Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Trephine biopsy of the bone marrow has wide application in clinical medicine; its greatest utility is in the evaluation of patients with malignant lymphoma, acute leukemias, myeloproliferative neoplasms (MPNs), myelodysplastic syndromes (MDSs), metastatic tumor, granulomatous disorders, myelofibrosis, aplastic anemia, and plasma cell dyscrasias. It also serves as the most reliable method for assessing marrow cellularity following the administration of antineoplastic drugs and in assessing the status of engraftment following hematopoietic cell transplantation. Marrow biopsy is also utilized in the investigation of patients with infectious disease and metabolic disorders.
The trephine biopsy should be viewed as one component of the bone marrow specimen that should also include smears and particle crush preparations from aspirated marrow and touch imprint preparations of the core biopsy specimen. In some instances, because of marrow fibrosis, the trephine biopsy specimen will be the only marrow tissue available for examination. Marrow biopsy can usually be done with relatively little discomfort to the patient and is accompanied by very low morbidity when performed by experienced individuals with the biopsy needles presently available. The posterior superior iliac spines are the preferred sites. Bilateral trephine biopsies may be useful in the staging of patients with some types of lymphoma, granulomatous disorders, and metastatic tumor, although improved imaging studies have replaced bilateral staging marrow biopsies in some institutions. This approach should be decided on an individual basis, considering both the reason for the procedure and the impact of positive findings for therapy. In general, severe thrombocytopenia is not a contraindication to marrow biopsy. Whenever a marrow biopsy is performed, careful attention should be directed to preventing hematoma formation by applying an adequate pressure bandage to the biopsy site following the procedure. Prior to performing a biopsy on a patient with a bleeding disorder or on anticoagulants, consultation should be obtained from a physician expert in coagulation disorders.
Considerable discussion has occurred about the relative merits of trephine biopsy of the bone marrow as opposed to sections of particles obtained by aspiration biopsy. Particle sections are of limited value in marrow disorders that are accompanied by fibrosis; these frequently result in inadequately aspirated specimens. In addition, assessment of cellularity, determination of the extent of marrow involvement by neoplastic processes, and the relationship of lesions to marrow structures such as bone trabeculae and vasculature can be accurately assessed only in trephine biopsies. Nevertheless, any particles obtained in a marrow aspirate should be processed for histologic examination, as this material provides additional sampling that may be important when disease involvement is focal.
Paraffin embedding is the preferred method for the routine processing of bone marrow biopsies, and the observations described in this chapter are based primarily on examination of specimens prepared in this manner. Plastic embedding offers some advantages over the paraffin method, such as excellent cytology and the ability to perform numerous histochemical reactions. However, plastic embedding techniques are more time consuming than the processing of paraffin-embedded tissue, and with careful attention to technical detail, excellent results can be obtained with specimens processed in paraffin. Additionally, immunohistochemistry with a wide range of antibodies can now be performed with excellent results on most paraffin-embedded specimens. For these reasons, plastic embedding is not routinely performed in most laboratories at this time.
Myelofibrosis is one of the more vexing problems in bone marrow histopathology because of the wide range of disorders that may cause marrow fibrosis and the usual difficulty in obtaining satisfactory aspirates for cytologic studies. Although marrow fibrosis occurs as a primary disorder, it is usually a secondary phenomenon; the most common causes are metastatic tumor and malignant lymphoma. Fibrosis also occurs relatively frequently in the evolution of chronic MPNs, such as chronic myeloid leukemia (CML) and polycythemia vera (PV). In general, fibrosis that occurs as a component of hematopoietic proliferations is characterized initially by the deposition of increased reticulin fibers; metastatic tumors such as breast and prostate are usually characterized by a severe desmoplastic reaction with collagenous fibrosis and possibly osteosclerosis.
In those instances in which the etiology for the marrow fibrosis is not apparent, several techniques may be used in an attempt to determine the cause. Immunohistochemistry, using paraffin-embedded specimens and the several antibodies described in the section on immunohistochemistry, may be particularly helpful in identifying lymphoma or metastatic tumor; antibodies to myeloperoxidase, lysozyme, CD14, CD68, and CD117 are particularly useful for identifying cells of granulocytic or monocytic origin. An additional procedure that may be useful in hematopoietic disorders associated with marrow fibrosis is the preparation of particle crush preparations from trephine biopsy specimens. This approach may necessitate a second biopsy unless the problem of fibrosis is anticipated before the initial procedure. As soon as possible after the trephine specimen is obtained and before it is placed in a fixative, small portions of the biopsy are cut away with a sharp scalpel blade and used for particle crush preparations in the same manner as particles from aspirated specimens. These crush preparations can be used for routine stains, cytochemistry, and immunocytochemistry. Portions of the biopsy specimen obtained in this manner may also be used for cytogenetics, molecular genetics, flow cytometry studies, routine electron microscopy, and ultrastructural cytochemistry. However, electron microscopy is generally no longer performed because of the availability of immunohistochemistry.
The use of special stains in bone marrow pathology should be determined following review of the routinely stained aspirate smears and biopsy and the patient's clinical history.
Several instruments are available for the bone marrow trephine biopsy procedure. The most satisfactory from the standpoint of safety, ease of performance, and overall quality of specimen obtained is the Jamshidi-type biopsy needle; several such instruments, both reusable and disposable, are commercially available. These instruments are produced in several sizes for both adult and pediatric patients. The 11-gauge needle is the most commonly used for routine procedures in adults and older children. The 8-gauge instruments are preferred by some for lymphoma staging procedures; this size may result in more postbiopsy discomfort. If difficulty is encountered with the 11-gauge needle in obtaining adequate specimens from patients with severe osteoporosis, an 8- or 9-gauge instrument should be used. Numerous modifications of the original instruments are available.
The importance of proper technique in performing the biopsy procedure cannot be overemphasized. Instructions for the use of the biopsy needles are included with the instruments, and some manufacturers provide audiovisual aids that illustrate proper technique. Accurate identification of body landmarks is crucial in obtaining satisfactory specimens; an improperly positioned needle may cause considerable discomfort to the patient and frequently yields an inadequate biopsy specimen. Individuals not acquainted with the biopsy technique are advised to familiarize themselves with the procedure on cadavers.
Optimally, the biopsy specimen should be at least 1.5 cm in length and should be free of distortion caused by crushing or other damage. Crush artifact and the deposition of fibrin in torn biopsies may render accurate interpretation difficult or impossible. In such instances the biopsy should be repeated. Aspiration through the biopsy needle prior to obtaining the trephine biopsy specimen should be discouraged because of the possibility of introducing hemorrhage or other artifact in the biopsy specimen.
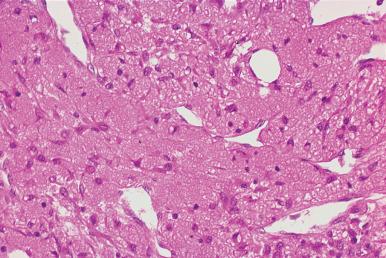
Occasionally, in patients undergoing frequent repeat biopsies, a specimen may be obtained from a recently biopsied site. In these instances, the changes will reflect the stage of healing. The presence of granulation tissue in the biopsy may result in misinterpretation of cellularity. The appearance of the marrow biopsy may be inconsistent with the marrow smears, which show recovering or normal hematopoiesis ( Fig. 39.1 ).
Imprint preparations should be routinely made from the biopsy specimen immediately after it is removed from the biopsy needle and prior to being placed in a fixative. These can be used for Romanowsky stains and special cytochemical and immunocytochemical procedures. Following the preparation of imprints, the specimen is placed in an appropriate fixative; the most satisfactory are B5, 10% buffered neutral formalin, and acetic acid–zinc-formalin (AZF). Zenker acetic acid gives excellent cytology but ablates several antigens that may be useful in evaluating neoplastic processes; importantly it and B5 contain mercury, which is environmentally hazardous. In laboratories where bone marrow specimens are processed with other tissues, buffered neutral formalin may be the preferred fixative. Because of the environmental concerns and disposal problems, some institutions prohibit the use of mercury-based fixatives. As noted in the section on immunohistochemistry, reactivity with some antibodies may be ablated by some fixatives, and the choice of fixative may be determined by the reason for the biopsy. Following fixation for an appropriate period of time, the biopsy is decalcified. Several decalcification solutions are commercially available. Most biopsy specimens will be adequately decalcified following 45–60 minutes in a rapid decalcifying solution. Most rapid decalcification methods, however, destroy nucleic acids making the samples unsuitable for molecular genetic studies. Formalin-fixed clot sections that do not undergo decalcification are commonly collected to overcome this limitation. Alternatively, slower decalcification with ethylenediaminetetraacetic acid (EDTA) allows for preservation of nucleic acids. A discussion of the relative merits and disadvantages of several fixatives and decalcifying agents used for bone marrow biopsies has been published.
The biopsies should be sectioned at 3–4 µm with a sharp knife that is checked frequently for the presence of defects. In those patients being evaluated for the extent of lymphomatous involvement, metastatic tumor, or granulomatous disease, the specimens should be completely sectioned and stepwise serial sections mounted for hematoxylin and eosin staining. The remaining ribbons should be numbered and retained in a manner that will facilitate the ready and accurate mounting of additional sections for special stains, immunohistochemical studies, and molecular studies. Most of the stains used for other fixed tissues are also applicable to bone marrow sections. However, tissue processed with acid fixatives, such as B5 and Zenker, or with acid decalcifiers will yield unsatisfactory results with the chloroacetate esterase stain.
Optimally, when interpreting the trephine biopsy, the pathologist should examine the trephine imprints, bone marrow aspirate, blood smears, and other pathology specimens. Knowledge of the patient's clinical history, hematology profile, immunoelectrophoretic studies, and radiographic findings may be of considerable importance and may greatly facilitate the interpretation of the biopsy specimen.
As in other areas of pathology, immunohistochemistry is an important resource in the evaluation of proliferative processes involving the marrow. The availability of antibodies reactive in paraffin-embedded tissue and the use of antigen retrieval have been of considerable importance in the application of immunohistochemistry in bone marrow pathology. Although cryostat sections of marrow may be used for immunohistochemistry, the procedure is difficult and is not routinely performed. In addition, cytologic preservation in cryostat sections is frequently of marginal quality.
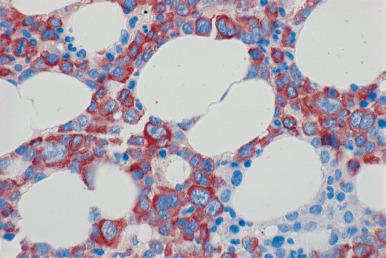
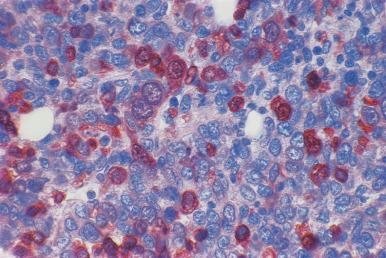
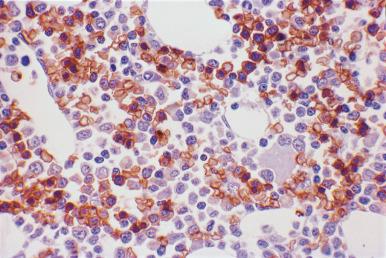
Although decalcification with rapid acid decalcifiers may result in ablation of some antigens, an increasing number of antibodies to membrane antigens and cytoplasmic constituents are reactive in paraffin-embedded decalcified marrow biopsies and can be of considerable aid in identifying the lineage of immature cell populations in the marrow; these include antibodies to kappa and lambda light chains, leukocyte common antigen (CD45), myeloperoxidase, CD33, CD117, glycophorin, hemoglobin A, E-cadherin, von Willebrand factor (factor VIII–related antigen), CD61, CD14, CD68, CD163, CD20 (L26), CD79a, PAX5, CD3, CD5, CD30, CD15, CD34, terminal deoxynucleotidyl transferase (TdT), and tumor-related antigens, and proliferation antigens ( Figs. 39.2–39.10 ). Antibodies to kappa and lambda immunoglobulin light chains are particularly useful for determining the relative proportions of kappa- and lambda-containing cells in immunoproliferative disorders such as multiple myeloma. Reactivity with these antibodies is generally restricted to processes in which the cells contain cytoplasmic immunoglobulin. The technique is not sufficiently sensitive to detect surface immunoglobulin on the lymphocytes in most B lymphoproliferative diseases. Occasionally the lymphocytes in a B-cell lymphocytic lymphoma contain cytoplasmic immunoglobulin that may be detected by this method. With some fixatives, in situ hybridization methods for immunoglobulin light chains work well in marrow specimens, often with less background staining than immunohistochemical methods. The antibodies to lymphocyte antigens are useful in determining the B- or T-cell origin of the lymphoproliferative processes and the extent of marrow involvement (see Fig. 39.9 ). These antibodies are not determinants of clonality. Polyclonal antibody to myeloperoxidase is a specific and sensitive antibody for cells of neutrophil origin that reacts with the myeloblasts in most cases of acute myeloid leukemia (AML; see Fig. 39.5 ). Some studies, however, have shown that in paraffin-embedded tissue, monoclonal antibodies to myeloperoxidase are more specific. Several panels for the immunohistochemical characterization of acute leukemia have been described. These generally include antibodies to CD68 (both PGM1 and KP1), myeloperoxidase, CD34, lysozyme, lymphocyte antibodies, and TdT, with addition of other antibodies as needed.
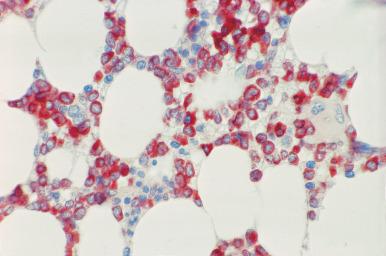
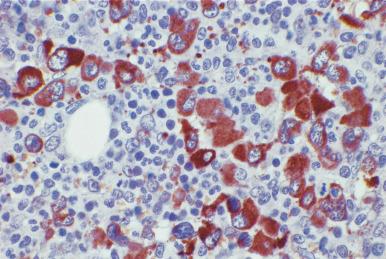
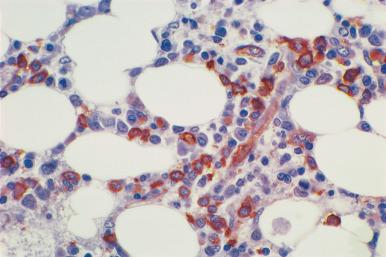
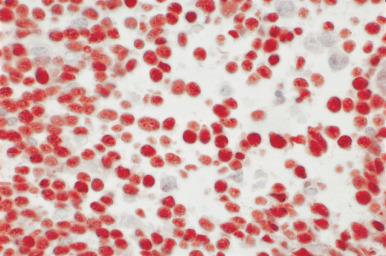
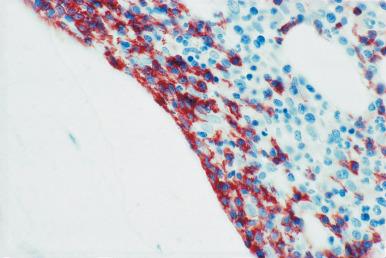
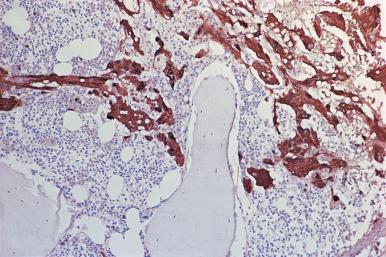
It is important that the reactivity pattern of all antibodies be determined by each laboratory. The range of reactivity attributed to an antibody by the manufacturer should be confirmed with lesions of known antigenicity. The pattern of reactivity of the antibodies to lymphoid cells is generally based on studies of lymph nodes fixed in B5 or buffered neutral formalin. The same reactivity results may not be applicable to marrow biopsies decalcified with a rapid acid decalcifier; L26, an excellent antibody to CD20, a pan B-cell antigen, works well in bone marrow biopsies decalcified with a rapid acid decalcifier but may not react in Zenker-fixed tissue decalcified in the same manner. The effects of decalcification on antibody reactivity should be determined by subjecting lymph node tissue to the same decalcification procedure employed for bone marrow biopsies as part of the antibody validation procedure.
Most large histopathology laboratories use automated instruments for immunohistochemistry; with careful attention to detail, generally very good results are obtained.
In addition to routine histopathologic studies, the bone marrow specimen is being increasingly utilized for molecular and cytogenetic studies. These special techniques should, if possible, be anticipated prior to obtaining the biopsy in order for the specimen to be processed in an appropriate manner. However, not infrequently, the need for these studies is not determined until a biopsy specimen is examined with routine stains. In these instances, it would be appropriate to repeat the biopsy for the necessary specimens. Fixed specimens embedded in paraffin may have been used for some of these studies. This may suffice if the reliability of the technique is ensured from quality control studies. Decalcified specimens may yield distinctly different, often inferior, results from nondecalcified fixed specimens.
This section is not intended to serve as a complete description of all of the antibodies available for bone marrow immunohistochemistry; the individual antibodies that may be useful will be noted in the discussion of the various disease conditions.
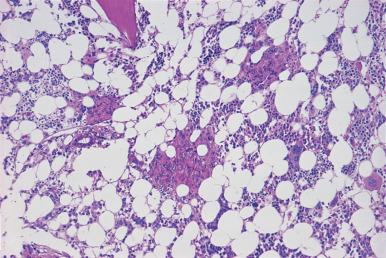
Assessment of marrow cellularity must take into account the age of the patient because the amount of hematopoietic tissue in bone marrow from normal individuals varies with age, as demonstrated in both histologic and radiologic imaging studies. The simplistic formula “100 minus the patient age” to estimate normal cellularity for age is relatively inaccurate and may overestimate or underestimate the normal cellularity of many patients because the decrease in cellularity is not constant throughout human life. In the first decade, the mean marrow cellularity is 79%; the mean cellularity in the eighth decade is 29%. In the first three decades of life more than half of the marrow is composed of hematopoietic cells. During this period, there is a gradual decrease in the amount of hematopoietic tissue with an increase in fat cells. From the fourth to the seventh decade, there is relative stabilization of the number of hematopoietic cells ( Figs. 39.11 and 39.12 ); beginning in the eighth decade, there is a renewed decrease. The increase in adipose tissue results from a decrease in both hematopoietic and bone tissue.
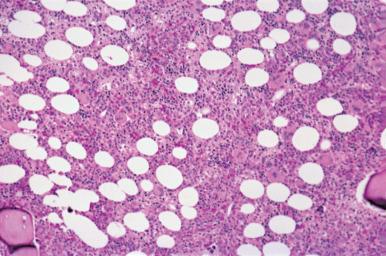
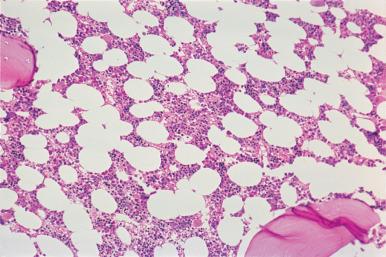
The immediate subcortical area of the bone marrow may normally be more hypocellular than the deeper medullary areas. As a result, specimens that contain a substantial amount of subcortical marrow may be inadequate for estimating cellularity. In addition, the immediate paratrabecular areas may be preferentially hypocellular.
Acquired aplastic anemia has historically been classified as idiopathic or secondary, the latter cases allegedly resulting from exposure to drugs, chemicals, viral infections, or ionizing radiation. Contemporary theories have focused on immunologic mechanisms as the causative factor in the majority of cases. These mechanisms involve activation of T-cell subsets that attack marrow stem cells and progenitor cells. In some instances, apparently acquired aplastic anemia is related to inherited genetic mutations and telomere shortening. Severe aplastic anemia is defined by a markedly hypocellular bone marrow (<25% of normal for age or 25%–50% of normal with <30% hematopoietic cells) accompanied by two of the following: granulocytes less than 0.5 × 10 9 /L; platelets less than 20 × 10 9 /L; or a corrected reticulocyte count less than 1%.
The biologic mechanisms underlying the occasional evolution of aplastic anemia to paroxysmal nocturnal hemoglobinuria (PNH) is not presently known, but the presence of small PNH clones in aplastic anemia may be associated with in improved response to immunosuppressive therapy.
The term “constitutional aplastic anemia” has been used collectively for all congenital aplastic anemias, familial and nonfamilial, with and without associated abnormalities of body structures. There are several inherited bone marrow failure syndromes that may manifest marrow aplasia or hypoplasia, including Fanconi anemia, Shwachman–Diamond syndrome, dyskeratosis congenita, and Diamond–Blackfan anemia.
Fanconi anemia is a syndrome of familial hypoplastic anemia occurring primarily in the first decade of life that is associated with multiple organ malformations, including hypoplasia of the kidneys and absent or hypoplastic thumbs or radii. It is associated with numerous gene mutations and is the most common cause of aplasia in the first decade of life. An association of hypoplastic bone marrow and pancreatic dysfunction (Shwachman–Diamond syndrome) is a rare disorder occurring in children. Diamond–Blackfan anemia is characterized by erythroid aplasia or hypoplasia related to mutations of genes encoding ribosomal structural proteins, manifesting usually in the first year of life.
Selective aplasia or hypoplasia of the megakaryocytes associated with missing radii (TAR syndrome) is a rare disorder. An inherited autosomal dominant hematologic disorder associated with proximal fusion of the radius and ulna has been reported; the hematologic manifestations are somewhat variable and include adult onset of generalized bone marrow failure and amegakaryocytic thrombocytopenia presenting in childhood. The latter presentation has been associated with a mutation in homeobox genes HOXA11 . The homeobox genes encode regulatory proteins that have a role in skeletal morphogenesis and hematopoiesis.
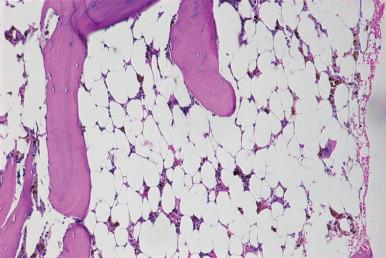
In the most severe form of aplastic anemia, the intertrabecular marrow space is occupied predominantly by adipose tissue with scattered lymphocytes, plasma cells, tissue mast cells, and hemosiderin-laden macrophages ( Fig. 39.13 ). In less severe processes, there is an increased amount of fat tissue and scattered small collections of erythroblasts, granulocytes, and megakaryocytes; in some instances, the decrease in megakaryocytes is disproportionate to other cell types. The blood findings in aplastic anemia are characterized by varying degrees of pancytopenia.
Uncommonly, the marrow biopsy in a patient with aplastic anemia contains aggregates of well-differentiated lymphocytes similar to the lesions that occur in a variety of immune disorders; these are described in this chapter as polymorphous lymphoid aggregates. Unusually large aggregates of benign T lymphocytes have been observed in aplastic marrows in patients with invasive malignant thymoma with an associated nonclonal T lymphocytosis in the blood ( Fig. 39.14 ).
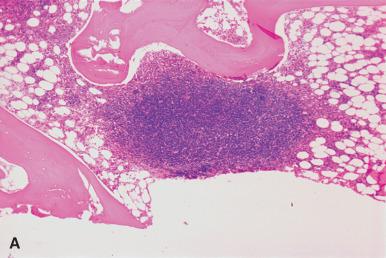
Hypoplastic marrows may be encountered in some newly diagnosed cases of acute leukemia and MDS. These can generally be distinguished from true aplastic anemia by the population of blasts and immature granulocytes, in contrast to the mature lymphocytes, plasma cells, and tissue mast cells in aplastic/hypoplastic marrows. The use of CD34 is an additional aid in identifying blast cells. However, it is important to note that not all blasts express CD34 and a negative reaction does not exclude a blast population. Concurrent staining for myeloperoxidase should be performed. Cytogenetic studies show distinct clinical outcomes for cytogenetic abnormalities arising in aplastic anemia.
The use of hematopoietic cell transplantation as a therapeutic approach to aplastic anemia in patients who do not respond to immunosuppressive therapy has gained wide acceptance. Evidence of marrow reconstitution usually is present in biopsies obtained 2–3 weeks following transplantation and consists of foci or islands of hematopoietic cells. Sequential marrow biopsies in the subsequent 5- to 10-week period show increasing numbers of erythroid precursors, granulocytes, and megakaryocytes in patients with engraftment. Impending rejection of engraftment may be heralded by a decrease in one marrow cell line. An association between high mast cell counts in post marrow transplant specimens from patients with aplastic anemia and marrow rejection has been reported, but this has not been a uniform observation.
Growth factor therapy and immunosuppressive and antibiotic therapy may alter the morphology of the proliferating engrafted cells, and evidence of dyserythropoiesis and dysgranulopoiesis may be present. At times, agranulocytosis with a “maturation arrest” of the proliferating neutrophil precursors at the promyelocyte stage may occur as a result of antibiotic or other drug therapy. The use of recombinant granulocyte growth factor may result in a marked shift to immaturity in the developing neutrophils. Selective hypoplasia of myeloid cell lines may occur and is frequently related to specific drug- or virus-related immune mechanisms. Selective erythroid hypoplasia may occur in patients with parvovirus B19 infection and in association with some drugs such as the immunosuppressive drug mycophenolate mofetil, used after solid organ transplantation.
Hyperplasia of one or more myeloid cell lines (erythroid, granulocytic–monocytic, megakaryocytic) may be found in several hematopoietic disorders. Several benign hematologic disorders are characterized by hypercellularity; these include cell maturation defects such as the megaloblastic and sideroblastic anemias or disorders with increased rates of destruction or utilization of various cell types in which the hypercellularity is due to compensatory hyperplasia. Hemolytic anemias are generally characterized by a marked erythroid hyperplasia. In immune thrombocytopenia in which there is an increased rate of platelet destruction, the megakaryocytes are normal to increased in number. One of the major problems in evaluation of marrow from patients with benign disorders, such as megaloblastic anemia in which the marrow may be very hypercellular and precursor cells may show striking nuclear changes, is that the proliferating erythroblasts or other myeloid cells may be misinterpreted as a leukemic proliferation; examination of the blood and marrow smears in combination with review of the clinical history and laboratory data should prevent the possibility of this type of error.
Infrequently, a posterior iliac spine biopsy may contain findings unrelated to the reason for which the biopsy is performed. One such finding is osteitis fibrosa cystica related to secondary hyperparathyroidism in patients with chronic renal disease ( Fig. 39.15 ). It is imperative for pathologists and hematologists to recognize the potential for diseases other than hematologic disorders that manifest in marrow biopsies.
Gelatinous (serous) transformation is a degenerative change of the marrow characterized by atrophy of the fat and hematopoietic cells and accumulation of serous fluid in the interstitium. It is an epiphenomenon associated with several disorders that are usually accompanied by extreme malnourishment and weight loss; it is potentially reversible with resolution of the underlying problem and restoration of normal nutritional status. The etiologic bases are somewhat age related: anorexia nervosa, human immunodeficiency virus (HIV) syndrome, and acute febrile illnesses are the most common factors in young individuals; alcoholism and lymphomas in middle age; and carcinomas, lymphomas, and congestive heart failure in older individuals. It may also be observed in the pediatric population. The majority of patients are anemic.
The areas of gelatinous transformation in the marrow biopsy may be focal with intervening areas of normal hematopoietic and fat cells, or the entire biopsy may manifest the transformation, which appears as a homogeneous lightly eosinophilic appearance in sections stained with hematoxylin and eosin; it is pale pink with periodic acid–Schiff (PAS) and slightly bluish with Giemsa ( Fig. 39.16 ). Bone marrow aspirate smears contain a dense metachromatic, mucoid-appearing material with Giemsa stain.

Osteopetrosis is a rare genetic disorder of osteoclast function and/or development, characterized by impaired bone resorption and increased bone sclerosis. There are several possible genetic mutations that may result in osteoclast dysfunction or lack of development. Several classifications have been proposed. Generally two major groups are recognized: autosomal recessive osteopetrosis and autosomal dominant osteopetrosis. A rare sex-linked form has also been reported. There is wide variation in the clinical severity of the disease, both in the autosomal dominant group (also known as Albers–Schönberg disease, which includes asymptomatic individuals and adults) and the autosomal recessive group. The most severe forms of the disease, which occur in young infants, are lethal unless treated with hematopoietic cell transplantation and are usually autosomal recessive (infantile malignant osteopetrosis). A less severe form of autosomal recessive osteopetrosis is caused by a mutation in the gene encoding carbonic anhydrase II, resulting in carbonic anhydrase II deficiency; in addition to the osteopetrosis, these patients have renal tubular acidosis and cerebral calcifications. The few X-linked cases have been associated with ectodermal dysplasia, lymphedema, and immune deficiency. The incidence of infantile malignant osteopetrosis is 1 in 300,000 births, except in Costa Rica where it is 3–4 in 100,000 births.
Most cases of osteopetrosis are associated with a failure of osteoclast function resulting in disturbances of intracellular and extracellular pH of the osteoclast resorption compartment which leads to impaired resorption of organic and inorganic bone matrix. In some cases there is a failure of osteoclast development; this autosomal recessive form is associated with mutations of a variety of genes in 70% of cases that disrupt osteoclast development, differentiation, and function. In the severe forms of the disease there is sclerosis of all of the bones. In the less severe forms there is sclerosis of the bones of the skull, pelvis, vertebrae, and phalanges.
The radiographs in osteopetrosis show increased bone density. The increased bone sclerosis results in reduced marrow space and impaired myeloid hematopoiesis, essentially a form of bone marrow failure; this is accompanied by extramedullary hematopoiesis with hepatosplenomegaly and a leukoerythroblastic blood picture. Reduction in the size of the foramina in the skull leads to optic and auditory nerve compression with visual and auditory defects. Although the bones are dense there is increased fragility and propensity for fractures. Dentition problems are also a part of the clinical picture. The only known cure for the infantile malignant form of the disease is hematopoietic cell transplantation. Many cases of the autosomal dominant form of osteopetrosis are asymptomatic; the most common clinical manifestation is increased fragility of bone leading to fractures.
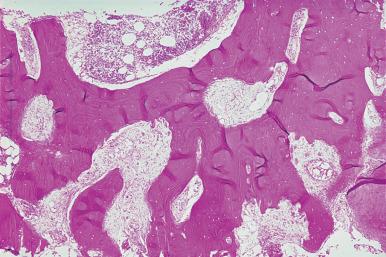
Posterior iliac crest biopsy may be performed prior to and following hematopoietic cell transplantation. The diagnostic features of the disorder include marked thickening of the bone trabeculae and marked reduction in the medullary cavity ( Fig. 39.17 ). There is usually increased connective tissue in the medullary space, although this may be difficult to judge because of the overall reduction in the space. The bone structures contain cartilaginous plates, which in nondecalcified specimens show calcium deposition. Osteoclasts are frequently numerous along the endosteal surface. The osteoclasts lack a normal ruffled border. In the small number of cases of failure of osteoclast production associated with a mutation of the RANKL gene, there is an absence of osteoclasts.
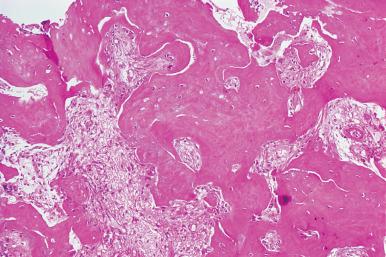
Following successful allogeneic transplantation there is gradual resorption of the abnormal bone structure, regression of the cartilaginous plates, and expansion of the medullary space with abundant hematopoietic tissue. This is accompanied by regression of splenomegaly and reversal of the leukoerythroblastic blood picture.
Bone marrow necrosis unrelated to chemotherapy or radiation therapy occurs occasionally in patients with acute leukemia, malignant lymphoma, and metastatic tumor ; it has also been observed in patients with sickle cell anemia, infectious processes, systemic lupus erythematosus, caisson disease, voluntary starvation, and megaloblastic anemia complicated by infection. The process may be accompanied by severe and generalized bone pain.
The aspirated marrow specimens from these patients frequently have a gelatinous consistency. The microscopic picture in the trephine section reflects the stage of necrosis; different stages are frequently found in the same biopsy specimen. In the early stages, the nuclei show pyknosis and karyorrhexis, and the cells have a granular appearance; this is followed by karyolysis. In advanced stages, all cell outlines disappear, and the marrow space is replaced by amorphous, granular, eosinophilic debris. The trabeculae may be involved and show loss of osteocytes. The necrosis may be patchy or involve virtually all of the cells in the biopsy specimen ( Fig. 39.18 ).
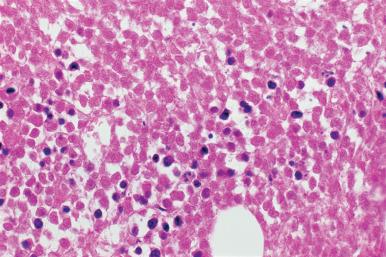
The inflammatory diseases that are most readily identified in marrow biopsies are those associated with a granulomatous reaction; the etiologic bases include fungi, Mycobacterium tuberculosis , Mycobacterium avium-intracellulare , sarcoidosis, Mycoplasma pneumoniae , and viral infections such as Epstein–Barr. Marrow granulomas have been observed in patients with cytopenias who are on the antidysrhythmic agent amiodarone. Granulomas may also be found in patients with Hodgkin disease and non-Hodgkin lymphomas, with or without marrow involvement by the lymphoma, and in occasional cases of a drug reaction. Perivascular granulomas may be related to hypersensitivity states. With a combination of studies, including histochemical stains, microbiologic culture, serology, enzyme immunoassay of body fluids, and molecular analysis, the etiologic basis for the granulomas should be established in a very high percentage of cases, approximately 80%–90%. In approximately 80% of the cases, the etiologic basis is identified by culture, stain, or molecular studies.
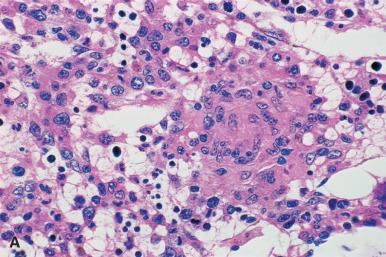
Granulomas in the marrow are similar to those in other sites; the most commonly encountered are composed only of a collection of epithelioid histiocytes that may be surrounded by a rim of well-differentiated lymphocytes. Others may contain large numbers of multinucleated giant cells. The number may range from a single lesion to numerous and confluent granulomas ( Figs. 39.19–39.21 ). An unusual granulomatous lesion referred to as a “doughnut” or ring granuloma, because of a central clear area or lumen, has been described in the bone marrow of some patients with Q fever. The appearance of these lesions, which is similar to those in other organs, such as the liver, varies from what appear to be vascular structures or fat globules encircled by a rim of fibrinoid material, polymorphonuclear leukocytes, and monocytes to a collection of epithelioid histiocytes surrounding a clear space (see Fig. 39.20 ). The vascular-associated granulomas observed in hypersensitivity states are similar in appearance. In addition to Q fever, these lesions may be observed in marrows from patients with a variety of diseases, both neoplastic and non-neoplastic. In one series the most frequent association was with Epstein–Barr virus (EBV) infection.
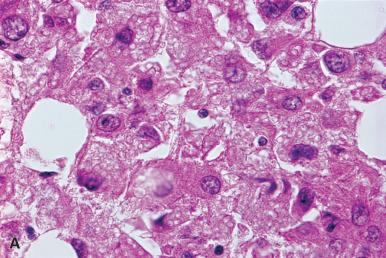
Cells with prominent intranuclear inclusions may be found in granulomas in the marrow of patients with cytomegalovirus or other viral infection.
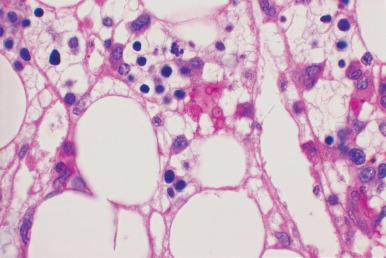
Acute parvovirus B19 infection in immunocompetent individuals may be associated with marked erythroblast hypoplasia and very immature giant erythroblasts; scattered erythroblasts may contain intranuclear inclusions ( Fig. 39.22 ). Recovery from the erythroid hypoplasia may be marked by a wave of regeneration with a large number of erythroblasts at an early stage of maturation, analogous to the proliferation of promyelocytes and myelocytes that occurs in agranulocytosis. Immunodeficient patients may develop a chronic parvovirus B19 infection; in these cases there may be an erythroid hyperplasia with numerous intranuclear inclusions in the erythroid precursors; the inclusions are most prominent in the basophilic and polychromatic stages of maturation (see Fig. 39.22C ). The parvovirus B19 relationship to the inclusions may be demonstrated with immunohistochemical or in situ hybridization studies.

As with other tissues, stains for acid-fast bacilli (AFB) and fungi should be performed in all cases of marrow granulomas. Failure to detect AFB does not exclude infection with M. tuberculosis ; organisms are found in approximately 25%–35% of marrow specimens from patients with documented disease by culture studies. Occasional patients with negative culture studies have AFB stain–positive biopsies. The need for culture of a portion of the bone marrow aspirate for AFB and fungi should be anticipated in all patients suspected of having a granulomatous disorder, particularly those with acquired immune deficiency syndrome (AIDS) or patients being investigated for a fever of undetermined etiology.
Bone marrow biopsies performed on immunosuppressed patients should always be thoroughly examined for opportunistic infections ( Figs. 39.23–39.25 ; see also Fig. 39.21 ). Typical granuloma formation may not be present in the marrows of some patients with disseminated fungal or mycobacterial disease. Marrow biopsies from patients with HIV may contain scattered macrophages with AFB in the absence of granuloma formation (see Fig. 39.25 ). Uncommonly, Pneumocystis jirovecii (formerly P. carinii ) may be observed in scattered macrophages in sections stained with PAS or methenamine silver (see Fig. 39.24 ). An increased number of macrophages with or without evident phagocytosis is sufficient reason to perform special stains for microorganisms.
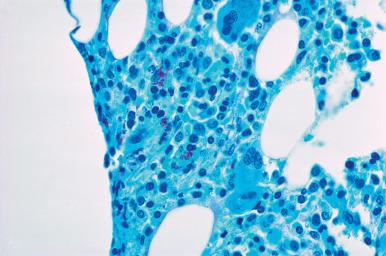
The presence of infection-related granulomas in the bone marrow sections may occasionally be accompanied by macrophages containing microorganisms in the Romanowsky-stained bone marrow smears or trephine imprints. The morphology of the organisms in these preparations is usually sufficient to establish a diagnosis.
Lipid granulomas, which have been reported to be the most frequent type of granuloma in bone marrow, are similar to those found in the liver, spleen, and lymph node. These granulomas range from 0.2 to 0.8 µm in size and usually are associated with lymphocytic aggregates or sinusoids. The loosely spaced macrophages contain fat vacuoles of varying size. The lesions also contain admixed lymphocytes, plasma cells, and eosinophils; giant cells are found in approximately 5% of cases. Some of these granulomas resemble those found in sarcoidosis.
An unusual form of granulomatous reaction can be found in the bone marrow of patients with the genetic disorder of glyoxylate metabolism, primary hyperoxaluria. This finding is secondary to the deposition of calcium oxalate crystals. The crystals, which have a slightly yellowish tinge, form a radial pattern, are encircled or engulfed by epithelioid and giant cells, and are doubly refractile with polarized light ( Fig. 39.26 ). Substantial portions of the marrow biopsy may be replaced by these lesions, which are similar to those found in the kidneys and other tissues.
Nonspecific inflammatory alterations may be noted in the marrow from patients with a variety of systemic disorders, including acute infection, malignancy, connective tissue disease, and immune disorders, most notably AIDS. These alterations generally are characterized by changes in both the vascular structures and parenchyma. The terms tumor myelopathy and myelitis have been applied to the nonspecific marrow changes that are observed in a high percentage of patients with malignant lymphoma. These changes include edema of the vessel walls, plasma cell and mast cell proliferations in the adventitia, protein deposits adjacent to the vessels, patchy edema, depressed erythropoiesis, and increased granulopoiesis and megakaryocytopoiesis. Acute necrosis of bone marrow tissue is reported in patients with tuberculosis and typhoid fever.
Marrow biopsies from untreated patients with HIV have been generally reported to be hypercellular, although normocellular and hypocellular specimens may be observed; nonspecific findings include marrow damage, increased plasma cells, myelodysplasia, serous degeneration, lymphocytic infiltration, increased reticulin and “naked” megakaryocytic nuclei. The marrow specimen may serve as a vehicle for the rapid diagnosis of febrile illnesses in HIV-infected patients. Opportunistic organisms including AFB and P. jirovecii and fungal organisms may be present, occasionally without granuloma formation ( Fig. 39.27 ; see also Figs. 39.24 and 39.25 ). Immune thrombocytopenia may be observed, but the frequency of severe thrombocytopenia in HIV-infected patients has decreased with the use of anti-retroviral therapy.
HIV-infected individuals may develop a persistent parvovirus B19 infection because of ineffective production of IgG parvovirus-neutralizing antibodies. This may result in chronic anemia and erythroid hyperplasia with all stages of maturation in contrast to the erythroid aplasia that may occur in acute parvovirus B19 infection. The erythroid precursors in these patients may show intranuclear inclusions primarily in the basophilic and polychromatic erythroblasts (see Fig. 39.22C ).
Negative stains for microorganisms and absence of granulomas do not exclude the possibility of infection by microorganism; a minority of patients with infection by M. avium complex have demonstrable granulomas or AFB in their marrow biopsies. In addition, although infrequent, marrow with only mild nonspecific changes may contain scattered macrophages with mycobacteria. These macrophages may resemble pseudo-Gaucher cells in hematoxylin and eosin–stained sections. Scattered macrophages containing P. jirovecii may also be present in a background of essentially normal-appearing or only slightly altered marrow. As a result, it is considered prudent by some observers to routinely stain marrows from HIV patients for AFB and fungi, regardless of the appearance of the marrow with routine stains.
The marrow damage that may be present in HIV patients is characterized principally by a loosely structured, hypocellular interstitium. Areas of fibrinoid necrosis may be present. The changes resemble those found in marrows from patients recovering from chemotherapy with myelotoxic agents and are distinct from serous degeneration, which usually occurs in severely malnourished individuals and which may also be present in marrow biopsies from patients with HIV. Both of these alterations may be accompanied by an increase in plasma cells.
Lymphocytic aggregates of varying size occur in the marrow of a relatively high percentage of patients with HIV. These are randomly distributed without any preferential paratrabecular distribution; in some cases the lesions appear to be preferentially perisinusoidal. They are composed primarily of small lymphocytes, some of which may have irregularly shaped nuclei. There are usually associated plasma cells, histiocytes, and increased vascular structures; eosinophils may be increased ( Fig. 39.28 ). Occasional immunoblasts may be noted. This type of lymphocyte proliferation, polymorphous reactive lymphoid hyperplasia, is not unique to patients with HIV and may be observed in marrow biopsies in a wide range of immunologic disorders. Because of the large size and cellular composition, these aggregates may be difficult to distinguish from the lesions of peripheral T-cell lymphoma; the latter lesions are frequently accompanied by numerous epithelioid histiocytes and scattered large transformed cells. In some instances, the distinction between the two processes based on morphologic criteria may not be possible. Peripheral T-cell lymphoma in HIV patients, however, is quite rare, and such a diagnosis should be established with considerable caution and only after review of all pathology specimens. Biopsy of other organ sites as well as molecular studies for T-cell receptor rearrangement may be necessary.
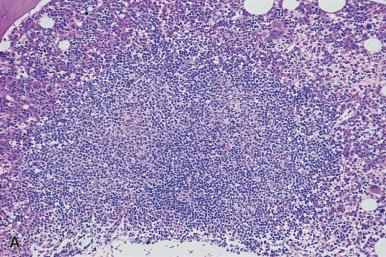
Occasionally, marrow specimens from patients with HIV contain benign lymphoid follicles with germinal centers.
In addition to reactive lymphocytic lesions, the marrow biopsies from AIDS patients may rarely exhibit a florid plasmacytoid immunoblastic proliferation that may resemble a neoplastic process because of the degree of replacement of marrow. The use of immunohistochemical studies with anti-kappa and anti-lambda antibodies can be very useful in demonstrating the polyclonal nature of these lesions.
Although it is important to recognize that marrow biopsies from patients with HIV may show a variety of reactive lymphocytic proliferations, it is equally important to appreciate that these patients have an increased incidence of malignant lymphoma, of either non-Hodgkin or Hodgkin type, and the marrow biopsy may be the initial diagnostic specimen. The most common HIV-associated lymphomas that may involve the marrow are B-cell lymphomas, including Burkitt lymphoma, diffuse large B-cell lymphoma, and plasmablastic lymphoma. A single biopsy may contain both reactive lymphocytic lesions and malignant lymphoma. In suspected cases of Hodgkin disease, adherence to established criteria for marrow involvement by Hodgkin disease should be observed.
Patients with HIV who are receiving zidovudine (AZT) may develop evidence of marrow suppression such as anemia and neutropenia. Marrow hypoplasia may be present. Abnormal megakaryocytes with sparse cytoplasm, “naked” megakaryocyte nuclei, may be numerous; this finding is not specific for AIDS and may be present in other disorders, including the MPNs. The incidence of immune thrombocytopenia is increased in individuals with HIV.
The diagnosis and classification of the acute leukemias are optimally established from examination of Romanowsky-stained blood and bone marrow smears in conjunction with appropriate immunophenotypic, cytogenetic, and/or molecular genetic and cytochemical techniques; this approach forms the basis for the World Health Organization (WHO) classification. Terminology from the French–American–British (FAB) Cooperative Group classification remains in common usage, but this classification system should no longer be used. Chromosome analysis has an important role in the evaluation of cases of acute leukemia, primarily in regard to prognostic significance, and is incorporated into the diagnosis of many categories of acute leukemia in the 2016 WHO classification. The ideal approach to the diagnosis and classification of acute leukemia requires correlation of clinical and peripheral blood findings, bone marrow aspirate smear morphology, and the bone marrow trephine biopsy. Immunophenotyping and cytogenetic or molecular genetic studies, which are now performed on all cases, are best performed on marrow aspirate material. Although smears and imprint preparations are generally superior to sections in classifying the majority of cases of acute leukemia, there are some types of acute leukemia that are accompanied by fibrosis or with unusual morphologic patterns, such as acute megakaryocytic leukemia with an associated t(1;22)(p13.3;q13.1) cytogenetic abnormality that occurs in infants in which the bone marrow sections are often more informative than the bone marrow smears ( Figs. 39.29–39.31 ). In addition, as noted in the section on immunohistochemistry, the development of antibodies reactive in paraffin-embedded tissue, most notably myeloperoxidase, lysozyme, TdT, and CD34 antibodies, has greatly enhanced the recognition of myeloid leukemia in trephine biopsy sections.



The 2016 WHO classification of acute leukemia includes general categories of AML, precursor lymphoid neoplasms, which include the related acute lymphoblastic leukemias (ALLs) and their corresponding lymphoblastic lymphomas, and acute leukemias of ambiguous lineage. AML ( Box 39.1 ) is further subdivided into AML with recurrent genetic abnormalities, AML with myelodysplasia-related changes, therapy-related myeloid neoplasms, myeloid proliferations of Down syndrome, and AML, not otherwise specified. AML with myelodysplasia-related changes may be diagnosed by the presence of: (1) a history of prior myelodysplasia; (2) presence of a myelodysplasia-related cytogenetic abnormality ( Box 39.2 ); or (3) multilineage dysplasia defined as dysplasia in 50% or more of two nonblast cell lines; and the absence of prior cytotoxic chemotherapy for an unrelated disease and absence of any of the genetic abnormalities of AML with recurrent genetic abnormalities. All cases of AML are defined by the presence of 20% or more blast cells in the peripheral blood, except for cases of AML with t(8;21)(q22;q22.1), AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), and acute promyelocytic leukemia with PML - RARA in which a diagnosis of AML can be made with less than 20% peripheral blood and bone marrow blast cells. A large number of gene mutations occur in AML and detection of some (NPM1, CEBPA, RUNX1) are critical for a complete diagnosis, whereas others assist in defining prognostic disease groups. Testing for multiple gene mutations using next-generation sequencing methods is quickly becoming routine in evaluation of AML.
AML with recurrent genetic abnormalities
AML with t(8;21)(q22;q22.1); RUNX1-RUNX1T1
AML with inv(16)(p13.1q22) or t(16;16)(p13;q22); CBFB-MYH11
Acute promyelocytic leukemia with PML-RARA
AML with t(9;11)(p21.3;q23.3); KMT2A - MLLT3
AML with t(6;9)(p23;q34.1); DEK-NUP214
AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2, MECOM
AML (megakaryoblastic) with t(1;22)(p13.3;q13.3); RBM15-MKL1
Provisional entity: AML with BCR-ABL1
AML with mutated NPM1
AML with biallelic mutations of CEBPA
Provisional entity: AML with mutated RUNX1
AML with myelodysplasia-related changes
Therapy-related myeloid neoplasms
AML, not otherwise specified
AML minimally differentiated
AML without maturation
AML with maturation
Acute myelomonocytic leukemia
Acute monoblastic/monocytic leukemia
Pure erythroid leukemia
Acute megakaryocytic leukemia
Acute basophilic leukemia
Acute panmyelosis with myelofibrosis
Myeloid sarcoma
Myeloid proliferations related to Down syndrome
Complex karyotype (three or more abnormalities)
Unbalanced abnormalities
−7/del(7q)
del(5q)/t(5q)
i(17q)/t(17p)
−13/del(13q)
del(11q)
del(12p)/t(12p)
idic(X)(q13)
Balanced abnormalities
t(11;16)(q23.3;p13.3)
t(3;21)(q26.2;q22.1)
t(1;3)(p36.3;q21.2)
t(2;11)(p21;q23.3)
t(5;12)(q32;p13.2)
t(5;7)(q32;q11.2)
t(5;17)(q32;p13.2)
t(5;10)(q32;q21.2)
t(3;5)(q25.3;q35.1)
ALL ( Box 39.3 ) is subdivided into precursor B- and T-cell types, with precursor B-ALL further subgrouped into cases with recurrent genetic abnormalities and B-ALL, not otherwise specified. ALL is biologically and morphologically identical to lymphoblastic lymphoma, although the clinical presentation varies between the two. For this reason, the two are combined in the WHO classification. While most cases of ALL are of precursor B lineage, lymphoblastic lymphoma is of precursor T-cell origin in approximately 80% of cases and B-cell precursor type in 20%. Precursor T-cell ALL/lymphoma has a high incidence of mediastinal mass, occurs in young adults, more frequently in males, and has a predilection for early blood and marrow involvement (see Fig. 39.31 ). Lymphoblasts of both precursor B and T lineage are TdT positive. The blood and marrow may be involved at the time of initial diagnosis and the designation of a case as lymphoblastic lymphoma or ALL is arbitrary. The presence of extramedullary tumor masses and evidence of sparing of marrow function as indicated by a normal platelet count, hemoglobin levels greater than 10 g/dL, and a normal number of neutrophils in the blood are more compatible with a diagnosis of lymphoma with marrow involvement as opposed to ALL. The Children's Cancer Study Group proposed distinguishing leukemia from lymphoblastic lymphoma on the basis of the percentage of lymphoblasts in the marrow. If there are less than 25% lymphoblasts, the case is classified as lymphoma; if there are 25% or more lymphoblasts, the case is classified as leukemia. In the cases of lymphoma, there may be substantial residual normal marrow with the lymphoma cells diffusely scattered in the interstitium. The therapeutic approach in these two fundamentally similar processes is the same. B lymphoblastic lymphoma appears to have lower propensity for blood and marrow involvement than T lymphoblastic lymphoma.
B-lymphoblastic leukemia/lymphoma, not otherwise specified
B-lymphoblastic leukemia/lymphoma with recurrent genetic abnormalities
B-lymphoblastic leukemia/lymphoma with t(9;22)(q34.1;q11.2); BCR/ABL1
B-lymphoblastic leukemia/lymphoma with t(v;11q23.3); KMT2A rearranged
B-lymphoblastic leukemia/lymphoma with t(12;21)(p13.2;q22.1); ETV6-RUNX1
B-lymphoblastic leukemia/lymphoma with hyperdiploidy
B-lymphoblastic leukemia/lymphoma with hypodiploidy
B-lymphoblastic leukemia/lymphoma with t(5;14)(q31.1;q32.3); IL3- IGH
B-lymphoblastic leukemia/lymphoma with t(1;19)(q23;p13.3); TCF3-PBX1
Provisional entity: B-lymphoblastic leukemia/lymphoma, BCR-ABL1 -like
Provisional entity: B-lymphoblastic leukemia/lymphoma with iAMP21
T-lymphoblastic leukemia/lymphoma
Provisional entity: Early T-cell precursor leukemia/lymphoma
Provisional entity: Natural killer cell lymphoblastic leukemia/lymphoma
The acute leukemias of ambiguous lineage ( Box 39.4 ) are divided into acute undifferentiated leukemia and mixed phenotype acute leukemias. The latter group also includes specific genetic subtypes.
Acute undifferentiated leukemia
Mixed phenotype acute leukemia with t(9;22)(q34.1;q11.2); BCR/ABL1
Mixed phenotype acute leukemia with t(v;11q23.3); KMT2A rearranged
Mixed phenotype acute leukemia, B/myeloid, not otherwise specified
Mixed phenotype acute leukemia, T/myeloid, not otherwise specified
In the majority of cases of acute leukemia, both myeloid and lymphoblastic, in children and adults, the marrow is markedly hypercellular because of the proliferation of leukemic cells, with replacement of normal hematopoietic cells. In a small minority of patients, particularly older individuals, AML and, rarely, ALL may present with a hypocellular marrow (i.e., hypoplastic or hypocellular acute leukemia). The marrow biopsies in these patients may, on low magnification, suggest the diagnosis of aplastic anemia ( Fig. 39.32 ). In contrast to aplastic anemia, in which the residual cells are well-differentiated lymphocytes and plasma cells, the cell population in the interstitium is principally blasts; some normal cells may be present but are markedly reduced. The diagnosis is confirmed by examination of blood and bone marrow smears and the use of appropriate immunohistochemical studies, particularly myeloperoxidase, lysozyme, CD79A, TdT CD117 (KIT), and CD34. In rare instances, ALL in children is preceded by an aplastic or hypocellular phase.

Myelofibrosis with a slight-to-moderate increase in reticulin fibers may be present in the initial or late stages of acute leukemia in a minority of cases; it may occur in both acute lymphoblastic and AML. The presence of myelofibrosis in acute leukemia may result in difficult and inadequate marrow aspiration and an erroneous diagnosis of aplastic anemia if trephine biopsy sections are not available. This emphasizes the importance of obtaining adequate marrow biopsies. The long-held view that difficult aspirations in acute leukemia are caused by “packed” marrows has little basis in fact. The vast majority of hypercellular marrows in acute leukemia are readily aspirated. If aspiration is difficult, it is probably a result of poor biopsy technique or an increase in reticulin fibers.
The trephine biopsy provides the only accurate assessment of marrow cellularity and is of considerable importance in monitoring changes following treatment for leukemia. The rapidity of development and degree of necrosis and aplasia following the institution of therapy will vary with the different chemotherapeutic agents or combination of agents used. In general, the sequence of histopathologic events is marked initially by nuclear karyorrhexis followed by karyolysis; the cells then disintegrate into relatively uniform granular, eosinophilic debris. Subsequently the marrow shows a somewhat irregular, loosely structured appearance with scattered fat cells, vessels, stromal elements, and distended sinusoids ( Fig. 39.33 ). Regeneration of fat cells is followed by regeneration of normal hematopoietic cells in the successfully treated patient. The erythroid cells are usually the first to recover and frequently manifest dyserythropoietic changes as the result of chemotherapeutic drugs. The regeneration of the erythroid cells is followed in sequence by that of the granulocytes and megakaryocytes. This sequence may be altered with different drug regimens and the use of recombinant growth factors. The marrow from patients receiving recombinant granulocyte growth factor may show an early marked increase in neutrophil promyelocytes and myelocytes. In some patients, particularly in the pediatric age group, the post-chemotherapy marrow may contain numerous hematogones, which may resemble lymphoblasts. These can usually be distinguished from lymphoblasts by careful morphologic assessment and immunophenotypic characteristics. The bone marrow biopsy can be particularly useful in this differential diagnosis, as leukemic blasts tend to cluster on biopsy sections, while hematogones represent a spectrum of B lymphocyte maturation dispersed throughout the biopsy. The infiltration pattern can be further assessed by immunohistochemical markers for CD34 or TdT to identify the clusters of leukemia cells versus the more scattered B cell progenitors (hematogones).

As noted, the course of events described in the preceding discussion is characteristic of effective therapy. In those patients in whom there has been partial or no response to treatment, the marrow will show varying numbers of leukemic cells. In the totally nonresponsive patient, the findings are essentially those of the pretreatment specimen; scattered isolated areas of necrosis may be present. In a partial response, the areas of residual leukemia will be intermixed with necrotic or regenerating normal marrow cells. In some patients, the areas of residual leukemia may be very small and difficult to distinguish from foci of regenerating normal cells, particularly early erythroblasts and promyelocytes. Careful comparison of suspicious foci with the cytologic pattern in the initial diagnostic biopsy should always be performed. Distinguishing foci of normal regenerative promyelocytes from the foci of leukemic promyelocytes in patients being treated for acute promyelocytic leukemia may be particularly troublesome. Normal promyelocyte regeneration is usually accentuated along the endosteal surface of the bone trabeculae and in perivascular locations. Acute promyelocytic leukemia in marrows with partial response may manifest as large or small focal lesions unrelated to bone trabeculae or vascular structures ( Fig. 39.34 ). The cytoplasm of the leukemic promyelocyte may be more abundant than in normal promyelocytes.

In addition to assessment of the effects of chemotherapy, post-chemotherapy biopsy specimens should always be carefully evaluated for the presence of granulomas or other evidence of infection. In some instances, a focus of microorganisms may be present only as an area of nonspecific necrosis with a few histiocytes. These foci may be very difficult to recognize in a marrow biopsy showing cell necrosis as a result of chemotherapy. Any suspicious lesion should be studied with special stains. Proliferation of histiocytes, with and without hemophagocytosis, may be prominent in infected patients. The histiocytes are widely dispersed throughout the interstitium and in the sinusoids; in some patients this may be a very prominent feature.
Patients being monitored for the effects of chemotherapy will usually have sequential marrow biopsies at relatively short time intervals. If a specimen is from the area of a recent biopsy procedure, there may be evidence of granulation tissue and new bone formation (see Fig. 39.1 ). The biopsy repair site will usually be relatively well demarcated from the remainder of the biopsy specimen but at times may lead to difficulties in interpretation.
Myeloid sarcoma (granulocytic sarcoma; extramedullary myeloid tumor) is an unusual variant of myeloid malignancy in which there is an extramedullary tumor mass composed of myeloblasts, with or without mature neutrophils. The tumor may occur as an isolated finding or may be associated with AML, CML, primary myelofibrosis (PMF), hypereosinophilic syndrome, and PV. An association of myeloid sarcoma and AML with the t(8;21) chromosome abnormality has been reported and appears to be more common in children. These tumors have also been associated with AML with abnormalities of chromosome 16. In earlier literature, the term chloroma was used for these lesions because of the green appearance of the freshly cut surface of the tumor. The green color, which is due to the presence of peroxidase in the leukemic cells, is not present in all tumors of this type, and the less specific designation of myeloid sarcoma is preferred. Although tumors of monocytes have not previously been classified with the myeloid sarcomas, they represent a similar process with a similar predilection to leukemic evolution, primarily acute monoblastic leukemia, and are now considered in the spectrum of myeloid sarcoma if they create a tumor mass.
The myeloid sarcomas are more frequent in children than in adults and are most commonly associated with the subperiosteal bone structures; the most common sites are the skull, paranasal sinuses, sternum, ribs, vertebrae, and pelvis; lymph nodes and skin are also relatively frequently involved. Orbital masses leading to proptosis and spinal canal lesions resulting in neurologic manifestations are two of the clinical presentations associated with these tumors ( Fig. 39.35 ). A high incidence of myeloid sarcomas involving the orbit has been reported in Turkish children with acute myelomonocytic leukemia. Myeloid sarcomas may present as a mediastinal mass and clinically resemble a mediastinal lymphoma.

A myeloid sarcoma may occur simultaneously with a typical blood and bone marrow pattern of AML or other type of MPN or may antedate leukemia by many months or rarely years. It may be the first evidence of relapse in a patient with AML on maintenance chemotherapy and may be the only evidence of recurrence. These tumors may also represent the initial manifestation of a blast crisis of CML, and an isolated tumor mass or enlarged lymph node in a patient with CML should be evaluated for this possibility, including cytogenetic studies for the t(9;22)(234.1;q11.2) or molecular studies for evidence of the BCR/ABL1 fusion gene.
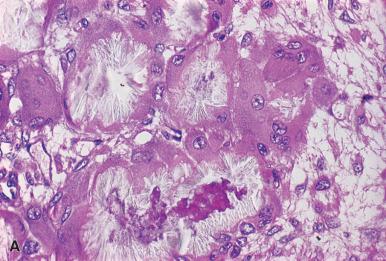
Histologically, the tumor is composed of a relatively uniform population of immature cells and may be misdiagnosed as an aggressive malignant lymphoma; this is particularly a problem with those lesions comprised predominantly of blasts. Occasionally, the presence of immature eosinophils and maturing neutrophils may indicate the true nature of the lesion. Attempts at histopathologic classification have generally resulted in three levels of differentiation: blastic, immature, and differentiated. The blastic type is composed primarily of myeloblasts with little evidence of maturation to the promyelocyte stage ( Fig. 39.36 ). The myeloblasts have a slight-to-moderate rim of basophilic cytoplasm, fine nuclear chromatin, and two to four nucleoli. Eosinophil myelocytes are not usually found with this degree of maturation. The immature type with an intermediate degree of differentiation contains principally myeloblasts and promyelocytes; eosinophil myelocytes are usually present. The differentiated type primarily consists of promyelocytes and later stages of maturation. Eosinophil myelocytes are most abundant in this type. Immunohistochemistry using antibodies to myeloperoxidase, lysozyme, CD68 (PGM1), CD34, and CD117 (KIT) are very useful in identification (see Fig. 39.35B ). The naphthol ASD chloroacetate esterase reaction, which is positive only in neutrophils and mast cells, is also useful for the diagnosis of some of the lesions; however, the reaction is partially ablated by mercury-based fixation and has largely been replaced by immunohistochemical reactions. In the unusual myeloid lesions composed of immature erythroid cells or megakaryocytes, antibodies to hemoglobin A, glycophorin A and E-cadherin for red blood cells, and von Willebrand factor (factor VIII-related antigen), CD41, CD61, and CD31 for megakaryocytes may be used. Monocytic lesions react with antibodies to lysozyme, CD14, CD68 (KP1 and PGM1), and CD163.

Imprint preparations of tumor masses may be particularly useful in identifying the myeloid nature of the cells. Auer rods may be found, and the myeloblasts may show intense staining with the myeloperoxidase cytochemical reaction.
Localized tumor masses occurring in the absence of blood or marrow involvement may respond to local radiation therapy. Eventually the process in the majority of patients will evolve into a form of AML or may be associated with additional tumor masses at other sites. The leukemic evolution may be characterized by a gradual increase in myeloblasts in the blood and marrow; frequently, blasts containing Auer rods are identified. Rarely the leukemic cells may be identified in body fluids due to the occurrence of myeloid sarcoma in one of these areas.
In 7 of 16 patients with isolated granulocytic sarcomas reported by Meis et al., the process did not show a leukemic evolution, although three of the seven patients developed granulocytic sarcomas at additional sites and died 2–8 months following initial presentation. The other four patients showed no evidence of recurrent disease from 3.5 to 16 years following presentation.
Unlike the myeloid sarcomas that occur in patients with established hematologic disorders, which are usually correctly diagnosed, the predominantly blastic myeloid sarcomas occurring as isolated lesions in the absence of some type of leukemia may be initially misdiagnosed as a malignant lymphoma or poorly differentiated tumor because of the lack of diagnostic features. In lymph nodes involved by myeloid sarcoma, the germinal centers are frequently preserved; the infiltrate is usually present in the sinuses and occasionally in the paracortical and medullary areas. In other tissues, the cells usually display an infiltrative pattern with the overall architecture remaining intact. The presence of immature eosinophils or cells with lobulated nuclei should evoke suspicion of a myeloid sarcoma.
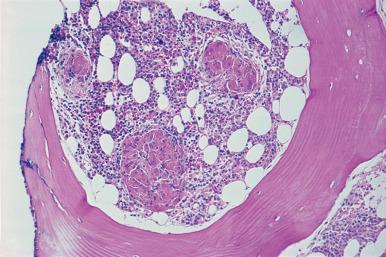
The MDS are a heterogeneous group of myeloid disorders with varying potential for bone marrow failure and evolution to AML. The MDS are basically disorders of ineffective myelopoiesis with hyperplastic marrows and cytopenias, dysplastic changes, and frequently pancytopenia with or without an increase in blasts. In a minority of cases, the marrow is hypocellular. The 2016 WHO Classification of hematopoietic neoplasms has changed the terminology in MDS, but most categories are similar to the prior classification. The major categories are: (1) MDS with single lineage dysplasia (previously termed refractory anemia); (2) MDS with ring sideroblasts (refractory anemia with ring sideroblasts); (3) MDS with ring sideroblasts and multilineage dysplasia; (4) MDS with multilineage dysplasia (refractory cytopenia with multilineage dysplasia); (5) MDS with excess blasts-1 (refractory anemia with excess blasts-1); (6) MDS with excess blasts-2 (refractory anemia with excess blasts-2); (7) MDS, unclassified; and (8) MDS with isolated del(5q). The classification is based primarily on examination of blood and bone marrow smears. However, marrow section specimens may add important diagnostic information, such as foci of blasts, increased fibrosis, and megakaryocyte dysplasia. Marrow sections in MDS with isolated del(5q) may be particularly informative in demonstrating megakaryocytes with hypolobulated nuclei characteristic of the disorder.
The WHO proposal also includes a category of myeloid disorders, referred to as MDS/MPN, for those myeloid processes that share features of both an MDS and a myeloproliferative process. This category includes chronic myelomonocytic leukemia, juvenile myelomonocytic leukemia, atypical CML, MDS/MPN with ring sideroblasts and thrombocytosis, and MDS/MPN, unclassified.
The trephine biopsy has a very important role in the evaluation of MDS and may provide important prognostic information. The cell population in biopsy specimens varies according to the subtype. In MDS with ring sideroblasts (sideroblastic anemia), there is generally a marked increase in erythroid precursors, with greatly increased iron accumulation in macrophages. In MDS with excess blasts 1 and 2, the marrow is hypercellular in the majority of patients, with an increase in neutrophil precursors. Chronic myelomonocytic leukemia, which is classified by the WHO as a myelodysplastic/MPN, is characterized by a hypercellular marrow with an increase in both monocytes and neutrophils. In any of these types of MDS, the marrow may be hypocellular in a minority of patients ( Fig. 39.37 ). This has been associated with an adverse prognosis in some studies.

Increased reticulin fibers may be observed in chronic myelomonocytic leukemia and MDS with excess blasts but is not usually a prominent feature. In approximately 10% of patients there is a marked increase in reticulin fibers ( Fig. 39.38 ). These cases usually satisfy the criteria for MDS with excess blasts. These cases have been associated with an adverse prognosis in some studies.

A finding referred to as abnormal localization of immature precursors (ALIPs), characterized by aggregates (3–5 cells) and clusters (>5 cells) of myeloblasts and promyelocytes in central areas of the marrow tissue away from the endosteal surface of the bone trabeculae and vascular structures, has been described in the MDS ( Fig. 39.39 ) ; the finding of three or more ALIPs in a bone marrow section has been reported to have predictive value for evolution to leukemia. Apoptosis may be a prominent feature in some cases ( Fig. 39.40 ).


Although the precise classification of the MDS is based principally on the evaluation of blood and marrow smears, there are some types of MDS in which the bone marrow biopsy findings are highly suggestive of a specific classification. The de novo MDS with isolated del(5q) occurs primarily in older women who present with a macrocytic anemia that is often severe, normal-to-increased platelet count, and usually prolonged survival; the blast percentage in the marrow is less than 5% and less than 1% in the blood. The marrow biopsy usually shows increased normal sized to slightly small megakaryocytes, many with hypolobulated nuclei ( Fig. 39.41 ). There is usually no significant dysplasia in the erythroid and neutrophil series. The presence of an isolated del(5q) cytogenetic abnormality with the described findings characterizes the del(5q) syndrome. Despite the name, patients with a second clonal cytogenetic abnormality (other than −7) have a similarly favorable prognosis to those with isolated del(5q) and may now also be diagnosed with this disorder. Three clonal abnormalities that included del(5q), however, are not allowed and are associated with a worse prognosis. Patients with this syndrome may respond favorably to treatment with the thalidomide analogue, lenalidomide. Patients with del(5q) and more than one additional cytogenetic abnormalities or blasts in excess of 5% in the marrow may also benefit from lenalidomide but have a generally worse prognosis and should not be diagnosed as MDS with isolated del(5q).

Increased megakaryocytes may also be observed in association with inv((3)q21.2q26.2) or t(3;3)(q21.3;q26.2), which may present as MDS or AML. The megakaryocytes in cases with this association are abnormally small, many with hypolobulated nuclei, as well as more pronounced dyspoiesis in other cell types. The very small megakaryocyte size and associated multilineage dyspoiesis are not features usually seen in MDS with isolated del(5q).
Similar to the AMLs and MPNs, extramedullary myeloid sarcomas may occur in the course of a MDS ( Fig. 39.42 ).

The therapy-related myeloid neoplasms (AML and MDS) occur in patients who have been treated with chemotherapy, radiation therapy, or both for a variety of malignant and nonmalignant conditions. Two major forms have been recognized: alkylating agent/radiation-related type and topoisomerase II inhibitor–related type, but many patients have received both types of therapeutic agents. The alkylating agent–related type is generally a panmyelopathy, which may evolve to acute leukemia; in either instance, AML or MDS, it is a poor prognosis lesion with relatively short survival. The topoisomerase II–related type usually presents as acute leukemia with specific cytogenetic abnormalities, notably abnormalities involving chromosome 11q23.3. The bone marrow cellularity in the therapy-related MDS related to alkylating agents is more variable than with de novo MDS: in approximately 50% of patients the marrow is hypercellular; in 25% normocellular; and in 25% hypocellular. In addition to changes characteristic of an MDS, the marrow specimen may show evidence of the initial lesion for which treatment was indicated. Megakaryocyte abnormalities may be particularly prominent in marrow biopsies ( Fig. 39.43 ). Reticulin fibrosis may be marked, and some of these cases have been described as acute myelodysplasia with myelofibrosis when there is a pronounced shift to immature cells.

Immunohistochemistry may be very useful in evaluating the MDS. CD34 may be particularly useful in identifying blast in cases of hypoplastic MDS that may resemble aplastic anemia. However, the absence of CD34 reactivity does not exclude blasts since not all myeloblasts are CD34 positive. Myeloperoxidase, CD15, CD117, and lysozyme antibodies are useful for recognizing myeloblasts and monoblasts. Antibodies to von Willebrand factor (factor VIII–related antigen), CD41, CD61, and CD31 may facilitate the recognition of small and abnormal megakaryocytes.
Cytogenetic studies performed on an aspirate specimen have a critical role in the evaluation of the MDS, both for determining clonality and for prognostic factors (see Box 39.2 ).
The WHO classification recognizes refractory cytopenia of childhood (RCC) as a provisional entity. Although this entity is reported to be the most common subtype of MDS in children, accounting for approximately 50% of MDS, it is very uncommon since MDS accounts for approximately 4% of hematologic malignancies in children. Patients present with persistent cytopenias; there are less than 5% blasts in the bone marrow and less than 2% blasts in the blood. Dysplastic features are present in all cell lineages in the smears. Evaluation of a marrow biopsy is critical for the diagnosis. About 75% of the patients have a markedly hypocellular marrow biopsy, which may have the appearance of aplastic anemia. The distinction may not always be possible on initial examination and repeat biopsies may be necessary.
The disorder occurs in all childhood age groups, and both sexes are affected with equal frequency. The evaluation of marrow biopsies is critical to the diagnosis.
The majority of children with RCC have marrows with a cellularity of 5%–10%. There are one to several foci of 10 or more immature erythroid precursors with increased mitoses. Megakaryocytes are usually markedly decreased to absent. Rare micromegakaryocytes may be found and are helpful in establishing a diagnosis; anti–von Willebrand factor (factor VIII–related antigen) and CD61 may facilitate their detection. Granulopoiesis is sparsely distributed and left shifted. In the minority of patients with RCC who present with normal or hypercellular marrows there is a slight-to-moderate increase in erythropoiesis, with a predominance of proerythroblasts with increased mitoses. Granulopoiesis is slightly to moderately decreased with a shift to immaturity. Blasts are less than 5% of the cells. Most cases show a normal karyotype; the most common cytogenetic abnormality when one is present is monosomy 7. The demonstration of a clonal abnormality is extremely helpful in establishing the diagnosis.
As noted, it may be very difficult to distinguish RCC from aplastic anemia and also, as noted, more than one observation may be necessary. In aplastic anemia there is generally no evidence of dysplasia in the smears. The erythropoiesis in the marrow sections is markedly decreased to absent; the several foci characteristic of RCC are usually not found, and if one is present it contains fewer than 10 cells that show evidence of maturation. Dysplastic megakaryocytes are not present. Granulocytes are markedly diminished and show normal maturation.
Considerable progress has been made in the classification of the MPNs in the past decade. This progress is the result of several discoveries of molecular alterations which may accompany these disorders. These discoveries have had impact on both the classification of these neoplasms as well as the recognition of entities, which previously were grouped under a nonspecific morphologic classification.
The discovery of the JAK2 V617F mutation has been the most significant contribution to the classification of the MPNs since the discovery of the Philadelphia chromosome in 1959 and subsequently of its molecular counterpart, the BCR/ABL1 fusion gene in CML. Since then, additional mutations in MPL and CALR have been reported, mostly in the JAK2 negative cases. The relationship of the these mutations to the specific MPNs will be noted in the diagnostic criteria for these individual disorders.
Although affecting a much smaller number of patients, the discovery of the genetic abnormalities in the PDGFRA , PDGFRB, and FGFR1 genes has important implications, at least in some of them, for possible therapy with tyrosine kinase inhibitors for a group of diseases previously refractory to most forms of therapy. The recognition of this group of diseases is based on cytogenetic studies, fluorescence in situ hybridization (FISH), and molecular genetic analysis. Most, but not all, of these patients present with marked eosinophilia.
CML is a stem cell disorder arising from fusion of the ABL1 gene on chromosome 9 with the BCR gene on chromosome 22. The altered chromosome 22 is referred to as the Philadelphia chromosome. CML, BCR/ABL1 positive, is distinct from other myeloproliferative disorders, which are BCR/ABL1 negative. CML usually has three clinical pathologic stages, not always clearly distinguished: chronic, accelerated, and blast. The vast majority of patients present in the chronic phase; uncommonly patients present in an accelerated or blast phase.
The initial presentation in the chronic phase is characterized by marked leukocytosis, thrombocytosis, and basophilia and usually splenomegaly. Atypical presentations such as marked thrombocytosis or basophilia without leukocytosis may occur. The number of myeloblasts in the blood and marrow smears in the chronic phase does not usually exceed 5%. The trephine sections are markedly hypercellular, primarily because of an increase in granulocytes and megakaryocytes. Macrophages resembling Gaucher cells, usually occurring singly, may be present in the bone marrow smears and sections; they are more prominent in perivascular locations. Increased reticulin fibers are present in approximately 30% of cases and megakaryocytes are increased in approximately 40% of cases. Many of the megakaryocytes are small with hypolobulated nuclei. The increase in reticulin fibers correlates with an increase in microvascularity.
The chronic phase, usually of 3–4 years' duration, is followed by the accelerated phase of shorter duration, characterized by increasing blasts in the blood and marrow, progressive basophilia, increasing myelofibrosis, and additional cytogenetic changes. The blast phase usually occurs abruptly and may occur without an intermediate accelerated phase; the blasts exceed 20% in the blood or marrow ( Figs. 39.44–39.46 ). Approximately 70% of blast crises are morphologically and immunophenotypically myeloid, and 30% are lymphoblastic. The myeloid type may be characterized by proliferation of any of the myeloid cell lineages, including myeloblasts, erythroblasts, and megakaryoblasts. Immunohistochemical studies may be very useful in identifying the distribution and lineage of blast populations. In some patients, blast transformation may be initially manifest in bone marrow sections as large, irregular, focal collections of blasts. Extramedullary manifestation of blast crisis also occurs, and the diagnosis should be suspected in any patient with CML who develops a tumor mass or lymphadenopathy.



The development of extensive myelofibrosis in patients with CML usually occurs late in the disease and has been associated with a more aggressive clinical course. Exceptions to this generalization have been reported and myelofibrosis may occur in the early stages of CML with the same prognostic implications as when it occurs late in the disease. The myelofibrosis in CML is usually characterized by an increase in reticulin fibers; collagenous fibrosis is not common but may occasionally occur (see Fig. 39.44 ). As noted, the increase in reticulin fibers correlates with an increase in angiogenesis. Reversal of myelofibrosis may occur following hematopoietic cell transplantation and imatinib therapy. A positive correlation between the degree of reticulin fibrosis and the number of CD61-positive megakaryocytes has been reported both pre- and post-allogeneic marrow transplant.
The introduction of therapy with the tyrosine kinase inhibitor imatinib mesylate (Gleevec), and now next-generation tyrosine kinase inhibitors, for CML has been one of the major success stories in the field of leukemia therapy; the agent induces hematologic remission in a high percentage of patients with CML in the chronic phase and accelerated phases of the disease. The hematologic remission is accompanied by morphologic normalization or near normalization of the bone marrow in most cases. The normalization of the marrow may occur even with persistence of the Philadelphia chromosome, or evidence of the BCR/ABL1 fusion gene. The normalization of the marrow usually may lag behind remission of blood findings and may not be complete for several months. Other therapies for CML are now less common. Treatment with interferon usually results in a decrease in overall marrow cellularity and an increase in erythroid precursors. There may be a concurrent increase in megakaryocytes and reticulin fibers. Hydroxyurea therapy generally results in a decrease in cellularity and no increase in megakaryocytes or reticulin fibers.
PV is an MPN in which the major diagnostic feature is an increased red blood cell mass. There is usually splenomegaly and some degree of leukocytosis and thrombocytosis. Precise criteria for the diagnosis of PV have been established by the Polycythemia Vera Study Group (PVSG); these were modified in the WHO proposal for the classification of hematopoietic neoplasms. An important addition to the diagnostic criteria for PV in the 2008 WHO classification is the recognition of the Janus 2 kinase gene mutation JAK2 V617F or similar mutations, such as JAK2 exon 12 mutation, present in over 95% of cases ( Box 39.5 ). The discovery of this mutation has also led to the identification of a marker for familial cases of PV.
Hemoglobin >16.5 g/dL in men; >16.0 g/dL women, or
Hematocrit >49% in men; >48% in women, or
Other evidence of increased red blood cell mass (>25% above mean normal predicted value). Bone marrow biopsy showing hypercellularity for age with trilineage growth (panmyelosis) including prominent erythroid, granulocytic, and megakaryocytic proliferation with pleomorphic mature megakaryocytes (differences in size)
JAK2 V617F or JAK2 exon 12 mutation
Subnormal serum erythropoietin level.
Diagnosis requires either all three major criteria or the first two major and the minor criterion
Three clinical phases of PV are recognized: (1) prodromal prepolycythemia phase with borderline to slight erythrocytosis; (2) overt PV with a significant increase in red blood cell mass; and (3) spent phase or post polycythemia with myelofibrosis.
The blood smear in the overt phase shows increased erythrocytes, reflecting the increased red blood cell mass, leukocytosis, and thrombocytosis. The bone marrow is usually markedly hypercellular. However, this is not an invariant finding; 13% of the patients enrolled in the National Cancer Institute Polycythemia Vera Study had marrow biopsies with cellularity of less than 60%. The cellularity of the marrows from patients in the study ranged from 37% to 100%, with a mean of 82%. The hypercellularity is due to a panhyperplasia of myeloid cells, principally erythroid precursors and megakaryocytes; the megakaryocytes range in size from small to unusually large, frequently with hyperlobulated nuclei and in clusters ( Fig. 39.47 ). The clusters may be accentuated in perisinusoidal and paratrabecular locations. Stains for iron usually show decreased or no hemosiderin deposits. A slight increase in reticulin fibers is present at the outset of the disease in 25% of cases; 11% of pretreatment biopsies show a marked increase in reticulin fibers. The increase in reticulin corresponds, in general, to an increase in marrow cellularity and the number of megakaryocytes. An increase in reticulin fibers in pretreatment marrow biopsies is not necessarily indicative of the spent phase of the disease. Similar to other MPNs, there is an increase in vascular structures in PV.

Approximately 20% of patients with PV have cytogenetic abnormalities; the most frequently detected are +8, +9, del(20q), del(13q), and del(9p). The incidence increases with disease progression.
PV may be difficult to distinguish from the other non-CML MPNs based solely on marrow examination. As a result, it is critical to adhere to established diagnostic guidelines when making a diagnosis. Although the JAK2 V617F mutation readily distinguishes PV from other causes of erythrocytosis, the mutation is shared by essential thrombocythemia and PMF, albeit less frequently. Morphology continues to have a central role in the diagnosis along with the clinical data.
The evolution of PV to the spent or post-polycythemic phase may be marked by a decrease in red blood cell mass with anemia and the development of myelofibrosis with a marked increase in reticulin fibers, increased angiogenesis, collagenous fibrosis, and clusters of abnormal megakaryocytes; the incidence of this complication varies from 9% to 20% ( Fig. 39.48 ). This is accompanied by a leukoerythroblastic blood picture and increased red blood cell poikilocytosis with numerous dacryocytes and increasing splenomegaly due to extramedullary hematopoiesis. An additional complication in some patients is the development of acute leukemia and myelodysplasia; the incidence is higher in patients treated with chemotherapy or radiation as opposed to those treated only with phlebotomy.

Essential thrombocythemia (ET) is a myeloproliferative disorder that is closely related to PV but lacks the essential diagnostic criteria of PV, most notably an increase of red blood cell mass. The principal morphologic findings relate to the increased megakaryocytes and thrombocytosis. The marrow is usually normocellular for age but may be hypercellular or hypocellular. The bone marrow findings are principally related to increased and large megakaryocytes with hyperlobulated nuclei ( Fig. 39.49 ). The megakaryocytes occur singly and in clusters. The erythroid and granulocytic cell lines are usually normal. Reticulin is usually normal but may be slightly increased, and there may be an associated increase in angiogenesis. There is no specific molecular marker for ET, such as the BCR/ABL1 fusion gene in CML, but half of patients with ET have the JAK2 V617F mutation, which may also be present in PV and PMF. Another approximately 25% of cases show mutations of CALR, and 3%–5% have MPL mutations, both usually in the absence of a JAK2 mutation. The WHO criteria for diagnosis of ET are listed in Box 39.6 .

Platelet count of ≥450 × 10 9 /L
Bone marrow biopsy showing proliferation mainly of the megakaryocyte lineage with increased numbers of enlarged, mature megakaryocytes with hyperlobulated nuclei. No significant increase or left shift in neutrophil granulopoiesis or erythropoiesis and very rarely minor (grade 1) increase in reticulin fibers
Not meeting WHO criteria for BCR-ABL1 + chronic myeloid leukemia, polycythemia vera, primary myelofibrosis, myelodysplastic syndrome or other myeloid neoplasms
Presence of JAK3 , CALR or MPL mutation
Presence of a clonal marker or absence of evidence for reactive thrombocytosis
Diagnosis requires meeting all four major criteria, or the first three and the minor criterion
The distinction between essential thrombocytosis and secondary forms of thrombocytosis may be difficult from routine morphologic studies. The clinical history is important in excluding possible causes for a secondary thrombocytosis. Demonstration of the JAK2 V617F or other ET-associated mutations would be definitive in excluding a secondary thrombocytosis. However, other mutations may be detectable in reactive cases, associated with so-called clonal hematopoiesis of indeterminate prognosis (CHIP), and the detection of a mutation alone does not equate with neoplasia.
ET is an indolent disease in most patients, with survival of 10–15 years. Transformation to AML occurs in less than 5% of patients and may be related to chemotherapy.
PMF (chronic idiopathic myelofibrosis, agnogenic myeloid metaplasia) is a clonal myeloproliferative disease of undetermined etiology characterized by myeloid cell proliferation and reactive fibrosis. It occurs primarily in adults; the average age at diagnosis is approximately 60 years. Rare cases have been reported in the pediatric population. There is some degree of detectable hepatosplenomegaly caused by extramedullary hematopoiesis.
PMF may, at times, be confused with CML or other types of MPNs because of occasional similarities in the blood findings; the important differentiating clinical and laboratory findings have been described in detail. The principal clinical and morphologic features have been described in the WHO Classification ( Boxes 39.7 and 39.8 ). The most important distinguishing biologic feature is the presence of the Philadelphia chromosome or molecular evidence of the BCR/ABL1 fusion gene in the hematopoietic cells in CML and its absence in the cells from patients with PMF and the possible presence of a JAK2 V617F (50%–60%), CALR (30%) or MPL (5%–10%) mutation in PMF. Marrow fibrosis in CML is generally a late occurrence, and its onset usually heralds a more aggressive evolution, unlike PMF in which marrow fibrosis is present to some extent from inception and is generally associated with a more prolonged clinical course. Approximately 30% of patients have a cytogenetic abnormality at presentation; the incidence increases with leukemic progression. The most common abnormalities are del(13q), del(20q), +8, +9, and abnormalities of chromosomes 1, and 7.
Megakaryocytic proliferation and atypia, without reticulin fibrosis > grade 1, accompanied by increased age-adjusted bone marrow cellularity, granulocytic proliferation and often decreased erythropoiesis
Not meeting the WHO criteria for BCR-ABL1 + chronic myeloid leukemia, polycythemia vera, essential thrombocythemia, myelodysplastic syndromes, or other myeloid neoplasms
Presence of JAK2 , CALR or MPL mutation or in the absence of these mutations, presence of another clonal marker or absence of minor reactive bone marrow reticulin fibrosis a
a minor (grade 1) reticulin fibrosis secondary to infection, autoimmune disorder or other chronic inflammatory conditions, hairy cell leukemia or other lymphoid neoplasm, metastatic malignancy, or toxic (chronic) myelopathies
Presence of at least one of the following, confirmed in two consecutive determinations:
Anemia not attributed to a comorbid condition
Leukocytosis ≥11 × 10 9 /L
Palpable splenomegaly
LDH increased to above upper normal limit of institutional reference range
Diagnosis of early/prePMF requires meeting all three major criteria, and at least one minor criterion
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here