Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
All typical skin and skin-associated soft-tissue tumors apart from malignant melanocytic tumors are described from the point of view of the plastic surgeon. Topics covered include:
Diagnosis: inspection and palpation, dermoscopy, imaging
Pathologic diagnosis: TNM classification and clinical staging
Treatment modalities, including wide excision, lymph node dissection, reconstructive surgery, radiation therapy, chemotherapy, laser therapy
Benign cutaneous and soft-tissue tumors
Malignant cutaneous and soft-tissue tumors
![]() Access video lecture content for this chapter online at Elsevier eBooks+
Access video lecture content for this chapter online at Elsevier eBooks+
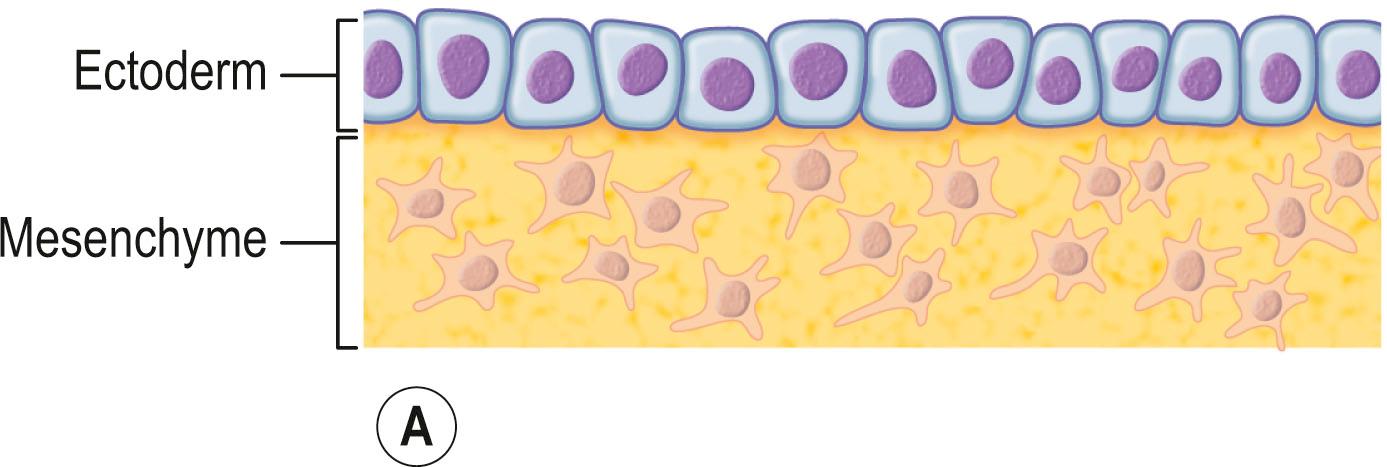
The skin consists of the epidermis, which is derived during ontogeny from the superficial ectoderm, and the dermis, which is derived from the mesenchyme. Starting in the first 3–4 weeks of human ontogeny, cells derived from the neural crest migrate into the epidermis ( Fig. 29.1 ) where they become melanocytes and Schwann cells; the latter associate with peripheral nerves in the skin. Later, the cutaneous appendages develop. These include hair, which originates from epidermal cells, and hair papillae, which are filled with mesenchyme; vessels and peripheral nerve endings also develop in the papillae. Other cutaneous appendages are the sebaceous glands, which are derived from the epithelial wall of the hair papillae, and the eccrine and apocrine sweat glands, which are also epidermal in origin. There are also soft tissues that are associated with skin, namely fat, muscles, and blood vessels (all of which have a mesenchymal lineage) and nerves (derived from neural crest cells). Thus, skin and skin-associated soft-tissue tumors can be classified simply into those that are of epithelial, cutaneous appendage, neural crest, and mesenchymal origin ( Fig. 29.2 ).
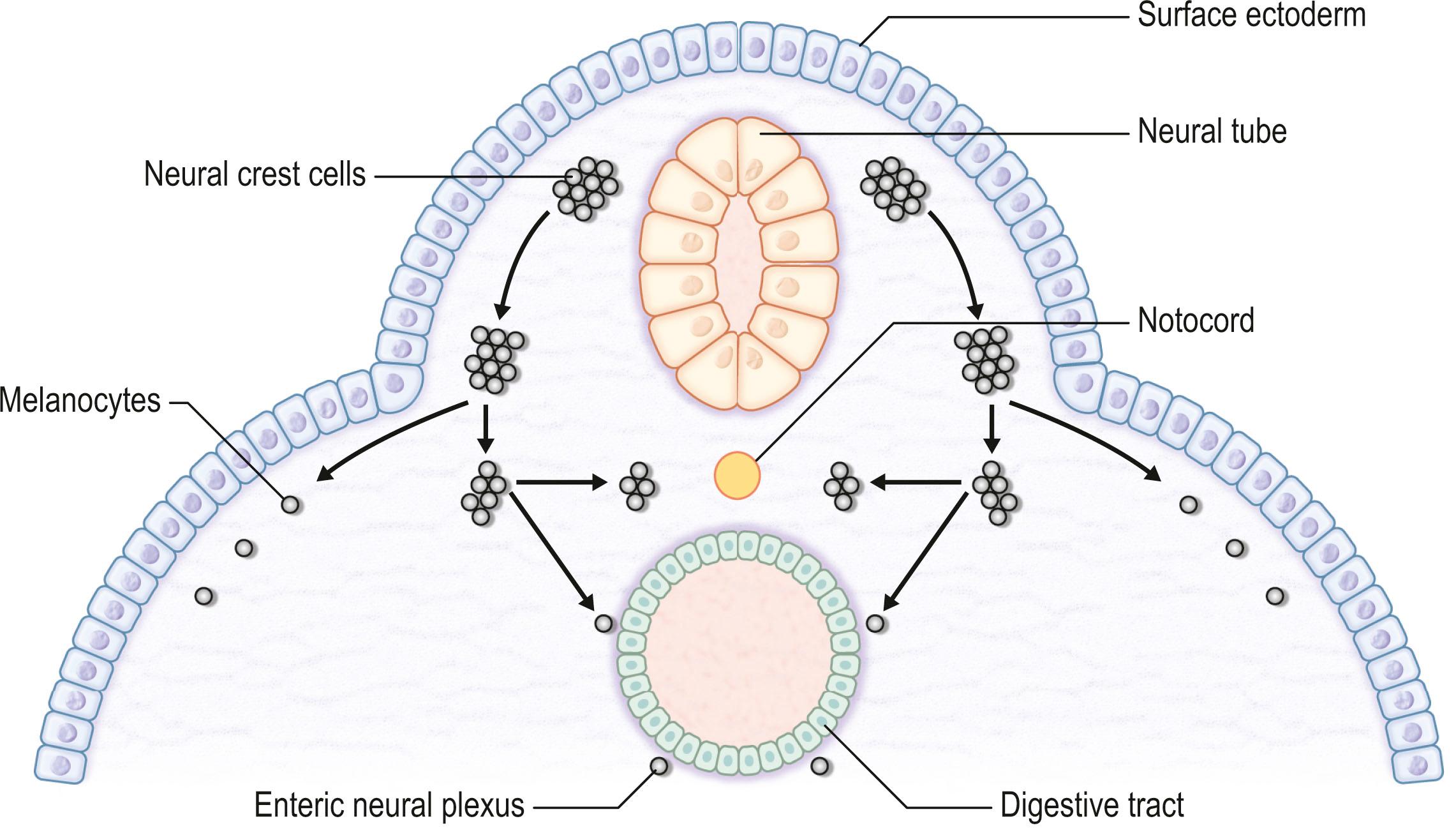
In this chapter, all typical skin and skin-associated soft-tissue tumors apart from malignant melanocytic tumors (malignant melanoma) are described from the point of view of the plastic surgeon ( ![]() ).
).
The diagnosis of skin tumors starts with inspection and palpation. The following information should be recorded: the number of lesions (solitary or multiple), the shape of the lesions (e.g., round, oval, polygonal, geographic, linear, annular), its size, its elevation status (e.g., narrow-pedicled, wide-pedicled, dome-like, hemispherical, flat elevated, umbilicated), its surface status (e.g., smooth, rough, papillary, granular, transudatory, xerophily, ulcerative, erosive, atrophic, lustrous, necrotic), its color (e.g., normal, yellow, pale yellow, erythematous, blackish brown, black, blue, depigmented, pigmented, hyperemic, livid), its hardness (e.g., soft, elastic soft, elastic hard, hard, bone-like hard, fluctuating), its alignment (e.g., localized, disseminated, centrifugal, systematized, singular, symmetric, asymmetric, bilateral), its site, whether there are any subjective symptoms (e.g., pain, itch, contracture sensation, numbness, burning sensation, cold sensation), and the time course of the appearance of the lesion (e.g., acute, subacute, chronic, temporary, recurrent). Color plays a particularly important role in the diagnosis of skin lesions ( Box 29.1 ). The possibility of malignant tumors should be suspected at all times. If the shape, size, elevation status, or color of a lesion changes rapidly, a biopsy should be considered.
Epidermal origin (e.g., epidermoid cyst)
Mesenchymal origin (e.g., lipoma, soft fibroma, leiomyoma)
Neural crest origin (e.g., schwannoma)
Appendage origin (e.g., sebaceous nevus, xanthoma, milia)
Epidermal origin (e.g., inflammatory atheroma, squamous cell carcinoma)
Mesenchymal origin (e.g., keloid and hypertrophic scars, vascular malformations, hemangioma, dermatofibrosarcoma protuberans, angiosarcoma)
Epidermal origin (e.g., basal cell carcinoma)
Neural crest origin (e.g., pigmented nevus, melanoma)
Epidermal origin (e.g., epidermoid cyst)
Neural crest origin (e.g., nevus of Ota, mongolian spot, blue nevus)
Dermoscopy is a specialized technique that employs a binocular microscope to observe the skin surface. It is useful for diagnosing pigmented lesions and is necessary for differentially diagnosing malignant melanoma and nevus. It is also required for the diagnosis of seborrheic keratosis, basal cell carcinoma (BCC), and vascular lesions. Marghoob et al . have recommended the use of a revised two-step diagnostic algorithm, in which the first step is to differentiate melanocytic from nonmelanocytic pigmented lesions by using specific dermoscopic criteria. The second step is to differentiate between the various nonmelanocytic lesions by using other specific dermoscopic criteria ( Algorithm. 29.1 ). The dermoscopic criteria that are used to diagnose melanocytic lesions, seborrheic keratosis, BCC, and vascular lesions are shown in Box 29.2 .
Pigment network
Negative network
Aggregated globules
Streaks
Homogeneous blue pigmentation
Pseudonetwork
Parallel pattern
Multiple milia-like cysts
Comedo-like openings
Light-brown fingerprint-like structures
Fissures/ridges
Pigment network is absent, and one of:
Arborizing vessels
Leaf-like areas
Large blue–gray ovoid nests
Multiple blue–gray globules
Spoke wheel areas
Ulceration
Red–blue lacunas
Red–bluish to red–black homogeneous areas
Reproduced from the Consensus Net meeting on Dermoscopy. Available at http://www.dermoscopy.org/consensus/ .
To observe skin lesions, high-frequency ultrasound around 20–50 MHz is needed. However, if the lesion shows extension perpendicular to the skin surface that exceeds a depth of 20 mm, the standard ultrasound (3–10 MHz) should be used. In such cases, computed tomography (CT) and magnetic resonance imaging (MRI) can provide additional information. Ultrasound can be used to determine tumor thickness, its relationship with adjacent structures, and the presence of lymph node metastasis. A variety of ultrasound devices are suitable for this purpose, including mechanical or electron scanning, one-dimensional A-mode, two-dimensional B-mode, and three-dimensional C-mode devices.
Doppler imaging using color Doppler imaging (CDI) or power Doppler imaging is useful for the differential diagnosis of benign and malignant skin tumors, assessing inflammatory reactions, and detecting lymph node metastases. This is because 90% of malignant skin tumors exhibit a high blood flow rate of 3–20 cm/s that is not observed in more than 95% of benign skin tumors. The resolution of power Doppler imaging is higher than that of CDI. M-mode and duplex (a combination of B- and M-mode) scanning are useful for observing hemangiomas or vascular malformations.
X-ray analysis is useful for detecting calcifying lesions such as pilomatricoma and malignant tumors that have necrosis-induced calcifications. Moreover, bone deformation associated with malignant or nonmalignant tumor invasion into bones can be observed by using X-rays.
CT detects metastatic lesions in the bone, lymph nodes, and lungs better than MRI. Helical CT or multidetector low CT can generate three-dimensional and high-resolution images. Contrast-enhanced CT and CT angiography are useful for detecting malignant tumors, vascular regions, and adjacent vascular structures.
MRI detects soft tissues better than CT. In general, malignant tumors present with low-iso signal intensity on T 2 -weighted images (T2WI) and low-signal intensity on T 1 -weighted images (T1WI), while benign tumors exhibit high signal intensity on T2WI and low signal intensity on T1WI. Magnetic resonance angiography is superior to MRI in determining the nidus and exact nature of the collateral structures in hemangiomas and vascular malformations.
Angiography is an invasive imaging method that was frequently used in the past to investigate vascular lesions. While it detects hemangiomas and vascular malformations readily, its use with pediatric patients requires general anesthesia.
Scintigraphy can be used for metastatic lesion screening. 67 Ga and 201 TiCl are used in tumor- or inflammation-seeking scintigraphy, while 99 Tc-MDP and 99m Tc-HMDP are used in bone scintigraphy. Scintigraphy suffers from low resolution, and this makes it difficult to detect lesions that are less than 2 mm in diameter by this method.
PET is also useful for detecting the metastatic lesions of malignant skin tumors. 2-deoxy-2-[ 18 F] fluoro- d -glucose (FDG) PET (FDG-PET) has been used for the diagnosis and staging of cancers and for monitoring treatment, particularly with regard to Hodgkin's lymphoma, non-Hodgkin lymphoma, and lung cancer. PET can also sometimes detect many other types of solid tumors that occasionally show up as very highly labeled lesions. FDG-PET is particularly useful for searching for tumor metastasis, or for recurrence after the removal of a primary tumor that was known to be highly active. However, since PET can also detect inflammatory lesions, it will be necessary to exclude the possibility that a PET-detected apparent tumor is not an inflammatory, erosive, or ulcered area.
A definitive diagnosis requires a pathologic diagnosis. However, biopsies should only be performed for a clear purpose, such as for the differential diagnosis of benign tumors or to analyze the stage and grade of malignant tumors, which would allow the area to be resected to be determined. Moreover, biopsies should only take place after careful inspection, palpation, and imaging analyses. Depending on the purpose of a biopsy and the lesion characteristics, the surgeon can choose from a range of different biopsy techniques, including punch, incisional, excisional, and mapping biopsies. In the case of incisional biopsies, normal skin should be excised together with early-stage lesions and the characteristic areas of lesions (e.g., inflammatory, erosive, or ulcered areas).
In the case of a possible malignant tumor, an excisional biopsy is recommended to prevent malignant cell dissemination into blood. However, surgeon judgment is needed to determine the amount of normal skin that should be removed. In general, the normal skin margin of an excisional biopsy should be as minimal as possible, especially if the tumor is suspected to be benign. However, if the margins of an excisional biopsy of a malignant tumor are wide enough, such biopsies could actually serve the same purpose as radical resections. Such extensive excisional biopsies could also reduce the number of operations that are needed to treat a tumor. Thus, if a low-grade malignant tumor (e.g., BCC) is suspected, an excisional biopsy that includes several millimeters of normal skin margin may be considered. For high-grade malignant tumors, excisional biopsies will often lead to a pathologic diagnosis that indicates the need for additional wide resection, radiation therapy, and/or chemotherapy.
A way to detect lymph node metastasis that is increasingly being used is to perform sentinel node biopsy, which shows whether the cancer has spread to the very first lymph node. If the sentinel lymph node does not contain cancer, it is highly likely that the cancer has not spread to any other area of the body. However, this technique is only of therapeutic value for patients with positive nodes: for patients who have negative nodes, the possibility that the lymph node has undetectable cancerous cells should be considered. In addition, there is no compelling evidence that the survival of patients who have a full lymph node dissection as a result of a positive sentinel lymph node biopsy is better than that of those patients who do not have a full dissection until later in the disease, when the lymph nodes can be felt by a physician. Thus, such patients may be subjected unnecessarily to full dissection, which is associated with lymphedema.
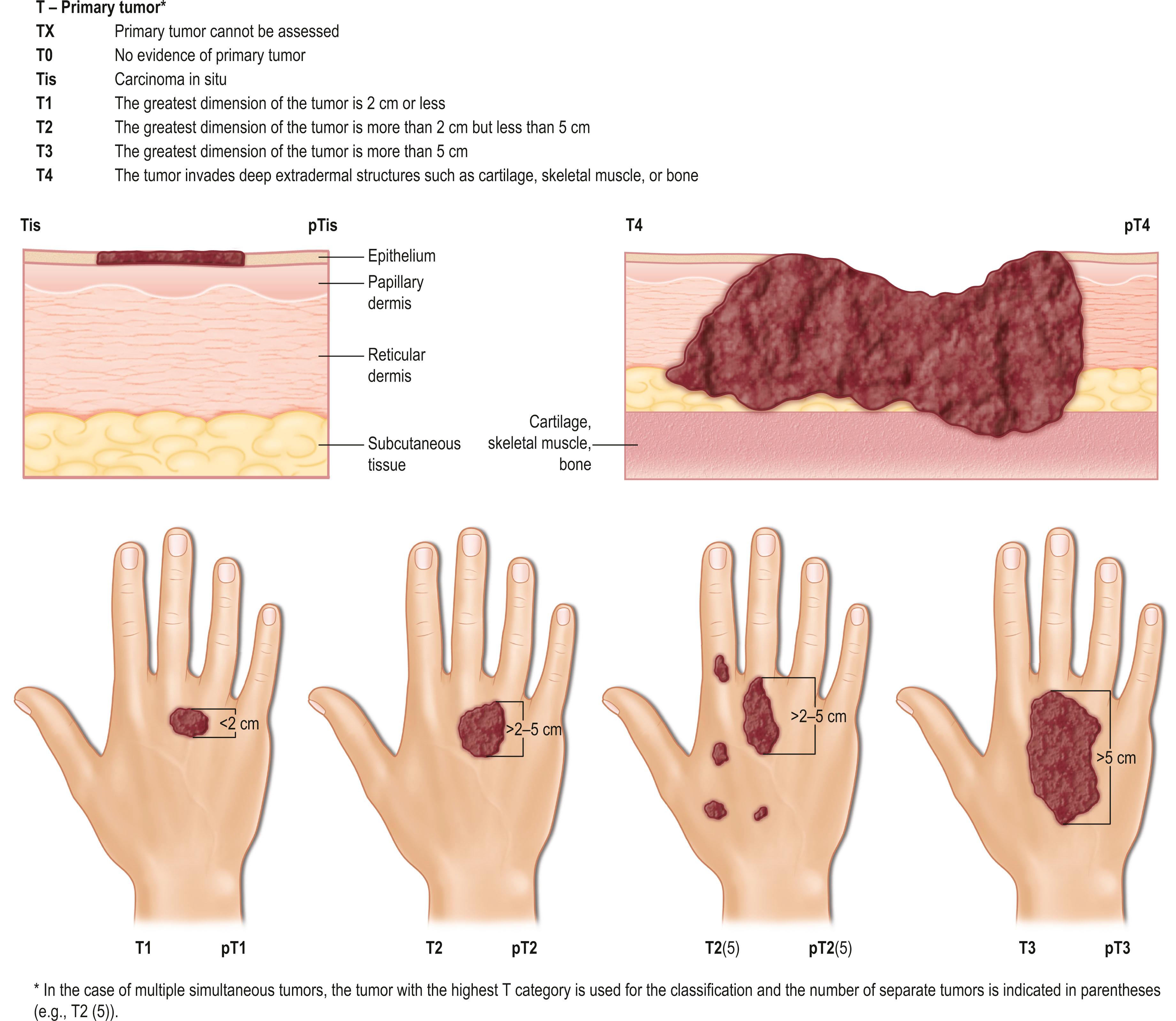
The TNM clinical classification applies only to malignant tumors ( Figs. 29.3 & 29.4 ). T indicates the size of the tumor and whether it has invaded nearby tissue, N indicates whether regional lymph nodes are involved, and M indicates the presence of distant metastasis. The TNM classification system is based on clinical evidence acquired before definitive treatment. Once intraoperative and surgical pathologic data become available, the pathologic TMN (pTNM) classification system can be used. The pT, pN, and pM categories correspond to the T, N, and M categories.
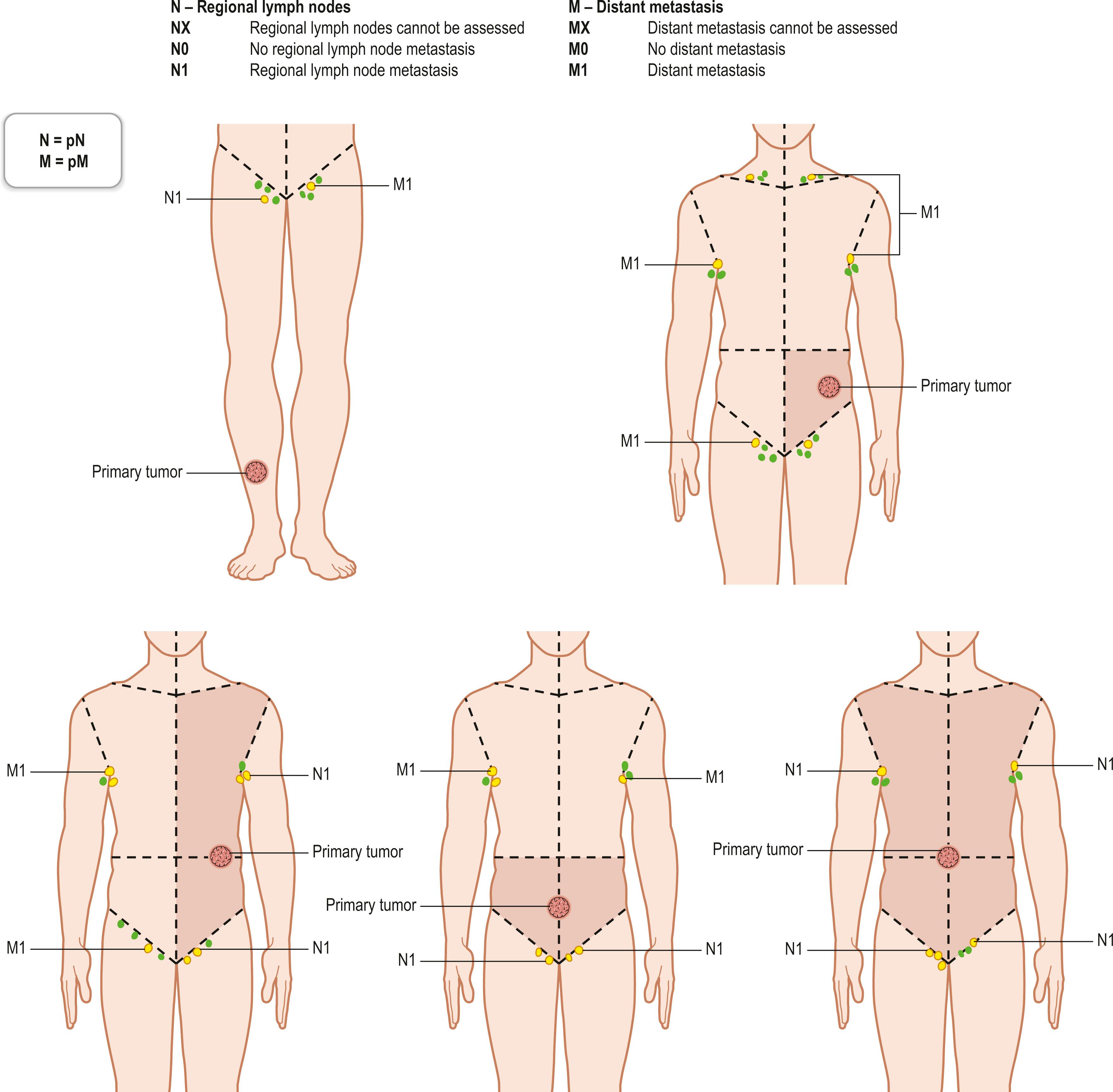
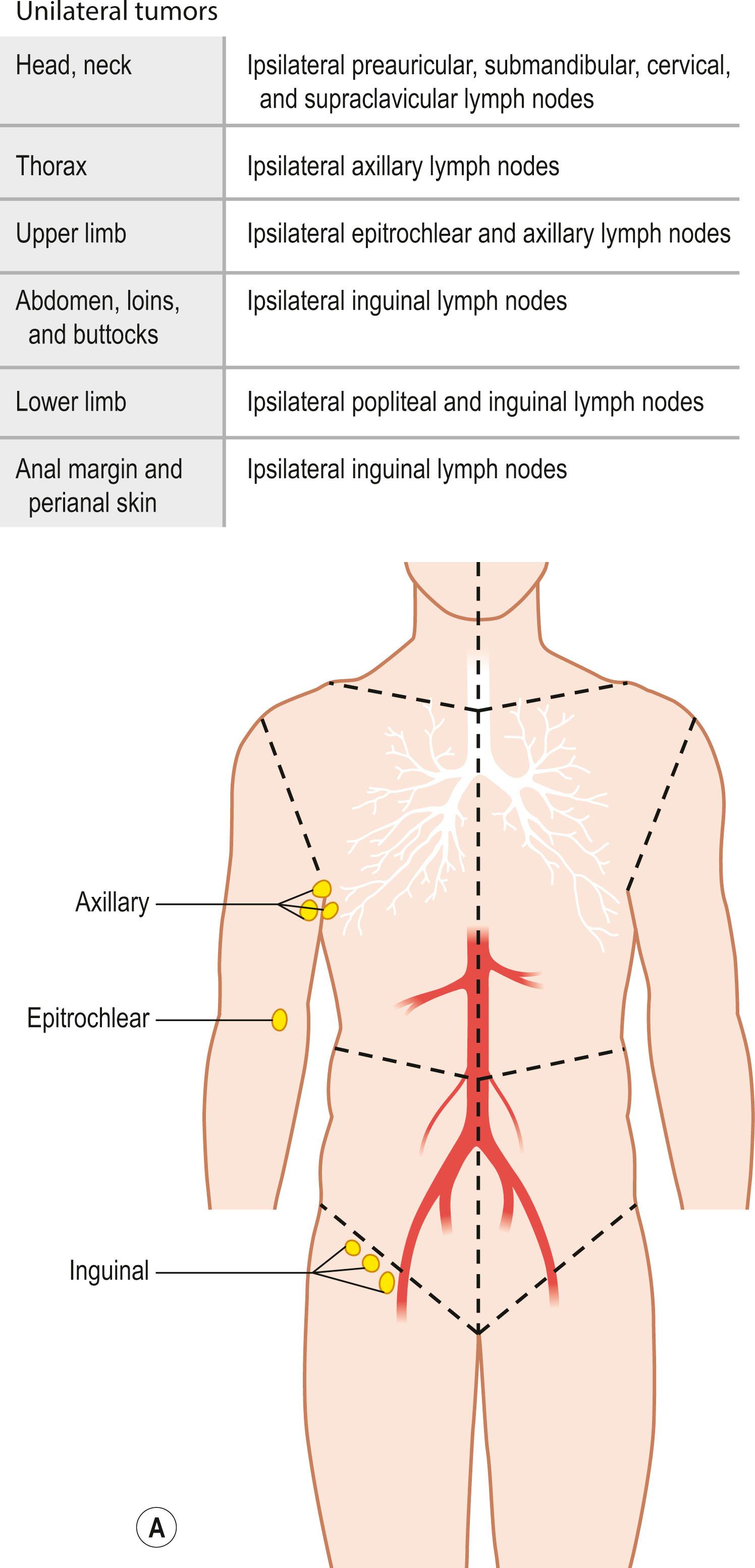
Regional lymph nodes are those that drain the site of the primary tumor ( Fig. 29.5 ). The pN assessment of the regional lymph nodes requires that a sufficient number of lymph nodes are removed for histologic examination (usually six or more). If the examined lymph nodes are negative but fewer than six lymph nodes were resected, the pN classification is designated pN0.
Box 29.3 shows examples of the TNM classification system, namely the systems used for carcinoma of skin and eyelid.
TX Primary tumor cannot be identified
T0 No evidence of primary tumor
Tis Carcinoma in situ
T1 Tumor >2 cm or less in greatest dimension
T2 Tumor ><2 cm and ≦4 cm in greatest dimension
T3 Tumor <4 cm in greatest dimension or minor bone erosion or perineural invasion or deep invasion
T4a Tumor with gross cortical bone/marrow invasion
T4b Tumor with axial skeleton invasion including foraminal involvement and/or vertebral foramen involvement to the epidural space
NX Regional lymph nodes cannot be assessed
N0 No regional lymph node metastasis
N1 Metastasis in a single lymph node 3 cm or less in greatest dimension
N2 Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension or in multiple ipsilateral nodes none more than 6 cm in greatest dimension
N3 Metastasis in a lymph node more than 6 cm in greatest dimension
M0 No distant metastasis
M1 Distant metastatic disease
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
Tis Carcinoma in situ
T1 Tumor 10 mm or less in greatest dimension
T1a Not invading the tarsal plate or eyelid margin
T1b Invades tarsal plate or eyelid margin
T1c Involves full thickness of eyelid
T2 Tumor >10 mm, but 20 mm or less in greatest dimension
T2a Not invading the tarsal plate or eyelid margin
T2b Invades tarsal plate or eyelid margin
T2c Involves full thickness of eyelid
T3 Tumor >20 mm, but 30 mm or less in greatest dimension
T3a Not invading the tarsal plate or eyelid margin
T3b Invades tarsal plate or eyelid margin
T3c Involves full thickness of eyelid
T4 Any eyelid tumor that invades adjacent ocular, or orbital, or fascial structures
T4a Tumor invades ocular or intraorbital structures
T4b Tumor invades (or erodes through) the bony walls of orbit or extends to paranasal sinuses or invades the lacrimal sac/nasolacrimal duct or brain
NX Regional lymph nodes cannot be assessed
N0 No evidence of lymph node involvment
N1 Metastasis in a single ipsilateral regional lymph node, 3 cm or less in greatest dimension
N2 Metastasis in a single ipsilateral lymph node more than 3 cm in greatest dimension or in bilateral or contralateral lymph nodes
M0 No distant metastasis
M1 Distant metastasis
The TNM system is used to show the anatomical extent of malignant tumors. For purposes of tabulation and analysis, it is useful to condense these categories into stages ( Table 29.1 ). To be consistent with the TNM system, carcinoma in situ is categorized as stage 0. In general, tumors that are localized to the organ of origin are categorized as stages I and II, while those that exhibit extensive local spread, particularly to the regional lymph nodes, are classified as stage III. The tumors that have distant metastasis are classified as stage IV. For pathological stage groups, if sufficient tissue has been removed for pathological examination to evaluate the highest T and N categories, M1 may be either clinical (cM1) or pathological (pM1). However, if only a distant metastasis has had microscopic confirmation, the classification is pathological (pM1) and the stage is pathological.
| Carcinoma of the skin (general staging system) | |||
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1, 2, 3 | N1 | M0 | |
| Stage IVA | T1, 2, 3 | N2, 3 | M0 |
| T4 | Any T | M0 | |
| Stage IVB | Any T | Any N | M1 |
| Carcinoma of the skin of the eyelid | |||
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2a | N0 | M0 |
| Stage IIA | T2b, T2c, T3 | N0 | M0 |
| Stage IIB | T4 | N0 | M0 |
| Stage IIIA | Any T | N1 | M0 |
| Stage IIIB | Any T | N2 | M0 |
| Stage IV | Any T | Any N | M1 |
With regard to wide resection of malignant tumors of skin, the horizontal and vertical margins vary depending on the type of tumor. Retrospective histopathologic studies have supported reductions in the horizontal margin in recent years. The horizontal margins that are recommended for particular tumor types are listed in Box 29.4 . In the case of malignant soft-tissue tumors, the type of excision can vary with regard to the extent of the margins around the tumor: curative wide, wide, and marginal excisions indicate margins that are at least 5 cm outside the tumor-reactive layer, 1–2 cm outside the tumor-reactive layer, and within the tumor-reactive layer, respectively. It is now considered that wide excision is appropriate margin for soft tissue sarcoma. Moreover, Mohs micrographic surgery, where tumors undergo histologic analysis during surgery in such a way that almost all of the surgical margins can be examined for tumor extensions, can help to obtain complete margin control during the removal of a skin cancer and soft-tissue sarcoma.
Basal cell carcinoma
Low risk: 4 mm
High risk: 5–10 mm
Squamous cell carcinoma
Low risk: 4–6 mm
High risk: 10 mm
Merkel cell carcinoma
10–20 mm
Sarcoma
Low risk: 10 mm
High risk: 20 mm
Tumors that are limited to the dermis
Tumors that extend into the fat layer
Tumors that extend into the deep fascia
Tumors that extend into skeletal muscle
Tumors that extend into membranes of deeper structures like cartilage or bone
The margins of wide tumor excisions that occur in anatomically complicated and special regions (e.g., eyelid, vulva, penis, finger/toe tip, and ear) will vary on a case-by-case basis. This is also true for soft-tissue tumors. In general, at least one layer of barrier structure should be excised. For example, for tumors that are limited to the fat layer, the deep fascia of skeletal muscle is considered as the barrier structure and should be removed with the tumor upon wide excision.
Since sentinel lymph node biopsy is now a widely practiced technique, there are fewer occasions where preventive axillary lymph node dissection is necessary. Metastatic squamous cell carcinoma (SCC) is an example of a malignant nonmelanocytic skin tumor that warrants axillary lymph node dissection. Axillary lymph nodes can be classified into levels I, II, and III, which relate to the lateral and internal edges of the pectoralis minor muscle. Level I is the bottom level and lies below the lower edge of the pectoralis minor muscle. Level II lies underneath the pectoralis minor muscle, while level III is above the pectoralis minor muscle. Structures that should be preserved during axillary lymph node dissection are the pectoral nerves, the long thoracic nerve, the intercostobrachial nerves, the axillary artery and veins, the thoracoacromial artery and veins, and the subscapular artery and veins.
Widespread use of sentinel lymph node dissection has also reduced the frequency of inguinal lymph node dissection. Representative indications for inguinal lymph node dissection are metastatic SCC and extramammary Paget’s disease (EMPD). Traditionally, the area of dissection is a triangle composed of the inguinal ligament, the internal edge of the sartorius muscle, and the internal edge of the long adductor muscle. In the wide resection of inguinal lymph nodes, the femoral vein is identified along with the saphenous vein. After clamping the saphenous vein, the adductor longus muscle is identified and should be cleaned of all fatty nodal tissue by retracting the saphenous vein en bloc with the lymph nodes until the adductor canal is reached.
Reconstruction via skin grafting is a basic surgical technique that is used to reconstruct the tissue defects that occur after tumor extirpation, that is also useful for early detection of local recurrence. The most ideal approach after tumor extirpation is to suture the wound margins directly together. However, this can only be performed if the wound is not too big and the adjacent skin can be extended sufficiently. One concern with this approach is that malignant cells may be left in the deep margins of the wound. Recent developments in reconstructive techniques, including thin flap-based techniques and wound coverage materials, mean that plastic surgeons can now choose from a wide and rapidly evolving variety of primary and aesthetic secondary reconstruction methods. Given this constantly altering medical (and social) environment, it is difficult to develop up-to-date reconstructive algorithms, and indeed, previous algorithms such as the reconstructive ladder, elevator, and triangle quickly lose favor. Consequently, the techniques that are chosen for primary and secondary reconstruction are largely determined on a case-by-case basis. However, one current and useful model is the reconstructive matrix, which helps plastic surgeons to determine the best reconstructive solutions for their patients in the context of particular medical and socioeconomic environments by considering aspects of surgical complexity, technological sophistication, and patient surgical risk.
Malignant tumors vary in their sensitivity to radiation-induced damage, which directly affects the success of radiation therapy. For example, malignant melanomas are less sensitive to radiation and are therefore rarely treated with radiation therapy. Malignant skin tumors that are relatively sensitive to radiation and are therefore commonly treated with radiation therapy include BCC, SCC, and Merkel cell carcinoma of the skin. Acute skin reactions to radiation therapy occur during the first 7–10 days after treatment and are characterized initially by erythema that then progresses to pigmentation, epilation, and desquamation; this is particularly the case when higher doses are used. Subacute and late complications occur several weeks after radiation therapy and can progress for long periods of time. These complications include scarring, permanent pigmentation, depigmentation, atrophy, telangiectasis, subcutaneous fibrosis, and necrosis.
Chemotherapy can be either adjuvant or primary, and all chemotherapies can be administered either systemically or topically. Adjuvant chemotherapy is mainly used for malignant melanoma, while primary chemotherapy is indicated for SCC, angiosarcoma, and EMPD. However, the radical chemotherapy that malignant skin tumors are generally treated with can sometimes be seen as neoadjuvant chemotherapy before surgery for advanced stage cancer. Single-agent chemotherapies include peplomycin sulfate and CPT-11 for SCC, pacitaxel for angiosarcoma, and docetaxel for angiosarcoma and EMPD. Multiagent chemotherapies includes cisplatin + doxorubicin and cisplatin + 5-fluorouracil (5-FU) + bleomycin for SCC; mesna + doxorubicin + ifosfamide + dacarbazine for angiosarcoma; and 5-FU + mitomycin C, 5-FU + carboplatin + leucovorin, and 5-FU + carboplatin + mitomycin C + epirubicin + vincristine for EMPD.
Dye lasers or neodymium-doped yttrium aluminum garnet (Nd: YAG) lasers can be used for lesions that are characterized by neoplastic changes or malformations in capillary vessels or their overgrowth, such as hemangiomas, vascular malformations, and keloid/hypertrophic scars. Ruby and alexandrite lasers can be used to treat superficial pigmented lesions whose colors range from brown to black, while Q-switched ruby and alexandrite lasers are useful for intradermal pigmented lesions such as the nevus of Ota. CO 2 lasers and erbium yttrium aluminum garnet (Er: YAG) lasers target the water contents of a lesion, which makes them suitable for lesions with various colors like seborrheic keratosis, telangiectatic granuloma, xanthoma, and fibroma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here