Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acknowledgment: I would like to acknowledge the excellent work of earlier authors who wrote and revised portions of this chapter in previous editions: Alexander C. Angeledes, Gordon B. McFarland, Jr., Waldo E. Floyd III, Richard J. Smith, Clayton A. Peimer, Harold M. Dick, and Owen J. Moy. The principles of management of aggressive and malignant tumors as described in great detail by these authors have evolved with over time. This chapter updates tumor assessment and patient management principles, which have changed as pathology, diagnostic imaging, and the effects of adjuvant therapies have become better understood.
The purpose of this chapter is to present the most recent knowledge on specific benign and malignant bone and soft tissue tumors that may appear in the hand. Tumors occurring in the hand and forearm generally have unique growth patterns and potential for metastasis that may be different from those seen elsewhere in the body. Tumors in the hand are relatively uncommon, and most surgeons encounter them infrequently. Knowledge regarding treatment and care of hand lesions is often based on case reports, small case series, retrospective reviews, and a few large general case studies. Data from controlled trials are limited but have advanced the use of chemotherapy and radiation therapy and the treatment of soft tissue sarcoma. At times, the information available is both contradictory and controversial. It is important for the treating surgeon to thoroughly understand the specific characteristics of the tumor being treated, as well as the systemic treatment of the disease. Of greater importance, however, is knowledge and practice of the principles and guidelines for the treatment of patients with tumors.
The reader is strongly encouraged to understand and review the principles of staging, biopsy, resection, and amputation before treating all tumors, particularly those that are malignant. In no other aspect of hand surgery are the implications for uninformed decisions, judgments, or actions more serious than in the treatment of malignant bone or soft tissue sarcomas. Conversely, treatment based on up-to-date knowledge and a thorough understanding of the principles of care for local and systemic cancer maximizes the potential to save the patient’s life. Only after this concern has been addressed can one focus on the task of salvaging or reconstructing a useful hand or limb. Regular review of treatment principles before treating patients for malignant tumors is imperative and helps ensure appropriate treatment.
Neoplasms are usually classified into two categories: benign and malignant; in musculoskeletal tissues, malignancies can also be subdivided into low- and high-grade. Cellular growth in benign neoplasms usually proceeds at a much slower rate than in malignant tumors.
Malignant neoplasia is characterized by a rapid growth rate, atypical cellularity, and poor cell differentiation. Local growth is aggressive and infiltrative; there are only “pseudocapsules” through which the tumor extends to form satellite lesions. Such tumors are likely to spread as blood-borne metastases, and local recurrence rates are high after excision unless a wide margin of normal tissue is included in the resection. Low-grade malignancies grow more slowly and infiltrate early but are less likely to metastasize than to recur locally. Neither all generic categories nor isolated case illustrations are consistent with these classifications, however.
Many benign tumors of the hand or forearm require no treatment, can be diagnosed clinically, and are asymptomatic. However, if a lesion increases in size or becomes symptomatic or if the physical or radiographic appearance suggests an aggressive process, appropriate staging studies, including obtaining tissue for diagnosis, must be performed. Unfortunately, lumps and growths that look innocent may not necessarily be so. Surgeons need to be familiar with the range of possible diagnoses and generate a differential diagnosis for the individual patient under consideration. A physician is not justified in advising a patient that a mass should be “left alone” until the proper diagnosis is established by all appropriate means. Any tumor with an unclear diagnosis on the basis of nonsurgical evaluation should be sampled. If the biopsy proves the tumor to be benign, no further treatment may be necessary. If a malignant or an aggressive nonmalignant lesion is identified, however, further management is required.
Tumors that are symptomatic need to be diagnosed and staged. The clinical and family history, physical characteristics of the tumor, and data from laboratory and imaging studies provide at least the basis of a clinical impression. If the precise diagnosis is unclear after a complete workup, a carefully planned biopsy is required to avoid the hazards of misdiagnosis and its complications.
Management of hand and upper extremity tumors does not differ significantly from management of tumors in other parts of the musculoskeletal system. Correct treatment must always take into consideration the size and location of the growth, its histologic grade and clinical behavior, and its potential for metastases. , , A thorough understanding of the general principles and guidelines is essential for accurate assessment and staging of tumors.
The histologic grade (G) of a neoplasm is determined by the malignant characteristics of tissue obtained by biopsy. An accepted classification is as follows:
G0: Benign
G1: Low grade: few cells, much stroma, little necrosis, mature cells, <5 mitoses per high-power field
G2: High grade: many cells, little stroma, much necrosis, immature cells, >10 mitoses per high-power field
Benign tumors can be classified into three stages :
Latent, stage I tumors do not usually require treatment; they may heal spontaneously or remain unchanged.
Active, stage II benign neoplasms grow within a limited zone and are contained by natural barriers; if surgery is required, these tumors are most often controlled by intralesional or marginal excision.
Locally aggressive, stage III benign tumors may both grow and spread beyond natural barriers; excision usually requires a wide surgical margin or en bloc resection for local cure.
To avoid confusion and differences of opinion about malignant bone tumors, it has become important to establish specific grading criteria for tumors. A classification scheme based on a modified staging system for bone sarcomas was established by the American College of Surgeons Joint Committee on Cancer and End Results Reporting in 1977. It was proposed by W. F. Enneking at the University of Florida and accepted by the Musculoskeletal Tumor Society (MSTS) in 1979 ( Tables 59.1 and 59.2 ). Although this staging system was developed for bone sarcomas, it has also been used to describe and stage soft tissue sarcomas, particularly in the orthopedic surgical community. A comparison of the MSTS system and the fourth and fifth editions of the American Joint Committee on Cancer (AJCC) system has found the AJCC system to be more predictive of systemic relapse. The AJCC staging system incorporates grade, size, and the presence or absence of metastasis to determine the final stage of the lesion. This system should be used preferentially for staging of soft tissue sarcomas ( Table 59.3 ).
| Stage | Grade | Site |
|---|---|---|
| IA | Low (G1) | Intracompartmental (T1) |
| IB | Low (G1) | Extracompartmental (T1) |
| IIA | High (G2) | Intracompartmental (T1) |
| IIB | High (G2) | Extracompartmental (T2) |
| III | Any (G) | Any (T) |
| Regional or distant metastasis (M) | Regional or distant metastasis (M) |
| Margin | Surgical Method | Planes of Dissection | Microscopic Appearance | |
|---|---|---|---|---|
| Limb Salvage | Amputation | |||
| Intralesional | Debulking, piecemeal excision/curettage | Translesional amputation | Within tumor (palliative) | Tumor at all margins |
| Marginal | Marginal en bloc excision | Marginal amputation | Within tumor “reactive zone” | Reactive tissue (± microextensions of tumor) |
| Wide | Wide en bloc excision | Wide through-bone amputation | Through normal tissue but within compartment | Normal tissue (± “skip lesions”) |
| Radical | En bloc resection of entire compartment | Extraarticular disarticulation | Normal tissue extracompartmental | Normal tissue |
| Description | |
|---|---|
| Stage I | |
| IA | Low grade, size <5 cm |
| IB | Low grade, size >5 cm |
| Stage II | |
| II | High or intermediate grade, size <5 cm |
| Stage III | |
| Stage IIIA | High or intermediate grade, size >5 and ≤10 cm |
| Stage IIIB | High or intermediate grade size >10 cm |
| Stage IV | Any metastasis to lymph nodes or distant sites |
The pathologist and surgeon must agree on a surgical grade (G1,G2) of the malignancy based primarily on the histologic features of the tumor and also on its gross pathologic appearance, the clinical setting, and its radiologic appearance. The histologic grade is determined only after careful scrutiny of a representative biopsy specimen by an experienced musculoskeletal pathologist. Clinical and radiologic status are also important parameters for determining the final tissue diagnosis and surgical grade.
The two grades of malignant tumors differ with respect to their rate of metastasis and recurrence: G1 is a low-grade malignancy with a low likelihood of metastasis and possibly more frequent local recurrence, and G2 is a high-grade malignancy with frequent blood-borne metastases. The so-called low-grade malignant tumors can also metastasize but are typically less likely to do so early in their course. Assignment of a grade to a particular tumor is neither easy nor exact because grading criteria are not quantifiable. Cellular morphology, anaplasia, necrosis, mitoses, and tissue of origin can also be influenced by clinical behavior. Tumors that are known to be exceptionally dangerous (e.g., synovial and epithelioid sarcoma) are most properly classified into a higher grade than their histology would indicate. Two other criteria are important: T, which represents the size and site of the tumor, and M, which designates the presence of detectable metastases.
Regardless of size, if the tumor is limited to a single anatomic compartment, it generally will be resectable. At the same time, it will be possible to preserve the extremity, as is the case with highly malignant tumors involving the distal phalanges and other acral parts. The basis of defining a compartment is the recognition that certain natural anatomic barriers exist that will temporarily contain and delay the spread of a pathologic process such as infection and neoplasia. For example, an intraosseous tumor contained by the cortices and intramedullary canal of a tubular bone would be considered intracompartmental. However, if this tumor perforates into the surrounding soft tissues, it would then be considered extracompartmental because it has already crossed a natural barrier. Computed tomography (CT) and magnetic resonance imaging (MRI) enable us to estimate the size and location of a tumor, thereby permitting more accurate preoperative planning than was possible previously.
This concept also applies to soft tissue compartments, but not quite so neatly as with bone tumors. Tumors that involve a flexor tendon in the digit have, in theory, violated the entire compartment of extrinsic muscle. Tumors may have a propensity to spread proximally along the tendon to the muscle, much as a hematoma may track proximally after rupture of a flexor tendon by following the natural barriers of the tendon sheath and muscle fascia.
The third criterion for classification of malignant tumors identifies patients in whom the tumor has already metastasized to other sites. The MSTS has three stages, with stage III including all patients with distant metastases, regardless of the other parameters (see Table 59.1 ). Lymph node involvement is always an important finding in that primary musculoskeletal neoplasms do not commonly spread to regional nodes. Because lymph node metastases develop in less than 5% of patients with sarcomas, the differential diagnosis of extremity tumors with possible nodal metastases must always be expanded to include carcinomas and melanomas. Metastases to lymph nodes are significantly more prevalent with rhabdomyosarcoma, epithelioid sarcoma, clear cell sarcoma, and angiosarcoma.
Once the appropriate staging studies are completed, the surgeon will know the anatomic characteristics (the size or “T”) of the lesion and whether metastases (the “M”) are absent or present. A differential diagnosis can then be formulated and the biopsy planned.
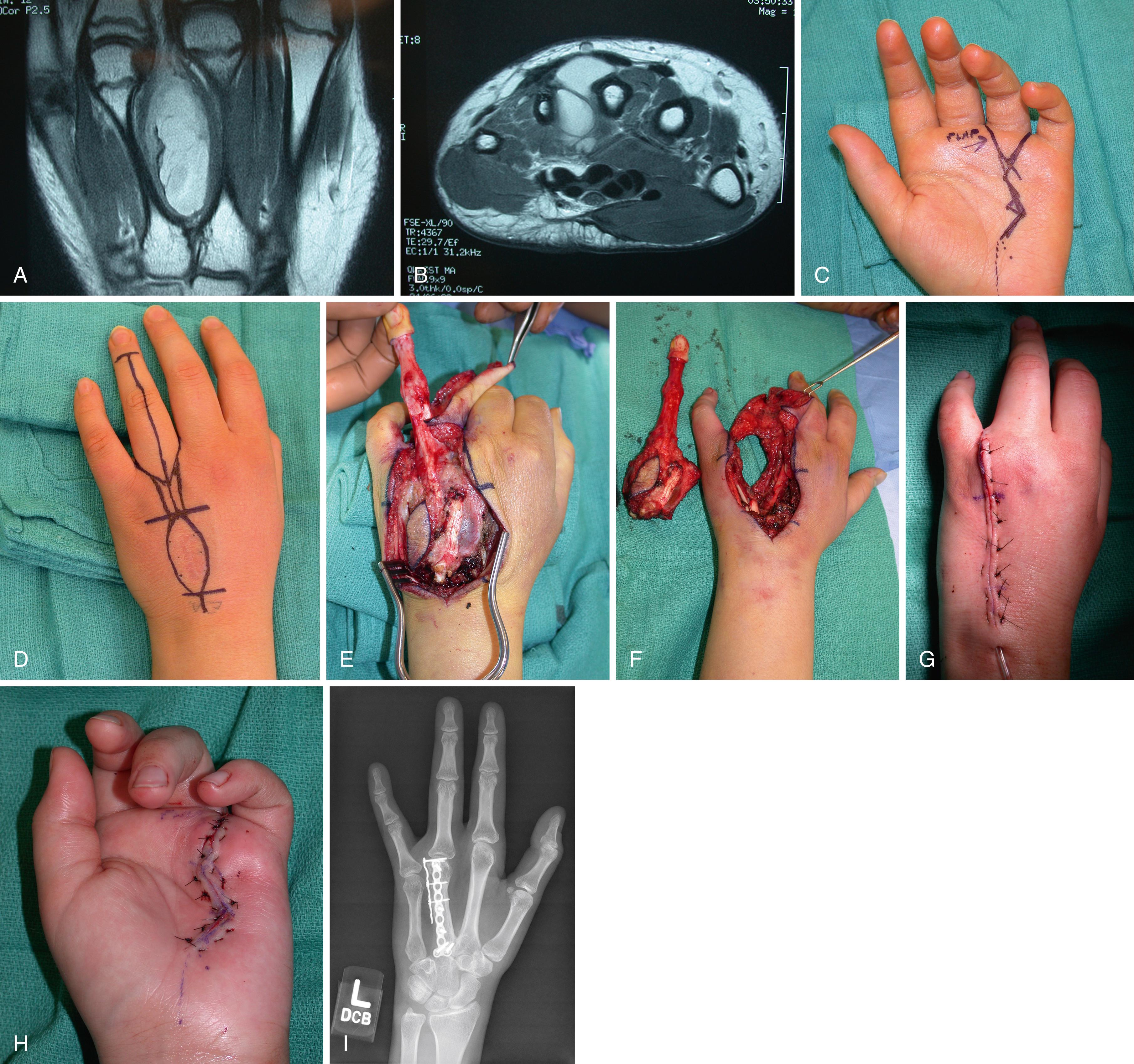
There are no precise physical characteristics that clearly distinguish aggressive and malignant tumors from benign and reactive lesions. The only definitive test is histologic evaluation of biopsy material. However, because hand tumors can produce such significant dysfunction and because inadequate or unnecessarily aggressive surgery also presents risks, biopsy must be the final step in obtaining a diagnosis. All nonsurgical studies should be completed before surgical biopsy ( Fig. 59.1 ).
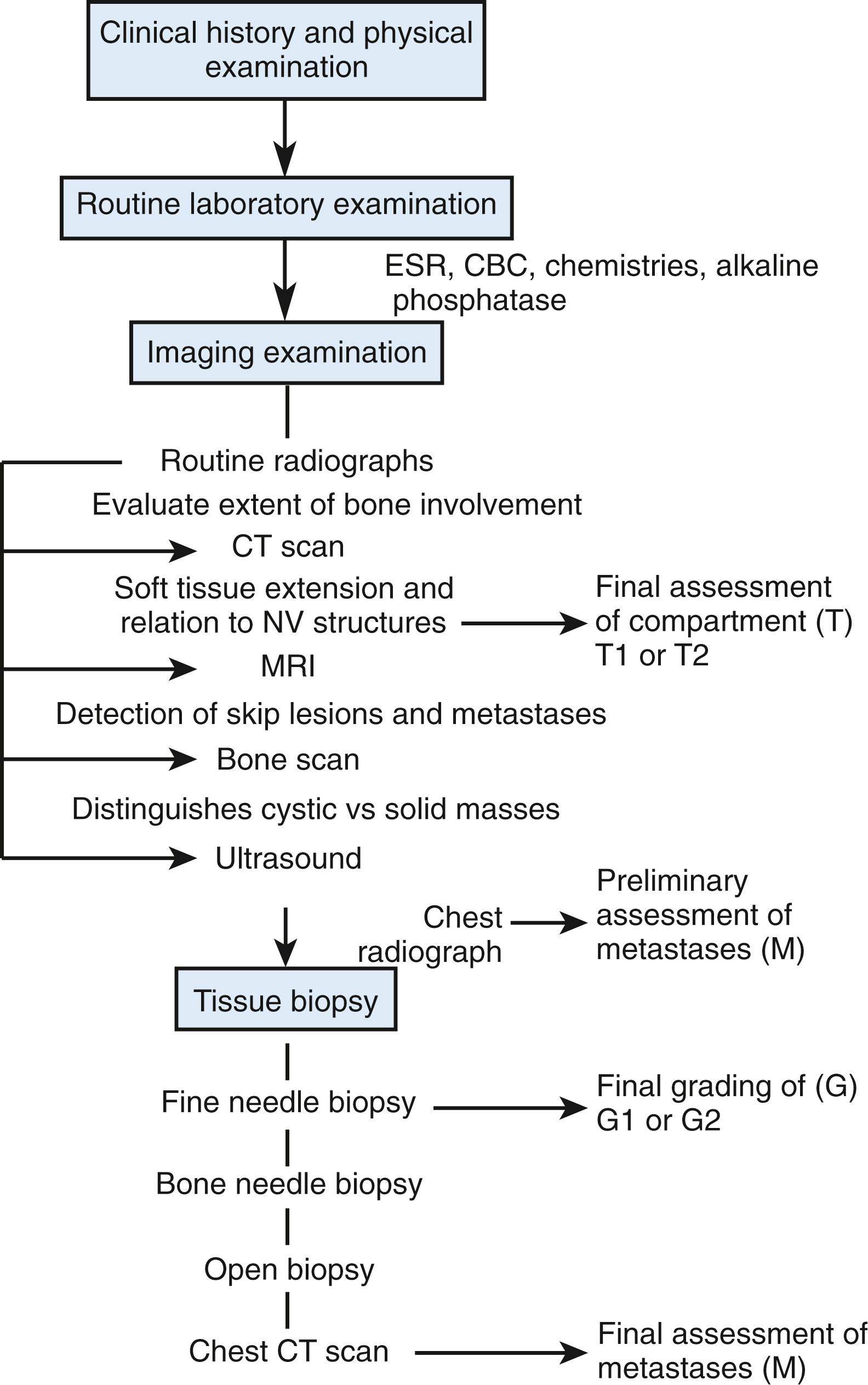
Laboratory studies include determination of the levels of serum calcium, phosphorus, blood urea nitrogen, and creatinine to evaluate the possibility of metabolic bone disease. Serum alkaline phosphatase and lactate dehydrogenase levels are elevated in some malignancies. Serum immunoelectrophoresis assists in determining whether multiple myeloma is present. The hematologic profile and the erythrocyte sedimentation rate are abnormal in the presence of many neoplastic and infectious processes. Urinalysis may detect an occult renal cell carcinoma that can metastasize to the hand. Antinuclear antibodies and rheumatoid factor may be positive in patients who have upper extremity swelling secondary to rheumatoid disease.
A variety of diagnostic imaging techniques permit analysis and localization of the size and extent of the lesion and its impingement on normal anatomy. Although imaging does not typically offer a specific diagnosis, it provides important clues in the evaluation, analysis, and workup of a specific patient.
Plain radiographs afford the best possible resolution and detail of bone and adjacent soft tissues. The anatomic region in question should be fully visualized on all radiographs, and this is achieved by taking an adequate number of views. Plain radiographs are initially performed to assess the presence and location of bone involvement. They may also be the most specific radiologic test for bone tumors.
Although conventional radiographs offer considerable spatial information, the higher resolution of CT produces images that are specifically useful in localizing small tumors within a bone and in identifying soft tissue extension or calcification within such growths. Because of its ability to discriminate varying densities, CT allows visual separation between the medullary canal, cortex, and surrounding soft tissues and thus often provides critical information concerning anatomic location (T) for tumor staging. Plain chest radiographs are always obtained before performing a biopsy on a suspected malignancy; chest CT scans are useful for staging histologically defined malignant tumors but are not routinely used for prebiopsy screening studies.
Radionuclide “bone scan” imaging can be very helpful in detecting primary and metastatic tumors. However, the phenomenon of increased radiopharmaceutical pooling (increased uptake) in a particular bone or soft tissue area is not at all specific or diagnostic. The technique has two time-variable phases. Within minutes after injection, conditions associated with increased vascularity show abnormally high uptake (i.e., trauma, infection, and neoplasia). Later, the isotopes are actively concentrated; in the skeleton, pooling occurs in woven (new) bone, so any process that forms immature bone would be associated with increased uptake on films taken 2 or 3 hours after injection. Radioisotope scans are probably the most helpful to demonstrate lesions that may not have been suspected clinically or in anatomic sites not seen on initial images that focused only on the symptomatic region. These findings can be important in patient management, and they have the potential to alter biopsy or therapeutic plans significantly.
MRI is a technique that depends on sophisticated computers to produce excellent delineation of soft tissue contrast, as well as images in the axial, coronal, and sagittal planes. For MRI studies, electromagnets generate strong fields that cause cellular nuclei to “wobble” or “vibrate” at specific frequencies. A number of technical factors affect the MRI signal, including echo time (TE), pulse or repetition time (TR), longitudinal or spin-lattice relaxation (T1), and spin–spin relaxation (T2).
The majority of soft tissue tumors have a low signal intensity (i.e., appear darker) on T1-weighted scans and a high signal intensity (i.e., appear brighter or whiter) on T2-weighted studies. Specific problems, such as hematoma, lipoma (or liposarcoma), hemangioma, or conditions that involve hemorrhage into an existing tumor zone, are known to have high signal intensity on Tl-weighted scans. Obviously, the potential value of MRI is diminished in a region recently subjected to biopsy, trauma, or other surgery.
MRI is a superior imaging technique for assessment of the soft tissue extent of disease and is particularly beneficial in the evaluation of soft tissue sarcoma. The extent of intramedullary bone involvement also can be readily determined. This facilitates preoperative planning of the bone transection level during resection and may aid in identification of skip metastases that might otherwise not be identified. High-resolution CT offers the benefit of more accurate assessment of cortical bone involvement and may be the preferred technique for evaluating lesions involving the cortex of a given bone. CT is particularly beneficial in identifying occult osteoid osteomas.
Sonograms produce images from a differential pattern of transmitted and received echoes. Compared with CT and MRI, sonography produces less detail with respect to precise anatomic relationships and tumor margins. The echo patterns of solid masses are nonspecific, but fluid-filled lesions can easily be differentiated from solid tumors. Sonograms are extremely inexpensive in comparison to the cost of CTs and MRIs and may be more useful than either for distinguishing reactive and cystic processes. Ultrasound guidance can be particularly useful for core needle biopsy of soft tissue lesions.
Biopsy is the last stage of diagnostic management. Such surgery should be planned as carefully as the definitive operation. , The exact technique used is influenced by the history, location, and size of the mass, as well as the experience of the surgeon and pathologist.
Core needle biopsy has a significant role in the diagnosis of lesions in the hand and upper extremity. It can be useful in the assessment of subcutaneous and some deep lesions or in confirming the histology of a recurrent or metastatic lesion. Ultrasound- or CT scan-guided core needle biopsy may increase the diagnostic yield of biopsy and also minimizes contamination risk. This my increase the utility of core needle biopsy in selected cases. Needle biopsy produces only a small tissue sample and at times may be impossible to diagnose or grade accurately. In this event subsequent formal incision biopsy may be indicated. Even if the core biopsy sample seems to clearly be diagnostic, the tissue volume may not be representative of the tumor and could lead to the possibility of overgrading or undermanagement.
Open surgical biopsy is a complex procedure but one that plays a critical role in determining treatment outcome for aggressive and malignant tumors. During the biopsy, the patient and surgeon must be prepared for the unexpected. Institutions or clinicians who are not prepared or able to complete all diagnostic studies and also provide definitive surgical and adjunctive medical management are best advised to refer patients before the biopsy is performed.
Hand surgery is most safely and efficiently performed in a bloodless field. The use of a pneumatic tourniquet is acceptable during both open biopsy and surgical treatment of tumors and neoplasms. Exsanguination of the limb before biopsy is contraindicated before inflation of the tourniquet because of the risk of seeding or dislodging tumor cells. An entirely satisfactory field is achievable by elevating the arm for 1 minute before inflating the tourniquet. Likewise, the use of intravenous anesthesia (i.e., Bier block technique), a method that also requires pretourniquet exsanguination, is not appropriate for tumor biopsy or treatment of aggressive bone or soft tissue neoplasms.
A frozen section is necessary at the time that the biopsy is performed to determine whether an adequate specimen has been sampled so that permanent light and special microscopic evaluations can be carried out subsequently. It is rare to find a pathologist who can consistently provide accurate histologic analysis of a frozen specimen dependable enough to begin treatment. , The definitive treatment plan for malignant lesions is best decided on only after the results of all permanent sections, electron microscopic studies, and special tissue techniques are complete and reported. All biopsy specimens should be cultured, and all cultures should have tissue sent for microscopic examination.
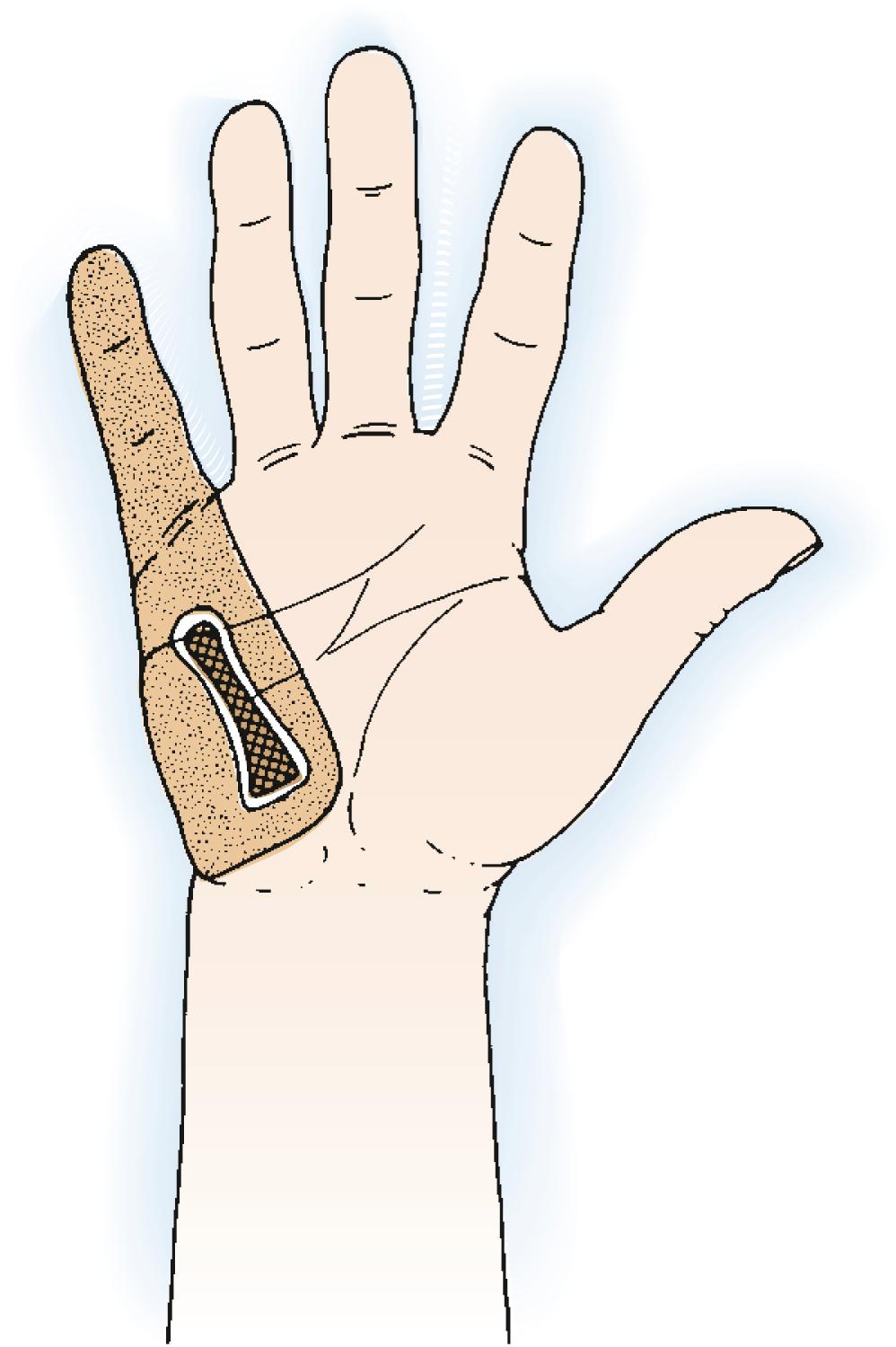
Although plain chest radiographs are taken before biopsy, chest CT is performed to stage a diagnosed lesion. If the tumor is aggressive or malignant, the biopsy tract itself will be contaminated with tumor cells and should later be excised en bloc with the subsequent resection or amputation specimen. Preferred biopsy incisions are therefore longitudinal and carefully placed to permit complete excision of them without having to extend a dissection margin simply to accommodate a badly placed incision. As a simple rule, the biopsy should be placed in line with or immediately parallel to the incision that may be required for later attempted limb salvage ( Fig. 59.2 ). Transverse and Bruner-type incisions are specifically to be avoided.
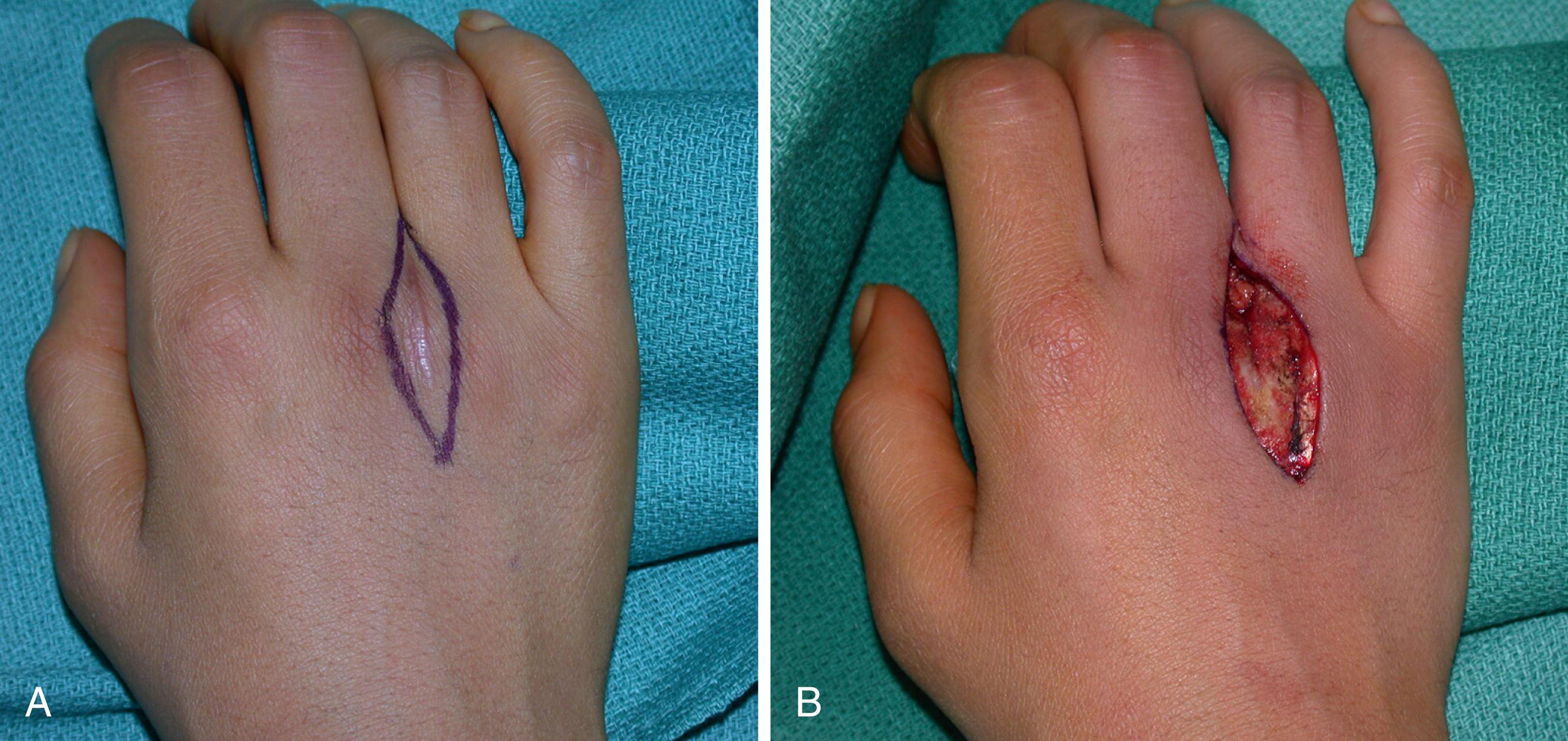
Incisional biopsy is frequently the most appropriate technique for the diagnosis of bone and soft tissue masses. It requires excision of an adequate tissue sample that is minimally manipulated. Frozen sections are important to determine the adequacy of the specimen. The biopsy incision is located so that it affords the most direct route to the tumor, thereby ensuring that the fewest tissue planes are disturbed or contaminated.
The correct surgical technique for biopsy is different from standard typical surgical dissections. Biopsy involves a direct approach using a longitudinal incision through muscle and other tissues that overlie the mass. Biopsy technique does not include spreading or extensive and vigorous retraction, which has the potential to contaminate a widened area. However, adequate visualization during biopsy, as in any operation, is critical. Because the biopsy tract is contaminated with cells, an approach through a muscle sacrifices only one plane, whereas spreading techniques (i.e., moving muscles aside) contaminate not only the muscle that is retracted but also all surrounding structures. Perfect postsurgical hemostasis is essential to avoid hematoma, which can lead to the spread of cells beyond the primary site and biopsy tract. Once the wound is dry and hemostasis is secure after release of the tourniquet, the wound is closed, not drained. Other than taking steps to avoid pathologic fracture, postbiopsy surgical care is routine while the surgeon awaits the definitive histologic findings.
Excisional biopsy involves complete removal of a lesion through the reactive zone. An example would be excision of a giant cell tumor of a tendon sheath or a lipoma. It is a technique that should be reserved for very small lesions (with a diameter of <2 cm) to avoid compromising subsequent surgical procedures. Extensive contamination of the operative field occurs with this type of biopsy. If this is done for a large tumor, the extent of contamination may increase the need for soft tissue coverage and increase the risk for both positive margins at definitive resection and subsequent local recurrence. Extensive contamination may preclude subsequent limb salvage and may commit the patient specifically to amputation as a result of the biopsy. This type of biopsy is either best avoided or used with great caution for lesions in or just distal to the carpal tunnel. Contamination of the carpal tunnel, or area just distal, during marginal excision of a malignant soft tissue sarcoma may commit the patient to amputation of the hand at definitive treatment specifically as a result of the biopsy.
This type of biopsy requires excision of the tumor with a wide margin or cuff of surrounding normal, nonreactive, healthy tissue. This is an excellent oncologic procedure but requires sacrifice of an extensive amount of normal tissue. Primary wide excision is usually best reserved for very small lesions in which suspicion of malignancy is high and the risk of soft tissue contamination with other forms of biopsy is excessive, as might occur in the carpal tunnel. The decision of when to perform this type of biopsy is generally best made with the assistance of or by a musculoskeletal oncologist.
Lesions that cannot be diagnosed as benign on clinical and radiographic grounds
Review the case with a pathologist before biopsy and confirm the availability of that person.
Draw limb salvage incisions or amputation flaps at the time of biopsy.
Place a longitudinal biopsy incision mark in line with the limb salvage incision.
Confirm that the biopsy incision will not compromise subsequent amputation flaps.
Elevate the arm only for exsanguination without using an Esmarch bandage.
Inflate the tourniquet to ensure a bloodless field.
Dissect through one anatomic plane or muscle to limit the contamination field.
Avoid extensive flaps or retraction to limit contamination.
Incise directly into the tumor to remove a wedge for frozen section and permanent analysis.
Culture all biopsy specimens.
Confirm the presence of adequate lesional tissue on the frozen section.
In most cases, defer definitive treatment of malignant lesions until permanent analysis confirms the diagnosis.
Obtain perfect hemostasis before closure.
If a drain is used, it should exit the patient immediately in line with the incision to limit contamination.
Place sutures close to the wound edges to avoid additional skin contamination.
Apply a bulky compressive dressing to avoid hematoma.
Limit use of the limb until definitive treatment.
Close coordination between surgeon and pathologist is critical because of the complexity of pathologic assessment of limb tumors, with many differential diagnoses to consider including sarcomas, carcinomas, and even melanoma. Radiology input is especially important for bone tumors in order to avoid known diagnostic pitfalls. Some of the information required by the pathologist includes the site of the tumor (bone versus soft tissue, anatomic site), radiologic characteristics, and relationship to surrounding structures likes nerves and muscle.
As mentioned earlier, frozen section should be used to confirm that lesional tissue has been sampled but cannot be relied on for a definitive diagnosis, especially in bone tumors.
Histologic diagnosis of limb sarcomas should be made according to the latest World Health Organization (WHO) classification for bone and soft tissue sarcomas. Pathologic assessment involves morphologic examination, looking at the architecture, cytomorphological features, and matrix of the tumor, complemented by ancillary immunohistochemistry and molecular diagnostic tests.
A panel of immunohistochemistry stains is often required for the diagnosis of most soft tissue tumors, though less so for bone sarcomas. Based on the pattern of uptake, subclassification of the tumor can be done.
The critical role that molecular tests play in the diagnosis of many sarcomas has been clearly established, with some arguing that they should be done when assessing all mesenchymal tumors thought to have a known molecular abnormality. Molecular tests are especially helpful in challenging situations. These include unusual clinical presentation and atypical morphology. This can also include tumors with similar morphology, as may be seen with round cell tumors and tumors with overlapping immunohistochemistry profiles.
Nonaggressive, benign neoplasms are usually removed simply by marginal or intralesional operations such as curettage. If there is a question about the adequacy of resection of the tissue margins and the diagnosis is already confirmed, a frozen section may be of great assistance for intraoperative sampling of the margins.
In the management of malignancies of the upper limb, preservation of function is secondary to eradication of the disease process. Hand surgeons who undertake management of a neoplasm must have a thorough understanding of the tumor’s biologic behavior, its patterns of local and metastatic spread, and its response to radiotherapy and chemotherapy. Radiation therapy and chemotherapy may allow the surgeon to use less radical tumor margins so that amputation can be avoided and function maintained. , However, the risk of unnecessary sacrifice of tissue must be balanced against the need for adequate resection because local recurrence of sarcoma sometimes carries a grave prognosis. One of the major problems in assessing outcome has been the lack of a common procedural language regarding surgical methods; however, uniform use of the Enneking terminology, as recommended by the MSTS, provides such a means of communication (see Table 59.2 ).
Treatment should not begin until a neoplasm has been diagnosed definitively and the results of all preoperative studies, including biopsy, are verified. The assumption that a problem can be “handled” by relatively simple extirpation in the absence of complete diagnostic data is neither justified nor defensible. The risks associated with soft tissue extension from a tumor-contaminated field are too great. Because most tumors are benign, cystic, and nonproblematic, the outcome of the few that are aggressive or truly malignant may be disastrous if surgeons become too casual or complacent. When a malignant tumor or aggressive nonmalignant lesion is identified, carefully planned treatment is required not only to preserve the maximal degree of hand function but also to save the patient’s life. Function is secondary to survival. , , ,
Hand surgeons who seek to treat a specific neoplasm must have not only a general understanding of the concepts and principles of tumor management but also detailed knowledge of the tumor’s biology, histologic and clinical behavior, tendency to spread locally and widely, and potential response to adjunctive measures. All these considerations can have a significant bearing on proposed surgical margins and long-term function. , , An inadequate resection that permits local recurrence may condemn the patient to a far worse prognosis than would otherwise have occurred.
According to MSTS criteria, compartmental resection and radical excision cannot always be applied to the hand. Most hand tumors occur in spaces rather than in compartments, and although metacarpal excisions may remove a compartment for a stage IA or IIA bone tumor, extirpation of the entire flexor surface or above-elbow amputation would be required to remove the compartment if the neoplasm arose on a digital flexor. Presently, there are no data to support a conclusion that the MSTS definitions should be applied uniformly to all parts of an acral compartment. Because the flexor tenosynovium is continuous in the thumb and little finger, but not in the index, middle, and ring fingers, there may be differences in management that can be used prudently with such variations in location.
A soft tissue tumor that develops along the dorsum of the wrist requires surgical extirpation of the extensors of several fingers or the thumb (or both), but not necessarily the entire muscle compartment proximally or distally. Functional restoration can be achieved with tendon grafts or transfers or with free muscle flaps. True radical excision may be impractical if function of any kind is to be salvaged in patients with lesions of the hand and forearm, and it may be that a 1- to 2-cm margin of normal tissue, especially with tumors that are sensitive to adjunctive measures, will be safe and allow secondary reconstruction. , , The extent of surgical resection for aggressive tumors is dependent on their histologic diagnosis, location, and size. Remote tissue reconstruction, except when provided by microvascular transfer, should be avoided because of the risk of transferring malignant cells using tissue or flap pedicles. If a local or pedicled flap is done it is imperative that the surgeon and entire surgical team adhere to strict measures to avoid cross contamination of the donor site.
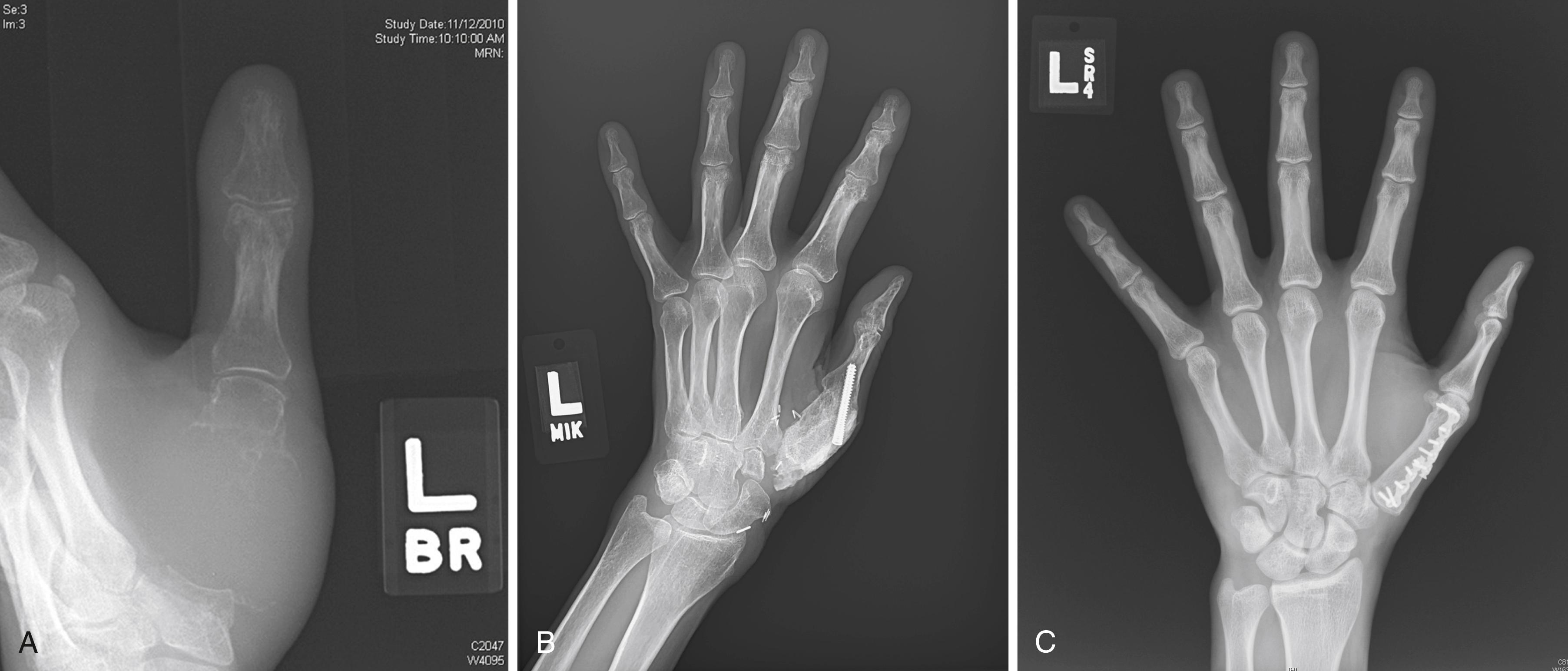
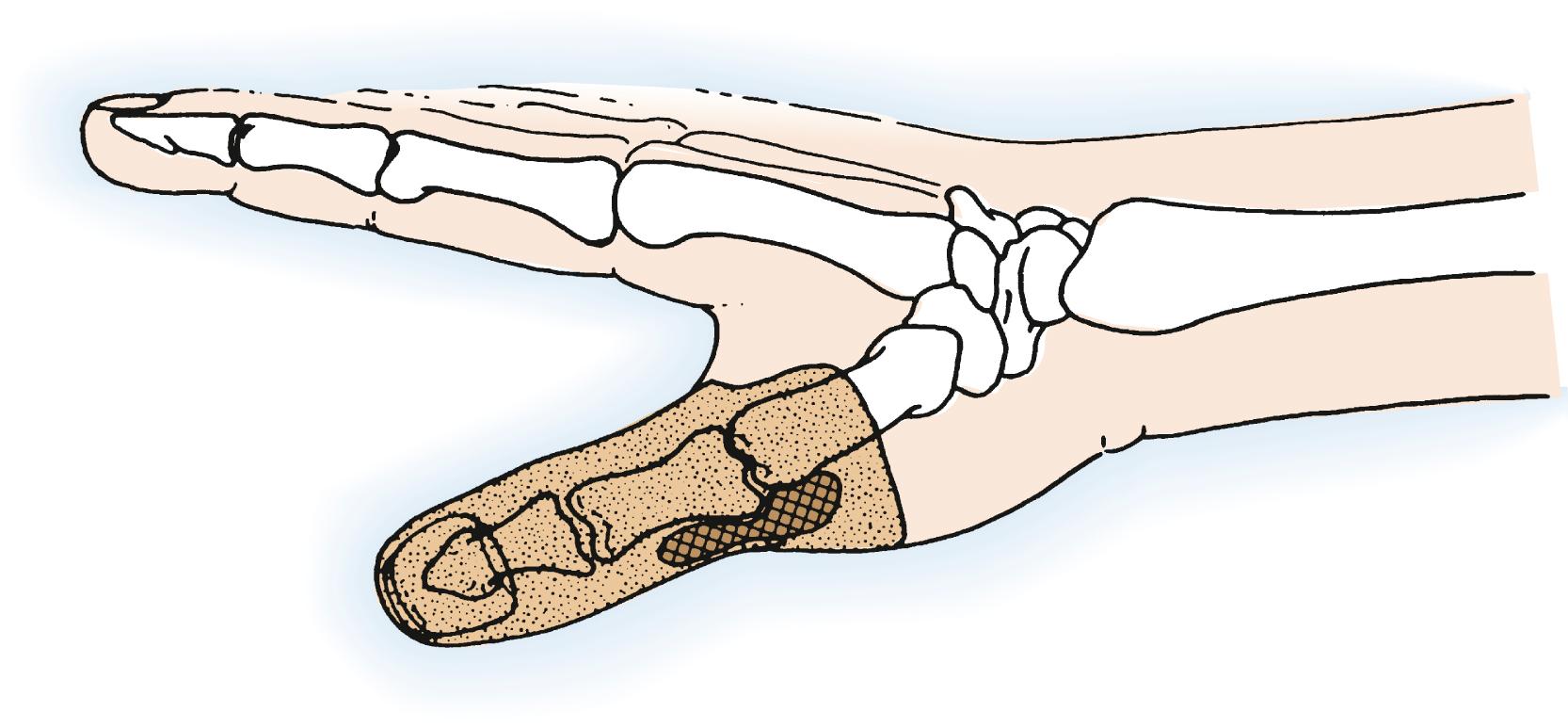
Malignant soft tissue tumors in the distal phalanx commonly involve skin and bone ( Fig. 59.3 ). Safe removal may require excision at the distal interphalangeal (DIP) joint; occasionally, a lesser segment may need to be excised when the lesion, such as a nail bed carcinoma, is more superficial and slow growing. A small portion of dorsal or palmar skin might be used as a local flap to cover the middle phalanx after disarticulation of the DIP joint. In such instances, the distal phalanx and DIP joint are sacrificed if the lesion involves or threatens enough tissue while retaining the tissues at a safe distance from the tumor site. Indeed, if the tumor involves both the dorsal and palmar surfaces of soft tissue as well as bone, a more proximal transosseous amputation through the middle phalanx is required. In every case, the proximal end of the excision margin should be sampled and inspected by frozen section at surgery and later by permanent-technique histology to ensure the safety of the level of removal.
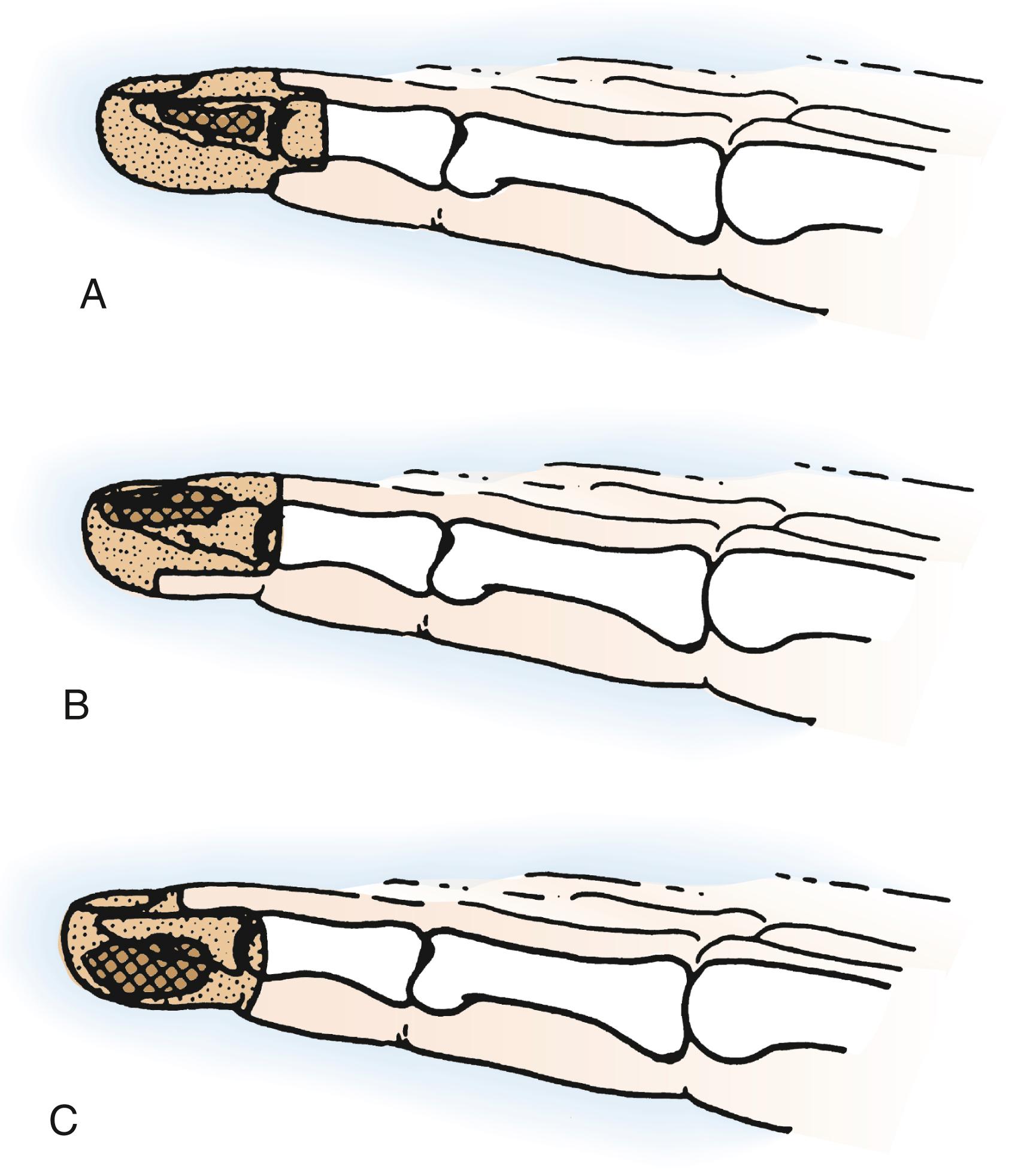
Malignant distal phalangeal lesions require removal with an appropriate cuff of contiguous soft tissue. Lower-grade bone tumors can be managed by distal phalangeal amputation that includes a soft tissue cuff proximal to the intraosseous tumor. This may require transosseous resection through the middle phalanx. It is unlikely that such lesions can be safely removed by DIP disarticulation alone. Patients with pathologic fractures or extracompartmental extension usually require removal of the finger at or proximal to the proximal interphalangeal (PIP) level or even ray resection.
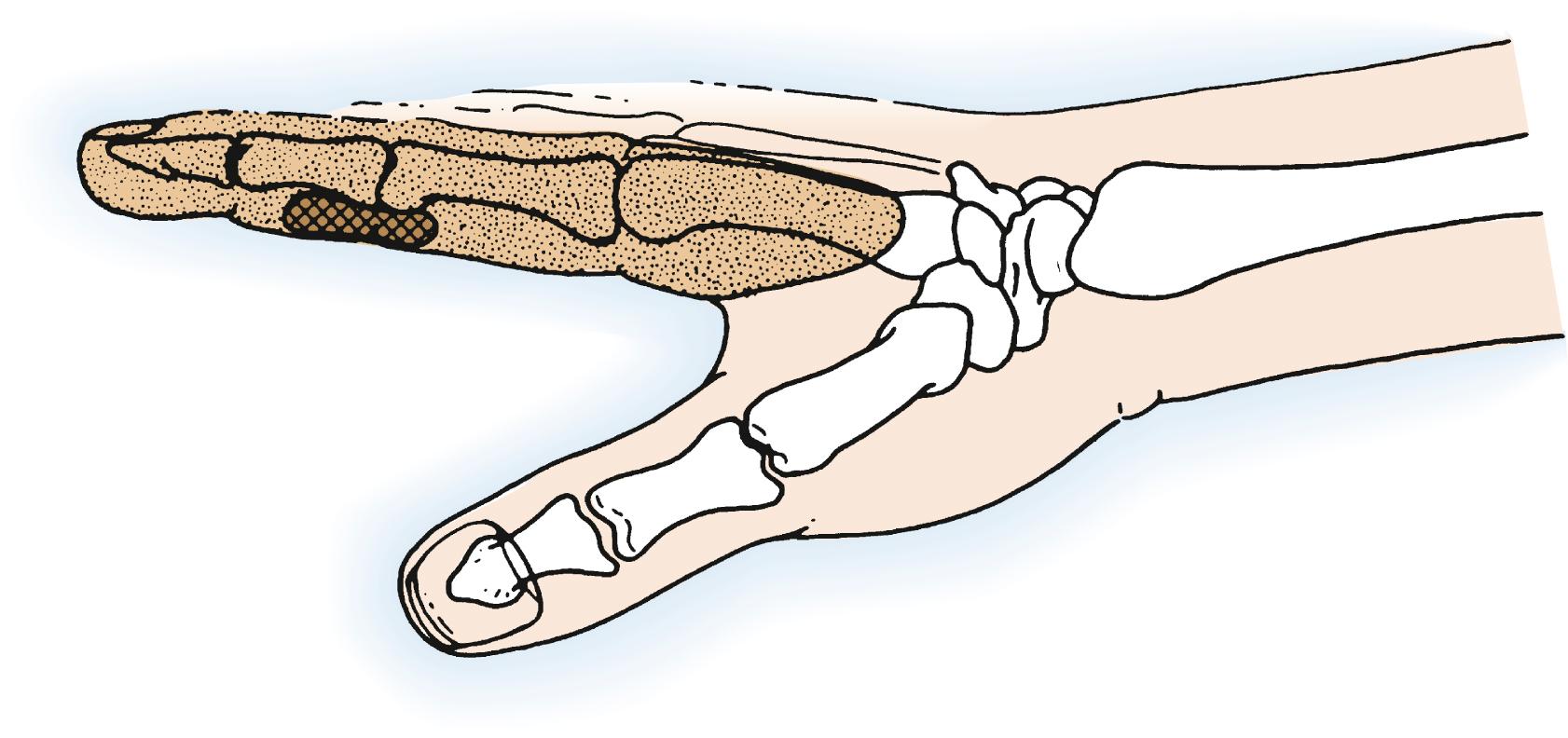
Malignant and aggressive soft tissue tumors in the middle phalanx and those proximal to the region of the DIP joint require amputation at the metacarpophalangeal (MCP) joint or metacarpal level or ray resection ( Fig. 59.4 ). In this instance MCP joint disarticulation should allow at least a 2- to 3-cm margin of normal tissue; however, functional and esthetic considerations strongly recommend ray resection ( Fig. 59.5 ). In theory, bone tumors involving the proximal and middle phalanges can be excised and replaced with bone graft if the lesion is not aggressive and has not extended into soft tissues. However, ray resection is actually a far more practical and functional alternative if both the proximal and middle phalanges are involved. For malignant tumors that have spread beyond bone because of fracture or slow, progressive extension, ray amputation is clearly indicated. MCP joint disarticulation is rarely a safe, practical, functional, or esthetic choice for treating tumors at these levels.
Thumb lesions are unique. Although lesions distal to the interphalangeal joint are often best treated by amputation, lesions at the proximal phalanx level may be amenable to wide excision and extensive reconstruction. This can be done safely while adhering to the principles of wide excision and subsequent extensive reconstruction, which may require bone and microsurgical soft tissue reconstruction.
First metacarpal malignancies can be treated by wide excision and autogenous or allograft replacement ( Fig. 59.6 ). Achieving wide margins may require excision of surrounding soft tissues and adjacent joints or bones. Reconstruction with metacarpal allograft, or by fibula or iliac autograft, may be considered in appropriate circumstances. Autografting should be done with a second, separate surgical setup and clean gowns and gloves to avoid cross contamination of the bone donor site. Because osteoarticular allografts may be unstable, arthrodesis may be required at the trapeziometacarpal articulation. Alternatively, suspension may be appropriate if trapezium resection is required.
With extensive soft tissue contamination, more radical resection may be required, including the entire first ray and possibly portions of the web and second metacarpal, to gain a safe margin. If part of the second metacarpal is resected, the “floating index finger” thereby produced can be considered for direct pollicization if sacrifice of the entire second ray is not required.
Aggressive and malignant bone tumors of the second through fifth metacarpals generally require en bloc bone excision. A more important treatment decision may be whether removal of one metacarpal alone is possible or whether removal of an entire ray or pair of rays is best. Ray excision is usually best for lesions of the second and fifth metacarpals ( Fig. 59.7 ). With the additional consideration of ray transposition, the principles governing removal of the third and fourth rays are no different from those governing the second and fifth. If bone graft has been used for reconstruction, carpometacarpal arthrodesis and MCP ligament reconstruction or silicone arthroplasty may be practical. Whatever level is chosen for reconstruction, it is essential that an adequate, safe margin of normal tissue first be excised en bloc with the tumor.
Aggressive tumors of the second through fifth metacarpals that have invaded soft tissue or sustained pathologic fractures often require excision of not only the involved ray but also the contiguous ray or rays radial and ulnar to the involved digit. A malignant tumor of the second metacarpal that extends into soft tissues may necessitate removal of the second and third rays or the first through third rays while preserving the soft tissue of the middle finger as a fillet flap to resurface the radial side of the hand. A similar technique can be used for tumors of the ulnar side of the hand. If the second, third, and fourth rays must be removed, the best strategy is usually to osteotomize and supinate the fifth metacarpal to provide effective oppositional pinch and grasp with the thumb. If a tumor of the fourth metacarpal requires removal of the third through fifth rays, the radial aspect of the middle finger or a fillet of that digit on its radial neurovascular pedicle can be used to close the ulnar side of the hand. Tumors of the fifth metacarpal may require excision of the fourth and fifth rays. Skin coverage may be possible by using a fillet flap from the ring finger.
If a malignant tumor has broken into the midpalm and extends across the metacarpals, removal of all digital rays may be needed to gain an adequate soft tissue margin. Retention of a sensate and relatively mobile thumb may be considerably more esthetic and functionally satisfactory than a forearm- or wrist-level amputation. However, if a tumor extends proximally from the metacarpal level, a more proximal level of hand, wrist, or forearm amputation is required for safe tumor management.
Malignant soft tissue tumors in the palm or carpal tunnel often require at least partial hand amputation. If treated by only local or limited excision, the chance of recurrence is extremely high particularly in the setting of a positive margin. Below-elbow amputation is necessary to treat larger tumors. Wide en bloc excision of soft tissue sarcomas with negative margins is required to achieve local control of the lesion. At a minimum, aggressive soft tissue tumors, such as bone tumors, require ray resection or removal of multiple rays ( Figs. 59.8 to 59.10 ). Central palmar lesions generally require sacrifice of three rays; those on the border are more likely than those in the center to be salvageable by removing just two rays. In the presence of proximal, broader, and larger lesions, all four digits or the entire hand may have to be sacrificed to save the patient.
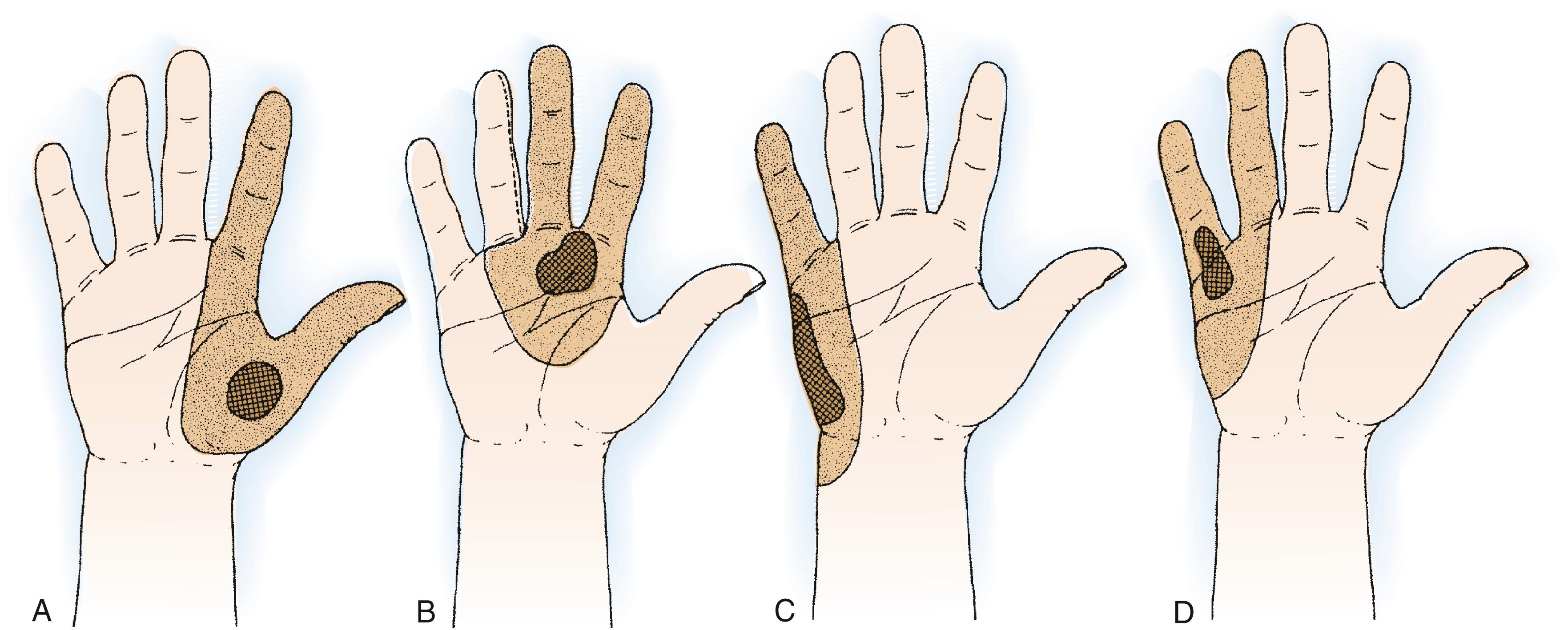
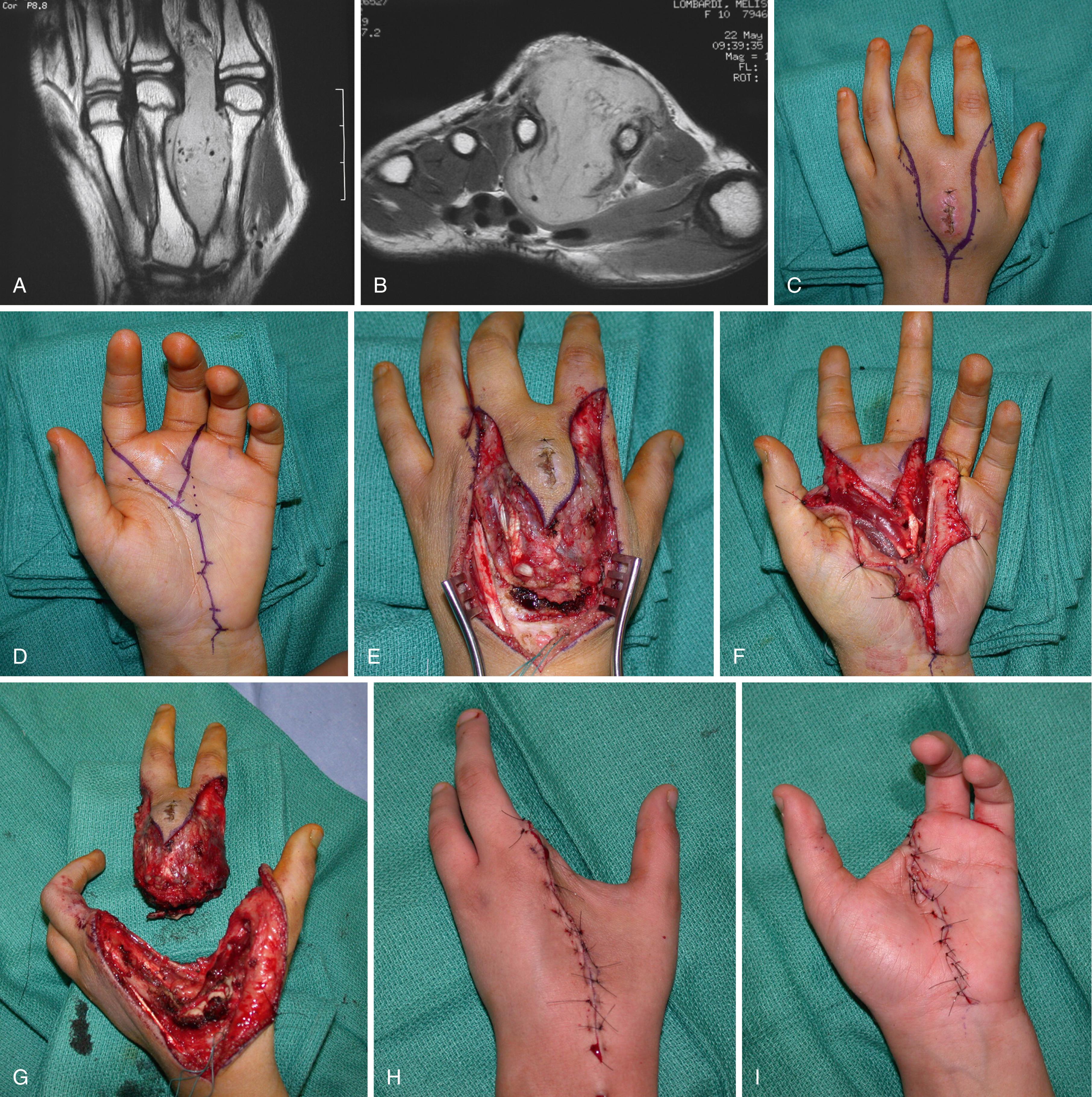
Malignancies of the proximal palmar surface of the hand and volar aspect of the wrist often require amputation. A dissection that attempts to “salvage” the median nerve or one or two flexors in the middle of an expanding tumor or reactive zone is only likely to spread the disease. Correct surgical and medical principles for tumor removal and care should be taken ( Figs. 59.11 to 59.13 ).
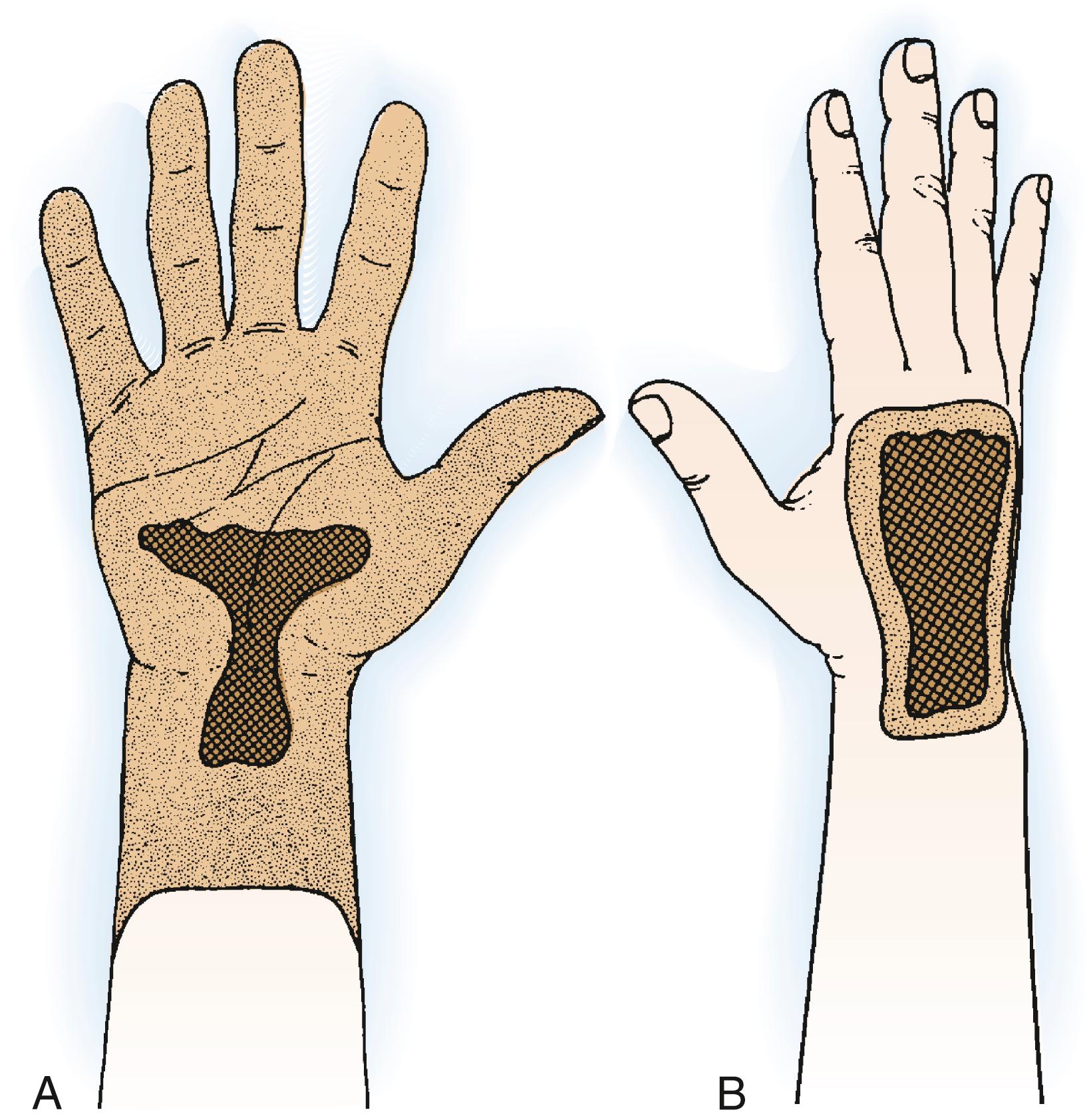
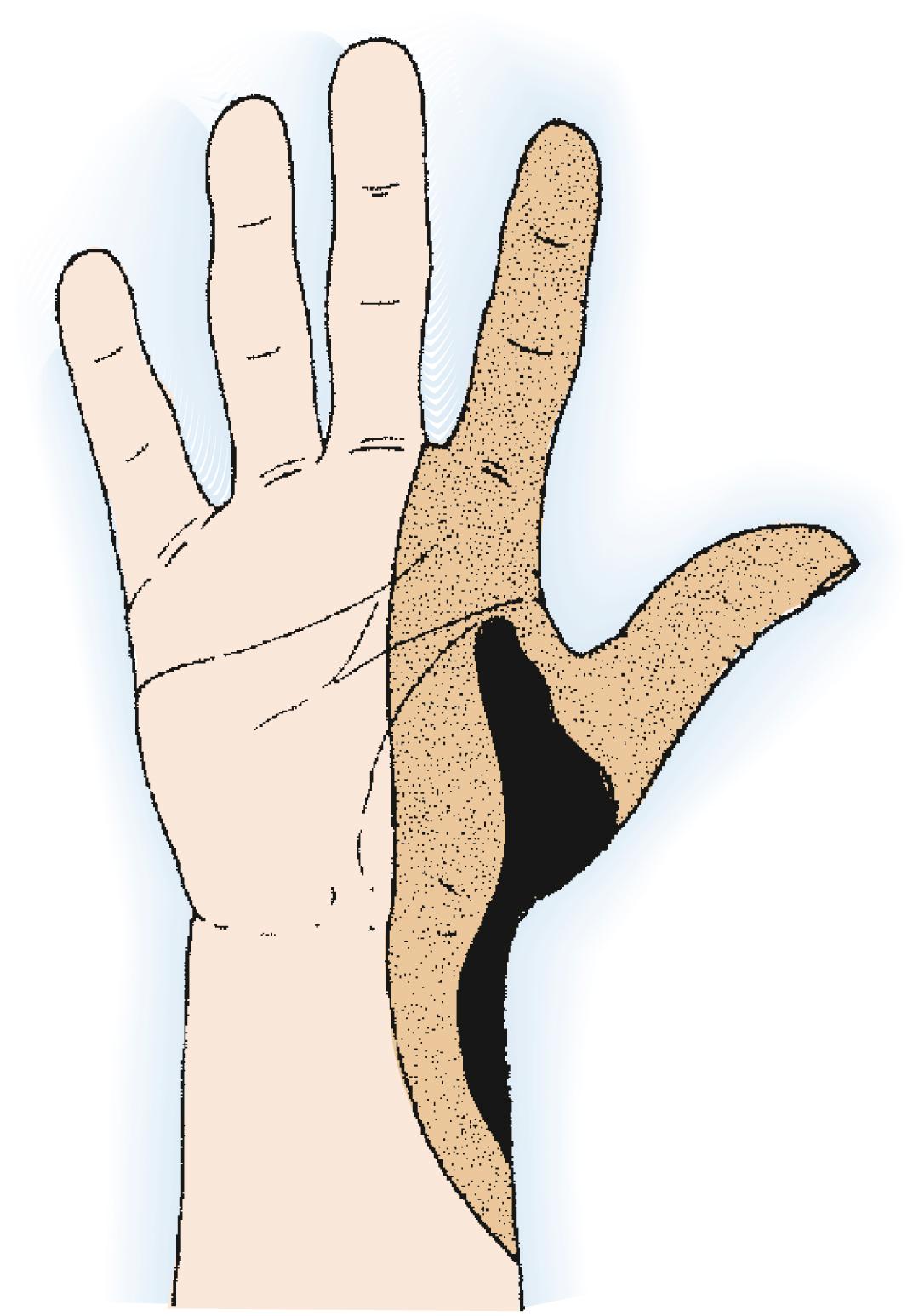
Tumors that arise on the dorsum may allow preservation of the hand if staging studies show that the lesion has not penetrated into the palm and the excision margin verifies a safe plane of normal tissue. Isolated intraosseous carpal lesions that have not invaded soft tissue are rare but can be excised locally. The carpal bones are intraarticular, so the onset of symptoms is generally associated with synovitis and joint invasion; therefore below-elbow amputation or complete en bloc excision of the entire radiocarpal articulation and carpus is likely to be necessary.
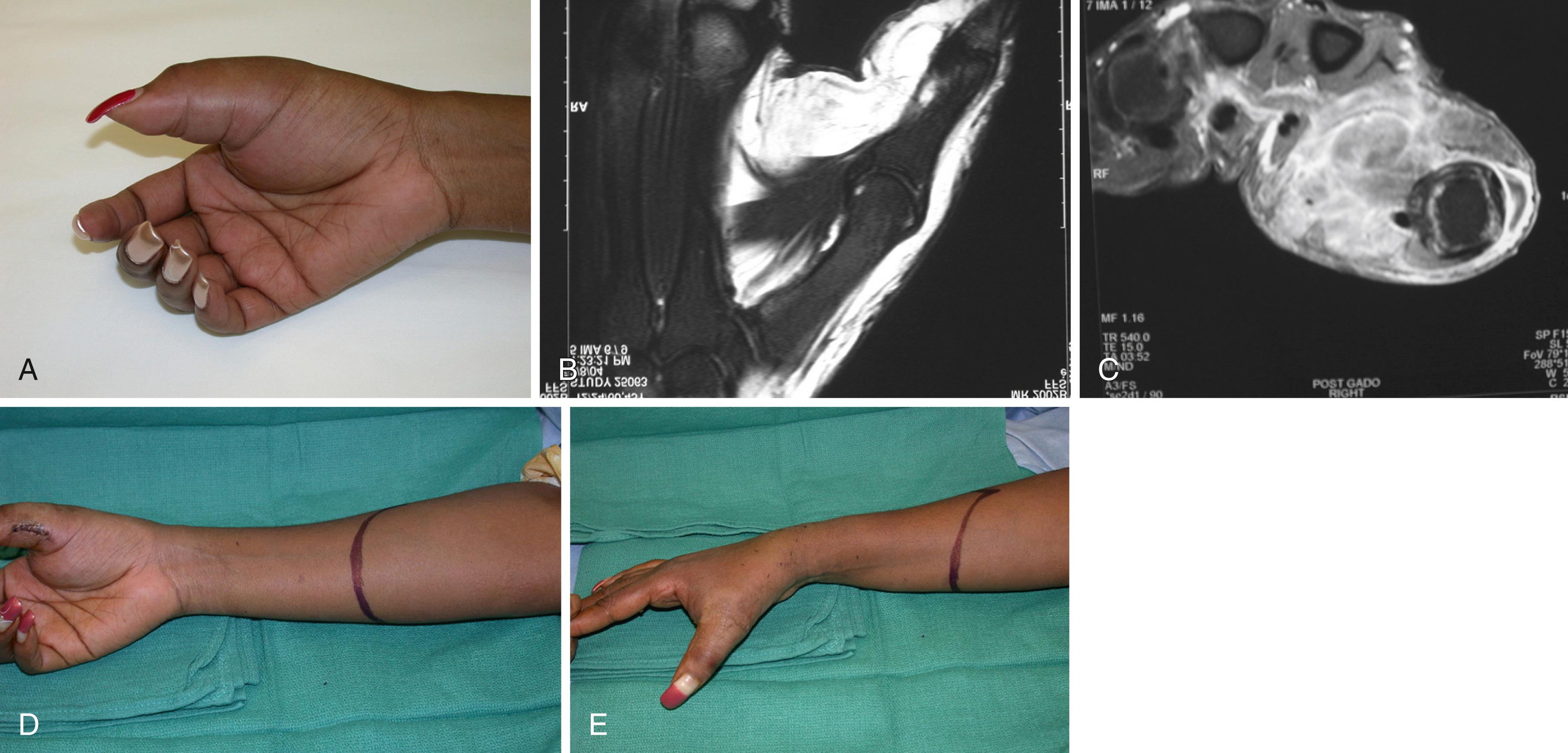
Growths on the volar aspect of the distal part of the forearm must be widely excised with negative margins. Most lesions can be treated with wide excision. At times below-elbow amputation is required. If a tumor arises in a location that does not specifically involve the ulnar nerve and artery, it may be possible to save a portion of the hand and wrist along with the neurovascular bundle and to consider later reconstruction after longitudinal hemiamputation. Treatment of tumors arising on the radial side of the distal end of the forearm varies according to the size and exact location of the lesion ( Fig. 59.14 ). Tumors on the extreme ulnar side of the wrist can be handled in a way that mirrors those on the radial aspect, although lesions in the extreme end of the ulna may be amenable to wide excision of the distal ulna ( Fig. 59.15 ).
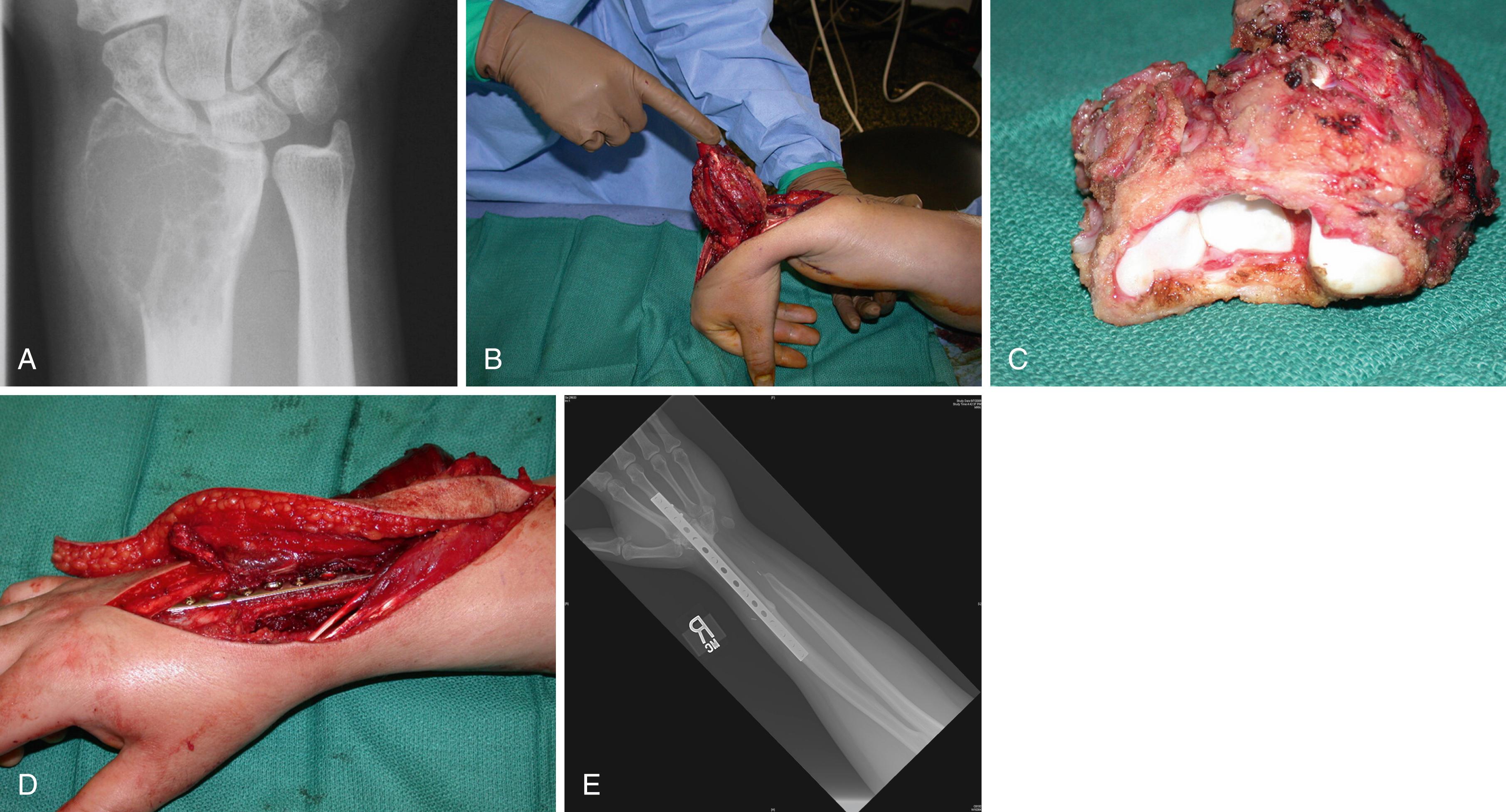
Intracompartmental lesions within the distal radius or ulna can be treated by wide excision of the bone and arthrodesis or autograft replacement. In such situations, transosseous excision must include biopsy of the medullary canal from the retained segment or segments. When tumors have invaded tissues or crossed compartments extensively, above- or below-elbow amputation may be required.
Wherever a tumor is located, treatment must be individualized to achieve the goal of functional restoration without risking local recurrence and later distant spread. Because of a lack of control over the site and the origin of the neoplasm, the surgeon’s responsibility is confined to intelligent management and treatment by adhering to the proper principles of care.
Ganglion cysts are the most common soft tissue tumors of the hand. These mucin-filled cysts are usually attached to the adjacent underlying joint capsule, tendon, or tendon sheath. Ganglions are most prevalent in women and generally occur (70%) between the second and fourth decades of life. They are not rare in children and have been reported from the first to the eighth decades. Ganglions usually occur singly and in very specific locations; however, they have been reported to arise from almost every joint of the hand and wrist ( Box 59.1 ). The less common ganglions are often associated with other conditions of the hand (e.g., bossing of the second and third carpometacarpal joints, de Quervain disease, and Heberden nodes of the DIP joint).
Dorsal wrist ganglion
Volar wrist ganglion
Volar retinacular ganglion (flexor tendon sheath ganglion)
Mucous cyst (ganglion of the distal interphalangeal joint)
Other ganglions
Carpometacarpal boss
Proximal interphalangeal joint
Extensor tendon
Miscellaneous locations
First extensor compartment (dorsal retinacular ganglion)
Carpal tunnel
Ulnar canal
Intraosseous ganglion
Ganglion cysts have also been reported to cause clinically symptomatic pressure on the median and ulnar nerves of the hand. Because of their high incidence and prevalence, ganglion cysts should readily be recognized by most hand surgeons. Other conditions that cause diffuse swelling over the dorsum of the wrist, such as extensor tenosynovitis, lipomas, and other hand tumors, also must be considered in the differential diagnosis. The history, physical examination, techniques of transillumination, and aspiration should allow a conclusive diagnosis in most instances.
Patients usually seek medical attention because of the cosmetic appearance of the mass, pain, weakness, and concern for potential malignancy. A specific antecedent traumatic event is present in at least 10% of cases, and repeated minor trauma may be an etiologic factor in their development. There is no obvious correlation with patient occupation. Malignant degeneration has never been reported; however, malignant soft tissue tumors are frequently misdiagnosed as ganglion cysts. Ganglions can appear quite suddenly or develop over a period of several months. They may subside with rest, enlarge with activity, and rupture or disappear spontaneously. Although recurrences are infrequent with proper excision, , >50% may recur if incompletely excised.
Radiographs of the involved region are often unremarkable, although intraosseous cysts are occasionally present at the wrist. Osteoarthritic changes are commonly seen with cysts at the DIP or carpometacarpal joints. Communication between the wrist joint and cyst has been demonstrated with arthrograms but not with cystograms. A one-way valvular mechanism has therefore been postulated to connect the wrist joint to the cyst.
The microscopic description of ganglions is well known. The main cyst may be single or multiloculated and appears smooth, white, and translucent. The wall is made up of compressed collagen fibers and is sparsely lined with flattened cells without evidence of an epithelial or synovial lining.
The capsular attachment of the main cyst reveals mucin-filled “clefts,” which have been shown by serial sections to intercommunicate through a tortuous continuous duct connecting the main cyst with the adjacent underlying joint. The stroma surrounding the intracapsular ducts may show tightly packed collagen fibers or sparsely cellular areas with broken collagen fibers and mucin-filled intercellular and extracellular lakes. No inflammatory reaction or mitotic activity has been noted.
The contents of the cyst are characterized by a highly viscous, clear, sticky, jelly-like mucin made up of glucosamine, albumin, globulin, and high concentrations of hyaluronic acid. In some cases, the mucin may be blood tinged. The contents of the cyst are decidedly more viscous than normal joint fluid.
Since Hippocrates offered the first recorded description of “knots” of tissue containing “mucoid flesh,” numerous speculative theories with little scientific basis have abounded. This became especially apparent when 18th- and 19th-century anatomists offered the following hypotheses: (1) synovial herniation (Eller, 1746) or rupture through the tendon sheath, (2) synovial dermoid or rest caused by “arthrogenesis blastoma cell nests” or embryonic periarticular tissue (Hoeftman, 1876), (3) new growths from synovial membranes (Henle, 1847), and (4) modifications of bursae or degenerative cysts (Vogt, 1881).
Until recently, the most widely accepted theory, initially postulated by Ledderhose (1893) and popularized by Carp and Stout, was mucoid degeneration. The fibrillation of collagen fibers, accumulation of intercellular and extracellular mucin, and decreased collagen fibers and stroma cells supported this theory. Mucoid degeneration does not, however, explain why the degenerative process is self-limited and solitary and generally occurs in adolescents and young adults and why the fluid recurs after aspiration or incomplete excision.
Nonsurgical methods of treatment are usually considered initially because of the limited associated morbidity and possibility of success. Nonsurgical treatment has included digital pressure, injections of hyaluronidase or sclerosing solutions, subcutaneous tenotome dissection, and cross fixation with a heavy suture. Historical folk medicine has mentioned rupture with a mallet or Bible, methods that need not be considered except for their historical interest. Observation has been advocated in the pediatric population because of the high likelihood of spontaneous resolution.
Aspiration of the ganglion, puncture of the cyst wall, and instillation of lidocaine (Xylocaine) and betamethasone (Celestone) into the capsule or tendon sheath attachments reduce the mass and may alleviate symptoms for varying periods. Aspiration may provide long-term relief and has been reported to be effective in 20% to 30% of patients with wrist ganglions. Injection and aspiration of volar wrist ganglions must be approached with caution because of the proximity of the radial artery. Marked subcutaneous atrophy and skin depigmentation may be seen after injection with triamcinolone (Kenalog), which should be avoided.
The most effective nonsurgical treatment is patient reassurance. An explanation of the condition and assurance of its nonmalignant nature are often the only treatment sought or required. Surgery is best reserved for patients with persistently symptomatic ganglions.
Open procedures are most commonly done for excision of ganglion cysts. Treatment principles include attempts to minimize scar formation and loss of range of motion. Data on arthroscopic approaches to both dorsal and volar carpal ganglion cysts have been published. The technique appears to be effective with a risk for local recurrence comparable to the historic risk for recurrence after open procedures. Recent comparative studies have evaluated the relative risks and benefits of open versus arthroscopic treatment and demonstrated similar outcomes. ,
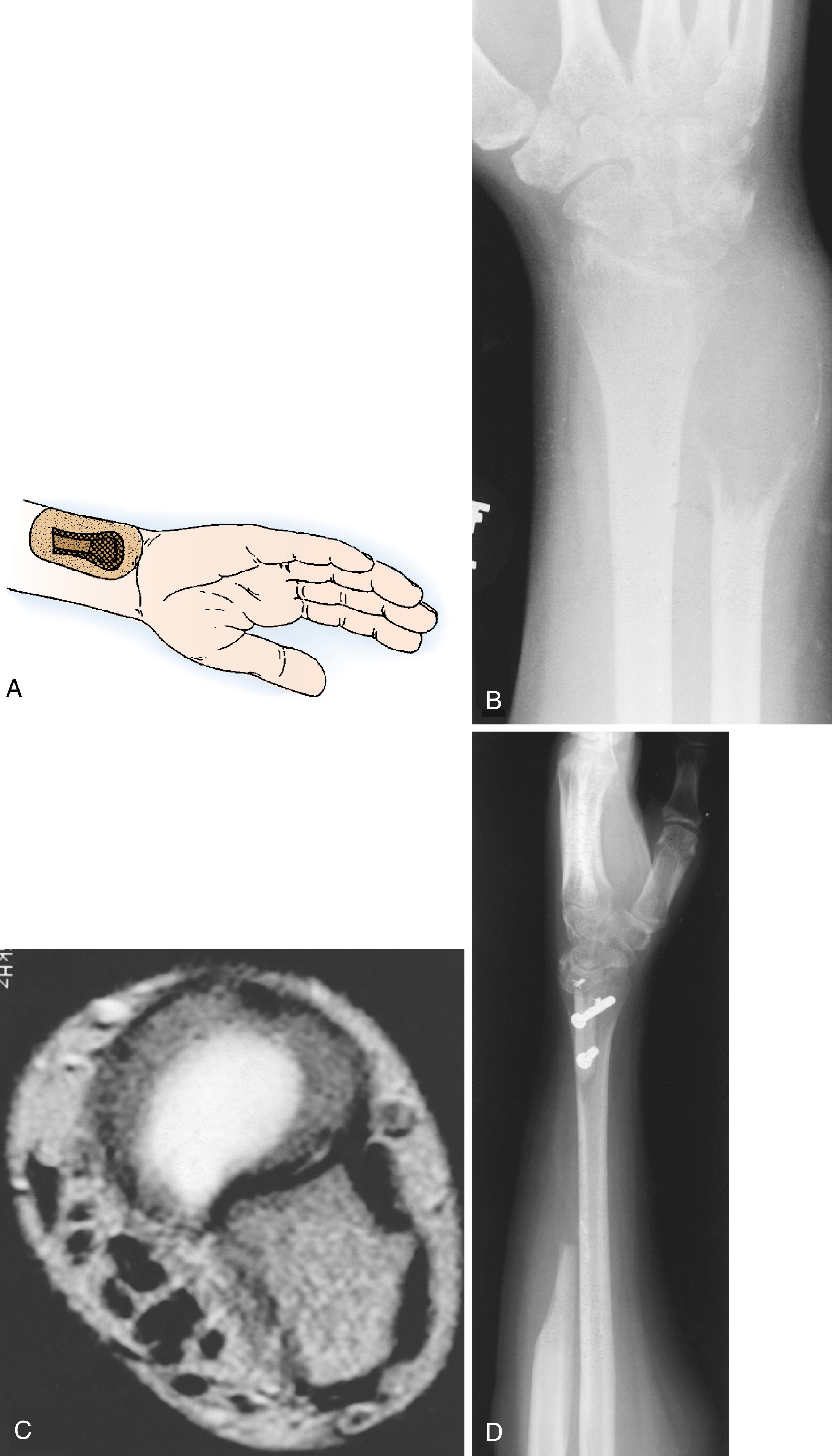
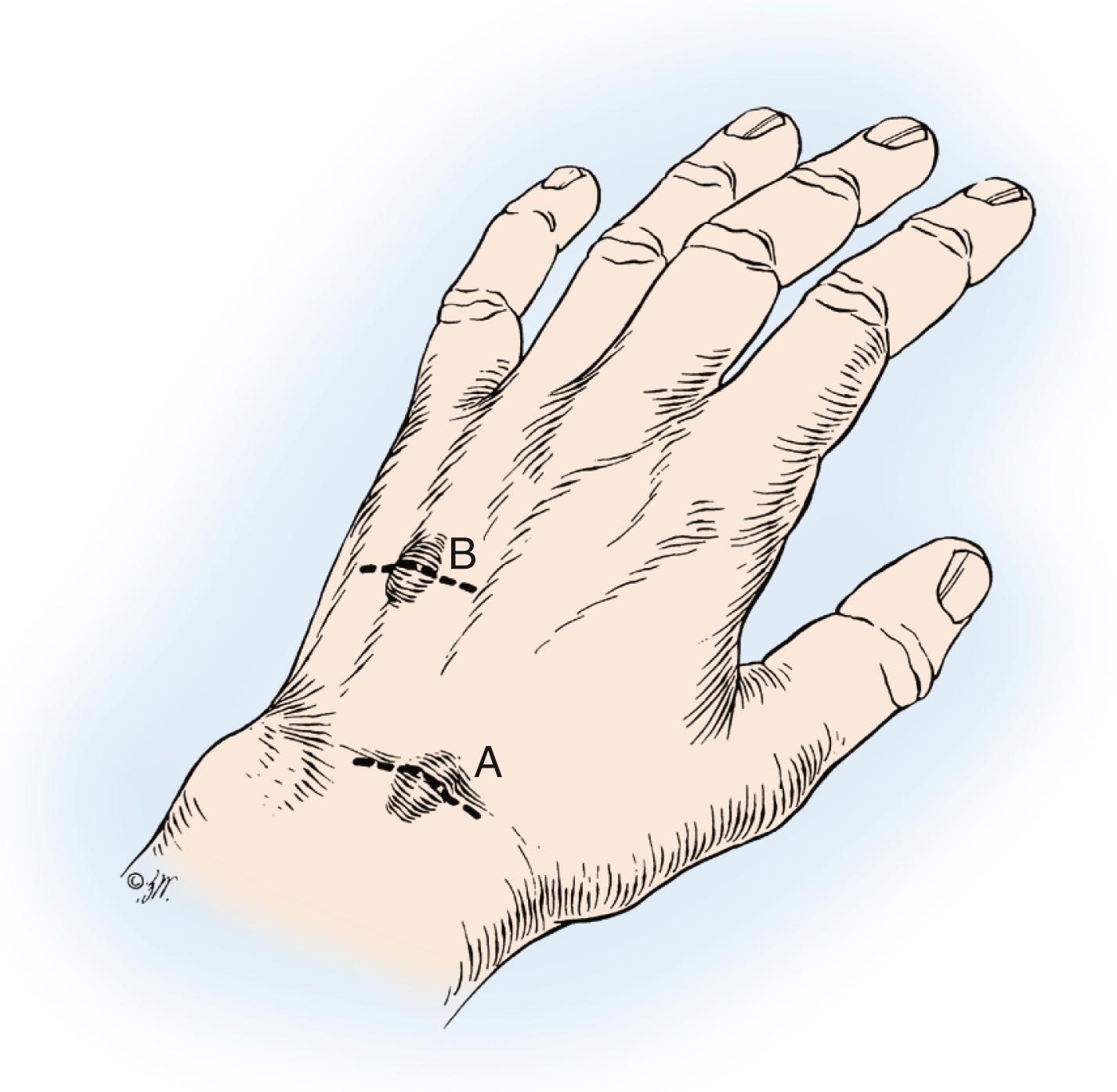
The prototype of all ganglions of the hand is the dorsal wrist ganglion, which accounts for 60% to 70% of all hand and wrist ganglions. The main cyst is usually located directly over the scapholunate ligament ( Fig. 59.16 ) and is easily seen and diagnosed. The cyst may occur anywhere else between the extensor tendons, however, and can be connected to the ligament through a long pedicle (see Fig. 59.16 ). Failure to identify this pedicle and excise its attachment to the scapholunate ligament increases the likelihood of recurrence. Careful preoperative palpation of the cyst with digital compression often reveals its extent and the direction of the pedicle. Transillumination or aspiration confirms the diagnosis preoperatively. Although ganglions have been reported in other carpal joints, they are rare and attachments to the scapholunate joint must be ruled out before a dissection is considered complete. Review of the patient’s preoperative radiographs to rule out an interosseous component is wise.
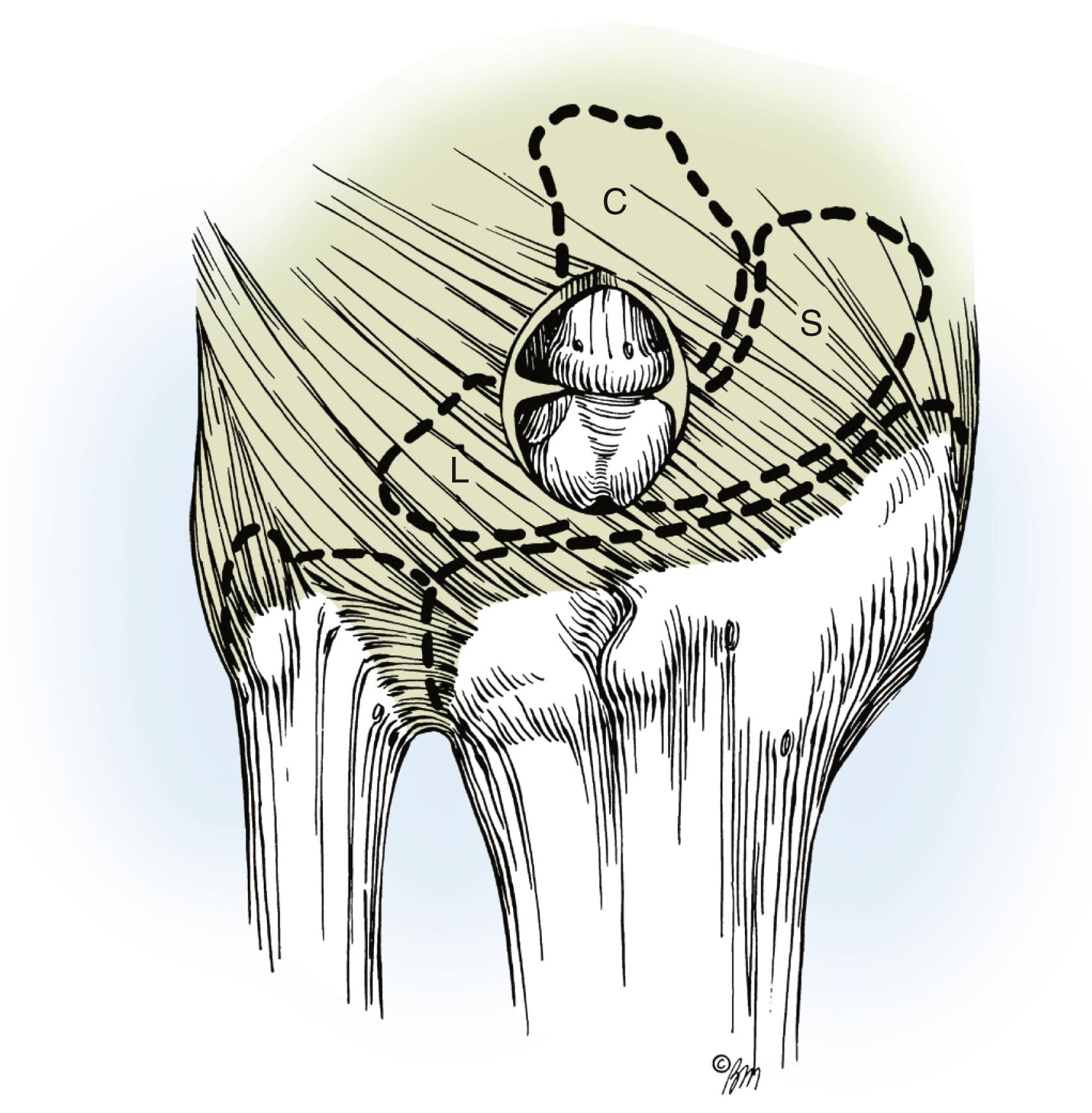
Most dorsal ganglions can be approached through a transverse incision over the proximal carpal row, but a modified incision or second transverse incision may be necessary for ganglions not directly over the scapholunate ligament ( Fig. 59.17 ). The diagnosis of ganglion cyst should be made before commitment to a transverse incision because this type of incision is not readily incorporated into a limb-sparing incision in the event of a subsequent diagnosis of a malignant soft tissue tumor. Typically, a dorsal ganglion appears between the extensor pollicis longus and extensor digitorum communis tendons, which are retracted radially and ulnarly, respectively ( Fig. 59.18 ).
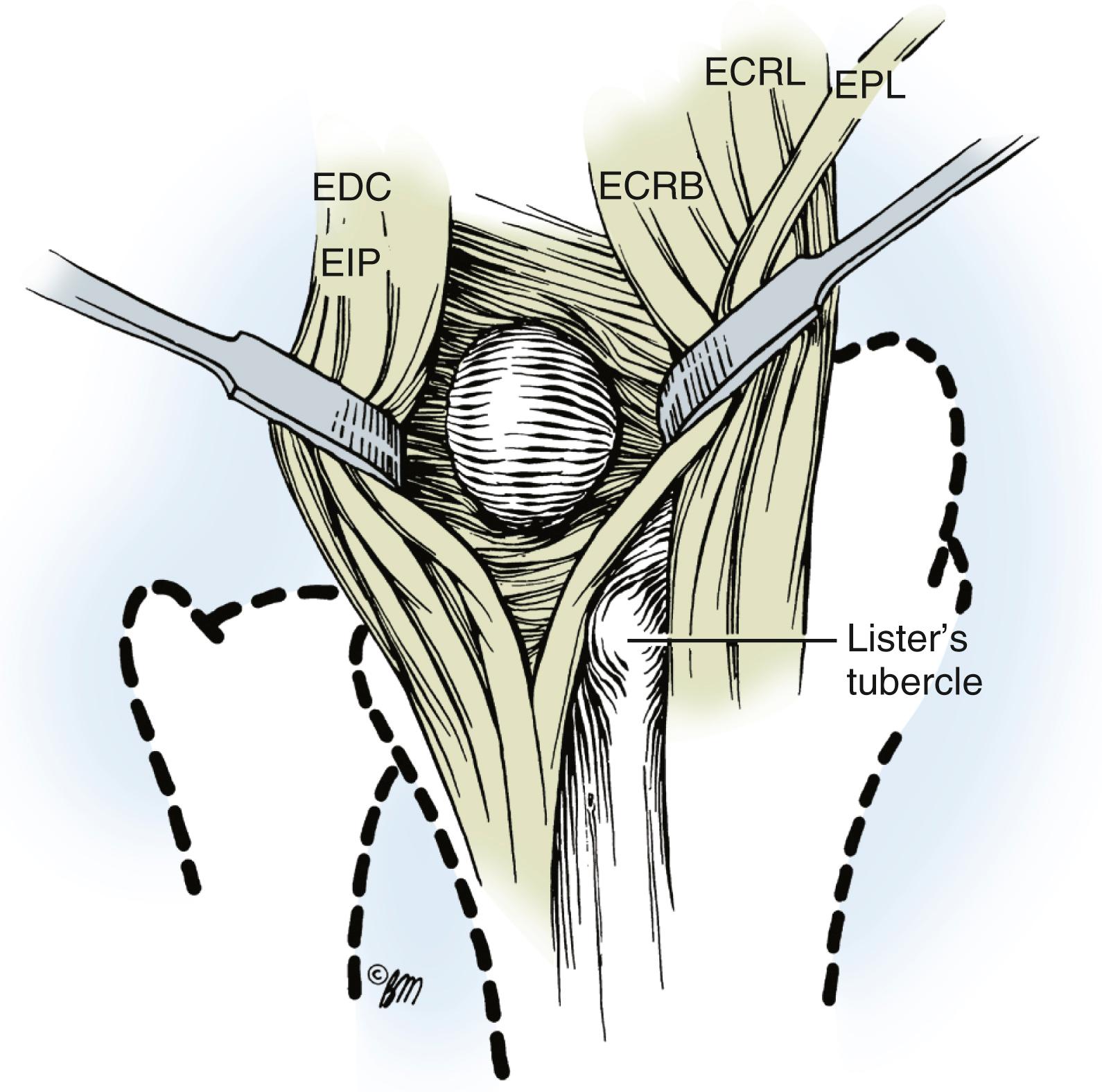
The main cyst and its pedicle are mobilized down to the underlying joint capsule. With the wrist in volar flexion, the joint capsule is opened along the border of the radius and scaphoid’s proximal pole ( Fig. 59.19 ). The capsule is elevated and retracted distally to expose the capsular attachments to the scapholunate ligament ( Fig. 59.20 ). Smaller intraarticular cysts are often seen attached to the scapholunate ligament. The capsular incision is then continued around the ganglion, but all capsular attachments to the ligament are left intact ( Fig. 59.21 ). The capsular incision is extended more laterally if any capsular ducts, which can be identified by small amounts of mucin drainage, are encountered during the dissection. The ganglion and its capsular attachments are then tangentially excised off the scapholunate ligament ( Fig. 59.22 ).
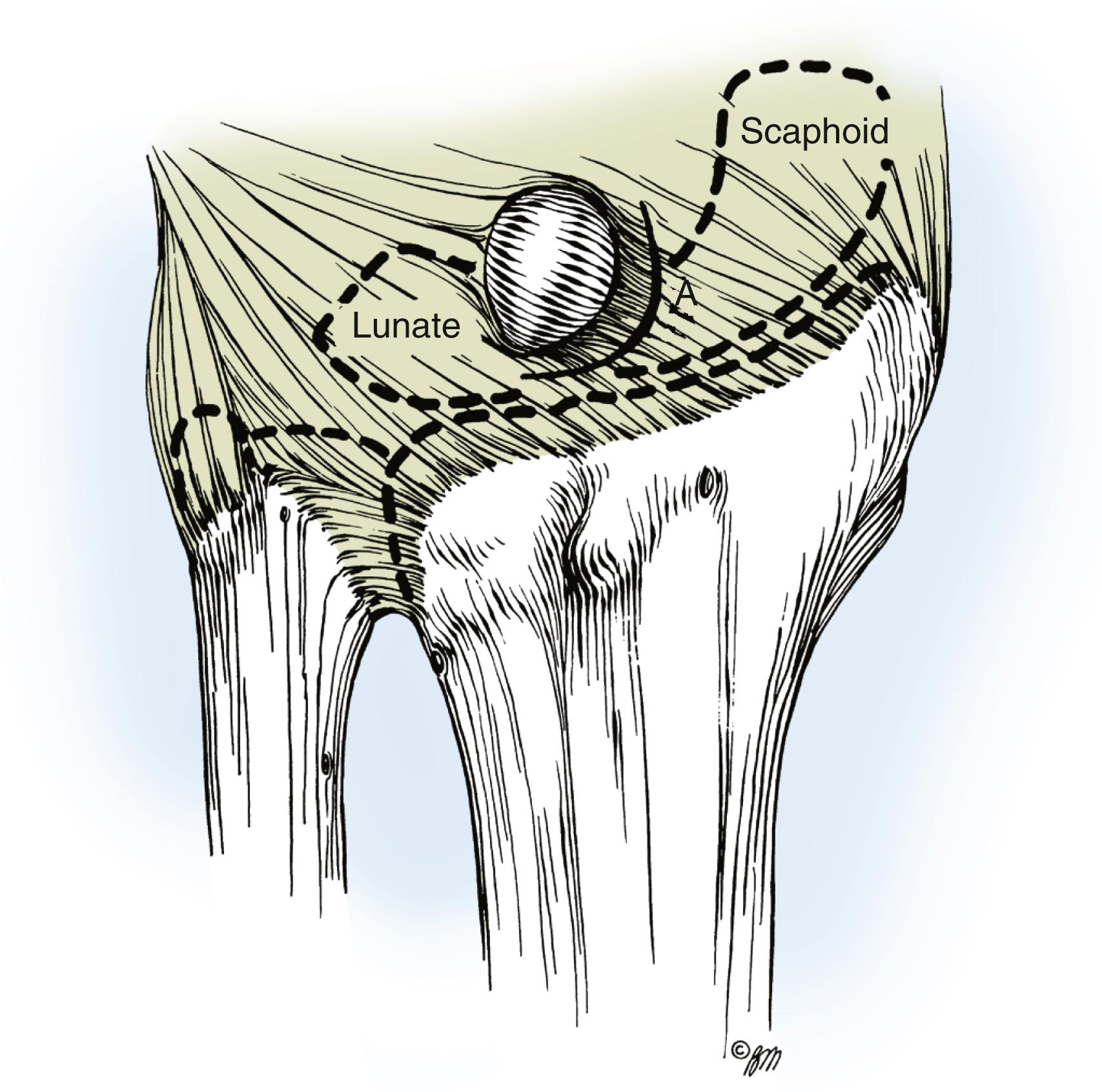
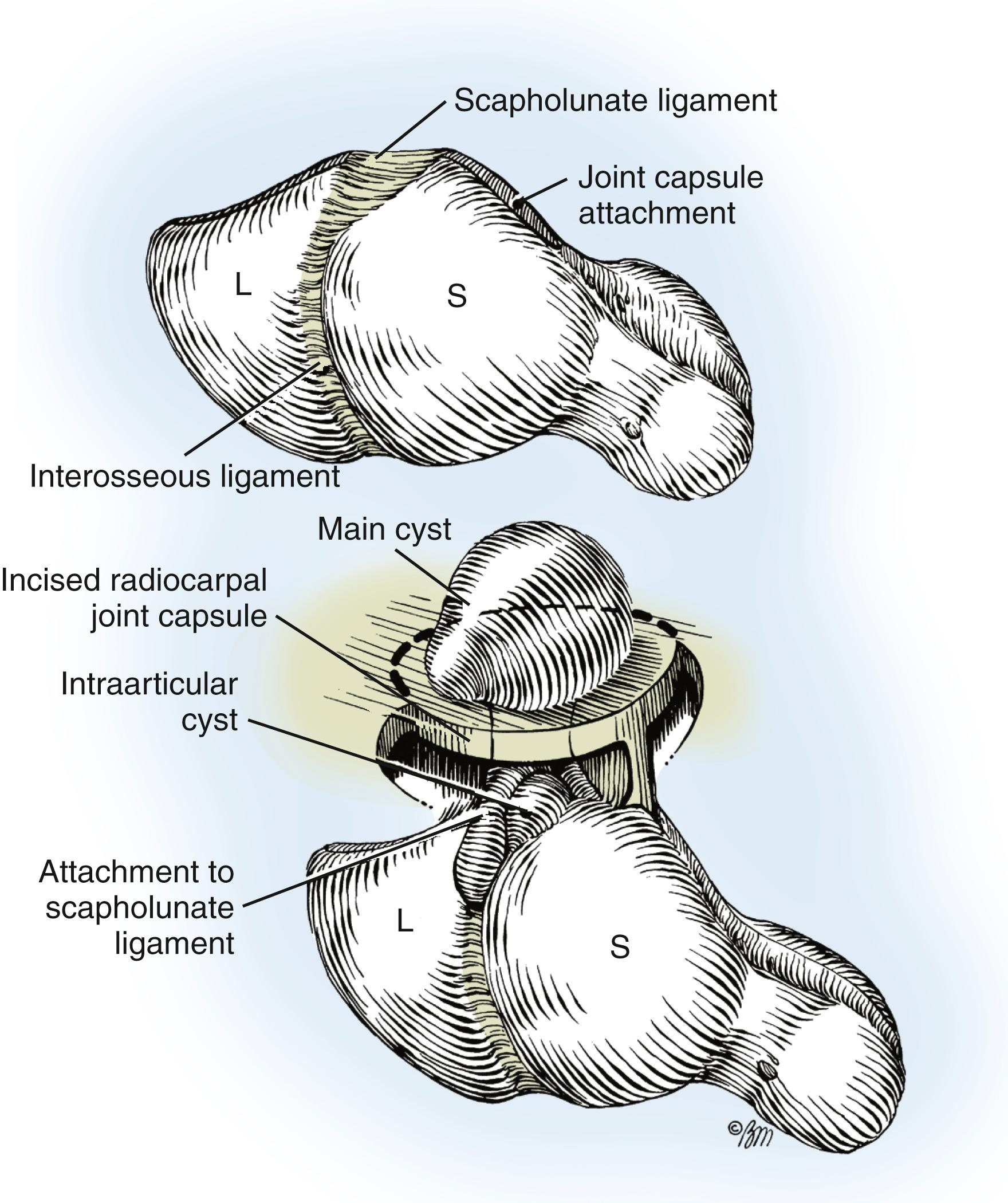
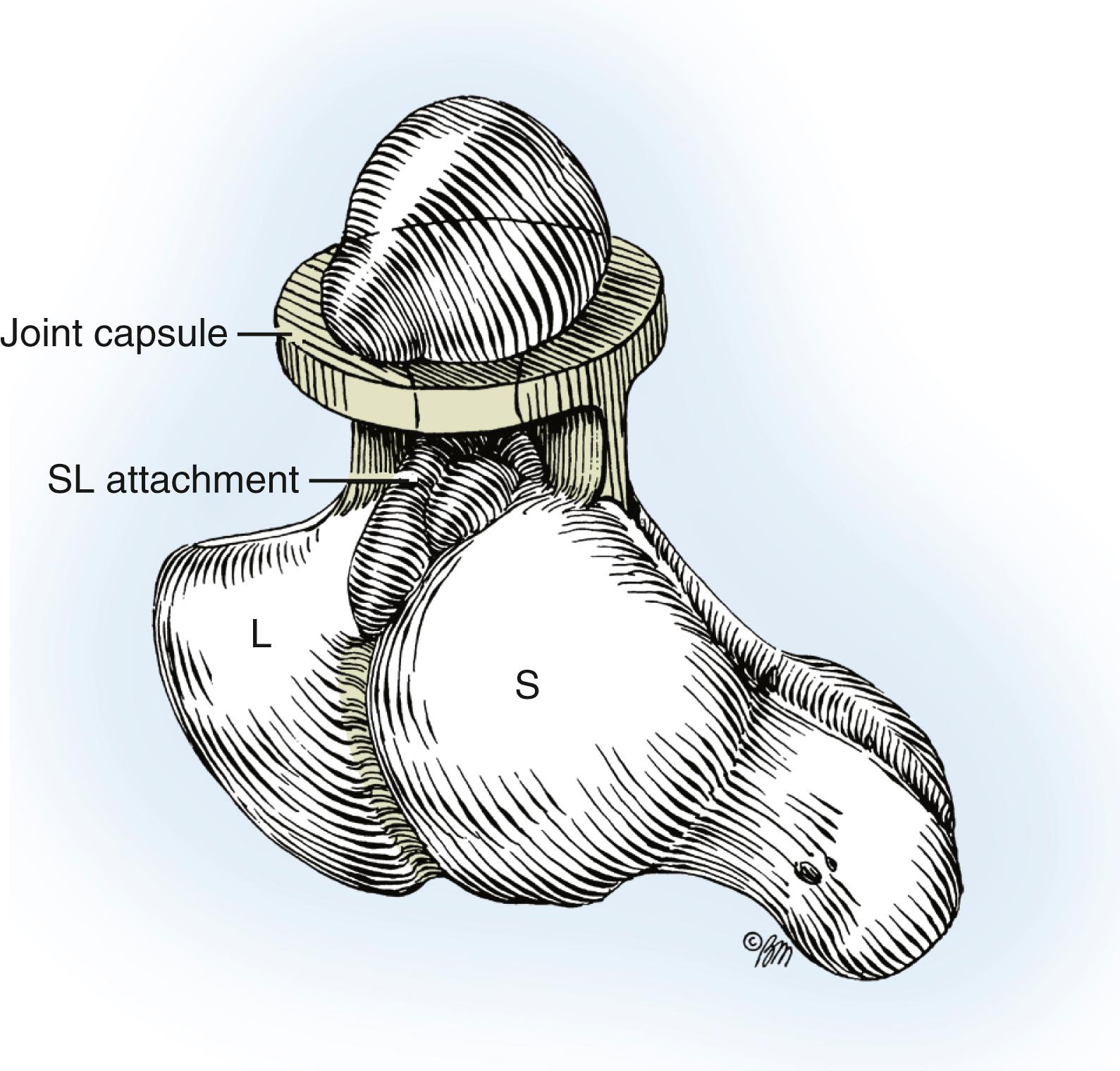
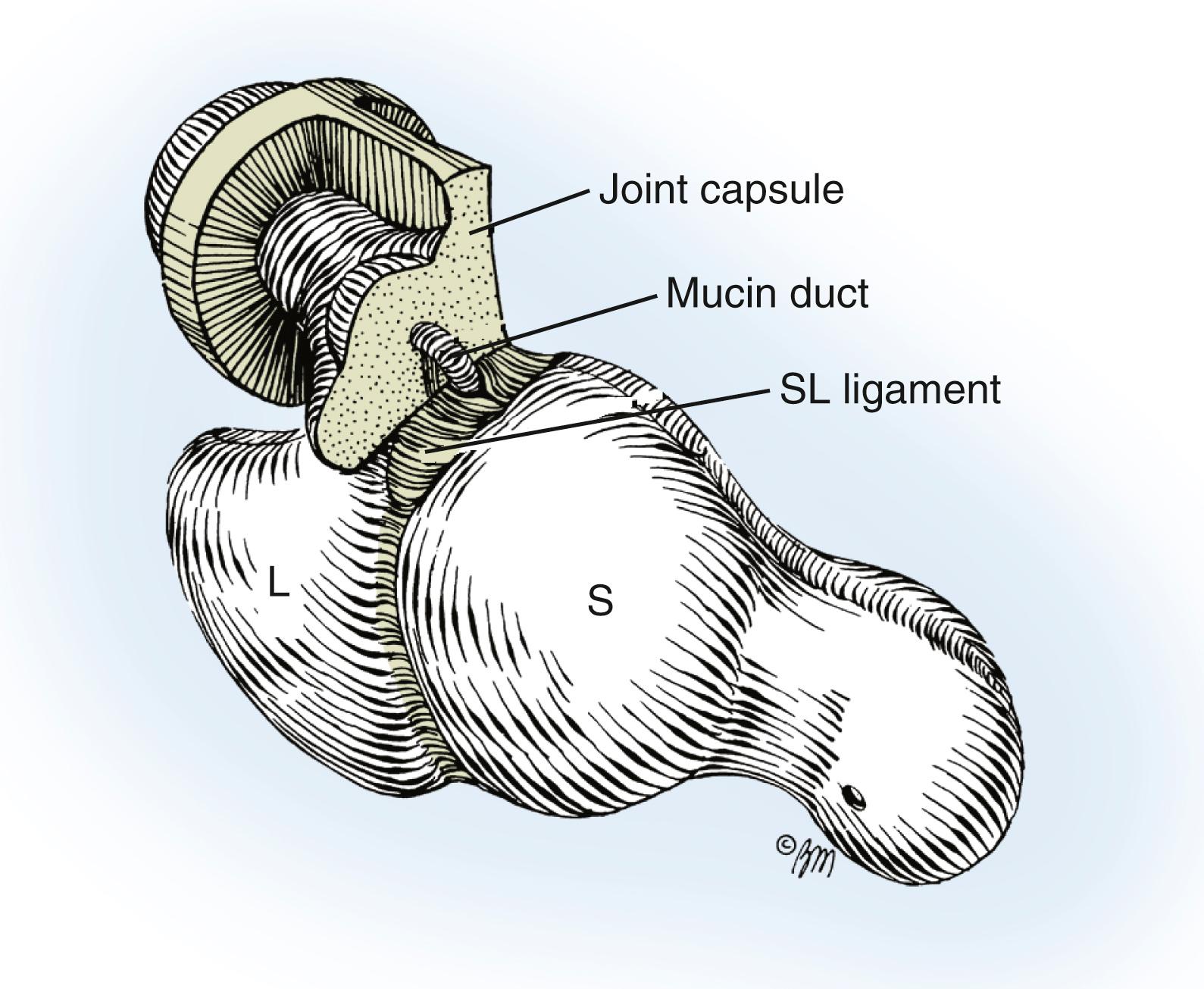
A small, mucin-filled duct is invariably seen piercing the transverse fibers of the scapholunate ligament. This duct appears to connect the underlying scapholunate joint with the main cyst. Synovial and capsular attachments along the distal margin of the scapholunate ligament are also excised to give an unobstructed view of the head and neck of the capitate ( Fig. 59.23 ). If the ganglion ruptures and its anatomic features are lost during the dissection, it should continue until all attachments to the scapholunate ligament have been excised. The excised portion of the joint capsule usually measures approximately 1.0 cm. It is neither necessary nor desirable to cut into the scapholunate ligament, nor is curettage of the scapholunate joint necessary.
The tourniquet is released and hemostasis obtained, then the wound is closed and a dressing applied. Attempts to close the joint capsule either primarily or with fibrous flaps are contraindicated. Subcuticular closure with a monofilament, nonabsorbable pull-out suture may minimize scar formation. Splinting the wrist in slight flexion may reduce postoperative flexion loss.
A bulky dressing extending from the proximal part of the forearm to the MCP joints is applied and the hand elevated. Early finger motion is encouraged. The dressing and sutures are removed between 7 and 10 days postoperatively. Wrist motion should be initiated and encouraged, especially volar flexion. Hand therapy is continued until a full range of motion has been obtained.
Early recurrences, the most common complication of ganglion surgery, are usually the result of inadequate and incomplete excision and rarely should occur. Ganglions appearing at the same site years after excision may in fact be new ganglions. Stiffness of the wrist can be avoided by early motion, physical therapy if necessary, and splinting of the wrist in slight flexion during the immediate postoperative period. It is imperative that early volar flexion be stressed. To avoid keloid or hypertrophic scars, longitudinal incisions across the wrist joint should be avoided. Awareness and respect for the sensory branches of the radial and ulnar nerves prevent neuroma formation, which often defies effective treatment. I have not seen avascular necrosis of either the lunate or scaphoid or scapholunate dissociation after the surgical technique just described. Ganglions and sprains of the intercarpal ligaments can occur concomitantly after trauma to the wrist and must be distinguished preoperatively. Identification of concomitant wrist pathology may be facilitated with arthroscopic treatment.
Unlike protruding dorsal ganglions, smaller, occult dorsal ganglions are easily overlooked and can often only be palpated with the involved wrist in marked volar flexion. Comparison with the opposite normal wrist is helpful. An occult ganglion may be the cause of unexplained wrist pain and is disproportionately tender. MRI is useful in confirming the diagnosis and differentiating pain related to scapholunate ligament injury. Dorsal ganglions occasionally do occur in association with an underlying scapholunate diastasis, and they may be blamed for the carpal instability after their excision.
Dorsal prominence of the proximal pole of the scaphoid secondary to intercarpal instability may be confused with a painful occult ganglion and must be diagnosed with appropriate radiographic studies to avoid a delay in proper treatment. Excising the ganglion alone might not alleviate all the patient’s preoperative pain. Excision of the posterior interosseous nerve at the level of the radiocarpal joint may help alleviate the pain and add to the patient’s postoperative comfort. If other causes of wrist pain and tenderness, especially directly over the scapholunate ligament, can be excluded, an occult dorsal ganglion is best initially treated conservatively by immobilization and steroid injections directly into the dorsal capsule, which can also aid in diagnosis.
In cases in which further diagnostic studies are necessary, some authors have found the use of MRI and ultrasonography helpful. Chronic tenosynovitis of the extensor tendons can be confused with a dorsal wrist ganglion but can be easily distinguished by the diffuse nature of the swelling and the puckering seen with digital extension, the so-called tuck sign.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here