Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Dr. Benditt is supported in part by a grant provided by the Dr. Earl E. Bakken family in support of heart-brain research.
The autonomic nervous system (ANS) controls multiple critical involuntary “background” functions in virtually all body organs. Some of these activities are controlled by central centers, whereas in other cases (e.g., gastrointestinal motility) there is substantial local autonomy. In any case, if moment-to-moment management of essential functions (e.g., heart rate [HR], respiration, body temperature, blood pressure [BP]) required conscious action, the effort would demand full committed attention, precluding accomplishing much else.
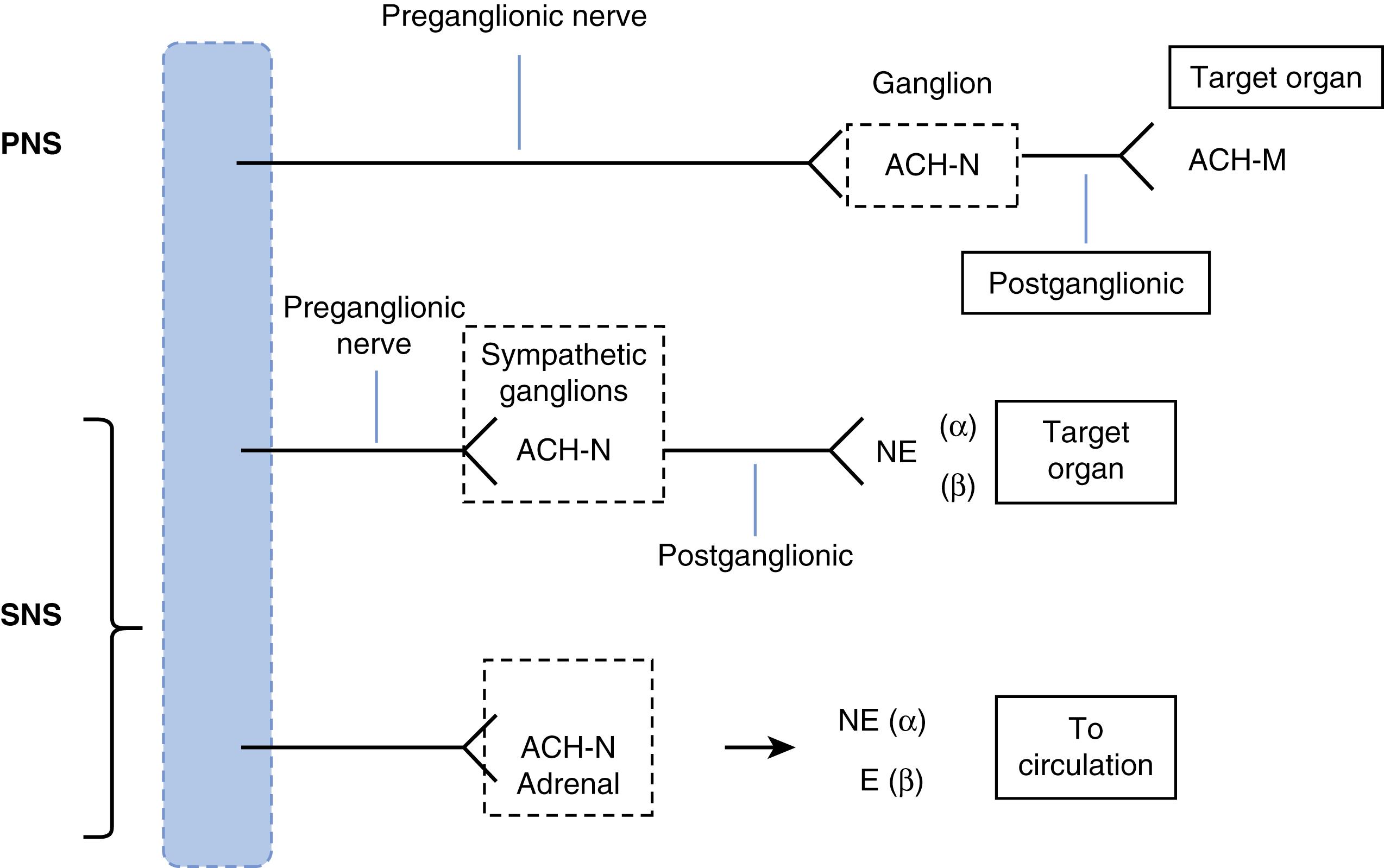
ANS cardiovascular system control is principally effected by vagal parasympathetic (parasympathetic nervous system [PNS]), sympathetic cardiac and vascular adrenergic (sympathetic nervous system [SNS]), and sympathetic cholinergic muscarinic sudomotor nerves ( Fig. 67.1 ); all of these use acetylcholine (ACh) for preganglionic transmission. The PNS nerves use ACh at all sites, whereas the SNS neurons use norepinephrine (NE) at postganglionic sites. However, although the PNS and SNS are the principal aspects of the ANS addressed here in the context of syncope and arrhythmia risk, the ANS is far more complex. It consists of multiple underappreciated specialized transmitters and modulators (e.g., dopamine, vasoactive intestinal peptide, serotonin, nitrous oxide, neuropeptide Y, and the purinergic transmitters adenosine and adenosine triphosphate).
This chapter examines various conditions in which the ANS is known to contribute importantly to either the occurrence of syncope/collapse, arrhythmia susceptibility, or both, recognizing that our understanding remains in its infancy.
SNS preganglionic fibers arise from the intermediolateral cell column of T1–L2 spinal cord segments with the majority passing to and synapsing with postganglionic neurons found in the paravertebral ganglia. The cervical sympathetic ganglia include the superior, middle, and inferior ganglia. The inferior ganglia fuse with the first thoracic ganglia on either side to form the stellate ganglia.
Postganglionic neurons from the superior, middle cervical, and stellate ganglia innervate the thoracic viscera including the heart, lungs, and great vessels, and the blood vessels and skin of the upper limbs. The upper four to five thoracic ganglia also innervate the thoracic viscera and blood vessels. Postganglionic fibers destined to innervate the thoracic viscera and blood vessels arising from these ganglia pass through cardiopulmonary nerves where they mix with similar nerves arising from the vagi. ,
Cardiovascular parasympathetic innervation is derived principally from both vagal nerves. The vagal preganglionic fibers arise from the nucleus ambiguous and dorsal motor nucleus of the vagus. These preganglionic fibers then travel through cardiac nerves arising from the recurrent laryngeal nerve (RLN) and from the vagus distal to the origin of the RLN and mix with post ganglionic fibers containing cardiopulmonary nerves arising from the paravertebral ganglia and the sympathetic trunk to form cardiopulmonary plexi that are distributed along the great vessels. Nerves arising from these plexi then form the dorsal and ventral cardiopulmonary plexi between the arch of the aorta and the pulmonary artery with nerves arising from these plexi being distributed to all ganglia of the intrinsic cardiac nervous system (ICNS).
The cardiac neuraxis is made up of essentially three levels : (1) central nervous system neurons including the preganglionic neurons in the medulla (parasympathetic) and spinal cord (sympathetic) and the modulating higher centers such as the thalamus/hypothalamus, prefrontal cortex, insular cortex, amygdala, and hippocampus; (2) intrathoracic extracardiac nervous system including paravertebral ganglia, vagus nerves, and cardiopulmonary plexi; and (c) ICNS including ganglionated plexi (GP) found in epicardial fat.
With regard to cardiac anatomic locations, GPs have consistently been described in relation to both the human atria and ventricles. Although neurons from each intrinsic cardiac ganglion are distributed widely to atrial and ventricular tissues bilaterally, these ganglia have their preferential spheres of influence with sinus node function primarily controlled by the superior right atrial (RA) GP, whereas atrioventricular (AV) node function is primarily controlled by the inferior RA GP, although some influence is also exerted by other atrial GPs. Further, the left stellate ganglion predominantly influences the lateral and posterior walls of the left ventricle (LV), whereas the right stellate ganglion predominantly influences the anterior wall of the LV.
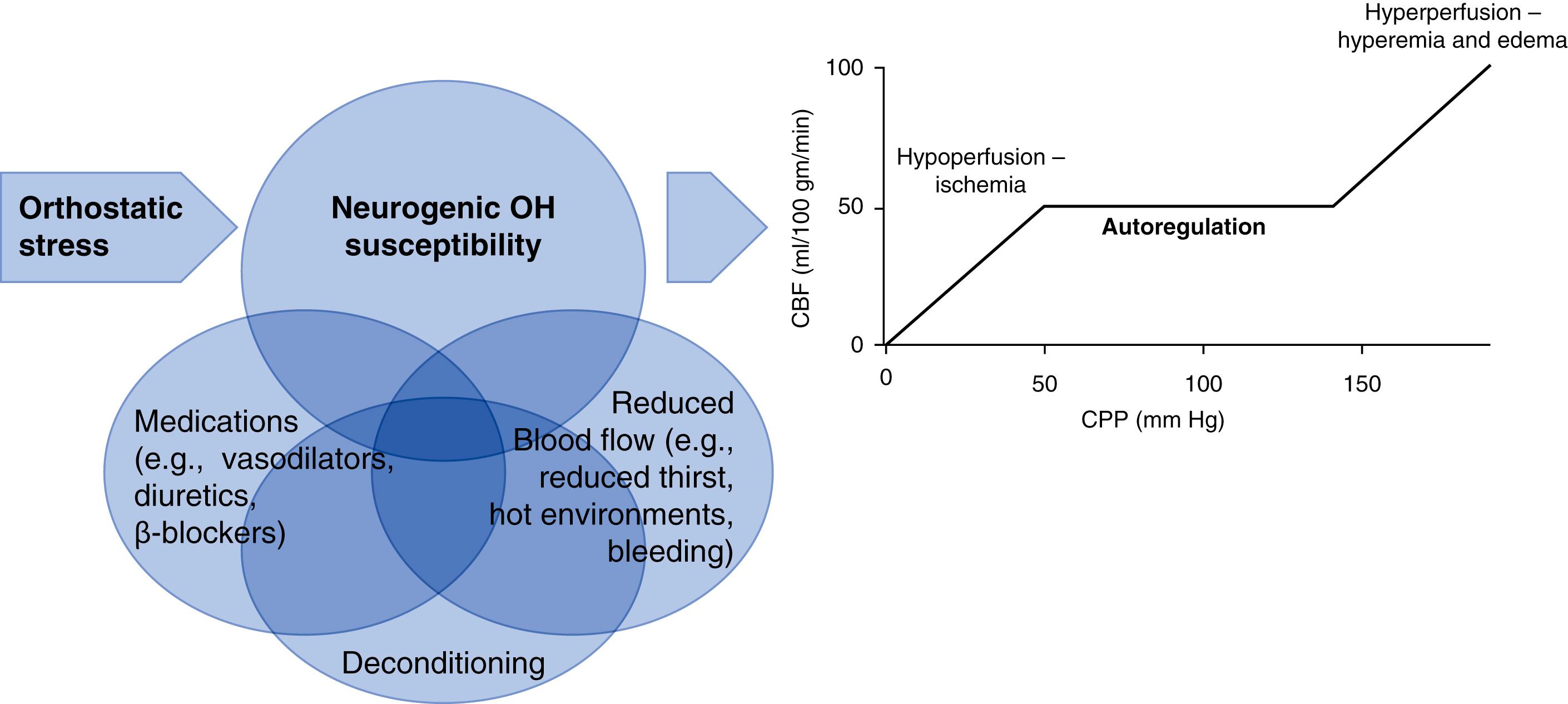
The ANS regulates beat-to-beat cardiac function to ensure cardiac output is able to meet cerebral blood flow (CBF) and metabolic needs. CBF is dependent on the difference between arterial and venous pressures, and on the balance between pressure within the lumen versus closing pressure imposed by both wall tension and tissue pressure. Within the skull, tissue pressure in the brain can increase dramatically and diminish CBF, such as often occurs because of cerebral edema in resuscitated cardiac arrest victims. In health, cerebral autoregulation allows CBF to remain relatively constant over a wide range of mean arterial pressures (MAPs), but the range may be altered by chronic disease (e.g., both hypertension and diabetes shift the curve to higher pressures). At MAP values below the lower autoregulatory limit, CBF falls off rapidly ( Fig. 67.2 ) and syncope or near-syncope may occur.
The ANS receives afferent information from the heart and vasculature via mechanosensitive and chemosensitive afferent neurons with mechanosensitive neurons contributing to short-term regulation of cardiac function (over seconds), whereas chemosensitive neurons provide longer-term regulation (minutes to hours). , , , Information from these afferent neurons is processed at all levels of the cardiac neuraxis giving rise to reflex loops with varied latencies. Information from baroreceptors (aortic and carotid) and chemoreceptors (central and peripheral) also contributes to the regulation of vascular tone and BP.
Evaluating ANS integrity and function clinically may be achieved in the clinical laboratory by application of various tests requiring approximately 1.5 to 2 hours to complete ( Table 67.1 ). The testing facility should be quiet and comfortable. The minimum necessary equipment includes continuous disclosure electrocardiogram (ECG) recorder and a noninvasive beat-to-beat BP recording system (not a sphygmomanometer). For selected cases an electroencephalogram (EEG) recorder should be available. All recordings should be archived.
| Test | Neural Component | Typical Method | Interpretation |
|---|---|---|---|
| Active standing | Baroreflex function HR response Adrenergic vascular constriction Muscle pump |
Quiet standing in front of a chair Max duration ≥10 min |
Chronotropic competence and orthostatic tolerance |
| Head-up tilt test | Baroreflex function HR response Adrenergic vascular constriction |
Head-up at 70 degrees Duration 20–30 min Loose protective straps |
Orthostatic tolerance and susceptibility to VVS |
| Valsalva maneuver | Baroreflex and adrenergic responses | 40 mm Hg for ∼15 seconds Measure HR and BP response and Valsalva ratio |
Baroreflex integrity Adrenergic vascular control integrity Limited by effort required |
| Respiratory sinus arrhythmia | Low-pressure atrial receptors Arterial baroreceptors |
Measure HR response on inspiration and expiration Difference typically >7 beats/min |
Baroreceptor function Sinus node intrinsic and neural control integrity Limited by effort required |
| Carotid sinus massage | Carotid sinus baroreceptor sensitivity | Vertical carotid massage for approximately 10 seconds on one side, then the other | Duration of cardiac pause (probably >5 seconds) Severity of vasodepressor hypotension |
| Heart rate variability | Cardiovagal function | Assess sinus rate variability over 24 hours by AECG | Evaluate vagal input predominance at sinus node |
| QSART | Assess sudomotor postganglionic axon reflex | Assess perspiration | Sweat response |
Testing usually focuses on three areas of essential ANS operation (see Table 67.1 ): (1) cardiovagal function, (2) adrenergic vasomotor function, and (3) sudomotor function (sweat testing). The first two are usually the most important for clinicians concerned with cardiovascular function.
Tilt-table testing, and to a much lesser extent, the active standing test, are the primary methods for assessing susceptibility to the vasovagal form of reflex syncope. Both tests can also be helpful to evaluate susceptibility to orthostatic hypotension (OH; particularly the delayed form), and postural tachycardia syndrome (POTS). However, testing may not be needed if the history is classic.
Heart rate variability (HRV), respiratory sinus arrhythmia (RSA), and Valsalva maneuver are also commonly tested, but they depend on the patient being in sinus rhythm with normal sinoatrial node function (unaffected by either intrinsic disease or drugs). HRV assesses variation of intervals between consecutive heartbeats, and this variation may be quantified using time domain or frequency domain analyses. A reduction in parasympathetic activity will result in an increased resting HR and a decreased HRV. Both have been associated with increased mortality risk, but HRV has received more attention.
RSA is primarily an indicator of parasympathetic cardiac control, and it can be measured by having the patient breathe at a fixed slow rate (usually approximately 6 breaths/min). In health, HR accelerates during inspiration and slows with expiration. The simplest measure of RSA is the difference between HR during inspiration and expiration. Normal values are age dependent (a difference of <5 beats/min is abnormal in all cases and <10 beats/min is abnormal in patients <40 years old). Abnormal RSA suggests ANS dysfunction, but predictive value for syncope/arrhythmia risk is unknown.
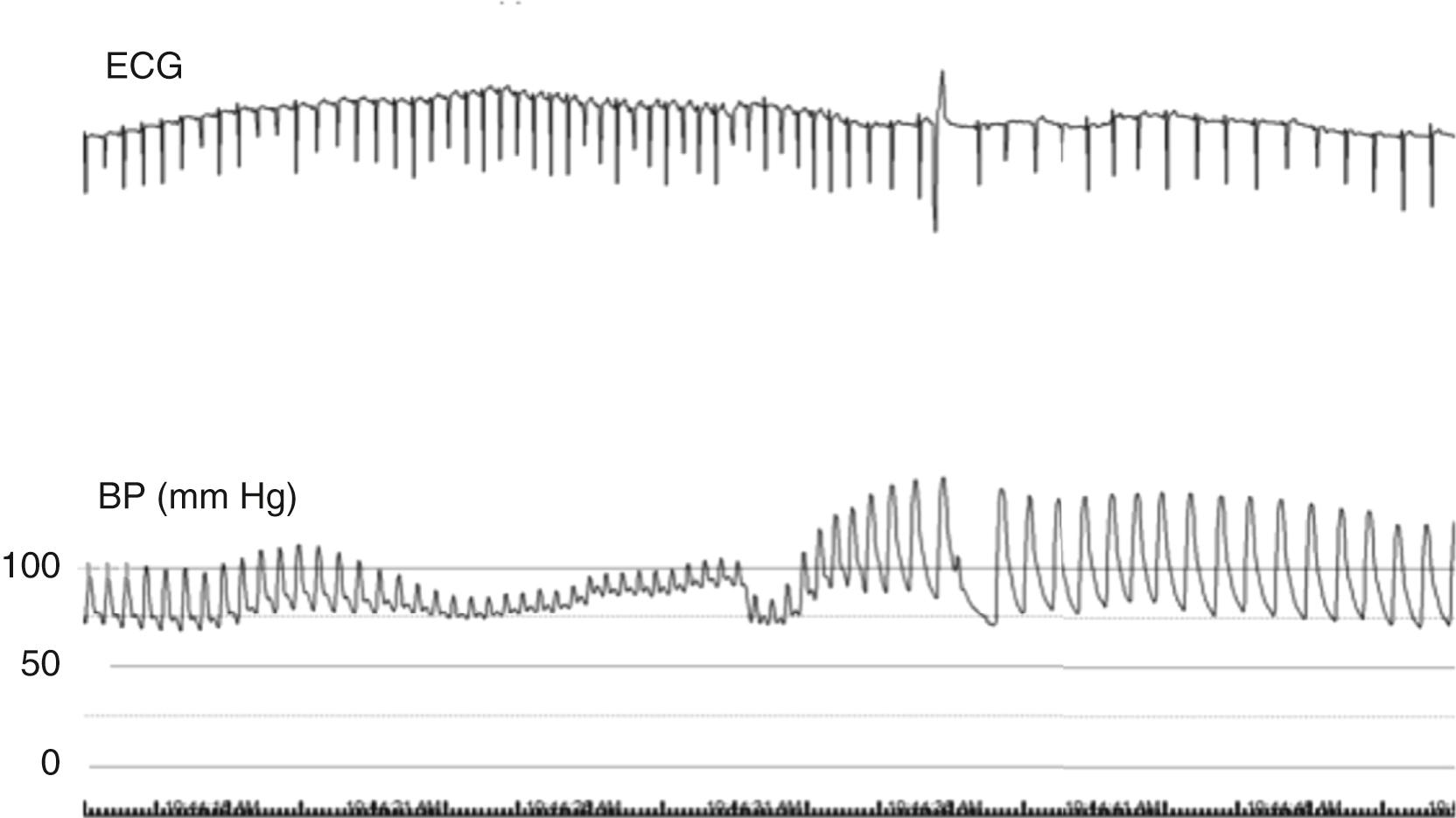
The Valsalva maneuver provides information on both sympathetic and parasympathetic function ( Fig. 67.3 ). Patients expire against a resistance of 40 mm Hg for ≈15 seconds while beat-to-beat HR and BP are recorded. A normal Valsalva response consists of four phases; the most important clinically are phase II, in which venous inflow to the heart is diminished and HR should rise, and phase IV, when return of cardiac output into a now sympathetically constricted circulation should result in a pressure overshoot and slowing of the HR. The Valsalva ratio is calculated by dividing the maximum HR during the expiratory phase by the minimum HR during the relaxation phase of the maneuver. A normal ratio is greater than 1.21. Values between 1.11 and 1.20 are borderline, whereas a ratio of 1.10 or less is deemed abnormal. Because patient effort is required, inadequate effort could lead to erroneous interpretation.
Sweat disturbances (increased or decreased) may occur in disorders affecting central sudomotor control (e.g., multiple sclerosis [MS], neurodegenerative syndromes) and in peripheral neuropathies (e.g., diabetes, paraneoplastic disorders). There are several tests of sudomotor function (sweating), and the quantitative sudomotor axon reflex test (QSART) is the most widely used , ; however, little is known of the utility of sudomotor testing in regard to syncope/arrhythmia risk assessment.
Additional specialized ANS testing procedures may be introduced as circumstances demand including cough, swallow, and laugh testing. Finally, if a seizure disorder or psychogenic pseudosyncope/seizure remain a diagnostic concern, then concomitant EEG recordings should be included. Interpreting the results of the battery of tests requires a physician familiar with ANS physiology and a neurologist with expertise in reading EEGs.
Reflex ANS disturbances are the most common causes of syncope and collapse across all age groups ( Box 67.1 ) and include, among others, vasovagal syncope (VVS), carotid sinus syncope (CSS), postmicturition syncope, cough syncope, and deglutition syncope ( Chapter 103 ). , A comprehensive discussion of these conditions is beyond the scope of this book; however, each condition is made up of two components, cardioinhibition and vasodepression. The clinician must ascertain to what extent these elements contribute to the patient’s hypotensive presentation because the choice of treatment strategy depends on that understanding; in most cases both elements participate.
Reflex faints
Vasovagal syncope, carotid sinus syndrome
Situational reflex faints (e.g., postmicturition, cough, deglutition)
Pure autonomic failure
Parkinson disease and Parkinson plus orthostatic hypotension
Multiple system atrophy
Dementia with Lewy bodies
Immune autonomic neuropathy
Dopamine β-hydroxylase deficiency
Diabetic neuropathy
Autoimmune ganglionopathy
Transthyretin amyloidosis
Alcohol abuse
Paraneoplastic syndromes
Toxins
Postural orthostatic tachycardia syndrome (syncope is not a common manifestation)
In general, in the setting of reflex faints, underlying ANS function is normal, although there remains some debate in regard to CSS. VVS is by far the most frequent of all causes of syncope. In VVS, the history provided by the patient and/or witnesses usually is sufficient to establish the diagnosis. However, in some cases (especially in older patients), the history alone may be inadequate. As noted previously, tilt-table testing may be helpful to establish the diagnosis, and more importantly to confirm for the patient that the physician has witnessed the patient’s symptoms and so is better able to understand and treat the patient’s problem. The active standing test may be used as well, but its utility in this setting is less well established and the test is currently not advocated. , Neither test can be used to assess the risk of future VVS recurrences.
For suspected CSS, carotid sinus massage (CSM) can be undertaken safely by experienced clinicians. The methodology is well described in practice guidelines and is best undertaken with the patient in a head-up position. , In terms of outcome, the test cannot predict future CSS recurrences, but if the induced pause is longer than 6 seconds, the finding may favor cardiac pacemaker treatment in some subjects ( Chapter 103 ). However, as noted earlier, the additional contribution of vasodepression to symptomatic hypotension must be taken into account. Finally, specialized tests have been described for certain situational faints. Although such tests help understand pathophysiology and may also help by permitting the physician to witness one of the patient’s episodes, they cannot at this time be used for predicting risk of future recurrences.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here