Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The exstrophy–epispadias complex (EEC) is a spectrum of embryologic abnormalities. Diagnoses within the EEC range in severity from those involving only one organ to others that are a part of a larger complex of defects. The spectrum includes:
Epispadias—the urethra is a partial or completely open “plate” dorsally on the penis or between the clitoral halves in a girl.
Classic bladder exstrophy (BE)—the urinary bladder is an open plate on the lower abdomen, always associated with epispadias.
Cloacal exstrophy (CE)—in which the bladder and the ileocecal junction of the bowel are an open plate on the lower abdomen, and the hindgut is truncated and terminates in the perineum. This condition is also known as the omphalocele/exstrophy/imperforate anus/spinal defect (OEIS) complex.
Exstrophy variants—in which partial manifestations of the above anomalies are seen and commonly lack symmetry in the sagittal plane.
Classic BE occurs in approximately one per 50,000 live births, with about an equal incidence in males and females. CE is rarer with an incidence of 1 in 300,000. Since the 19th century, various efforts to manage BE have been described. Because the condition is rare, these approaches were empiric and usually unsuccessful. Until the 20th century, there was no effective surgical approach. Even now, optimal management is elusive and surgical reconstruction may require multiple operations.
Children with BE typically have an anteriorly located anus. Also, the female genital anatomy is altered with a more vertically oriented vaginal opening following repair, and a wider and shorter vagina than normal. The anterior component of the penis is also foreshortened in males compared with the general population. Classic BE, however, is rarely associated with other organ system malformation.
BE can be diagnosed antenatally, although many affected fetuses are not identified until birth. Ultrasound (US) can usually detect BE before the twentieth week of gestation by noting an absence of the urinary bladder as a fluid-filled structure within the fetal pelvis. Other US findings include:
A semisolid mass protruding from the abdominal wall
A lower abdominal protrusion
An anteriorly displaced scrotum with a small phallus in male fetuses
Normal kidneys in association with a low-set umbilical cord
An abnormal iliac crest widening
Findings such as low umbilical cord insertion and the location of the genitalia will be seen only if the fetus is examined in the sagittal plane. The iliac angle will be about 110° rather than the usual 90°. Since kidneys and urine production are typically normal in these fetuses, amniotic fluid levels are usually normal. Any abnormalities seen on US should prompt a fetal magnetic resonance imaging (MRI) scan, which provides better visualization and the ability to characterize the exstrophy or variant, and distinguish exstrophy from other abdominal wall defects.
Prenatal diagnosis helps provide for prenatal counseling, optimal perinatal management, and the chance to be delivered near a pediatric center where skilled newborn care is available to treat these babies. This counseling should include the expertise of a fellowship trained pediatric urologist experienced in the care of children with exstrophy.
Experts disagree on the embryologic explanation for the development of BE. In the prescientific era, the cause of BE was attributed to trauma to the unborn child causing ulceration of the abdominal wall with subsequent bladder herniation. Today, we know that the developing human embryo does not normally pass through a stage that corresponds to exstrophy. This knowledge excludes an arrest in development as a possible etiology and implicates an error in embryogenesis involving the cloacal membrane. The mesodermal membrane folds to form and separate the coelomic cavity from the amniotic space late in the third week of development. The intermediate layer of this mesoderm forms the urogenital system. Disruption in this part of the membrane is thought to lead to EEC. Normally by the fifth week, the mesoderm has formed lateral folds that tubularize into the gut tube and cloaca. However, if the mesenchymal cells do not migrate appropriately during the fourth week of development, then EEC can develop. The actual reason why the intermediate mesoderm does not migrate appropriately to separate the cavities and form the abdominal wall is not known. Several hypotheses have been raised, including physical obstruction of the mesodermal migration, premature rupture of the cloacal membrane, and cellular dysfunction that limits migration of the mesoderm.
To explore the theory that early disruption of the cloacal membrane results in diagnoses within the EEC, a model of CE in the developing chick embryo was created by using a CO 2 laser to create an early dehiscence in the tail bud caudal to the omphalomesenteric vessels. This model suggested that exstrophy may result from failure of the mesodermal ingrowth between the ectoderm and endoderm of the cloacal membrane, which then later ruptures prematurely. It is hypothesized that such an event could be caused by early hypoxemic infarction in the region of the tail bud followed by subsequent cellular loss of the mesoderm and herniation of the developing bladder or cloaca. This type of ischemic injury has also been implicated as the cause of gastroschisis and could explain the EEC spectrum.
Another possible mechanism resulting in a similar pathophysiology could be a defect in a genetic switch that results in premature senescence of the infraumbilical membrane (analogous to an ischemic injury). This hypothesis implies an epigenetic basis for exstrophy.
Animal models to study BE have been difficult to create. In a BE sheep model, a significant increase in the ratio of collagen-to-smooth muscle was noted in exstrophic versus normal control bladders ( P < 0.05). These histologic changes in the ratio of collagen to smooth muscle content are similar to changes seen in human BE specimens.
Recently a genetic murine model of BE was created using p63 knockout mice that phenotypically demonstrated the complete spectrum of BE, including exstrophy, epispadias, separation of pubic bones, as well as imperforate anus and exomphalos. This model, combined with previously demonstrated mesenchymal-epithelial signaling, is leading to further hypotheses of the pathways involved in the development of BE.
While historically BE had been thought to be potentially due to environmental exposure or even an infectious pathogen, the exact underlying cause of exstrophy remains in question. There is clearly a genetic component. Siblings of children with BE have an incidence of BE that ranges from 0.3–2.3%, much higher than normal. The incidence in children in whom one parent had exstrophy has been reported to be 1.4%, or 400-fold higher than the general population. However, the numbers on which these statistics are based are small. Only 37 familial cases of BE have been reported, the most recent of which describes a mother and son with BE. Four other cases of an affected parent–child pair have also been described. Another 18 cases with BE have been found in twins.
Prenatal counselors estimate the risk of recurrence in a sibling of a patient with exstrophy at about 1% with a 1:70 chance of transmission to the progeny of an affected parent. A Florida population-based study found multiple births had a 46% increased risk of birth defects, with BE being the fifth highest adjusted relative risk. These findings support a multifactorial etiology with evidence for genetic predisposition. More recently, an epidemiologic survey of families with BE found no link between exstrophy and parental age, maternal reproductive history, or periconceptional maternal exposure to alcohol, drugs, chemical noxae, radiation, or infections. Periconceptional maternal exposure to smoking has been noted to be significantly more common in patients with EEC. Specific genes and copy number variants (CNVs) have been potentially associated with BE. A recent genome wide association study has implicated a gene ( ISL-1 ) on chromosome 5q11.1, which may be a susceptibility gene for BE. However, in other studies, evaluation of CNVs showed that single genomic CNV are unlikely to be the cause of BE, although this cannot be completely excluded. Whole exome sequencing and genome wide association studies are in progress to begin to identify candidate genes that may contribute to the likely multifactorial etiology of BE.
The initial goals of BE repair are to close the bladder and urethra, and to reconstruct the genitalia in order to create functional organs for continence, voiding, and sex. By achieving a successful primary repair, the bladder can cycle and grow in capacity and can provide safe storage under low pressure. The goal of closure is also to create a competent bladder neck that can coapt to provide continence, and also can relax to allow a sustained detrusor contraction, resulting in normal voiding with complete emptying. Achieving normal bladder storage and emptying minimizes the risk of upper urinary tract deterioration, prevents urinary tract infections (UTIs) and vesicoureteral reflux (VUR), and decreases the risk of urinary calculi.
Children with BE can survive untreated. However, significant morbidity can include skin breakdown secondary to total urinary incontinence, tumor development within the chronically exposed bladder plate, and significant psychosocial morbidity. In contrast, when these patients receive effective surgical and medical treatment, they can lead productive, healthy lives with minimal and manageable morbidity.
Very early efforts in BE management were directed at partial reconstruction of the abdominal wall to allow the application of a urinary receptacle to collect urine. Early attempts at closing the bladder were fraught with complications, which led to urinary diversion through the creation of ureterosigmoidostomies (USO) with equally poor results. It was only with a progressive understanding of urinary tract and bladder physiology, the effect of BE repair on urine storage and emptying, and the concept of clean intermittent catheterization (CIC) that the management has evolved to its current manageable, yet imperfect state.
A wide range of operations have been developed to repair BE. These can be grouped as (1) urinary diversion or (2) anatomic reconstruction. Anatomic reconstructions include single and multistaged repairs with progressive degrees of delayed reconstruction. Surgeon preference and experience, patient anatomy, history of previous operations, availability of tertiary care facilities, and access to medical care and resources all play a role in choosing a specific operative procedure for a particular patient.
Primary urinary diversion is not commonly performed in the United States or in most of Europe. Urinary diversion has largely been abandoned in favor of primary bladder closure. However, continent urinary diversion approaches produce a more consistent degree of dryness with fewer required operations and fewer early complications than that achieved with anatomic reconstruction. Techniques for constructing urinary diversions are listed in Table 58.1 .
| External Diversion (Continent Urinary Reservoir) | Internal Diversion (Rectal Sphincter-Based Continence) | Incontinent Diversions |
|---|---|---|
| Indiana pouch (cecal reservoir with ileal catheterizable channel) | Sigma pouchGhoneim reservoir | Ileal conduitColon conduit |
| Mainz pouch | Gersuny | Ileocecal conduit |
| Penn pouch (ileocecal reservoir with appendiceal catheterizable channel) | Heitz–Boyer–HovelacqueRectal bladder with proximal colostomy | |
| Kock pouch | Ureterosigmoidostomy | |
| Ileocecal ureterosigmoidostomy |
Continent diversions can be completely internalized and rely on the rectal sphincter complex, or can be partially externalized, requiring catheterization of a continent stoma. Incontinent diversion can also be employed. Incontinent diversion avoids the complications associated with continent reconstruction such as a failed continence mechanism resulting in persistent incontinence, urinary retention, stomal complications, and dependence on CIC to empty the bladder.
Diversion can be combined with cosmetic and functional reconstructive procedures for the external genitalia. Because of the difficulties encountered with functional bladder reconstruction, especially in resource poor settings, early urinary diversion advocates argue that diversion achieves the primary goals of dryness with fewer operations and higher success rates than are achieved with bladder closure and urethral reconstruction.
One of the earliest forms of diversion used in BE reconstruction was the USO. However, this diversion allows the high pressure of the contractile sigmoid to reflux infected urine or feces back into the urinary system resulting in severe infections. Long-term complications includes hyperchloremic metabolic acidosis, chronic pyelonephritis, bladder calculi, and a 250- to 300-fold increased risk of adenocarcinoma developing at the anastomosis of the ureter(s) to the colon. As a result of these complications, USO was replaced by incontinent urinary diversions such as colonic and ileal conduits. A significant disadvantage to these conduits is the associated incontinent abdominal stoma and the need for a receptacle/appliance as well as a high rate of bowel segment stenosis/obstruction and infection.
The Mainz II pouch (a detubularized sigmoid pouch) provided a significant improvement to the USO. This detubularized pouch reduces reservoir and ureteral pressures, and improves nighttime continence. In one study, renal preservation rates in children treated primarily with a urinary rectal reservoir (Mainz II pouch) approached 92% with continence rates up to 97%.
The Heitz–Boyer–Hovelacque procedure involves isolation of a rectal segment for ureteral implantation followed by posterior sagittal pull-through of the sigmoid colon through the anal sphincter. A small series using this approach reported continence rates of 95% with an acceptable complication rate. A recent long-term evaluation of patients treated with continent anal urinary diversion (CAD) found a 97% daytime urinary continence rate and a 65% nighttime continence rate with minimal urine loss during sleep or sexual activity. An alternative rectosigmoid bladder reservoir was recently evaluated for continence. This approach was used both for primary treatment and salvage repairs. Complete rectal continence was only 52% in this cohort, while the remainder had leakage during passage of flatus or abdominal straining.
Frequent bladder emptying can reduce the risk of metabolic electrolyte imbalances since frequent emptying of the rectal reservoir reduces the contact time between the urine and the absorptive rectal mucosa. The standard treatment of metabolic acidosis is with oral bicarbonate replacement. While reconfiguration of the pouch and frequent emptying may reduce the risk of infection and wetting, the risk of malignancy still remains a concern. Various modifications of the rectal reservoir to prevent admixture of feces and urine may decrease the incidence of cancer. In a 45-year experience with CAD, Rubenwolf reported 8/82 patients developed neoplasms, most often at the ureterointestinal anastomosis. Four of these patients died shortly after diagnosis, while the remainder were managed with resection and revision of the diversion.
The first efforts at anatomic reconstruction for BE were unsuccessful but set the stage for the current anatomic approach. In 1881, Trendelenberg described an exstrophy closure emphasizing the importance of pubic reapproximation in front of the reconstructed bladder in order to achieve continence and prevent dehiscence. However, because of discouraging results, anatomic reconstruction was largely replaced by urinary diversion in the early part of the 20th century. In the latter half of the 20th century, there were several series of patients who underwent single-stage reconstruction, with most reporting continence rates of 10–30%. The most concerning complication was the high incidence of renal damage, reported as high as 90% in generally secondary to bladder outlet obstruction.
As a result of devastating upper tract damage and the low rate of urinary continence with single-stage approaches, reconstructive efforts were modified toward staged bladder reconstruction. This approach was pioneered in the 1970s and further refined to what is now known as the modern staged repair of exstrophy (MSRE). Later advances in single-stage reconstruction, combined with the advent of CIC, led to a resurgence of this approach and the development of the complete primary repair for exstrophy (CPRE), which has now developed alongside the MRSE.
The primary goal of both approaches is to reconstruct the abdomen and genitalia to achieve anatomic and functional normalcy with minimal operative morbidity. Reconstruction of the newborn female with BE is similar in both the CPRE and MSRE techniques with the end result being closure of the bladder, urethra, and abdominal wall. For the CPRE technique, the main difference is a more aggressive mobilization of the vagina and urethral plate posteriorly into the pelvic diaphragm in order to gain bladder outlet resistance that allows for normal voiding with continence rather than the need for later bladder neck reconstruction (BNR), as is the case for MSRE. In contrast, closure of the male with BE is quite different in the CPRE and MSRE techniques. The CPRE closes and repositions the bladder and entire urethra in one surgical procedure. In contrast, the MSRE closes and repositions the bladder and posterior urethra at the first stage with the remainder of the urethra closed (epispadias repair) at a later stage, followed by BNR as the last stage.
Traditionally, primary BE closure was performed in the immediate newborn period, prior to 72 hours of life. This potentially allows for anatomic closure without the use of osteotomies and reduces the time of bladder exposure. McMahon and colleagues feel there are definite advantages to immediate postnatal exstrophy closure, reporting that infants closed before 7 days of age required fewer bladder augmentations, while continence rates were the same in their early or delayed closure group. Another study reported that closure of infants <72 hours old with a pubic diastasis <4 cm and without osteotomy has the same failure rates as those performed with osteotomy. In the past, proponents of early closure pointed to decreasing bladder exposure that can lead to histologic changes such as acute and chronic inflammation, squamous metaplasia, cystitis glandularis and cystitis cystica, and muscular fibrosis, which may adversely impact bladder capacity and compliance. A recent study showed that primary polyps did not change outcomes in bladders closed early or later. Other early studies of the anatomy and physiology of the newborn exstrophy bladder showing an “immature” physiology with fewer and smaller nerve fibers and less smooth muscle have helped to theorize that early closure could help to “mature” the bladder with bladder cycling.
After delivery, to reduce trauma to the bladder plate, the umbilical cord should be ligated with suture rather than a plastic or metal clamp. A hydrated gel dressing or plastic wrap can be used to protect the exposed bladder from superficial trauma from a diaper. The baby should undergo an US to evaluate the kidneys and to establish a baseline examination for later US studies, as well as an anterior–posterior radiograph of the pelvis to assess the degree of pubic diastasis. Preoperative spinal US examination should be considered if sacral dimpling or other signs of spina bifida occulta are noted on physical examination. Although an associated spinal abnormality is common with CE, it is rare with BE.
If closure is performed beyond the first 72 hours of birth, the baby can be discharged from the hospital with the mother, thus providing time for bonding with the parents. Preoperative antibiotic prophylaxis is not required. However, perioperative and postoperative antibiotics are used to decrease the risk for infection following reconstruction.
Several recent studies have advocated for delayed primary closure, especially in contrast to the CPRE approach, either in primary or failed exstrophy closures. Proponents of delayed repair argue that it is safer for the child, allows a more coordinated surgical effort, and allows an elective operation so that the most expert team of clinicians and personnel are available. Delay in closure can allow time for the baby to become more robust and stable, and allows for parental bonding before the child is placed in traction for 4–6 weeks. Moreover, in an era of the development of high-volume centers, delayed closure allows for consultation and transfer if needed. A recent study found no difference in the bladder capacity in babies who underwent newborn closure versus closure at 1–9 months of age. Others have similarly shown it is possible to wait months after birth with good results. Such a delay may be useful to stimulate the penis with testosterone with the goal of reducing the chance for glans injury, and there have been good short-term outcomes reported with this approach in six male infants.
The successful use of a delayed closure approach is predicated on adequate protection of the exposed tissue. However, Nelson et al. found an increased cost of as much as 50% with delayed closure Long-term outcomes will take years to mature.
Other considerations during general anesthesia include minimizing abdominal distention, which can increase intra-abdominal pressures postoperatively and can lead to compartment syndrome, which may compromise renal function and also increase the risk of wound dehiscence. Also, nitrous oxide should be avoided, as it can cause bowel distension. An epidural catheter can decrease the need for narcotics and inhaled anesthetics during the operation, and keeps the baby comfortable postoperatively. Tunneling the epidural may reduce the risk of infection if it will be used for prolonged time after repair. Postoperatively, maximal urinary drainage with ureteral stents and a suprapubic tube are critical to divert urine away from the bladder as it heals ( Table 58.2 ).
| Intraoperative | Postoperative |
|---|---|
| Avoid abdominal distention—nasogastric tube for continuous decompression, avoid nitrous oxide | Tunneled epidural for pain management |
| Epidural to avoid narcotics | Maximal urinary drainage |
| Careful fluid management | Immobilization with traction or spica cast |
| Antibiotics |
Indirect inguinal hernias are commonly associated with BE in both boys and girls. They arise as a consequence of enlarged internal and external inguinal rings combined with compromised fascial support and lack of obliquity of the inguinal canal. To assess the benefits of preemptive hernia surgery, a single institution recently reviewed its experience with 43 patients with BE. Of the 25 that did not undergo inguinal hernia repair at the time of original closure, 9 needed hernia repair later, while no children who had a hernia repair at the time of BE closure suffered any complications from the repair. Thus, they have advocated for inguinal exploration and repair of an inguinal hernia at the time of exstrophy closure. Alternatively, Lavien et al. reviewed a series of 136 patients with a history of BE and found that closure with osteotomies decreased the need for hernia repairs.
Due to the high incidence of VUR after exstrophy closure (up to 75%) some surgeons perform ureteral reimplantation at the time of initial closure. The cephalotrigonal reimplantation has been described in patients with bladder exstrophy. This approach implants the ureter in a safe way, accounting for the inferior insertion using an almost right angle into the bladder, thus providing a more gradual course through to the neohiatus, which provides more distance from the bladder neck to the ureteral orifices. Braga et al. also have demonstrated the safety and efficacy of ureteroneocystostomy at the time of initial closure. We have adopted Braga’s approach and perform ureteral reimplantation, particularly in the female, if the bladder plate is adequate.
Infants with BE have a wide and flattened pelvis that is laterally displaced, thus leaving little support for the genitourinary organs. There is external rotation of the posterior pelvis, shortening and external rotation of the pubic rami, and a wide pubic diastasis. Approximation of the externally rotated bony pelvis is critical to decrease tension on the abdominal wall closure, to help to reapproximate the pelvic floor musculature, and to place the bladder and urethra deep within the pelvic diaphragm. Effective reapproximation of the pubic symphysis decreases the rate of abdominal wall dehiscence and improves rates of continence in children closed outside of the immediate newborn period. In order to aid in the closure of the pelvis, osteotomies are performed.
Osteotomies offer several advantages when performing the anatomic approach to BE closure including (1) optimizing pubic symphysis apposition, diminishing tension on the fascial repair; (2) optimizing placement of the bladder, bladder neck, and urethra in the pelvis; (3) improving the reapproximation of the corporal and clitoral bodies; and (4) decreasing the chance for later uterine prolapse. Traditionally osteotomies have been done for any child >72 hours old, in newborns with an exceptionally wide diastasis, and in reoperative BE closure/repair. However, there are data supporting osteotomies as being valuable even in the first 72 hours. Osteotomies are usually performed at the same setting as bladder closure to help secure the closure.
Bilateral iliac osteotomies can be performed through either an anterior or posterior approach. Posterior iliac osteotomies are performed with the patient prone, after which the patient is then repositioned for the bladder closure. Anterior iliac osteotomies are performed with the patient supine. The use of anterior osteotomies avoids repositioning the patient between the osteotomies and the bladder closure. Anterior osteotomies can be done using an anterior diagonal technique or a combination of anterior diagonal and vertical iliac osteotomies, which have had excellent initial and long-term results compared with anterior iliac osteotomies alone in some series. Other less commonly employed methods include a diagonal mid-iliac osteotomy performed through the same incision as the exstrophy closure, and division of the superior pubic ramus, which can be used in the newborn period.
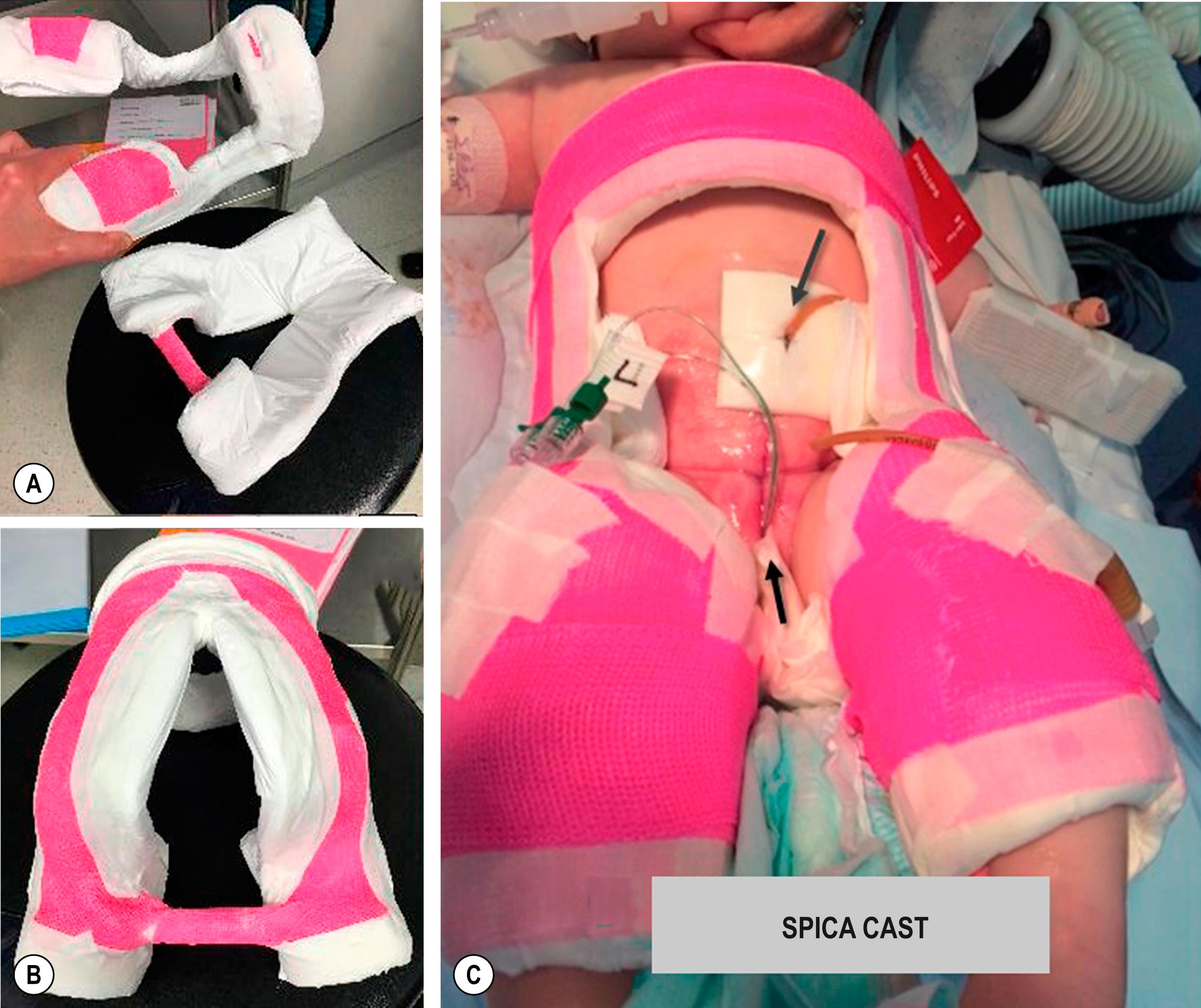
After the primary reconstructive procedure for exstrophy, either with or without osteotomy, the patient must be immobilized to decrease stress on the closure. There are various types of immobilization used, with no one optimal method. Options include (1) modified Bryant’s traction, (2) external fixation, or (3) spica cast. Each of these has its benefits and drawbacks. Modified Bryant’s traction immobilizes the baby in a bed and the hospital for 4–6 weeks, and the bindings on the legs can cause injury to the skin. External fixation also immobilizes the patient but carries a risk of external wound infections along the pin sites. Therefore, the pin sites require daily cleaning. In some centers, external pinned fixation is recommended for older children in whom the bones are mature enough to hold a pin. The spica cast allows for more mobility and earlier discharge from the hospital and is associated with decreased length of hospitalization and lower cost. Another option when using a spica cast is creation of a “window” over the epidural catheter entry site. This facilitates inspection of the site and management of the catheter as needed. We have moved to using a hinged spica cast, which is easier to remove (only the outer wrap can be cut and then replaced, or the two halves are kept together by Velcro wraps so the skin can be checked daily) ( Fig. 58.1 ) . Modified Buck’s traction has also been used by many groups with success. A posterior lightweight splint can be used in newborns when the child is out of traction to maintain hip adduction. Internal fixation may be necessary in older patients. Femoral nerve palsy is a possible complication with fixation that must be monitored and can be reduced by gradually tightening the fixator. Osteotomies are not a component of the classical Kelly technique (discussed later).
CPRE was introduced by Grady and Mitchell in 1998. This technique includes the combination of bladder closure, anatomic bladder neck narrowing, urethral elongation, and epispadias repair in a single operation in order to provide an environment for bladder cycling. At times, anatomy permitting, bilateral ureteral reimplantation is performed at CPRE in order to achieve the goals of urinary continence and preservation of renal function. Timing of the operation, whether or not to use osteotomies, the technique for the osteotomies, postoperative pain management, and postoperative immobilization are all factors to consider. Important elements of the surgical technique apply, with consideration given to limiting tissue injury, skin hook(s) versus forceps, scissors/knife versus electrocautery, topical dilute epinephrine, fine absorbable suture versus electrocautery (bipolar preferred) for hemostasis, and loupe magnification.
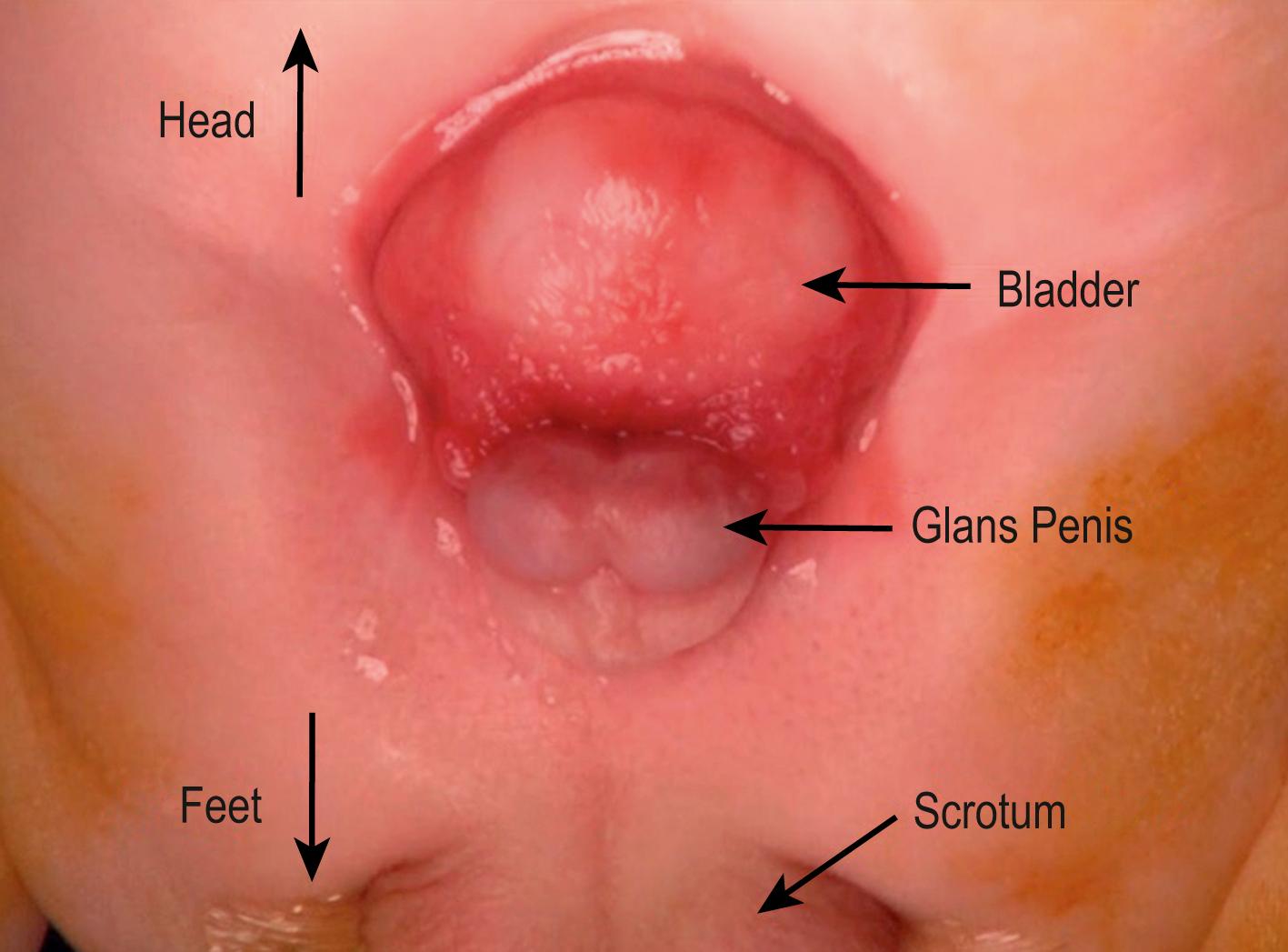
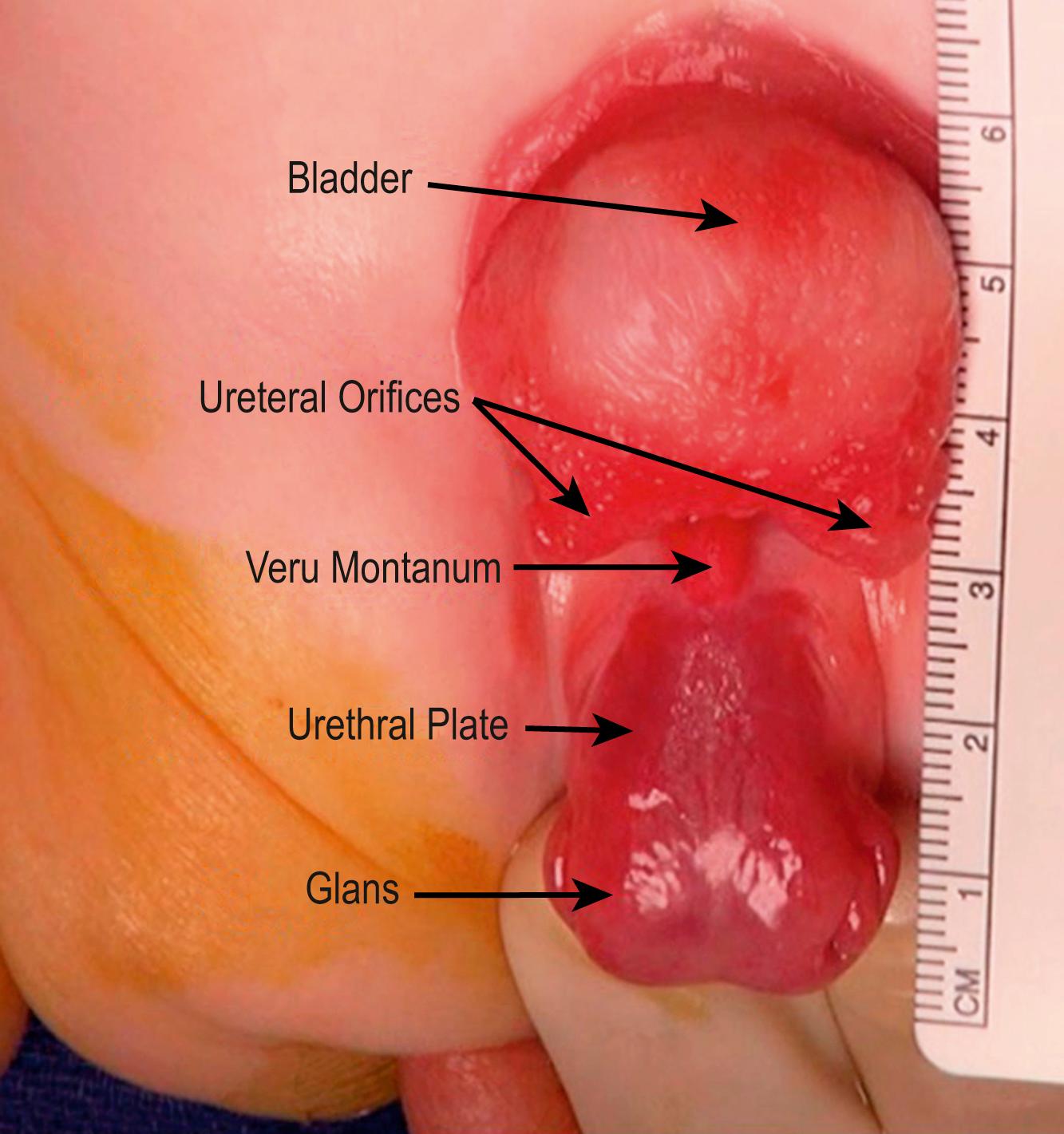
Total body preparation is performed. Bilateral iliac osteotomies are completed prior to sterile prep if a posterior approach is used, or after prepping if an anterior approach is chosen. The anatomy should be evaluated at the outset of the procedure ( Figs. 58.2 and 58.3 ). Tegaderm (3M) is placed over the anus. A stay suture is placed transversely in each hemiglans of the penis. Measurements of the bladder and urethral plate are recorded. Identifying and lightly marking the verumontanum and ureteral orifices facilitates orientation and assessment of dimensions, and clarifies important landmarks. Each ureteral orifice should be intubated with a small caliber feeding tube and secured with an absorbable suture ( Fig. 58.4 ). Assessing the bladder surface for polyps, with excision of large polyps as well as those near a ureteral orifice or at the bladder neck to eliminate irregularity, should be performed as this often facilitates bladder closure. Depending on the size of the polyp, approximation of tissues and hemostasis at the base of the polyp is achieved with a running absorbable suture. Pubic bones are marked bilaterally, and incision lines are marked at the perimeter of the bladder, bladder neck, and urethral plate. The future neoumbilicus site is also marked in the infant’s midline at the level of the iliac crest. The umbilical stump or scar is incorporated with the bladder via an inverted “V” incision marked at the cephalad extent of the skin incision caudal to the neoumbilicus. This tissue will eventually be discarded but functions nicely as a handle during dissection. Also, the incision planned in this manner will promote cosmesis at the time of closure. Initial dissection of the bladder, penis, and urethral plate then follows:
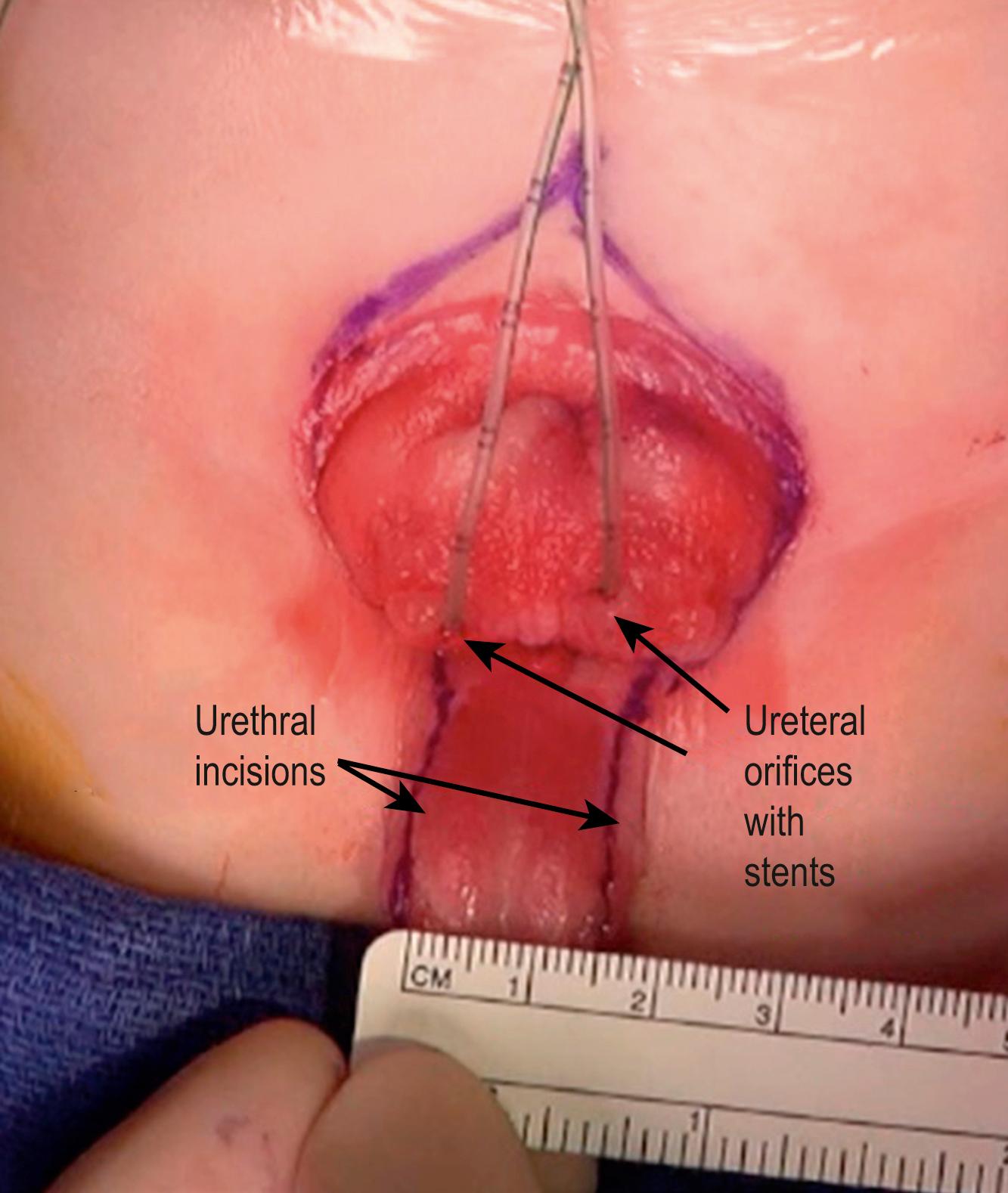
The incision begins at the cephalad extent of the defect (where the inverted “V” is marked in Fig. 58.4 ) and is carried along the perimeter of the bladder and urethral plate using fine needle point electrocautery. The incision is deepened at its cephalad extent, and care is taken to identify, isolate, and ligate the obliterated umbilical arteries and vein. The peritoneum is carefully separated with sharp and blunt dissection from the external surface of the bladder dome, and the posterior and lateral walls. A traction suture is placed in the umbilical stump/scar to facilitate dissection. In order to identify the often difficult plane between the bladder wall and the rectus muscle and fascia, one can first identify the plane between the skin and the anterior surface of the anterior rectus fascia. The dissection is carried along the anterior surface of the fascia, exploiting the “fat is your friend” concept in developing this important plane. In the thin infant with a paucity of abdominal wall fat, beginning this dissection at the level of the pubic bones, where typically a fat layer exists, and progressing in a cephalad direction, may be helpful. Accurately identifying this initial plane, and thus the medial edge of the anterior rectus fascia, facilitates accurate identification and dissection of the plane between the medial and posterior aspects of abdominal wall/rectus fascia and bladder edge. Adherent rectus and detrusor muscle fibers can be distinguished by the longitudinal course of the rectus component. Continued dissection along the perimeter of the bladder is facilitated by inserting the (right-handed) surgeon’s left index finger inside the bladder and inverting it. This allows better appreciation of the correct plane of dissection.
The dissection, as described earlier coursing caudally along the lateral aspects of the bladder wall, will eventually lead to the intersymphyseal band attachments that tether and displace the bladder trigone and bladder neck anteriorly. As the dissection progresses and tissue permits, blunt dissection along the lateral bladder wall will exploit the plane by identifying and entering the perivesical fat plane. This facilitates “pushing” of the distal aspect of the bladder medially and creates space underneath the intersymphyseal band on either side of the bladder neck. Using the medial edge of the pubic bone as a reference, the intersymphyseal bands are now divided. This is perhaps the most important part of the operation as it will allow appropriate placement of the bladder, bladder neck, and posterior urethra deep in the pelvis. This can be continued in a caudal to cephalad direction following urethral plate dissection.
This step is optimized via an initial ventral approach. Preputial skin adhesions to the glans are released, and the skin incision is marked ventrally parallel to the corona. The penile shaft is degloved of its skin. At the lateral aspects of this dissection, care is taken to avoid injury to the corpora and neurovascular bundle (NVB) as these structures are often adherent to the thin skin in this area. The ventral approach continues along the medial aspect of the corpora cavernosa using sharp dissection and bipolar electrocautery in order to develop this plane, allowing both hemostasis and preservation of the blood supply to the urethra ( Fig. 58.5 ). Visualization and dissection of this plane is facilitated for the right-handed surgeon positioned on the patient’s right by applying gentle lifting pressure with the left index finger underneath the dorsally reflected penis. This technique turns both corpora cavernosa laterally and elevates the corpus spongiosum/ ventral urethral plate. This dissection proceeds proximally to the penoscrotal junction where the corpora separate.
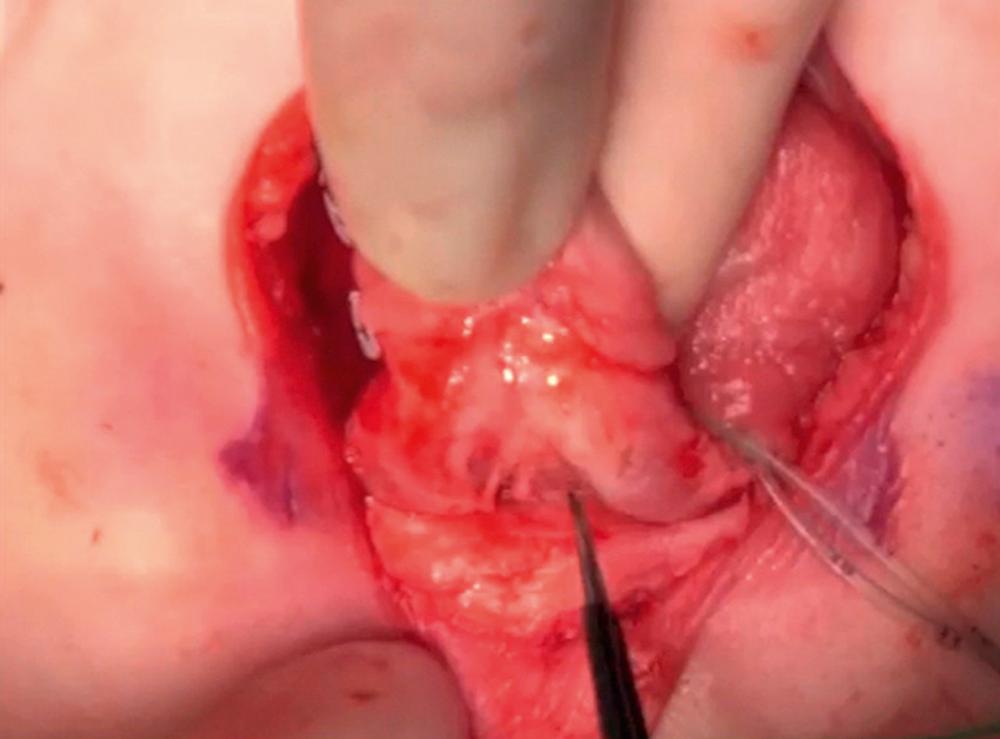
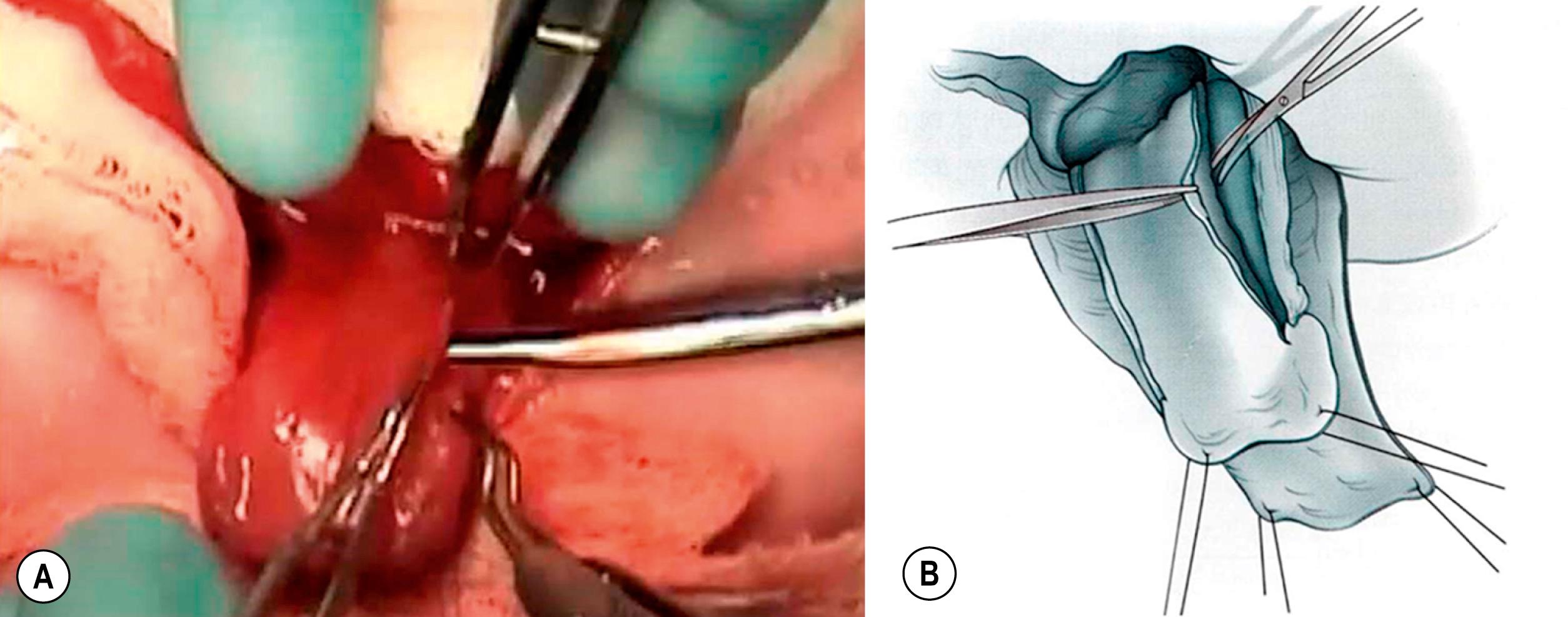
The dorsal urethral dissection begins with scissors at the lateral edge near the longitudinal midpoint of the urethral plate ( Fig. 58.6 ). The sharp dissection is medial and parallel to the neurovascular bundle. Dissection is carried medially alternating from dorsal to ventral until the corpora begin to be separated from the urethral plate, usually at the midpoint of the dissection. At this point, the surgeon passes a vessel loop between the corporal body and the urethral plate. The urethral plate dissection continues proximally toward the bladder neck and distally toward glans. The vessel loop gently reflects the urethral plate away from the midline ( Fig. 58.7 ). In a modification of the complete penile disassembly technique, the glans is instead kept in continuity, which seems to reduce the risk of venous stasis and glanular ischemia in the first few postoperative hours. In the absence of dorsal “bowing” secondary to a short urethral plate, the urethra is kept in continuity with the glans in order to preserve the urethral plate vascular communication and symmetry. A urethral width of approximately 15 mm is maintained throughout the entire length of the dissection all the way to the bladder neck. If the urethral plate is short and the “bowing” effect is enough to compromise penile length, the distal urethra can be detached from the glans as long as the left and right hemiglans remain intact.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here