Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
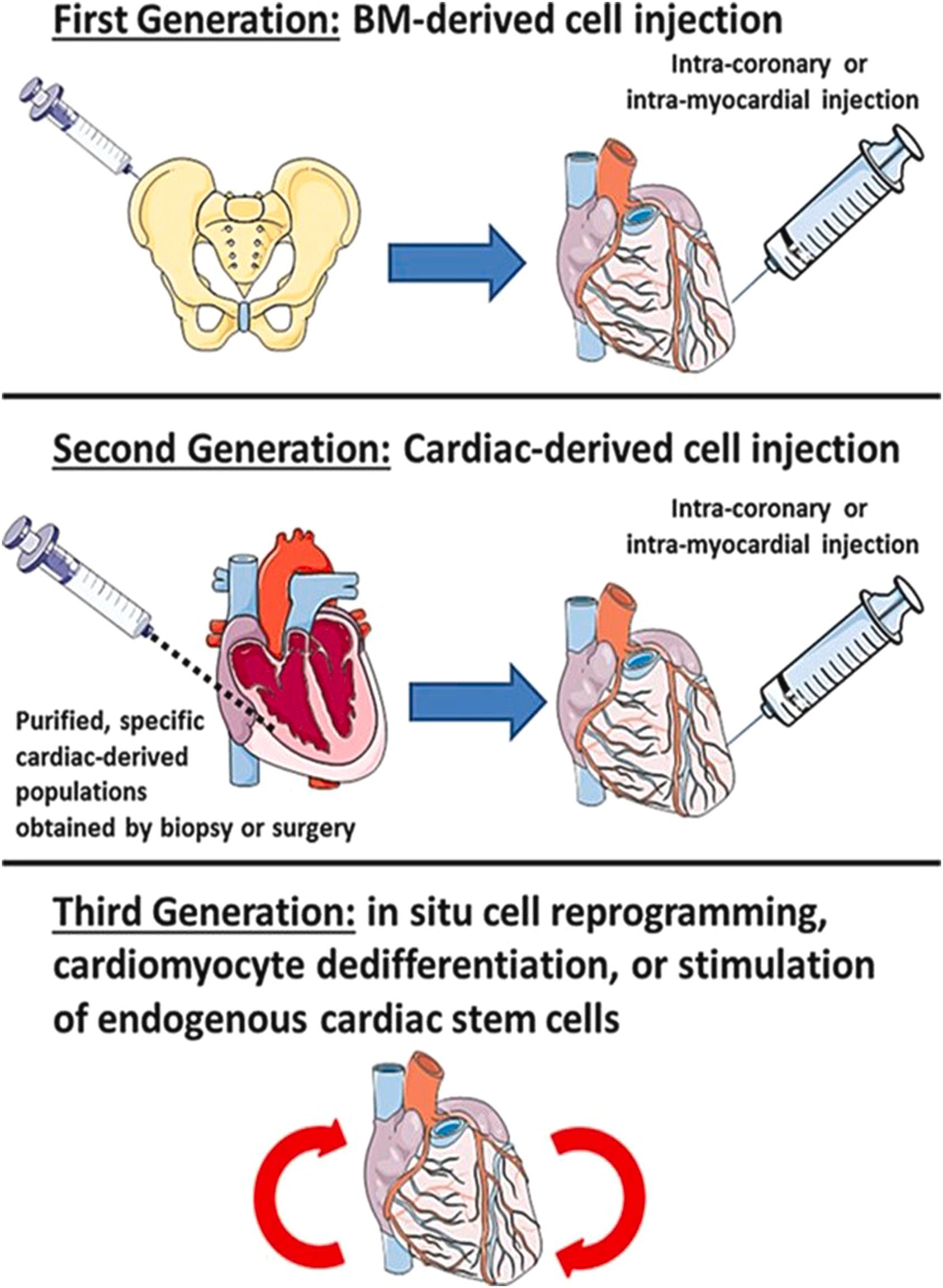
The late 1990s and early 2000s was a time period when major advances in stem cell biology were occurring . In this milieu, the concept of cell-based therapy to regenerate the damaged heart gained momentum . The need for definitive restorative therapy of the heart was compelling given the growing population of patients with advanced heart failure amenable only to implantation of ventricular assist devices and cardiac transplantation. This rationale drove the development of the field of cardiac regenerative medicine, and the field has stimulated a reassessment of fundamental myocardial biology . Cardiac regenerative medicine has since evolved through the three phases shown in the Central Concept Figure . Nevertheless, much is yet unknown, and even after 25 years, definitive regenerative therapy remains more aspirational than realistic in reversing heart failure and restoring normal cardiac structure and function .
Stem cells are undifferentiated cells with the key properties of self-renewal, pluripotency (the ability to differentiate into multiple cell types), and immunomodulation ( Fig. 21.1 ). Stem cells from the early embryo exhibit totipotency—they can generate the full gamut of cell types in the body. In comparison, adult stem cells that occur in various tissue niches (e.g., skin stem cells in the bulge area of the hair follicle, or intestinal stem cells in the crypts of the small bowel) have a more limited repertoire regarding the possible cell types they can become. In the 1990s and early 2000s, hematopoietic stem cells (HSCs) were shown to self-renew, continually giving rise to mature blood cell types, and be capable of restoring the bone marrow of patients with hematological diseases via autologous transplantation . Later, procedures to isolate totipotential human embryonic stem cells (hESCs) were developed, and differentiated adult cells were shown to be capable of reprograming to gain totipotency .
![Figure 21.1, Biological and immunological properties of stem cells. General properties of stem cells include self-renewal, in vitro and in vivo replication and expansion, and in vivo differentiation and tissue regeneration. Naive embryonic stem cells and mesenchymal stem cells function to modulate and dampen the native and acquired immune responses of the host through cytokine-mediated interactions with various populations of lymphocytes (B cells, T helper cells, cytotoxic T cells, and natural killer [NK] cells) and dendritic cells and macrophages. Stem cells also respond to perturbations of the host by proliferation and differentiation, which may result in tissue repair and regeneration. Concomitantly, stem cells are exposed to several host factors leading to their activation. In the process, the phenotype and functional properties of the stem cells are changed, as mediated and manifested by upregulation of major histocompatibility complex (MHC) class I and II molecules, costimulation factors, and conventional antigen epitopes via alternative gene splicing. One consequence is that the stem cells undergo stimulation-responsive splicing for the selection of autoantigens and self-tumor antigens. However, if the stem cells are nonself (allogeneic or xenogeneic), they also become subject to targeted immune and inflammatory responses by the host. IL , interleukin; INF , interferon; TNF , tumor necrosis factor. Figure 21.1, Biological and immunological properties of stem cells. General properties of stem cells include self-renewal, in vitro and in vivo replication and expansion, and in vivo differentiation and tissue regeneration. Naive embryonic stem cells and mesenchymal stem cells function to modulate and dampen the native and acquired immune responses of the host through cytokine-mediated interactions with various populations of lymphocytes (B cells, T helper cells, cytotoxic T cells, and natural killer [NK] cells) and dendritic cells and macrophages. Stem cells also respond to perturbations of the host by proliferation and differentiation, which may result in tissue repair and regeneration. Concomitantly, stem cells are exposed to several host factors leading to their activation. In the process, the phenotype and functional properties of the stem cells are changed, as mediated and manifested by upregulation of major histocompatibility complex (MHC) class I and II molecules, costimulation factors, and conventional antigen epitopes via alternative gene splicing. One consequence is that the stem cells undergo stimulation-responsive splicing for the selection of autoantigens and self-tumor antigens. However, if the stem cells are nonself (allogeneic or xenogeneic), they also become subject to targeted immune and inflammatory responses by the host. IL , interleukin; INF , interferon; TNF , tumor necrosis factor.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Basicpathobiologyofcellbasedtherapiesandcardiacregenerativemedicine/1_3s20B9780128222249000165.jpg)
Based on these initial developments, an attractive hypothesis was advanced that all adult stem cells were highly plastic and, when transplanted into new environments, could transdifferentiate into cells of the new host tissue. An enthusiastic flurry of manuscripts reported that transplanted adult stem cells, including HSCs, could transdifferentiate into parenchymal cell types of solid organs . However, these reports ran counter to the substantial body of work in hematopoiesis and transplantation biology, as well as more rigorous contemporaneous research. Indeed, HSCs had been shown to self-renew and also to give rise to all blood cell types, but there was absolutely no solid evidence that HSCs could transdifferentiate into tissues other than blood cell types .
In this environment, a handful of groups published several reports that challenged the generally accepted paradigm that the mature mammalian heart lacked replicative capacity. Rather, they asserted that the heart was a self-renewing organ and that the function of damaged myocardium could be enhanced by exogenously administered stem cells. Beginning in 2001, a series of papers were published stating that human hearts contain large numbers of mitotic cardiomyocytes (CMC) and that endogenous and circulating progenitor cells can become CMC (based on studies of female-to-male heart transplant patients) . The assertions were based on application of powerful new cell biology techniques and microscopy imaging methodologies, extrapolating from scattered apparent cardiomyocyte mitoses to the total populations in an intact heart. The claim was also made that the injured mouse heart could regenerate through either the transplantation of appropriately cytokine-stimulated bone marrow stem cells expressing the surface marker c-kit , or preparations composed of culture-expanded cardiac stem cells (CSC) . Although these conclusions were based on limited murine studies and other investigators were having difficulty reproducing key aspects of the original experimental findings , the animal work was rapidly followed by publication of the first human clinical trial of CSC therapy . The original report was instrumental in generating a wave of enthusiasm among cardiologists, leading to several clinical stem cell trials, in part enabled by a loose regulatory environment. The preclinical and clinical studies of cell-based therapy used a variety of stem cells ( Table 21.1 ) , and applied a variety of approaches (Central Concept Figure) . Concurrently, there was a persistent drumbeat of experimental and clinical evidence that did not support the regenerative capacity of the various stem cell preparations ( Fig. 21.2 ) . For example, in 2004, at least three groups published that transplanted bone marrow cells did not transdifferentiate into cardiac muscle, despite original reports .
| Skeletal myoblasts |
| Advantages: easy access from autologous muscle biopsies; low ethical concerns; rapid in vitro expansion; resistant to ischemic conditions; low risk of tumorigenicity |
| Disadvantages: no transdifferentiation into functional cardiomyocytes; hazard of ventricular arrhythmias due to the lack of electromechanical coupling |
| Hematopoietic stem cells/endothelial progenitor cells (HSCs/EPCs) |
| Advantages: easy access from autologous bone marrow or blood; low ethical concerns; proof of safety in clinical trials; straightforward and standardized isolation procedures of HSCs; promotion of vasculogenesis; therapeutic secretome; low risk of tumorigenicity |
| Disadvantages: low cell quality; limited differentiation potential; undefined phenotype of EPCs; heterogeneous cell populations; potential encouragement of inflammatory processes; inconsistent results regarding therapeutic effects |
| Mesenchymal stromal/stem cells (MSCs) |
| Advantages: easy access from several tissues; low ethical issues; transplantation of autologous and allogenic cells due to low immunogenicity; proof of safety in clinical trials; rapid in vitro expansion; therapeutic secretome; beneficial immunomodulative properties; low risk of tumorigenicity |
| Disadvantages: limited cell quantity; limited differentiation potential; undefined in situ phenotype; heterogeneous cell population; inconsistent results regarding therapeutic effects |
| Cardiac stem cells (CSCs) |
| Advantages: suitable for autologous transplantation; proof of safety in clinical trials; endogenous cardiac localization; low risk of tumorigenicity |
| Disadvantages: limited cell quantity; access from invasive myocardial biopsies; insufficient characterization; contradictory results concerning cardiovascular differentiation potential |
| Embryonic stem cells (ESCs) |
| Advantages: pluripotent differentiation potential; unlimited quantity; easy generation of cell lines; allows generation of off-the-shelf cell products; ESC-derived cardiomyocytes integrate electromagnetically into the host myocardium |
| Disadvantages: difficult to generate pure and mature cardiomyocytes in large quantities; ethical concerns; risk of tumorigenicity; genomic instability; lack of availability; risk of immunologic rejection and immunosuppression required |
| Induced pluripotent stem cells (iPSCs) |
| Advantages: pluripotent differentiation potential; low ethical concerns; suitable for autologous transplantation; easily accessible source tissue; unlimited quantity; iPSC-derived cardiomyocytes integrate electromagnetically into the host myocardium |
| Disadvantages: difficult to generate pure and mature cardiomyocytes in large quantities; risk of tumorigenicity; risk of immunologic rejection due to genomic instability; Lowe induction efficiency; lack of standardized generation procedure |
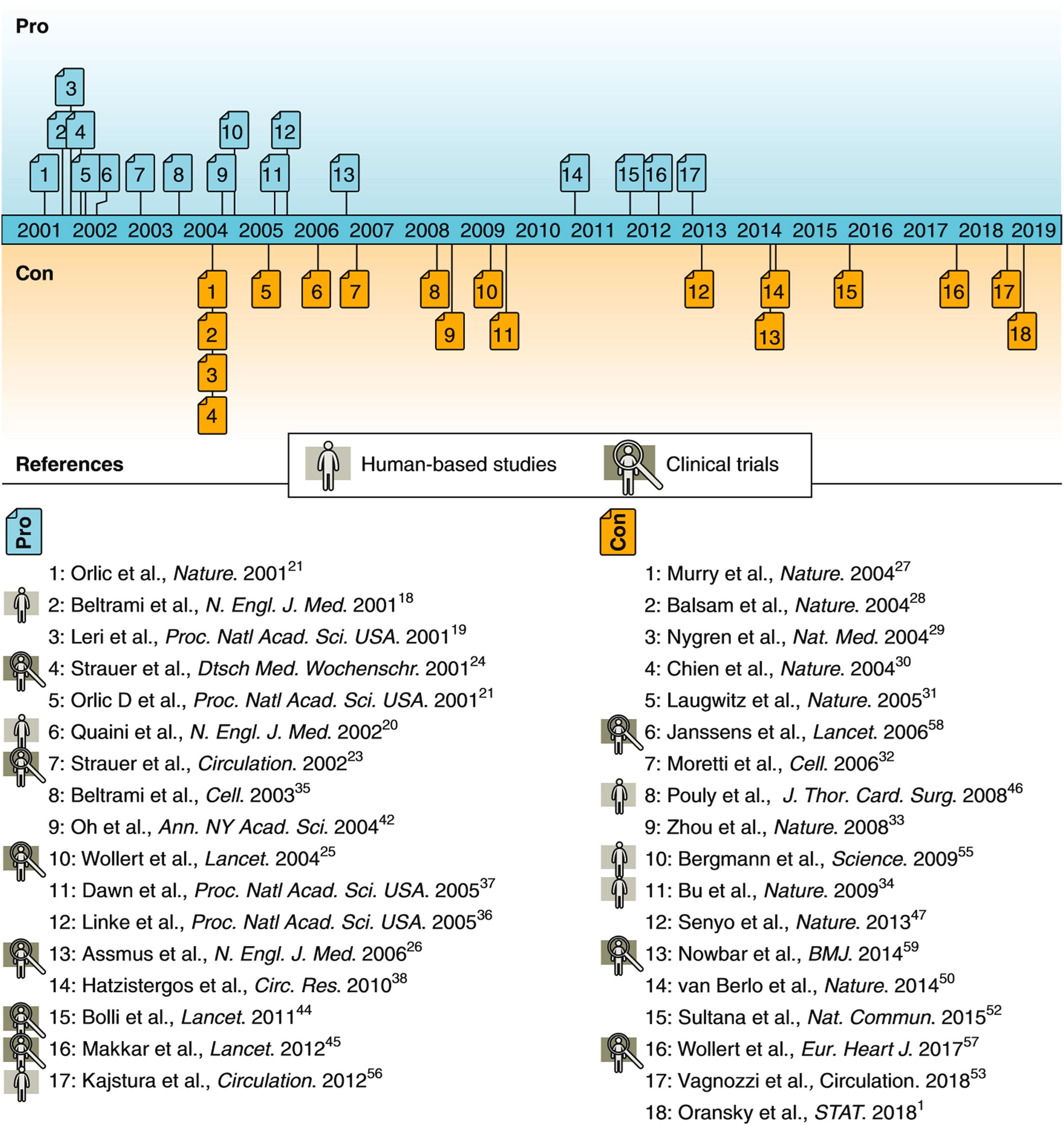
After 20 years of intense effort spawning an extensive body of experimental studies and clinical trials, uncovered inaccuracies in several of the early publications and their retraction from scientific journals led to a sober re-evaluation of the conceptual and evidentiary basis for cardiac regenerative medicine . The current state of the field warrants a critical analysis of issues pertinent to myocardial regeneration, including the putative role of stem cells in myocardial pathobiological processes and therapeutic interventions. This chapter takes into account incongruities between basic myocardial pathobiology, preclinical experimental studies, and clinical trials of stem cell therapy, and addresses current and future directions, based on the best available contemporary evidence regarding basic biological properties and pathological responses of the mammalian myocardium ( Tables 21.2 and 21.3 ) .
| Myocardial regeneration |
|---|
| Proliferation and regeneration of cardiomyocytes |
| Transdifferentiation of stem cells into cardiomyocytes |
| Fusion of stem cells with mature cardiomyocytes followed by cell division |
| Vasculogenesis (new blood vessels formed by stem cells) |
| Paracrine effects by factors produced and released from stem cells |
| Increased contractility of native cardiomyocytes |
| Activation of secretory and (possible) proliferative capacities of cardiomyocytes and cardiac stem cells |
| Angiogenesis (proliferation of blood vessels induced by factors produced by stem cells) |
| Increased healing of infarcted myocardium |
| Enhanced remodeling of ventricle subject to stress or infarction |
| 1. Definition of CMC renewal The ability to replace lost CMCs by new ones; distinct from turnover of cardiac proteins or generation of polyploid or binucleate CMC |
| 2. Naturally occurring CMC renewal and proliferation |
| A. During normal mammalian development |
|
| B. After cardiac injury in adult mammals |
|
| C. After heart or bone marrow transplantation (chimerism) |
|
| 3. Mechanisms of endogenous CMC renewal |
| (There is no infallible means of tracking cell renewal in any organ system. However, in experimental models of CMC renewal in mouse and fish, genetic fate-mapping studies provide the strongest level of scientific evidence, but require rigorous attention to critical components of the methodology and controls). |
| A. Cardiomyocyte proliferation |
| 1. The majority of studies suggest that CMC renewal in the uninjured adult heart derives from a modest level of preexisting CMC mitosis. |
| B. Resident stem/progenitor cells |
| i. Resident stem/progenitor cells contribute to multiple cell types within the ventricle, including CMC. However, estimates based on genetic fate-mapping in rodents suggests their contribution under basal conditions or after cardiac injury is low, at a rate of <0.01% per year. |
| C. Extracardiac stem/progenitor cells |
|
| 4. Therapeutic manipulation of cardiomyocyte renewal |
| Variable results with a spectrum of magnitude of effects have been reported with several types of cell preparations and protocols involving: |
| Bone marrow-derived cells |
| Cardiac-derived stem/progenitor cells |
| Pluripotent cells |
| Stimulated endogenous CMC preparations |
After robust embryonic proliferation of immature CMC, the ensuing early postnatal period in the mammalian myocardium is characterized by a rapid decrease in CMC replication—to virtually nondetectable levels based on standard histological examination. This is a classic manifestation of terminal differentiation , where a precursor cell formerly capable of cell division permanently leaves the cell cycle, dismantles the cell cycle machinery, and typically differentiates to express a range of genes characteristic of the cell’s final function (e.g., myosin and actin for a muscle cell). The cytology of proliferating and differentiating CMC has been well characterized . Thus the mature mammalian heart appears to contain a population of CMC that remain relatively constant during the life of the organism (unless lost due to injury or age). This is the basis for the paradigm developed in the 20th century that the mature mammalian heart is a four-chambered pumping organ composed of terminally differentiated CMC with supportive fibrovascular stroma.
Comparative anatomic studies have determined the following general characteristics of the mammalian heart: (1) the ratio of heart weight to body weight is species specific; (2) the number of all myocardial cells (CMC and nonmyocytes) varies directly with the size of the heart (rat heart, 9 × 10 7 ; human heart, 1.4 × 10 10 ; blue whale heart, 2 × 10 13 cells); (3) ventricular CMC of various species have similar mean diameter and size irrespective of the size of the hearts; (4) for most species, the ratio of CMC to nonmuscle cells is 25%:75% by number of cells and 75%:25% by volume of myocardium; (5) the ratio of CMC to capillaries is 1:1; and (6) heart size is related to physical activity, with relative heart size being larger for active versus sedentary species, wild versus domesticated strains, and strains bred for speed versus normal strains . The human heart is estimated to contain some 10–20 billion CMC, and young adults from 17 to 30 years of age have an average of 6.0 × 10 9 and 2.2 × 10 9 CMC in the left and right ventricular myocardium, respectively ( Fig. 21.3 ) The quantity of CMC appears to remain stable in adult life until a decrease is seen in the hearts of the elderly.
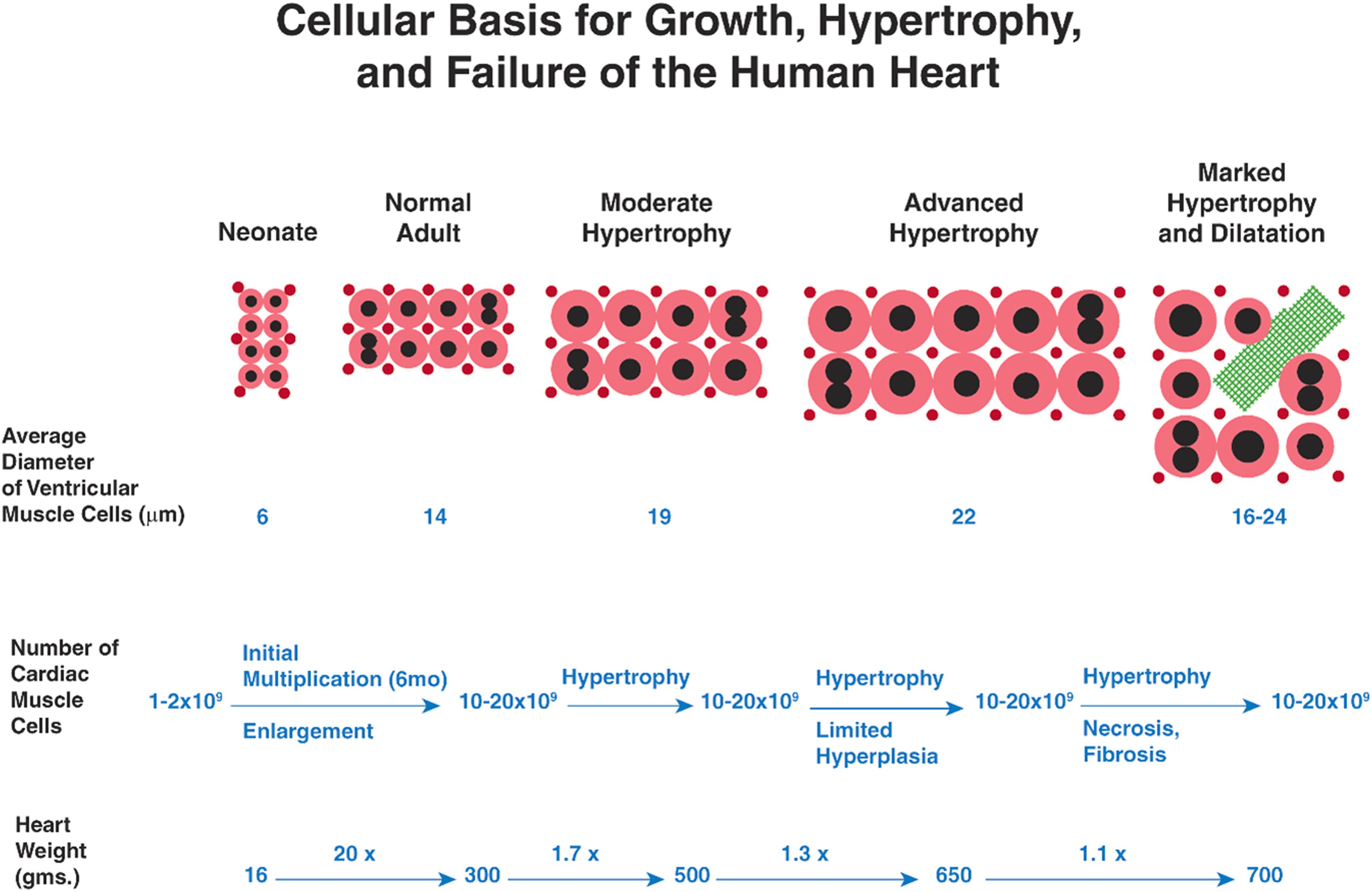
The population of CMC, and the one-to-one ratio of CMC to capillaries, remains stable during postnatal physiological growth of the mature heart and also during physiological and pathological enlargement in response to external stimuli . Terminally differentiated CMC are metabolically active, with ongoing degradation, synthesis, turnover, and repair of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), subcellular proteins, and organelles to maintain viability and to sustain contractile activity. Importantly, contemporary research has demonstrated that terminal differentiation of CMC is not absolute—allowing for the possibility of modulation to effect repair and possible regeneration.
Cardiovascular disease occurs when myocardium can no longer adapt to stressors (e.g., pressure or volume overload), with deterioration of cardiac function leading to progressive heart failure. In classic studies in the 1960s and 1970s, pathologists Linzbach and his colleague, Hort concluded that chronic cardiac stress results in progressive myocardial hypertrophy that eventually exceeds the capacity of the coronary vasculature to adequately perfuse the myocardial mass ( Fig. 21.3 ). This pathophysiological process leads to myocardial ischemia, particularly in the subendocardium, with associated myocyte necrosis, “slippage” of CMC, foci of replacement fibrosis, and “structural dilatation.” The process manifests as cardiac hypertrophy exceeds a “critical heart weight” of 500 g (critical left ventricular weight of 200 g). In contemporary terms, this fixed structural dilation represents the end result of a complex process termed myocardial remodeling . Interestingly, in hearts with advanced hypertrophy, Linzbach also determined that the size, that is, the cross-sectional diameter, of CMC was less than expected, and that the number of CMC per cross-sectional wall area was greater than expected. He concluded (incorrectly) that cardiomyocyte hyperplasia must be occurring (as opposed to eccentric cardiomyocyte remodeling) as part of the response leading to fixed structural dilation in hearts with eccentric hypertrophy. Linzbach did not observe mitotic figures and concluded that the cardiomyocyte hyperplasia must be due to the longitudinal cleavage of CMC, an observation which also could be explained by the lateral growth of side branches of existing CMC. Although the cellular and mechanistic basis for cardiomyocyte hyperplasia was speculative, Linzbach’s work, nevertheless, was influential in renewing the intellectual interest in the potential for cardiomyocyte proliferation and renewal in the mammalian heart.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here