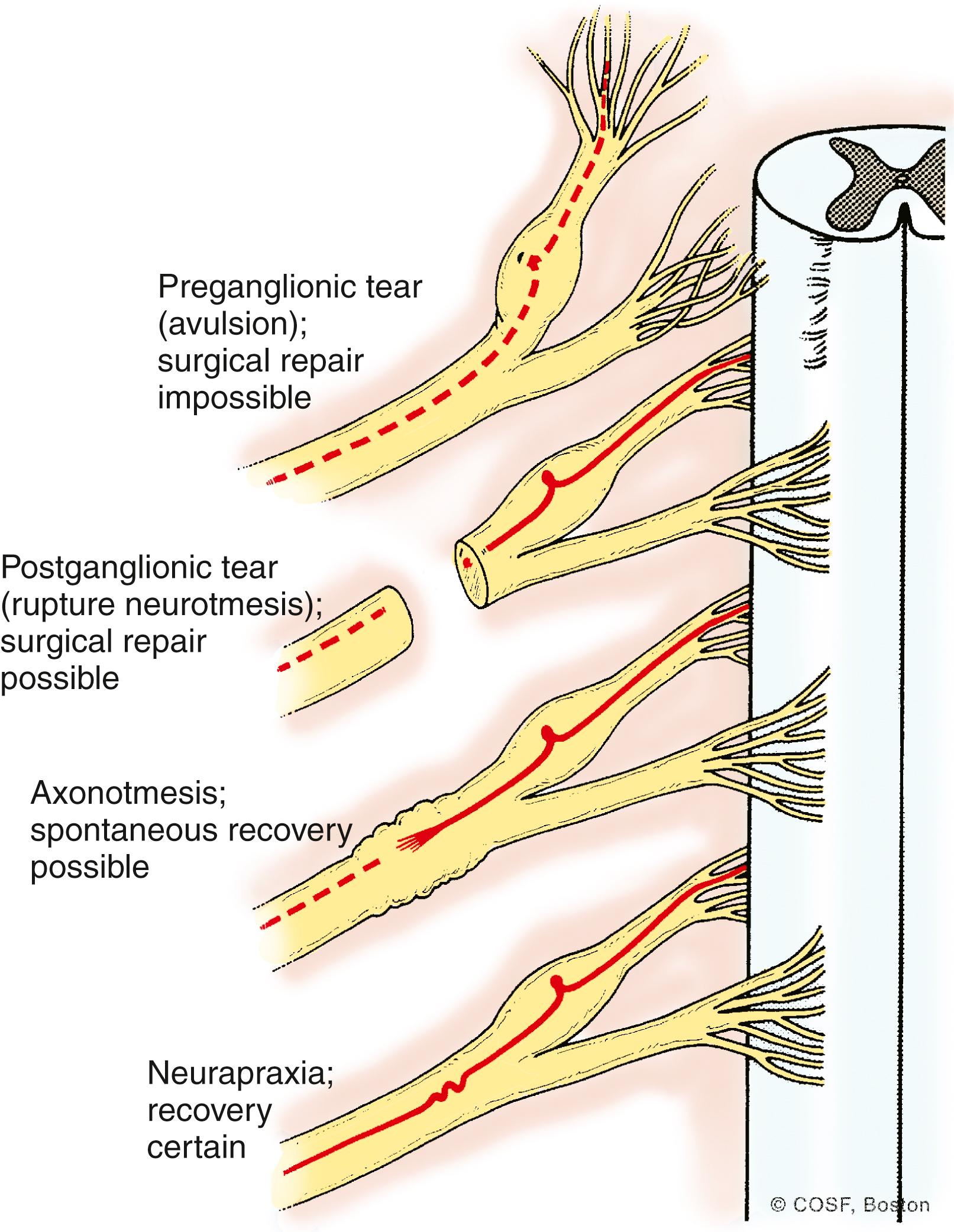
Most commonly, a BPBI involves the upper trunk (C5-6 or Erb’s palsy), potentially in combination with injury to C7 (extended Erb’s palsy); less often, the entire plexus (C5-T1 or global palsy) is injured. Injuries are described classically as neurapraxia (Sunderland type I), axonotmesis (Sunderland types II to IV), neurotmesis (Sunderland type V), or avulsion ( Fig. 40.2 ). Mechanically, lesions have been described as stretch (Sunderland type I), varying degrees of rupture (Sunderland types II to V), and avulsion. Upper trunk extraforaminal ruptures are more common with vertex delivery and shoulder dystocia. The right upper limb is involved more often because of the more frequent left occiput anterior vertex presentation, which places the right shoulder behind the symphysis pubis. C5-6 root avulsions are particularly frequent with breech deliveries and can at times be bilateral. , , When the entire plexus is involved, a combination of stretch, rupture, and avulsion injuries can occur in various portions of the plexus.
A mechanical cause is presumed as the etiology of BPBI. The theory is based on extensive work that began with original descriptions of infantile paralysis during delivery by Smellie (1764) and Duchenne (1872) and anatomic studies by Metaizeau (1979). , , However, there are rare reports of possible congenital causes of a birth palsy such as the hypoplastic plexus. , In addition, it has been postulated that there may be abnormal in utero forces (maternal propulsive forces) acting on the posterior shoulder region and the plexus as the fetus passes over the sacral promontory before obstetric delivery. The pushing forces of uterine contractions in a dystocia situation have been studied to determine their contribution to neonatal nerve injury in these deliveries.
Increased in utero pressure and subsequent traction across the plexus have been proposed as a possible root cause. In addition, the presence of an anomalous uterus, such as a bicornuate or fibroid one, may decrease the space available for the infant and lead to compression across the brachial plexus. These alternative causes have become contentious issues in a litigious society because the presence of a brachial plexus birth palsy may result in a malpractice suit against everyone participating in the birth of that infant, including the obstetrician, midwife, labor nurse, and hospital. ,
The most common brachial plexus birth injury involves the upper trunk (C5-6) and is also known as Erb’s palsy.
The second most common pattern involves C5-7 and is known as extended Erb’s palsy.
Involvement of the entire plexus (C5-T1) is called global or total brachial plexus injury. This is the least common pattern.
Erb’s palsy has the best prognosis for spontaneous recovery. In contrast, global palsy has the worst prognosis for spontaneous resolution.
The diagnosis of a BPBI is predominantly by physical examination. The differential diagnosis includes pseudoparalysis as a result of fracture. Less common differential diagnoses included infection, injury to the central nervous system (cervical spinal cord), neuromuscular disorders, and congenital anomalies of the upper limb that result in limited motion and strength. Clavicle fractures can occur concomitantly with a BPBI, and radiographs are clinically indicated. A humeral shaft fracture can mimic a BPBI, although the two injuries do not tend to coexist in the same limb. The injuries are usually on opposite sides, and the posterior arm maneuver to deliver the infant can result is a spiral fracture of the humerus. A severely displaced humeral shaft fracture can result in an isolated radial nerve palsy.
Generally, the diagnosis of BPBI is readily apparent immediately after birth. The spectrum of clinical findings is dependent on the extent of nerve injury and the timing of examination. The presence of Horner syndrome (i.e., ptosis, myosis, enophthalmos, anhidrosis) is worrisome for an avulsion injury in the lower roots and carries a poor prognosis ( Fig. 40.3 ). The recovery after birth is often dynamic with changing physical findings over the first few weeks and months of life. Patience is required to perform a reliable examination of an infant ( ![]() ). Observation of spontaneous movement, testing of neonatal reflexes, and tactile stimulation for motor activity are necessary for an accurate examination. The most important aspect of the examination is to determine the prognosis for recovery. , Therefore, serial examinations on a 1- to 3-month basis during infancy are critical for predicting of outcome and determination of indications for surgical intervention.
). Observation of spontaneous movement, testing of neonatal reflexes, and tactile stimulation for motor activity are necessary for an accurate examination. The most important aspect of the examination is to determine the prognosis for recovery. , Therefore, serial examinations on a 1- to 3-month basis during infancy are critical for predicting of outcome and determination of indications for surgical intervention.
Narakas and Slooff attempted to clinically categorize the continuum of brachial plexus injury into four groups. , , The mildest clinical group (I) is represented by the classic upper trunk C5-6 (Erb’s) palsy, with initial absence of shoulder abduction and external rotation, elbow flexion, and forearm supination. Wrist and digital flexion and extension are intact. Successful spontaneous recovery is believed to occur in as many as 90% of infants in this group. Group II represents an extended upper trunk lesion and includes additional involvement of C7, with the absence of wrist and digital extension added to the limitations noted for group I. These infants have the classic “waiter’s tip” posture of their hand and wrist. The prognosis is worse with C5-7 involvement. Group III consists of a flail extremity without a Horner syndrome. The most severe involvement (group IV) is manifested as a flail extremity with a Horner syndrome. These infants may have an associated phrenic nerve palsy with an elevated hemidiaphragm. The status of the diaphragm is best assessed via dynamic study such as a chest fluoroscopy or ultrasound during breathing. Group IV infants have limited chance of meaningful spontaneous recovery.
For prognostic reasons, it is important to determine whether the level of injury is preganglionic or postganglionic (see Fig. 40.2 ). Because of the proximity of the dorsal root ganglion to the spinal cord and the fact that the motor cell body is in the spinal cord, preganglionic lesions are avulsions from the cord that will not spontaneously recover. By assessing the function of several nerves that arise close to the midline and the ganglion, one can frequently determine the level of the lesion by physical examination ( Table 40.1 ). Specifically, the presence of an elevated hemidiaphragm (phrenic nerve, C5 nerve root), the absence of rhomboid major and minor (dorsal scapular nerve, C5 nerve root), a winged scapula (long thoracic nerve, C5-7 nerve roots), and the presence of a Horner syndrome (sympathetic chain, T1 nerve root) all raise concern about a preganglionic lesion.
| Finding | Implication |
|---|---|
| Paraspinal muscle injury | Dorsal rami injury |
| Rhomboid injury | Dorsal scapular nerve (C5) |
| Scapular winging | Long thoracic (C5, C7, C8) injury |
| Horner’s syndrome | Cervicothoracic sympathetic injury |
| Hemidiaphragm paralysis | Phrenic nerve injury |
| Pseudomeningocele | Dura and arachnoid avulsion injury |
Preganglionic lesions can be reconstructed only by nerve transfers, most commonly with the thoracic intercostal nerves, a branch of the spinal accessory nerve, contralateral C7 nerve root transfers, or partial peripheral nerve transfers. Postganglionic ruptures have reconstructible proximal and distal nerves beyond the zone of injury. Thus a postganglionic injury is a complex peripheral nerve lesion that can be reconstructed with intercalary nerve grafts. Peripheral nerve transfers can also be used to reconstruct these injuries. However, functional spontaneous recovery is also possible in postganglionic injuries, likely due to partial ruptures and redundant innervation pathways present at birth. Narakas group I and those in Narakas II with complete biceps recovery by 4 months have a high likelihood of full function. Infants in groups III and IV are unlikely to achieve full function without surgical intervention.
The majority of BPBIs involve the upper trunk. The classic Erb palsy involves C5-6 (46%). The next most common injury (an extended upper trunk lesion or extended Erb’s palsy) involves C5-7 (29%). With these injury patterns, the injury is most frequently postganglionic. When the lower plexus is involved, preganglionic avulsions of C8-T1 are most common, with additional avulsion of C7 often present. The exception to this situation is an upper trunk lesion seen with a breech delivery. These nerve injuries tend to be preganglionic C5-6 avulsions from the spinal cord and are more commonly bilateral in presentation compared with vertex deliveries.
Most authors use the timing of specific motor function recovery and the absence of recovery as an indication for surgical intervention. , Wyeth and Sharpe in 1917 advised surgical intervention if no recovery was seen by 3 months of life. Gilbert and Tassin concurred with the 3-month time interval and used recovery of antigravity biceps function as the key indicator of spontaneous recovery of the brachial plexus. Waters similarly found biceps recovery to be statistically reliable. Laurent and colleagues advised monitoring biceps, triceps, and deltoid function.
Clarke and associates described the timing of return of elbow flexion and elbow, wrist, finger, and thumb extension as discriminators of outcome and used a combined threshold score of motor recovery of these muscles as an indication for nerve surgery. , Ultimately, Clarke advised that recovery at 9 months be assessed with a “cookie test” (i.e., inability to bring a cookie to the mouth with the arm at the side) to predict outcome and determine the need for microsurgical intervention in difficult cases with mixed recovery.
Grading recovery of specific muscles in an infant is difficult. Many centers use the Medical Research Council (MRC) muscle grading system to define results. The schema classifies muscle strength as 0 (no contraction), 1 (trace contraction), 2 (active motion with gravity eliminated), 3 (active motion against gravity), 4 (active motion against gravity and resistance), and 5 (normal power). However, this system requires volitional contraction and participation, which is not feasible in an infant and difficult in a young child. Understanding these difficulties, Gilbert and Tassin modified the MRC grading system to a four-grade classification using 0 (no contraction), 1 (contraction without movement), 2 (movement with gravity eliminated), and 3 (complete movement against the corresponding weight of the extremity).
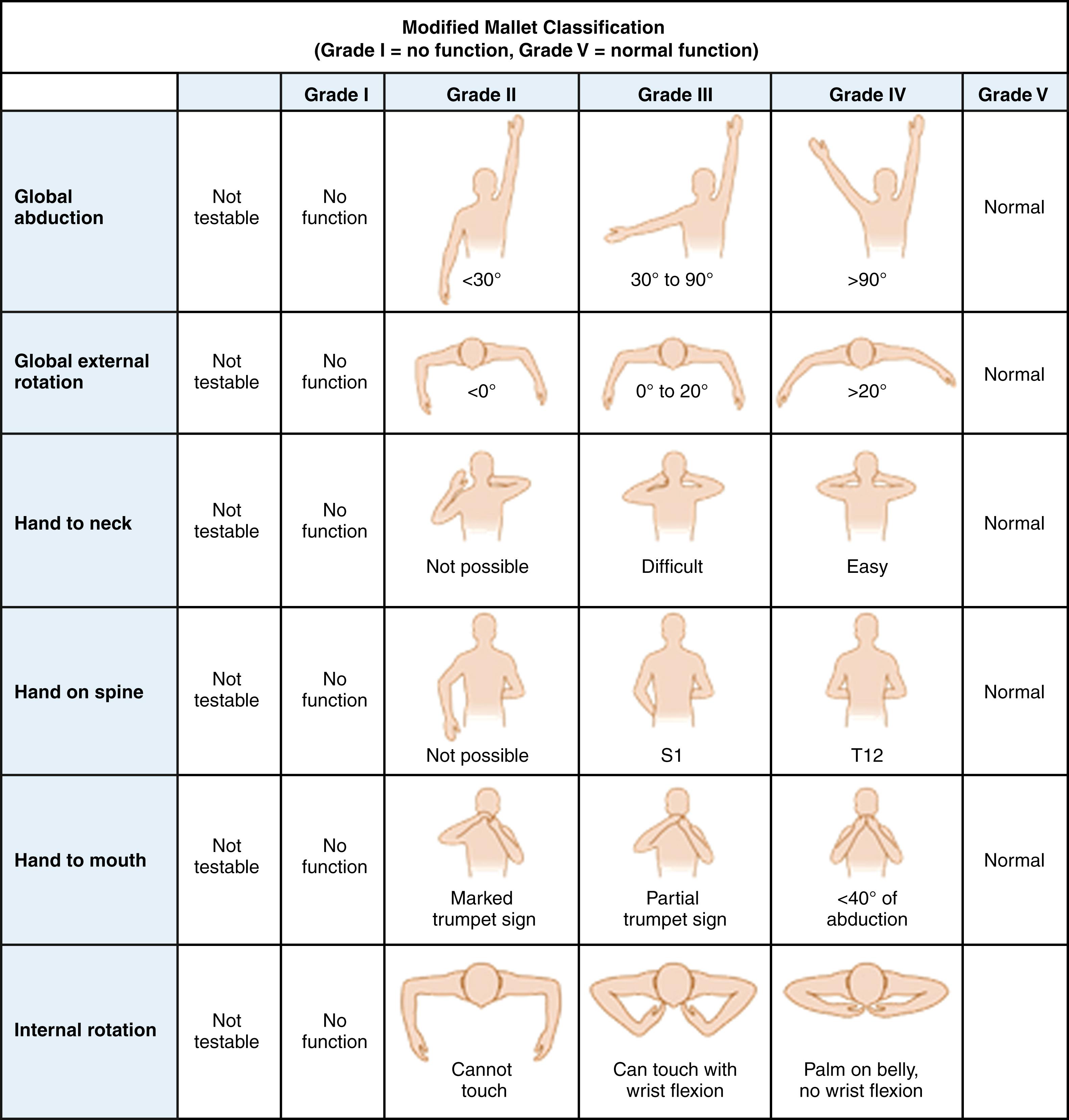
The modified Mallet classification ( Fig. 40.4 ) provides a more global motor functional assessment of the upper trunk as opposed to isolated muscle testing. This global shoulder motion system assesses hand-to-mouth, hand-to-neck, hand-to-spine, and hand-to-belly activities on a scale of 1 to 5. A sixth category of “internal rotation” has been added to better balance the Mallet classification with regard to external and internal rotation. A score of 1 is no function, 5 is normal function, and grades 2 to 4 denote progressive improved strength, as shown in Fig. 40.4 . The majority of late 20th century papers on infantile brachial plexus have used these classification schemes to determine the results of spontaneous recovery and surgical intervention.
More recently, Clarke and coworkers advocated use of the Hospital for Sick Children Active Movement Scale (AMS) ( Table 40.2 ), a grading system that divides muscle strength into movement with gravity eliminated (grades 0 to 4) and movement against gravity (grades 5 to 7). This system requires that full active motion with gravity eliminated to occur (grade 4) within the available range of passive motion before scoring antigravity muscle strength (5 to 7). The Toronto Test Score, a similar grading of specific muscle functions at the elbow, wrist, and hand, is used only in infancy to predict the need for surgical nerve reconstruction, and it is not designed to track progressive recovery over time ( Table 40.3 ). Thus no scoring system is without limitations.
| Gravity Eliminated | Score |
|---|---|
| No contraction | 0 |
| Contraction, no motion | 1 |
| <50% motion | 2 |
| >50% motion | 3 |
| Full motion | 4 |
| Against Gravity | |
| <50% motion | 5 |
| >50% motion | 6 |
| Full motion | 7 |
| Shoulder abduction | 2 |
| Shoulder adduction | 7 |
| Shoulder flexion | 2 |
| Shoulder external rotation | 0 |
| Shoulder internal rotation | 7 |
| Elbow flexion | 0 |
| Elbow extension | 7 |
| Forearm supination | 0 |
| Forearm pronation | 7 |
| Wrist flexion | 7 |
| Wrist extension | 2 |
| Finger flexion | 7 |
| Finger extension | 7 |
| Thumb flexion | 7 |
| Thumb extension | 7 |
| Total | 97 |
| Grade | Weight | |
|---|---|---|
| No joint movement | 0 | 0.0 |
| Flicker | 0 | 0.3 |
| <50% ROM | 1− | 0.6 |
| =50% ROM | 1 | 1.0 |
| >50% ROM | 1+ | 1.3 |
| Good but not full | 2− | 1.6 |
| Full ROM | 2 | 2.0 |
| Elbow flexion (0–2) | ||
| Elbow extension (0–2) | ||
| Wrist extension (0–2) | ||
| Finger extension (0–2) | ||
| Thumb extension (0–2) | ||
| Total | 7 | 6.8 |
The Mallet, Toronto Test Score, and AMS are statistically reliable in terms of interobserver and intraobserver analyses. , As might be expected, intraobserver reliability is better than interobserver, and less-complicated systems (Mallet) have higher reliability than more complicated schemes (AMS). The Pediatric Outcomes Data Collection Instrument (PODCI), which assesses parent and patient-reported function, has also been shown to be a reliable tool for measuring baseline function in children with chronic brachial plexus injuries against normative per age data, as well as for assessing postoperative changes in these children. , However, parent reports of their child’s function can be confounded by litigation against the delivery team, as this injury carries with it a strong emotional component that affects perceptions of function. This information is critical for comparing results and for assessing multicenter studies of therapeutic interventions for BPBI.
Recently the recognition that objective measurements and legacy measures may not correlate with enhanced function has resulted in a paradigm shift in outcome assessments. The initiative toward patient- or caregiver-reported outcomes has resulted in the development of reliable subjective measures. For children with brachial plexus injury, Shriners Hospitals for Children has developed patient-reported outcomes using the computer adaptive test (CAT) approach. , This methodology has been shown to correlate with legacy measures, discriminate among brachial plexus impairment levels, possess minimal floor and ceiling effects, and be less burdensome compared with previous measurement systems.
Invasive and noninvasive radiographic studies, such as myelography, combined myelography and computed tomography (CT), and magnetic resonance imaging (MRI), , have been used in an attempt to distinguish between preganglionic avulsion injuries and postganglionic extraforaminal ruptures. Kawai et al. compared all three techniques with operative findings in infants. Myelography had an 84% true-positive rate with 4% false-positive and 12% false-negative rates. The addition of CT to myelography increased the true-positive rate to 94%. The presence of small diverticula was only 60% accurate in diagnosing an avulsion; however, the presence of large diverticula or frank meningoceles was universally diagnostic. MRI had a true-positive rate similar to that of CT myelography but also allowed extraforaminal assessment of the plexus. MRI allows evaluation for possible double-crush injuries. High spin echo MRI, MR myelography, and MR neurography improve the resolution of MR analysis. MRI has the potential advantage of sedation only inasmuch as myelography requires general anesthesia for an infant. These radiographic studies may improve the quality of preoperative planning, but the final decision regarding the presence or absence of an avulsion injury is determined at the time of surgery.
Electrodiagnostic studies with electromyography (EMG) and nerve conduction velocity (NCV) have also been used in an attempt to improve diagnostic accuracy of the severity of the nerve injury. The presence of normal sensory nerve conduction in the absence of motor nerve conduction is diagnostic of root avulsion. Absence of reinnervation at 3 months is suggestive of an avulsion. The H-reflex has been shown to help prognosticate outcome. Unfortunately, the presence of motor activity in a muscle has not been accurate in predicting an acceptable level of clinical motor recovery in that muscle. With EMG, the presence of partial reinnervation can confuse the clinical picture. , ,
Studies have shown that nearly normal findings on EMG can be seen in infants with a severe lesion or even root avulsion. , , , Frequently, there are substantial discrepancies between preoperative electrodiagnostic testing (EMG and nerve conduction), intraoperative somatosensory-evoked potential testing, and surgical findings. A potential source of this discrepancy is the plasticity of the infantile nervous system. For example, Slooff documented innervation of the deltoid and biceps from C7 in the presence of avulsions of C5 and C6. At this stage, it is clear that neurophysiologic studies may underestimate the severity of injuries and provide a false optimism regarding recovery. It has been estimated that 20% to 25% of infants with a BPBI will require microsurgical intervention and that the clinical information is the decisive factor in determining indications for surgery. At present, most centers and brachial plexus microsurgeons ultimately rely on the physical examination findings over time to assess recovery and decide on surgical intervention. A regression analysis of data from several Brachial Plexus centers demonstrated that certain early physical examination findings independently predicted microsurgical reconstruction. The rate of surgery increased with the number of factors found on physical examination. These factors included a Horner’s sign and less than antigravity (AMS <5) hand/wrist flexion, elbow flexion, and wrist extension. Patients having 0/4 factors never underwent surgery, while patients with 1 (22%), 2 (43%), 3 (76%), and 4 (93%) had increasing rates of surgical reconstruction.
Despite difficulty in assessing muscle strength in infants, serial physical examinations remain the best method for predicting prognosis for recovery.
Current imaging and electrodiagnostic studies have been unreliable in predicting recovery.
The natural history of BPBI was initially thought to be uniformly favorable; however, recent studies portend a more guarded prognosis. Assessing history is complicated by the lack of true natural history studies. In a systematic review of 76 studies discussing the natural history of BPBP, no paper met all four established criteria for an unbiased study and only two studies met even two criteria. In these papers, residual neurologic deficits existed in 20% to 30% of children. Subsequently, the rate of permanent neurologic deficit has been found to be as high as 40%. A true natural history likely may never be elucidated as doing so would require withholding beneficial treatment that would optimize outcomes. Despite limitations in natural history studies, there is sufficient evidence to draw certain conclusions, as follows.
The majority of brachial birth palsies are transient. Infants who recover full function within the first 1 to 2 months of life have normal development of upper limb function.
Infants who recover partial antigravity upper trunk muscle strength during the first 2 months typically improve gradually over the first 1 to 2 years of life and most often do not require surgical intervention.
Infants who do not recover antigravity biceps strength by 5 to 6 months of life should undergo microsurgical reconstruction because successful surgery would result in a better outcome than the natural history alone.
Infants with partial recovery of C5-7 antigravity strength during months 3 to 6 of life will have permanent limitations in motion and strength, as well as risk for the development of progressive joint contractures.
Infants with global injuries and avulsion injuries of the lower trunk with have the worst prognosis and most limitations, with lifelong dysfunction in the arm and hand. These infants are considered candidates for microsurgical reconstruction as early as 3 months of age if no substantial recovery has been identified.
The major clinical dilemma resulting from the uncertainty regarding spontaneous neurologic recovery is to determine whether infants without antigravity return of C5-7 function at 3, 4, 5, or 6 months of age warrant surgical exploration and nerve reconstruction. These infants have varying degrees of nerve injury (Sunderland types II to IV), and their ultimate neuromuscular recovery without intervention or with just tendon transfers has still not been compared with their recovery after microsurgery plus tendon transfers. Ideally, this question would be addressed in a prospective randomized clinical trial with sufficient enrollment ; however, such a study has been difficult to perform due to varying clinical practices and beliefs among surgeons at different centers.
Parents, primary care physicians, and therapists are under substantial emotional pressure to do what they believe is best for the affected infant. Unfortunately, despite strong opinions and even solicitous pressure from specific medical centers, data are still insufficient to definitively answer the question. Comparative analysis has shown that elbow flexion alone is an insufficient criterion to warrant nonoperative care and that useful motor function has been observed in patients without elbow flexion at 3 months of age. ,
Disagreement among surgeons is common regarding surgical indications. This lack of clarity creates large regional variations in care (also known as unexplained clinical variation) and makes decision making difficult for parents. Accessing information from support groups and the Internet is common. This input affects parents’ decision making regarding care of their child. , The quality of that information, however, can be variable. The long-term outcome with regard to function, activities of daily living, pain, career choice, and personal life is limited. The minimal data collected by support groups is often biased and infers ongoing difficulties in performing activities of daily living. Pain, as evidenced by self-mutilating behavior, is rare in infants, occurring about 3% to 4% of the time. In older children, pain has been variably reported in the affected limb, with most studies demonstrating mild pain that does not interfere with function. Chronic pain in adulthood is thought to be uncommon but the true incidence has yet to be documented. There are limited studies that have concluded that pain in the affected limb or back is common and may worsen as patients age.
The role, timing, and technique for nerve reconstruction are controversial issues. , The original surgical interventions on the brachial plexus at the turn of the 20th century consisted of resection of the neuroma and direct repair. Kennedy initially described three cases in 1903 with subsequent reports by Wyeth and Sharpe in 1917 and Taylor in 1920. In a report on 1100 infants with brachial plexus injury in 1925, Sever was uncertain of the benefit of surgical intervention. By the 1930s, brachial plexus nerve surgery had fallen out of favor. The advent of microsurgical techniques and extensive work in the 1970s and 1980s by Narakas, , Millesi, and Gilbert , , in Europe; Kawabata , , and others in Asia; and Boome and Kaye in South Africa led to the resurgence in brachial plexus microsurgical reconstruction. Now, medical centers throughout the world have plastic surgery, neurosurgery, hand surgery, and orthopedic surgery subspecialty brachial plexus centers actively performing brachial plexus nerve reconstruction.
The spectrum of nerve surgery includes neurolysis, neuroma resection and nerve grafting, and nerve transfers. Direct repair is rarely performed because of the extensive nature of the lesion and inability to achieve a tension-free nerve repair without grafting. Although neurolysis had been performed extensively, , most centers have abandoned its independent use. Clearly, there is no role for neurolysis in the presence of an avulsion injury. Neurolysis has been shown to have results no different from the natural history following global brachial plexus injury, and the evidence is similar (although less conclusive) for upper trunk ruptures.
Gilbert and Whitaker strongly believe there is no role for neurolysis alone. Laurent and colleagues advocated its use in conjunction with intraoperative electrodiagnostic studies. A neuroma-in-continuity was maintained if greater than 50% of a muscle action potential was found after neurolysis. Otherwise, the neuroma was resected and grafted. However, recovery of muscle strength following nerve grafting was superior to neurolysis despite the fact that the preoperative status of the neurolysis patients was better compared with the nerve grafted patients.
Capek and associates described better long-term results after resection and grafting of both conducting and nonconducting neuromas compared with neurolysis despite initial worsening of the situation following resection and grafting. Superiority of nerve grafting over neurolysis has also been shown by others. The topic of neurolysis has been recently revisited, although without clear evidence that neurolysis alone affects the natural history as many children would have improved spontaneously without surgery. Based on the present information, neurolysis alone is viewed as having little therapeutic benefit, and most authors have abandoned it in favor of neuroma excision and grafting. ,
The standard nerve reconstruction strategy is resection of the neuroma and sural nerve grafting with extraforaminal ruptures. In an upper trunk (C5-6) rupture with an extraforaminal neuroma-in-continuity, resection of the neuroma and sural nerve grafting are performed from the C5 and C6 roots to the most proximal healthy nerve tissue of (1) the upper trunk anterior division, lateral cord, or musculocutaneous nerve; (2) the upper trunk posterior division, posterior cord, or axillary and radial nerves; and (3) the suprascapular nerve ( Fig. 40.5A ). If the middle trunk is involved, additional grafting is necessary from C7 to the posterior cord (see Fig. 40.5B ). Nerve transfers are becoming more popular , , for reconstruction of C5-6 and C5-7 injuries, with transfer of (1) the intercostals or ulnar nerve fascicle to the biceps motor branch, (2) a branch of the spinal accessory nerve to the suprascapular nerve, and (3) the radial nerve long head of the triceps motor branch (if available and expendable) to the axillary motor branch. Results of nerve transfers for upper plexus lesions appear to be equivalent to nerve grafting at the shoulder and elbow.
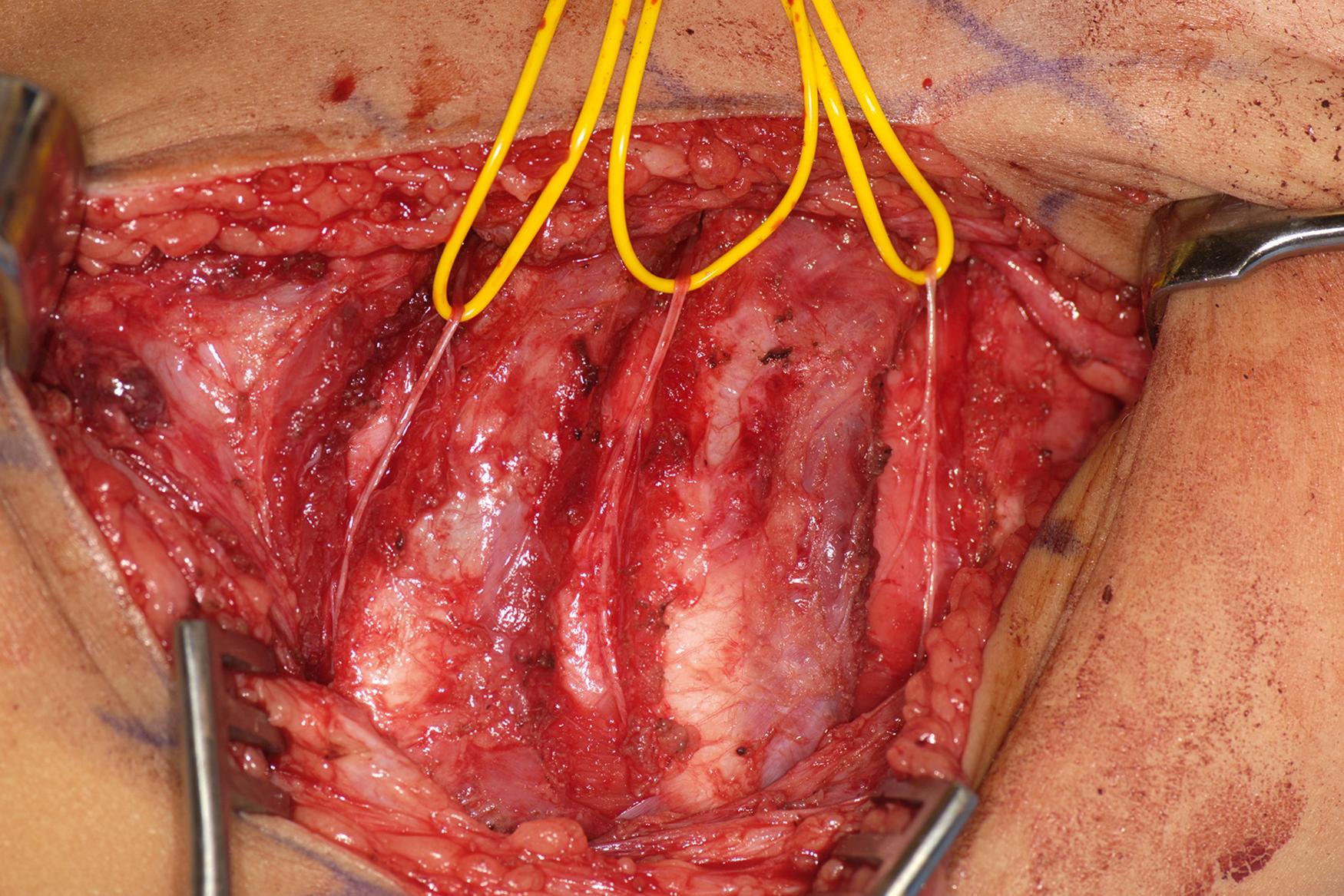
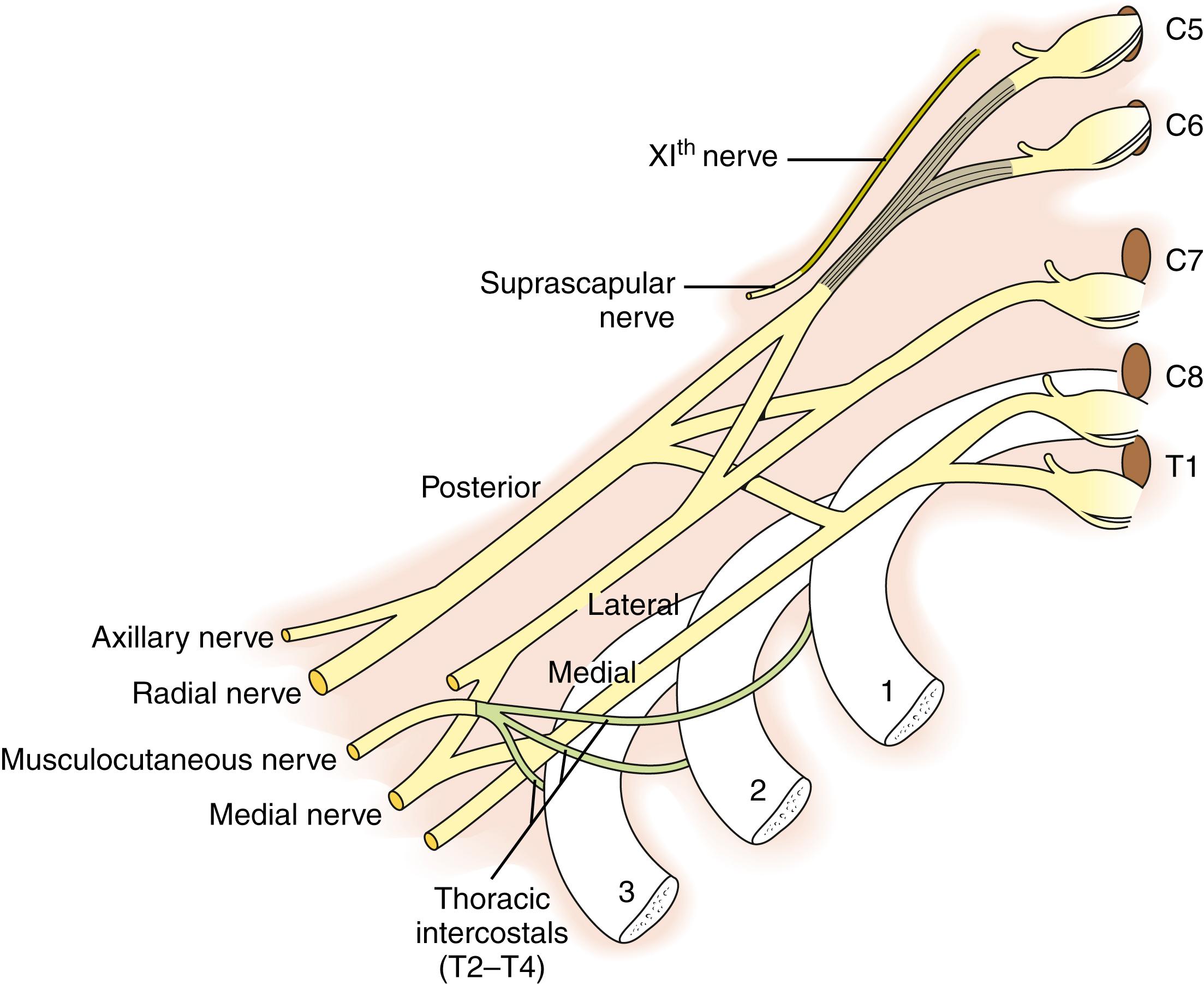
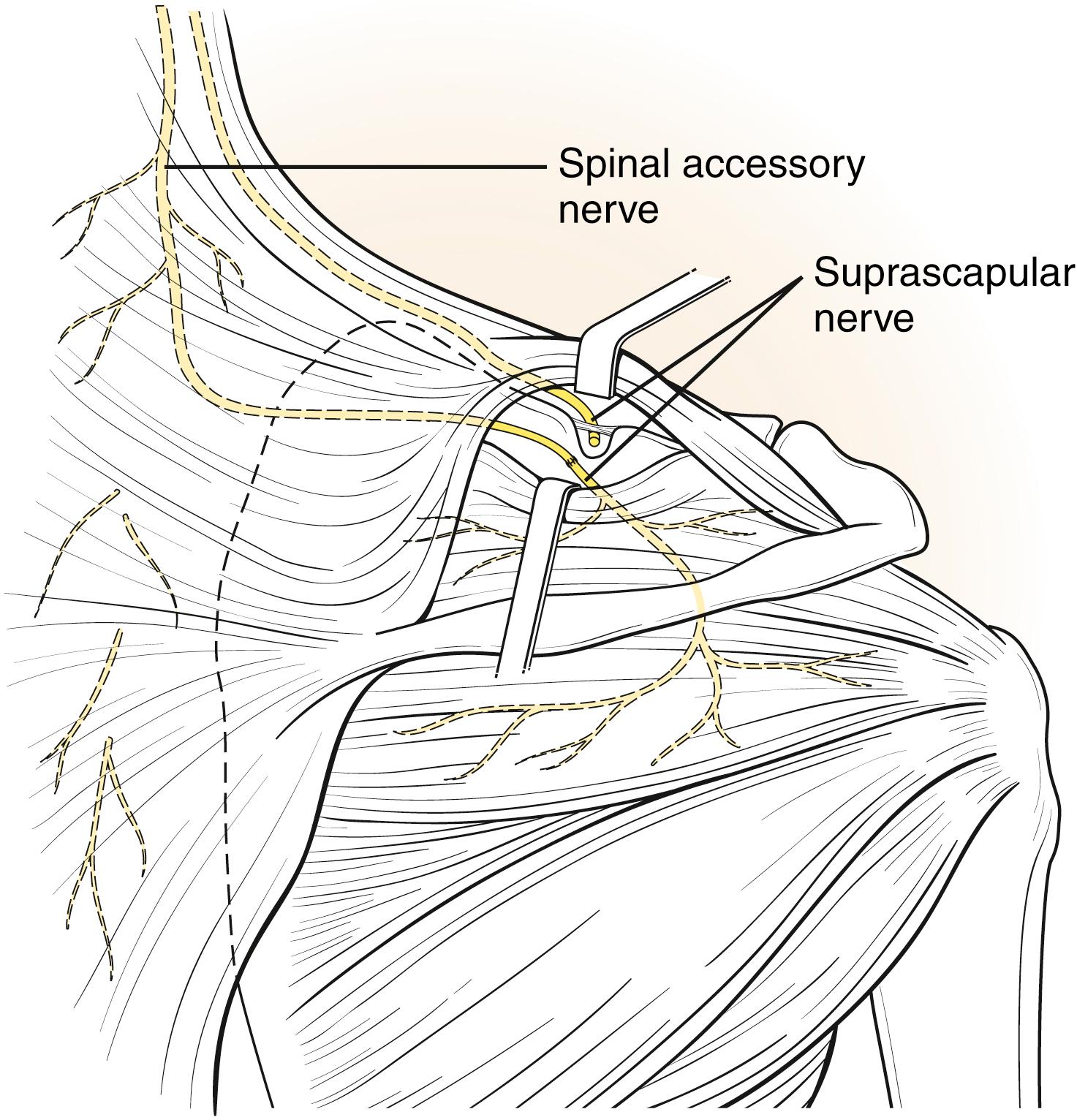
In the case of ruptures and avulsions, nerve transfers in conjunction with nerve grafting are performed by using the thoracic intercostals (T2-4) ( Fig. 40.6 ) or a branch of the spinal accessory nerve (cranial nerve XI) after it innervates the upper trapezius ( Fig. 40.7 ). The spinal accessory can be harvested from an anterior or posterior approach ( Fig. 40.8 ); however, there is the potential of a second area of injury to the suprascapular nerve at the suprascapular notch, which is best visualized using the posterior approach ( ![]() ). Nonetheless, the results appear to be similar with either approach, and additional surgery to later augment shoulder function is rare following this transfer. In patients with good shoulder abduction and elevation above shoulder height, transfer of the spinal accessory nerve directly to the infraspinatus branch of the suprascapular nerve has been performed. The results indicate early recovery of external rotation, which appears to be more consistent compared with transfer to the suprascapular nerve proper. , With total plexus avulsions, nerve transfers are the only reconstructive option and may include the intercostals, spinal accessory, and contralateral C7. Carlstedt and coworkers have performed experimental and limited clinical work on direct reimplantation or grafting into the spinal cord. Currently, the suboptimal results and the potential risks (cervical instability following laminotomy or nerve injury to the uninvolved limbs) preclude its use.
). Nonetheless, the results appear to be similar with either approach, and additional surgery to later augment shoulder function is rare following this transfer. In patients with good shoulder abduction and elevation above shoulder height, transfer of the spinal accessory nerve directly to the infraspinatus branch of the suprascapular nerve has been performed. The results indicate early recovery of external rotation, which appears to be more consistent compared with transfer to the suprascapular nerve proper. , With total plexus avulsions, nerve transfers are the only reconstructive option and may include the intercostals, spinal accessory, and contralateral C7. Carlstedt and coworkers have performed experimental and limited clinical work on direct reimplantation or grafting into the spinal cord. Currently, the suboptimal results and the potential risks (cervical instability following laminotomy or nerve injury to the uninvolved limbs) preclude its use.
Gilbert and Whitaker as well as Slooff advocated that microsurgical priority be given to hand reconstruction in infants with extensive avulsions and limited nerve options. , Unlike adults, infants with global brachial plexus injuries have the potential to regain hand function after nerve grafting or nerve transfers, due to the shorter distance from the injury to the target muscles. In each microsurgical case, the plan must be individualized depending on the time from injury, extent of the palsy, and available reconstructive options.
Although there is ongoing debate about the timing of microsurgical intervention, the most common criterion is the absence of return of biceps muscle function associated with (1) global brachial plexus injury and Horner’s syndrome at 2 to 3 months or (2) an upper trunk lesion at 5 to 6 months. Reconstruction is performed between 3 and 9 months of age at various centers. The degree of subsequent muscle recovery is debatable and interobserver error confounds comparative analysis. Gilbert and others advocated for microsurgery when recovery of antigravity biceps function is not seen by 3 months of life. , , The reasons for early intervention include less risk for irreversible loss of motor endplates secondary to prolonged denervation and better parental acceptance of surgical intervention when the limb is flail or has minimal motion.
Prospective studies by Al-Qattan and Waters, however, have indicated that recovery of antigravity biceps function by 4 and 5 months of age, respectively, results in equivalent outcomes compared with microsurgery, especially when combined with secondary tendon transfers to improve shoulder motion. Similarly, others have demonstrated functional equivalent results (ranging from 12% to 55% of children lacking biceps function at 3 months old) to children undergoing nerve grafting. , Finally, Clarke advocated microsurgery as late as 9 months for infants who fail the “cookie test” and thus have less than grade 6 elbow flexion strength on the AMS. Microsurgical reconstruction at that relatively late time was overwhelmingly positive. A recent review of the Treatment and Outcomes of Brachial Plexus Injury (TOBI) database analyzed the recovery of patients following microsurgery after 9 months of age. All patients had improved function, and there were no differences between those patients who underwent neuroma excision and nerve grafting and those that underwent nerve transfers. Additionally, a recent comparison cohort from the same TOBI database demonstrated equivalent outcomes from microsurgical reconstruction performed at less than 6 months of age and those aged 6 to 22 months of age, even when controlling for injury severity. Therefore, either the motor endplates of infants may be more resilient to irreversible demise than those of adults or incomplete denervation of the affected muscles due to redundant innervation preserves motor endplate viability.
Ultimately, the best time for microsurgical intervention in children with extraforaminal nerve rupture is still unknown. Comparative analysis indicates that biceps function alone is an insufficient criterion for operative management as some patients without biceps function by 3 months old recover useful limb function without microsurgery. , , , There is little consensus regarding the timing and type of procedures performed. Economic analysis indicates that microsurgical intervention at 3 months for nerve rupture is unlikely to be successful enough to produce cost savings for the payor. Most centers and surgeons recommend that nerve surgery be indicated at 3 months of age for total plexus lesions and delayed for an isolated injury of the upper plexus.
The problem with reviewing the results of microsurgery is that few patients (1) have long-term follow-up or (2) undergo microsurgery alone. Gilbert and Tassin’s original study compared microsurgery with spontaneous recovery. For C5-6 lesions, 100% recovered average Mallet grade III shoulder motion in the spontaneous recovery group. In the nerve surgery cohort, 37% regained grade III and 63% obtained grade IV Mallet scores. With C5-7 lesions, 30% recovered grade II and 70% grade III in the spontaneous recovery group. With nerve surgery, 35% regained grade II, 42% grade III, and 22% grade IV. Gilbert and Whitaker reported that 81% of patients achieved Mallet scores of III, IV, or V for abduction following C5-6 nerve reconstruction. When combined with secondary shoulder reconstruction, these results improved to 70% of patients achieving Mallet grades IV or V at 5-year follow-up. These results are superior to their previously reported outcomes following total plexus reconstruction, where 64% achieved Mallet scores of grade III or IV at greater than 2-year follow-up.
When combined with secondary shoulder reconstruction, at 5 years these results increased with 70% gaining a Mallet abduction grade of IV or V following C5-6 reconstruction. Similarly, with total brachial plexus injury reconstructions that prioritized hand function, there were fewer gains in shoulder function. At 2-year follow-up, there were only 25% of patients with grade III or IV shoulder function; 70% with grade III, IV, or V elbow function; and 35% with grade III or IV hand function using Gilbert’s classification scheme. With the addition of secondary procedures (shoulder and hand), this recovery increased to 77% in the shoulder and 75% in the hand at 6-year follow-up. Gilbert maintains that nerve surgery improves function compared with the natural history and increases the possibility for secondary tendon transfers. Long-term data from Finland indicate that microsurgical patients have persistent deficits and require assistance in activities of daily living. Approximately 50% of patients will benefit from further reconstructive surgery, mostly to augment function in the shoulder and forearm. In patients with C5-6 injuries, 75% of patients had good/excellent outcomes following nerve reconstruction. The most consistent factor associated with lack of recovery was severity of injury.
Recently, Manske and colleagues reviewed functional recovery of patients who underwent microsurgical reconstruction. Patients were offered an operation at 3 months of age for Narakas group III and IV injuries and at 6 months of age with insufficient elbow flexion recovery, and followed for a minimum of 2 years. Antigravity elbow flexion was recovered in 91% of patients and 67% regained antigravity shoulder abduction. The recovery of external rotation (19%) and wrist extension (37%), however, was much more limited. Additionally, the timing of recovery was variable, with elbow flexion and shoulder abduction recovering much sooner than shoulder external rotation (30 months). The authors noted that 77% of patients underwent one or more secondary surgeries, including tendon transfers for shoulder external rotation (49%), forearm pronation (21%), and wrist extension (21%). For their patients with a global palsy, hand function recovery was disappointing with 81% (13/16) recovered some active finger flexion and only 38% (6/16) regained grasp. These results should be compared with the natural history data available. Smith and coauthors published a study of 170 patients to assess the natural history of BPBI with regard to timing of biceps recovery. Twenty-eight patients with absent biceps at 3 months of age were followed long term. Biceps contractions were observed in 20 patients (71%) by 6 months of age. Ultimately, 27 out of 28 had at least antigravity biceps muscle function. Patients who regained biceps between 3 and 6 months had better Mallet scores than patients who achieved biceps muscle function after 6 months of age. Twelve (55%) of the 22 patients who did not have brachial plexus surgery had grade IV shoulder function. The authors concluded that the long-term function was favorable for the majority of C5-6 infants without nerve surgery. Similarly, a longitudinal study from Scotland followed 232 patients born prior to 2008, when their brachial plexus center initiated microsurgery. Of those patients, 80 had a full spontaneous recovery and were discharged. One hundred forty-nine of the 152 patients remaining were classified according to Narakas (58: I, 55: II, 24: III, 12: IV). Perusal of these patients noted that spontaneous elbow flexion began around 4 months in the Narakas I group, 6 months in group II, 8 months in group III, and 12 months in group IV. Furthermore, all of their patients, except for one in group IV, recovered the ability to reach their month, indicating antigravity elbow flexion. However, the authors did not report on shoulder, wrist, or hand function. In addition, the minority of patients had recorded range of motion of the entire arm and elbow flexion strength measurements.
Zancolli and Zancolli found that 82% of affected infants had biceps function recovery and 75% began between 4 and 5 months. Waters addressed the same issue and found that of the 49 infants with no biceps recovery at 3 months, 42 recovered biceps function by 6 months. Reassessing function at 2 years of age noted that infants with recovery of biceps function between 3 and 6 months had a progressive decrease in Mallet grades (abduction, external rotation, hand-to-mouth, and hand-to-neck) stratified by month of biceps recovery. In infants with biceps recovery between 3 and 6 months of age, recovery of function by Mallet grade was as follows: (1) global abduction II (3%), III (52%), and IV (45%); (2) global external rotation II (54%), III (31%), and IV (15%); (3) hand-to-neck II (39%), III (33%), and IV (28%); and (4) hand-to-mouth II (33%), III (24%), and IV (43%). These results are similar to Gilbert’s published microsurgical results.
In both natural history and nerve surgery patients, secondary shoulder transfer and osteotomies improve function. For patients with recovery of biceps function between 3 and 6 months of life and later shoulder reconstruction surgery, there will be an expected improvement for all Mallet movements that involve external rotation and abduction to an average grade IV. However, in both natural history and nerve surgery patients with incomplete recovery, there may be some deterioration in function with time. , Other studies, however, have demonstrated that AMS scores are maintained at 10-year follow-up. A driving remaining question is, How different are patients who undergo nerve surgery at 3 months from those who recover biceps function between 3 and 6 months and undergo secondary reconstruction? This answer is critical to resolve the question as to whether unnecessary surgery is being performed or whether some centers are failing to perform necessary nerve surgery at a young age. This controversy remains unresolved because of lack of comparable data. , ,
The increasing use of nerve transfers further complicates the indications for nerve grafting. Nerve transfers utilize direct neurorrhaphy at a site closer to the target muscle. The more distal location of the neurorrhaphy allows a shorter regeneration time, faster recovery, and a longer window of time to await spontaneous recovery before fearing irreversible motor endplate demise. Therefore surgeons may prolong the decision to intervene when viable nerve transfer options are available. Ladak et al. reported results of 10 patients treated with three nerve transfers (i.e., spinal accessory-to-suprascapular, radial-to-axillary, and ulnar or median fascicle-to-musculocutaneous) at 10 to 18 months of age. Recovery progressed between 6 and 24 months following surgery with ultimate function equivalent to published results of nerve grafting that was perfomed at a younger age. More recently, O’Grady et al. reported on the results of a prospective cohort study that assessed the treatment of 26 patients who had failed the “cookie test” at 9 months of age. Twelve patients underwent brachial plexus exploration, neuroma excision, and sural nerve grafting, while 14 patients underwent triple nerve transfers as described by Ladak et al. above. Both groups demonstrated similar recovery of motion, with the nerve transfer group having improved external rotation and supination, as well as lower rates of secondary surgery, lower hospital costs, and overall faster recovery.
Tse demonstrated equivalent restoration of shoulder external rotation between patients treated with nerve grafting from C5 to the suprascapular nerve and those treated with spinal accessory nerve to suprascapular nerve transfer, noting that the latter patients had more severe brachial plexus injuries. Similar encouraging results of spinal accessory-to-suprascapular nerve transfer have been found by others, , even in patients treated as late as 30 months of age. , The superiority of nerve transfer surgery compared with nerve grafting to the suprascapular nerve was recently demonstrated utilizing a review of the TOBI database. However, the overall recovery of shoulder external rotation was notably poor with both techniques. The average postoperative AMS recovery was 2.7 in the nerve graft group and 3.2 in the nerve transfer group. Only 5% of patients in the nerve graft group compared with 24% in the transfer group achieved a functional recovery of AMS of 6 or 7. Additionally, secondary shoulder intervention including botulinum toxin injections (37% versus 10%) or tendon transfer surgeries (53% versus 40%) were more common in the nerve graft group.
Similarly, Segal et al. reported on their results of spinal accessory-to-suprascapular nerve transfer either when combined with nerve grafting surgery or as an isolated transfer in older infants. While all patients improved, the overall AMS recovery was low with only 58% of patients recovering antigravity (AMS >5) function of either shoulder forward flexion, abduction, or external rotation. However, they reported that results continued to improve up to 3 years, and less than 25% of patients required further surgical intervention to augment shoulder function.
Nerve transfers can be performed directly to the infraspinatus branch of the suprascapular nerve, distal to the spinoglenoid notch, to maximize shoulder external rotation recovery. This nerve transfer should be performed in patients with good shoulder abduction, as the transferred axons are directed to the infraspinatus muscle and will not augment supraspinatus function. The other surgical prerequisites are a concentric glenohumeral joint without dysplasia and passive external rotation (ER) ≥45 degrees. , This transfer has been performed with good success, even outside the traditional “window” for nerve surgery, likely due to some partial muscle regeneration that maintains some infraspinatus motor endplate viability. Subsequently, direct transfers with sufficient axonal ingrowth provide adequate power for active external rotation. ,
Elbow flexion can also be restored via nerve transfer. In a multicenter study, Little and colleagues outlined the indications and results following for ulnar and/or median fascicle transfer to restore elbow flexion and supination. Elbow flexion could reliably be restored in children as old as 18 months of age. Supination recovery was more dependent on age and better supination followed double-fascicle transfer (median and ulnar to both biceps and brachialis) compared with a single-fascicle transfer to the biceps.
Ultimately, the decision between nerve grafting and nerve transfers should be individualized for each patient. Clearly, C5-6 avulsion injuries that cannot be treated with grafting are ideal candidates for nerve transfers. Similarly, dissociative recovery can occur, where only some of the muscles innervated by the upper trunk have recovered sufficient function, and the surgeon’s goal is to restore innervation of the remaining paralyzed muscles without resecting the upper trunk. In such cases, a la carte nerve transfers can be targeted to specific distal motor nerves. Nonetheless, precise indicators for nerve transfer surgery instead of brachial plexus microsurgical reconstruction remain unclear at the present time.
The imperfect outcomes following nerve reconstruction in BPBI may be related to the susceptibility of the developing neonatal nervous system to deleterious effects following peripheral nerve injury. Importantly, peripheral axotomy in neonatal animal models causes death of approximately half the motor neurons in the spinal cord at the relevant level. In contrast, virtually no motor neuron cell death follows adult peripheral axotomy. , Nerve root avulsion causes death in approximately 70% of motor neurons in neonates and adults; however, neuron loss occurs three times faster in neonates. Similarly, afferent neurons undergo similar apoptosis in the dorsal root ganglion following peripheral axotomy. , These effects are not only simply localized to the neuron corresponding to the cut axon but also involve contralateral spinal cord neurons as well as interneuron connections. Although the mechanisms of this cell death remain incompletely understood, prevention of neuronal cell death following peripheral nerve injury in neonates is a potential opportunity for improving outcomes following nerve reconstruction. Nonetheless, the surgeon reconstructing a brachial plexus lesion in an infant must be aware that even a seemingly viable root may lack 50% or more of its proximal neurons in the spinal cord.
The major determinant for surgical intervention is the lack of recovery based on repeated physical examination.
The role, timing, and technique of nerve surgery remain controversial issues.
The spectrum of nerve surgery includes neurolysis, neuroma resection and nerve grafting, and nerve transfers. Direct repair is not typically possible because of the etiology and extent of the lesion.
Neurolysis has a limited role in brachial plexus birth palsy as long-term studies have not supported its efficacy. Most brachial plexus centers have abandoned neurolysis in favor of neuroma excision and sural nerve cable grafting.
Neuroma resection with sural nerve grafting is the standard microsurgical care for extraforaminal ruptures, although nerve transfers have an evolving role. A combination of nerve grafting and nerve transfers is performed on infants with severe lesions.
The outcome after microsurgery is difficult to decipher, but patients do improve function, although many require further secondary surgery to maximize function.
Studies imply that successful nerve surgery performed in infants who do not recover between 5 and 8 months of age will have a better outcome compared with the natural history.
Multicenter studies are necessary to truly elucidate the natural history and the microsurgical results.
In contrast to adults, nerve grafting to the lower trunk can achieve useful hand function and should be a priority for children.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here