Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The gastrointestinal (GI) tract performs the essential function of receiving, digesting, and absorbing nutrients from our diet. Digestion is the mechanical and chemical enzymatic breakdown of the food we consume. Absorption is the mobilization of digestion products across the gut to the bloodstream, from where they are destined to be utilized. The digestive system is also equipped to recycle the significant volume of fluid generated by the aerodigestive system, which is rich in vital electrolytes, proteins, and bile acids, thereby minimizing waste in feces. Our gut is also home to a microbiome consisting of bacteria, viruses, and yeasts. There are an estimated 100 trillion microorganisms in the human body, outnumbering human cells by a factor of 10 8 . , The microbiome genomes encode around 3 million different genes, a genetic pool dwarfing that of humans. The activity of our GI flora, its interactions with our immune system, and its impact on various health and disease states have become an area of increasing focus. Our digestive physiology is a fascinating reflection of our human evolution and a product of the environmental and dietary challenges faced by our ancestors.
An alteration in the physiology of the GI system, whether congenital or acquired, can result in significant morbidity and mortality. A better understanding of the various aspects of digestion and absorption can enable us to better appreciate how disease states occur and how to manage them appropriately. Since the discovery of the sodium-glucose cotransport mechanism in 1960, the use of both molecules in oral rehydration solutions was widely successful in decreasing mortality from acute diarrhea, which had resulted in 5 million deaths annually. , This chapter provides an overview of the basic aspects of digestion and absorption.
The main components of our diet are water, electrolytes, carbohydrates (CHO) (starches and sugars), proteins, fats, nucleic acids, vitamins, and minerals. These nutrients are in the form of complex structures that need to be digested into smaller molecules in order to be absorbed from the gut lumen, through the intestinal barrier layers, into the enterocyte, and subsequently into the body circulation. Absorption requires the molecules to be small enough in size, able to move down (or in some instances against) their concentration gradient, soluble in an aqueous or lipid medium, and charge-compatible (depending on the barrier to be crossed). Chewing and churning allows for the mechanical breakdown of large, complex food particles into smaller pieces with larger surface area for better exposure to enzymatic action. In addition, intestinal folding down to the villus and microvillus level allows for a wide absorptive surface area. Digestion and absorption processes are energy consuming and our metabolic rate increases after a meal.
The mouth and pharynx constitute the gateway to the GI tract and play a critical role in the initial mechanical (i.e., chewing) and enzymatic digestion of food. Salivary components moisten the food to ease swallowing and also protect the mouth against pathogens, irritants, and toxins. Saliva contains amylase, which initiates the digestion of starch. The esophagus then propels solids, now in puree form, and liquids from the hypopharynx to the stomach. The lower esophageal sphincter helps to prevent gastric contents from freely refluxing back into the esophagus.
The ingested meal is temporarily stored in the stomach, allowing gastric and salivary fluids and their enzymes to blend through the peristalsis-driven churning action. The end product is a thick liquid: chyme. The enzymes only partially digest proteins, CHO, and fats in the stomach. Salivary amylase can partially digest starch in the stomach until the acidity of gastric contents inactivates the enzyme. Antral grinding further breaks up any residual soft food particles to a size small enough (2 mm) to traverse the pylorus. Pepsinogen is secreted by fundic chief cells and cleaved in the gastric lumen to yield pepsin, which incompletely hydrolyzes protein to smaller polypeptides. The stomach secretes hydrochloric acid from parietal cells to keep the pH less than 4 during digestion, which is optimal for the activity of pepsin and absorption of iron and calcium. Parietal cells in the gastric fundus also produce intrinsic factor, which is essential for vitamin B12 absorption in the ileum (after it combines with the vitamin in the proximal small bowel). Gastric lipase, secreted by fundic chief cells, is active at acidic pH. Its lipolytic activity is less than 20% of pancreatic lipase but may become important in compensating for loss of pancreatic lipase in pancreatic insufficiency. The emptying of gastric contents through the pylorus is regulated by duodenal feedback to avoid overwhelming the digestive and absorptive processes in the small bowel. Acidic, fatty, and hypertonic chyme in the proximal small intestine inhibits antral motility. This inhibition is mediated by hormones released from small intestinal mucosa and mucosal receptors, which trigger vagal afferent fibers.
The essential function of the small bowel and colon is to digest chyme and absorb water, electrolytes, and broken-down nutrients ingested in one’s diet, in addition to retrieving bile salts and vitamins via the enterohepatic circulation. The small intestine is where chyme is mixed with the various digestive juices and propelled distally. The digestive enzymes of the pancreas and enterocytes digest luminal contents to absorbable small molecules, which are then transported into the enterocytes by active and passive processes. The duodenum and upper jejunum render their initially molecule-rich, hypertonic contents isotonic (290 mOsmol/kg) with plasma by having loose tight junctions (TJs), resulting in a relatively permeable epithelium, and by coupling the absorption of sodium with absorption of nutrient monomers. Normally, most nutrients are absorbed in the duodenum and proximal half of the jejunum. However, when proximal absorption is impaired due to duodenojejunal disease or resection, the distal jejunum and ileum increase their absorptive capacity to minimize malabsorption. The right colon absorbs salt and water and recovers nutrients not absorbed in the small bowel. These nutrients are first fermented to absorbable short-chain fatty acids by anaerobic colonic bacteria. The left colon further extracts salt and water to produce formed stool.
CHOs account for around 50% of the ingested calories in the Westernized adult diet. They come in three forms: simple sugars, starch, and fiber. The forms of consumed CHOs change with age as an infant’s intake evolves beyond breast milk or formula. The dominant consumed dietary CHOs ( Table 2.1 ) are monosaccharides (fructose) and disaccharides (mainly maltose, lactose, and sucrose) and the plant starches amylose and amylopectin, which are the dominant form of plant CHO storage. Consumption of high fructose corn syrup in sweet beverages has certainly increased. Our intake of animal starch (glycogen, a glucose polymer) is limited. Some CHOs such as cellulose cannot be broken down in the human body (see Nondigestible Carbohydrates later in this chapter).
| Disaccharides | Sucrose | Glucose + Fructose |
| Lactose | Glucose + Galactose | |
| Maltose | Glucose + Glucose | |
| Plant starch | Amylose | Glucose (α1,4 bonds) |
| Amylopectin | Glucose (α1,4 and α1,6 bonds) | |
| Animal starch | Glycogen | Glucose (α1,4 bonds) |
| Nondigestible CHO | Cellulose, hemicellulose, lactulose, sorbitol, sucralose | Various |
Lactose is the main CHO in breast milk and standard cow’s milk–based infant formula. Soy-based formulas and hypoallergenic formulas are lactose free and instead contain corn syrup (maltose and larger oligosaccharides), starch, or sucrose. Fructose imparts the sweet taste of fruits and vegetables. Fructose and glucose are the main ingredients in honey. Compared to glucose and sucrose, we perceive the fructose sweetness earlier after consumption and with more intensity. In addition, fructose can enhance the flavor and color of other food components. High fructose corn syrup differs from corn syrup in that glucose molecules are enzymatically converted to fructose in the latter, thus changing the taste and other properties. Sucrose (table sugar) is derived from cane or beets.
As infant weaning starts, the amount of consumed starch (amylopectin > amylose) increases to 50% of the total CHO intake. Amylopectin is the larger molecule of the two (molecular weight 10 9 vs. 10 6 ), as its α1,6 bonds allow for branching of the polysaccharide units. Plant starch granules vary in size (e.g. potato > wheat > rice) and shape. Wheat is a unique form of starch in that the CHO component is encased in a protein shell. Differences in size and molecular packaging of the starch account for the variable degrees of digestion and absorption. , The mechanical breakdown of these molecules by chewing also affects such variables, whereas food processing and preparation may alter the susceptibility of the molecular bonds within starch to enzymatic digestion. ,
The breakdown of CHO takes place in the gut lumen as well as at the enterocyte membrane level ( Fig. 2.1 ).
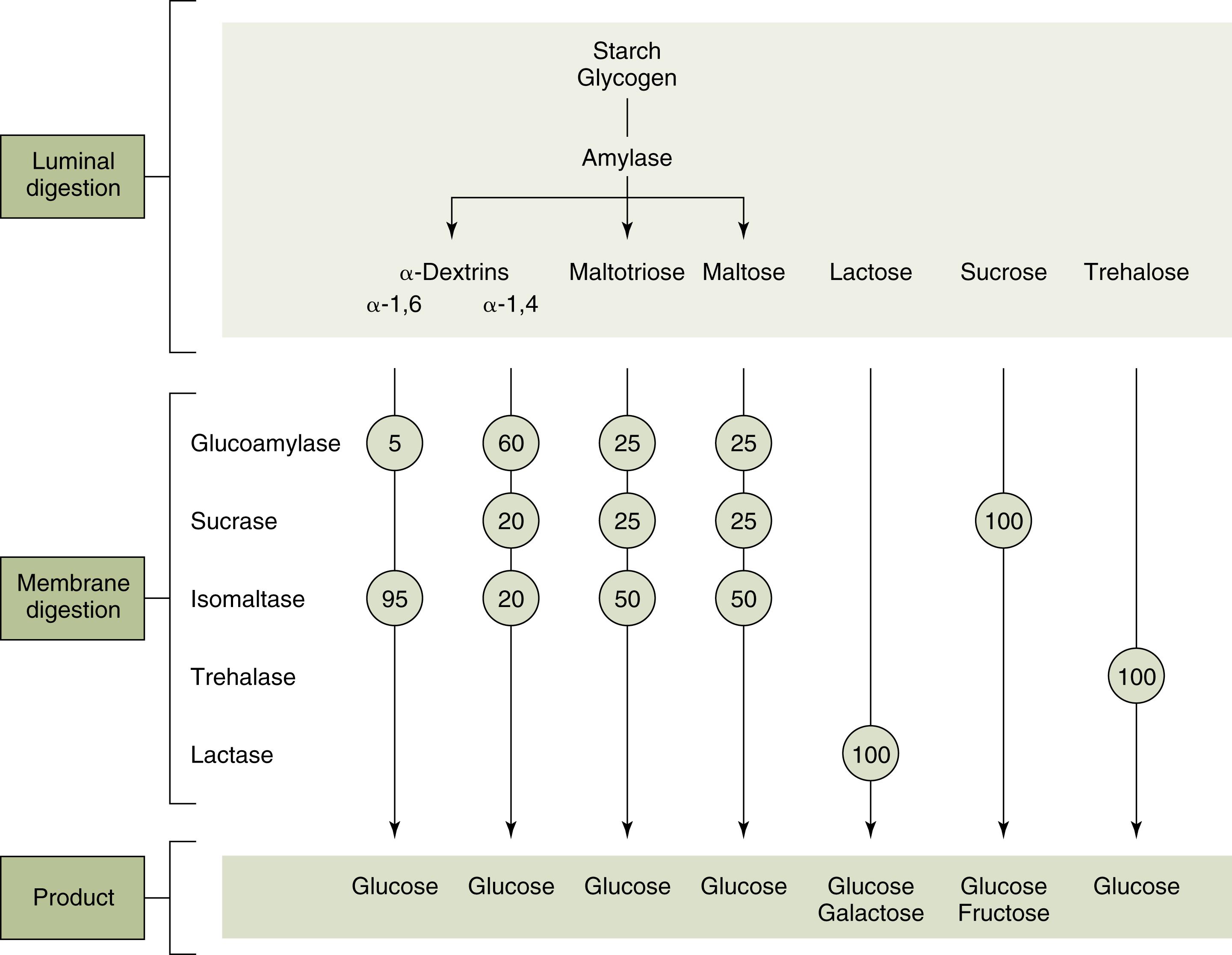
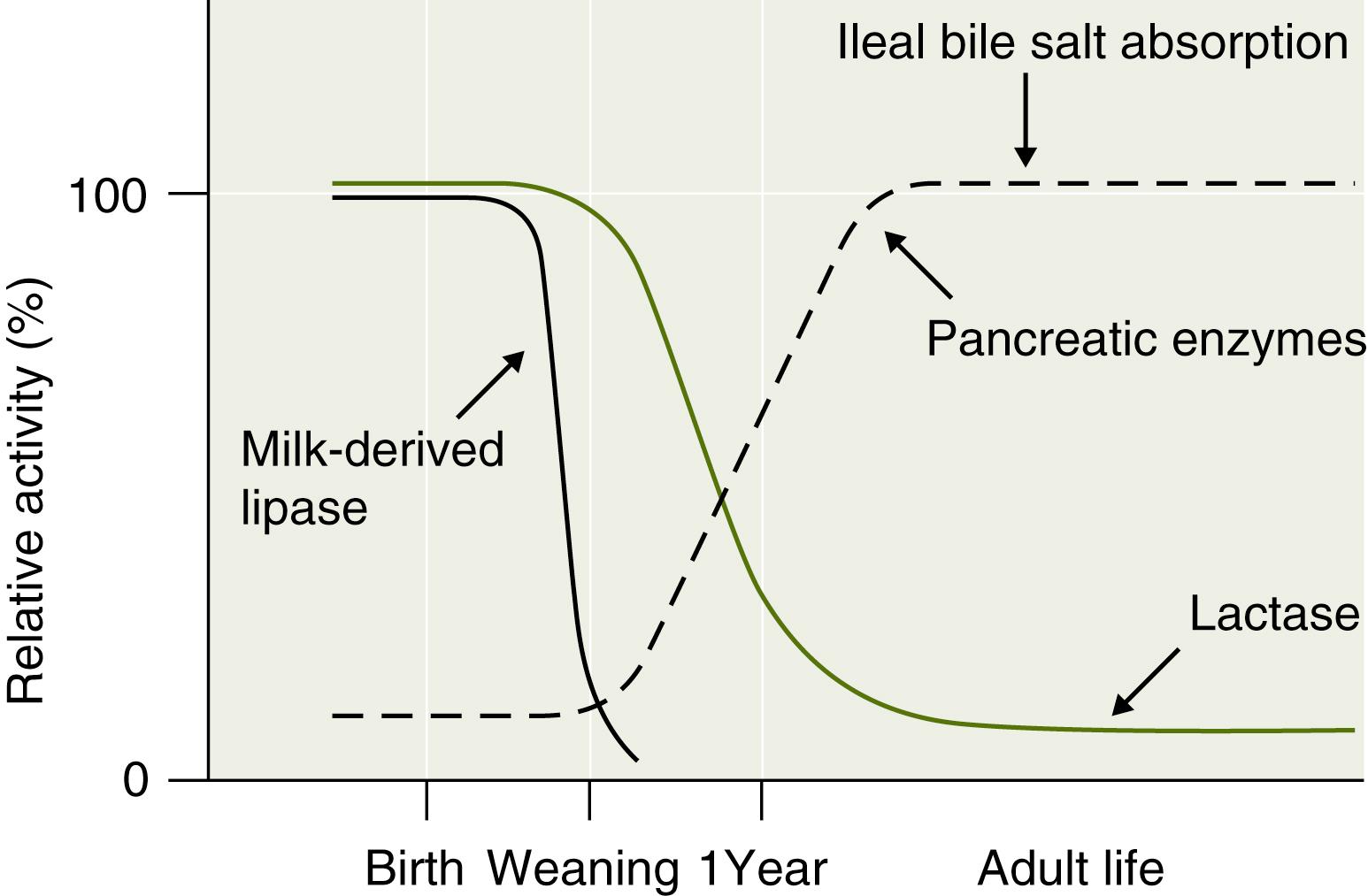
In the gut lumen, the dietary polysaccharides are hydrolyzed by salivary and pancreatic amylases, resulting in mostly oligosaccharides containing 5, 3, or 2 glucose molecules. The main enzyme that digests starch in the upper half of the small intestine is α-amylase, an endoenzyme that cleaves the α1,4 internal links in amylose and amylopectin, leaving oligosaccharides: maltose (two glucose molecules) and maltotriose (three glucose molecules). Only severe pancreatic insufficiency that leaves less than 10% normal amylase levels can affect starch breakdown. Amylase is also present in breast milk and plays a more significant role in premature neonates, in whom pancreatic amylase production is low ( Fig. 2.2 ). Since only monosaccharides can enter the enterocyte, the oligo- and disaccharides must be hydrolyzed to monosaccharides by oligosaccharidases within the microvillus membrane (brush border).
Maltase (glucoamylase) breaks the α1,4 links in oligosaccharides (maltose and large maltose-containing oligosaccharides) that are 5 to 9 glucose molecules long. Isomaltase (also called α-dextrinase) acts as a debranching enzyme as it breaks α1,6 bonds. It functions in conjunction with sucrase ( Fig. 2.3 ), both having their genetic coding on chromosome 3. Sucrase breaks sucrose into glucose and fructose. Sucrase-isomaltase complex cleaves its substrate by a ping-pong bi-bi mechanism: the first enzyme reacts with one substrate to form a product and a modified enzyme. The modified enzyme then acts on the second substrate to form a final product, and then the original enzyme is regenerated. ,
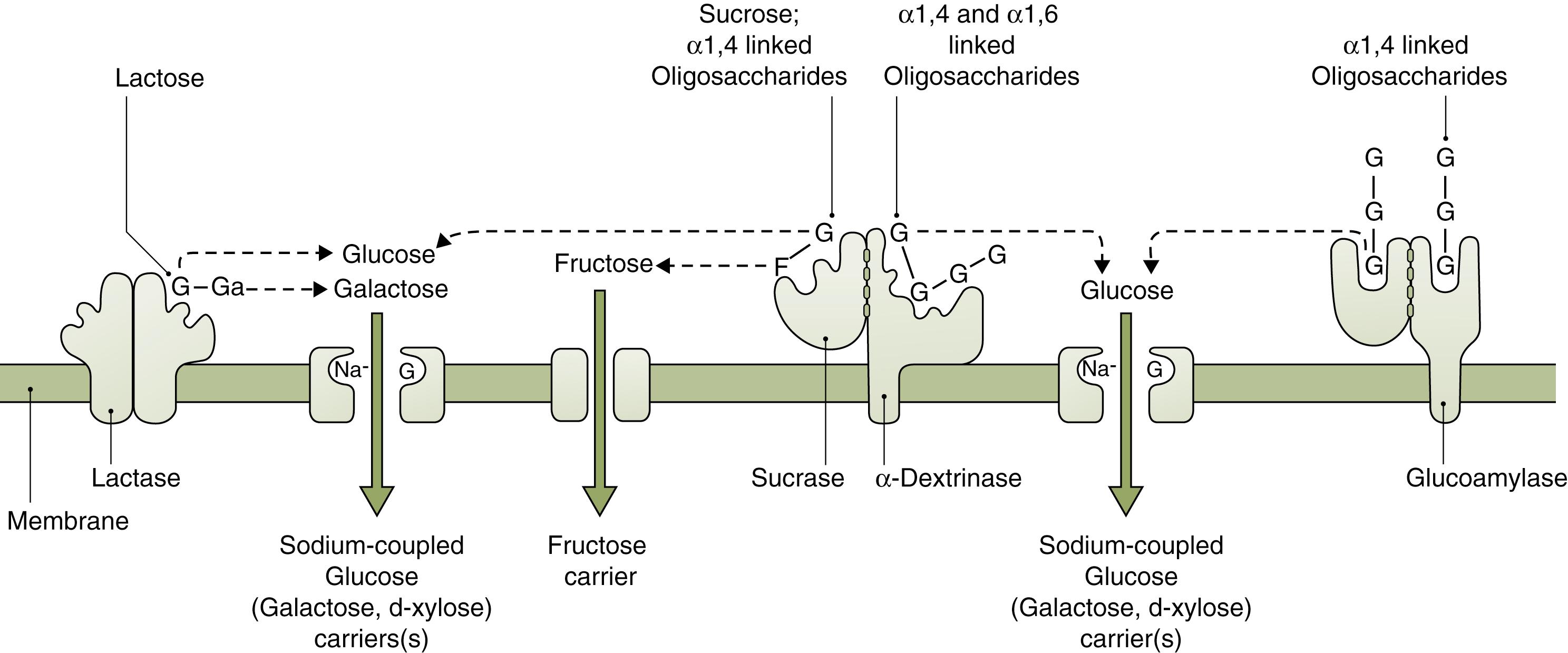
Lactase breaks lactose into glucose and galactose; its gene is located on chromosome 2. Lactose digestion in the premature neonate may be incomplete in the small intestine but partially salvaged through colonic fermentation. In childhood, lactase levels decline from a peak at birth to less than 10% as dietary lactose consumption falls with weaning (see Fig. 2.2 ). The decline in lactase in other mammals occurs even if weaning is prolonged. In certain human populations (e.g., Northern and Central European Caucasians), brush border lactase activity may persist. This phenotype is the result of a mutation that is inherited as an autosomal recessive trait, with intermediate activity levels in heterozygotes. Thus, the aberrant allele in the human population is considered to be the one that leads to persistence of the enzyme, not the deficiency. It has been hypothesized that lactase persistence, which allows more dairy-based vitamin D consumption, conferred an evolutionary advantage to northern latitude populations where limited sun exposure could compromise the body’s vitamin D levels. In such individuals, temporary lactase deficiency often occurs following injury to the intestinal mucosa (e.g., following viral or bacterial infections).
One of the brush border enzymes is trehalase, which breaks down the disaccharide trehalose, a sugar present in high concentration in insects and fungi. Interestingly, since trehalose is for most not a significant dietary component today, the presence of trehalase might offer a hint about the CHO-abundant sources in the diet of ancient human.
Disaccharidases are synthesized in the endoplasmic reticulum of the enterocyte, modified in the Golgi apparatus, and integrated into the brush border membrane, anchored by a hydrophobic portion in their structure. Pancreatic enzymes play a role in the modification and turnover of these enzymes. The half-life of sucrase-isomaltase drops from 20 hours during fasting to 4.5 hours after meals. There is generally an ample supply of disaccharidases; thus the rate of uptake of CHO monomers is the limiting step for their absorption. This implies that the quantity of the ingested CHO and the rate at which it is consumed and trafficked affect its absorption and potentially malabsorption. With the exception of lactase, brush border hydrolases are inducible by the presence of the substrate. Activity of mucosal carbohydrases is maximal in the duodenum and jejunum, decreasing distally along the small intestine. Most CHO digestion is complete by mid-jejunum.
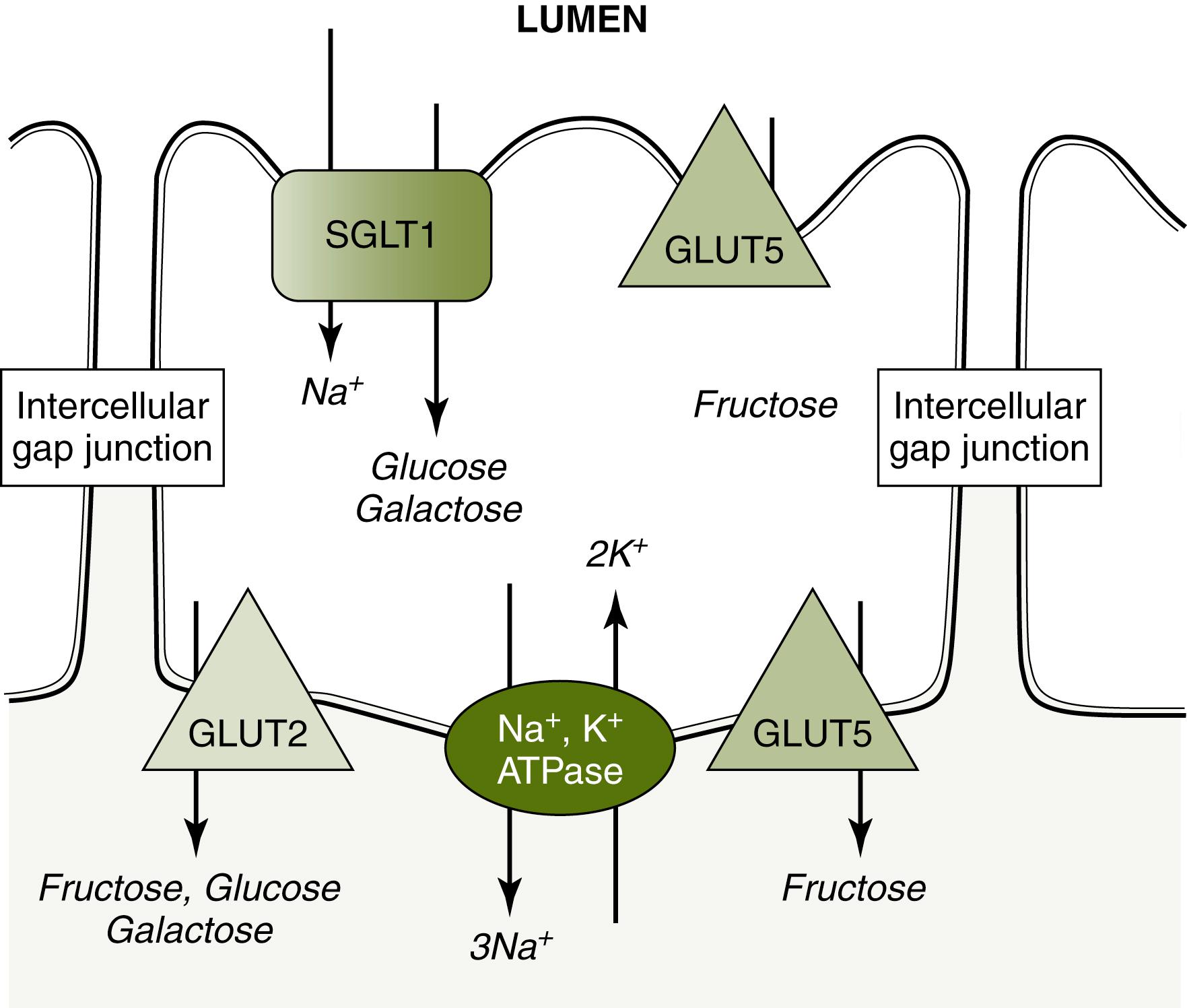
Monosaccharides cross the enterocyte apical membrane via carrier-mediated transport because they are too large to allow for adequate passive diffusion. There are two families of such transporters that are responsible for the movement of monosaccharides: sodium-coupled cotransporters sodium-glucose linked transporter (SGLT) (SLC5 gene family) and the glucose transporter (GLUT) (SLC2 gene family; Fig. 2.4 ). SGLT1 is located in the brush border membrane and cotransports glucose/galactose along with Na + down a sodium gradient generated by a Na + , K + ATPase pump in the basolateral membrane. GLUT5 is also in the brush border membrane and carries fructose from the lumen into cytosol. GLUT5 allows for fructose absorption by facilitated diffusion down its concentration gradient, which occurs at a faster rate than simple diffusion. It may also be present in the basolateral membrane. GLUT2 is within the basolateral membrane and transports fructose, galactose, and glucose from the enterocyte cytosol into the bloodstream by facilitated diffusion. Activation of the Na + -dependent cotransport protein allows water, electrolytes, and possibly smaller digested molecules (including glucose and oligopeptides) to pass into the intercellular space through relaxation of the tight junctions. , A small amount of hexoses may be utilized within the cell for metabolism.
Approximately 10% of ingested starch is not digested in the small intestine. Digestion-resistant starch includes complex molecules that resist amylase activity or are physically shelled and inaccessible, as in intact grains. Poor chewing of large digestible molecules may compromise enzymatic exposure. Some lactose and fructose may escape complete digestion or absorption and pass to the large intestine, along with other saccharides such as lactulose, sorbitol, and sucralose, which cannot be digested or absorbed in the human intestine. Cellulose and hemicellulose are present in fruits and vegetables. Cellulose is a polymer of glucose molecules linked by β1,4 bonds that, unlike α1,4 bonds, resist digestion by α-amylase. Hemicellulose is a polymer of pentose and hexose molecules in straight and chained forms. Resistant starches constitute dietary “fiber” together with nondigestible noncarbohydrate components present in the plant cell wall (e.g., phytates, lignins). Nondigestible CHOs are fermented by colonic bacteria, leaving short-chain fatty acids that are readily absorbed and may account for a minute caloric source in the healthy state, in addition to possibly having cellular trophic properties. By-products of this process are lactic, acetic, propionic, and butyric acids with methane and hydrogen accounting for flatus. Although excessive consumption of nondigestible CHO can result in undesirable GI symptoms, dietary fiber may offer multiple health benefits.
Proteins are essential for an array of enzymatic, immunologic, and structural functions within the human body. Dietary proteins are the primary source of amino acids and provide 10% to 15% of the energy intake in the average Western diet. The quality of dietary protein relates to its content of essential amino acids (valine, leucine, isoleucine, phenylalanine, lysine, tyrosine, methionine, tryptophan, and histidine) that cannot be synthesized in humans. Proteins from animal sources have a high content of essential amino acids. An egg, for example, has a high-protein biologic value because it is rich in essential amino acids. Plant proteins are less digestible than animal proteins and contain fewer essential amino acids. A cooked protein will have a simpler structure due to denaturation, which can be achieved through heat, mechanical pressure (e.g., pounding), or exposure to acid and sodium chloride. Coingesting the protein with reducing sugars such as fructose can alter its molecular structure and affect digestibility. , Proteins with high proline content (e.g., casein, gluten, collagen, and keratin) are incompletely digested by pancreatic proteases. , Other proteins that escape digestion include secretory immunoglobulin A (IgA) and vitamin B12 binding intrinsic factor. In addition to dietary protein, the GI tract recycles endogenous proteins in digestive juices (including enzymes, hormones, and immunoglobulins present in salivary, gastric, pancreatic, biliary, and jejunal secretions) and sheds epithelial cells and their proteins in an amount as much as 65 g daily in adults.
Digestion of proteins begins with gastric proteases, followed by pancreatic proteases. Unlike the digestive enzymes for CHO and lipids, proteases are secreted as proenzymes that require conversion to their active form for protein hydrolysis to occur. Gastric hydrochloric acid denatures protein and activates pepsinogen to pepsin. Pepsinogen is secreted by chief cells and acts as an endopeptidase, breaking peptide bonds within the polypeptide and leaving shorter polypeptides with a small number of free amino acids. Three pepsin isoenzymes have been identified, all optimally active at a pH range of 1 to 3. The duodenal alkaline medium irreversibly inactivates pepsin. Both pepsin and gastric acid production and secretion are stimulated by gastrin, acetylcholine, and histamine. The gastric phase does not seem critical in protein breakdown, because patients with decreased acid output and/or gastrectomy do not necessarily excessively lose protein.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here