Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Atrial tachycardia (AT) is an important entity in the differential diagnosis of narrow complex tachycardia. It is defined as an arrhythmia that originates in atrial tissue that does not encompass the sinus node. Although nonsustained asymptomatic episodes are frequently seen in routine Holter recordings, the prevalence of this arrhythmia is approximately 5% to 15% in patients referred for routine invasive electrophysiologic evaluation.
ATs are regular atrial rhythms at a constant rate of 100 beats/min or greater that originate in the atrium and do not require participation of the atrioventricular node (AVN) for maintenance. ATs constitute an important cause of supraventricular tachycardia. The mechanism of AT can be focal or macroreentrant. This chapter reviews information about the classification, mechanisms, electrocardiographic localization, and electrophysiologic characterization and invasive treatment of ATs.
An expert consensus group from the European Society of Cardiology and the North American Society of Pacing and Electrophysiology published a classification of atrial flutter (AFL) and regular ATs based on mechanism and anatomy. AT was classified as tachycardia with a regular atrial rate arising from the atrium and was further categorized as either focal or macroreentrant. Focal AT may be caused by automatic, triggered, or microreentrant mechanisms. Focal ATs are characterized by centrifugal spread of activation from a single focus or point source and lack of electrical activation spanning the tachycardia cycle length (TCL). Macroreentrant ATs are caused by reentry through relatively large, potentially well-characterized circuits that are typically greater than 2 cm 2 . Patterns that have been described include single loop, two reentrant loops creating a figure eight, and reentry through narrow channels adjacent to scars or natural anatomic barriers. Typical AFL, lower loop reentry, double-loop reentry, left atrial macroreentrant AT, scar-related and incisional AT, reverse typical AFL, and right atrial free wall macroreentry are classified as macroreentrant ATs (see also Chapter 78 ). Reentrant arrhythmias can also be microreentrant and the circuit can be as small as a couple of millimeters. Microreentrant arrhythmias are characterized by electrograms that span most of the tachycardia circuit in a very small area.
Under this classification scheme, a few ATs are left unclassified. For example, inappropriate sinus tachycardia and sinus node reentry cannot be easily classified. Despite the limitations of this classification scheme and the fact that most clinical arrhythmias are not classified by mechanism, it is useful to think of ATs as being either focal or macroreentrant. This differentiation has important implications for ablative therapy, because macroreentrant ATs often require mapping large segments of the circuit using techniques of “entrainment mapping” or “scar mapping” and delivery of multiple radiofrequency (RF) lesions for termination of tachycardia.
One of the first steps in the clinical diagnosis of these arrhythmias is to review the 12-lead electrocardiogram (ECG). A careful analysis will clarify the atrioventricular (AV) relationship and provides important information about the origin of the arrhythmia from the P wave morphology. In patients who have not undergone previous ablation or surgical procedures, a distinct isoelectric interval between the P waves (atrial diastole) is suggestive of a focal arrhythmia. In focal AT the total atrial activation time is generally shorter than the A-A interval; hence, the isoelectric interval represents an electrically silent isoelectric period without any atrial depolarization. In contrast, throughout a macroreentrant atrial rhythm some part of the atria is depolarized resulting in the absence of an isoelectric interval. However, surface ECGs are notoriously inadequate for identifying the mechanism of AT.
Sustained, focal ATs arise frequently in the absence of significant, preexisting structural heart disease, at any age with a greater incidence in the middle age with no gender predilection. They originate most commonly from the right atrium (RA) and a second focus can be found in 10% to 15% of patients.
Atrial arrhythmias frequently complicate heart failure and atrial enlargement. The autonomic nervous system likely plays a critical role in initiating or triggering some ATs. ,
Focal ATs may be caused by abnormal automaticity, triggered activity, or microreentry. Precise characterization of the mechanism of ATs might be a difficult exercise, but understanding the principles may help with certain therapeutic decisions. The electrophysiologic features of these causative mechanisms are summarized in Table 71.1 .
| Arrhythmia Mechanism | Clinical Features |
|---|---|
| Abnormal automaticity |
|
| Triggered activity |
|
| Macroreentry |
|
| Microreentry |
|
Studies to categorize the mechanisms of focal ATs based on their responses to pacing and pharmacologic maneuvers are difficult to perform and interpret and, given the lack of definitive criteria to identify the mechanism of focal AT in the electrophysiology laboratory, these studies remain largely descriptive and are rarely performed today. Further complicating appropriate mechanistic classification of focal ATs, programmed stimulation may initiate and terminate ATs caused by triggered activity and microreentry. Use of newer, multipolar catheters with closely spaced electrodes has allowed very high density omnipolar mapping recordings in small regions of interest. In combination with three-dimensional (3D) electroanatomic mapping, these catheter mapping techniques are shedding new light on the role of microreentry versus nonreentrant mechanisms in focal ATs. Recent studies with ultra-high-density mapping have shown that localized atrial reentrant (microreentrant) circuits may have multiple sequential zones of very slow conduction and occur in low-voltage areas of the atria. Additionally, observations with ultra-high-density mapping have also suggested that microreentry circuits can span just a few millimeters, as opposed to the traditional descriptions of less than 2 cm ( Fig. 71.1 ). The most frequent sites of origin of AT are depicted in Fig. 71.2 .
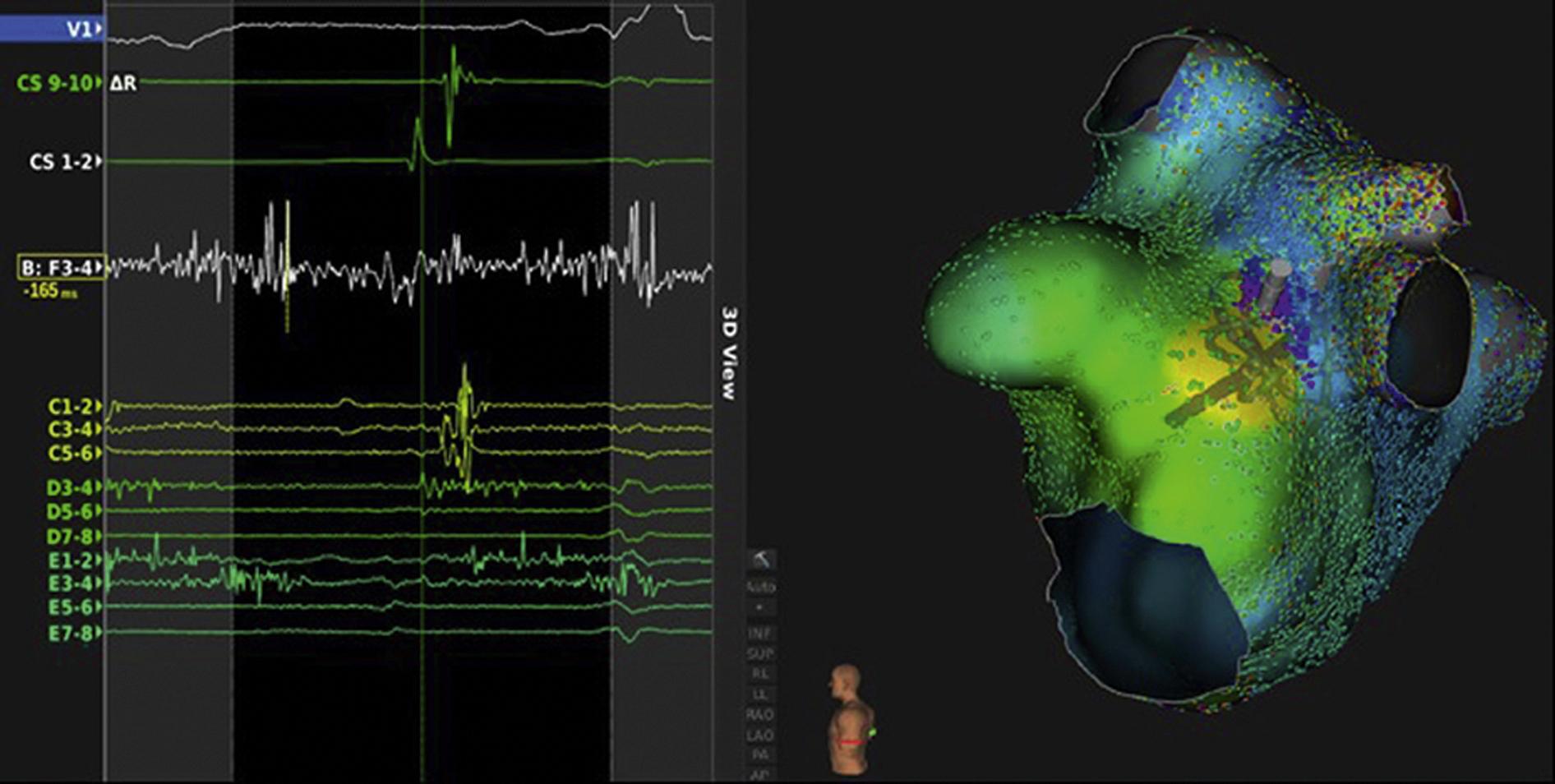
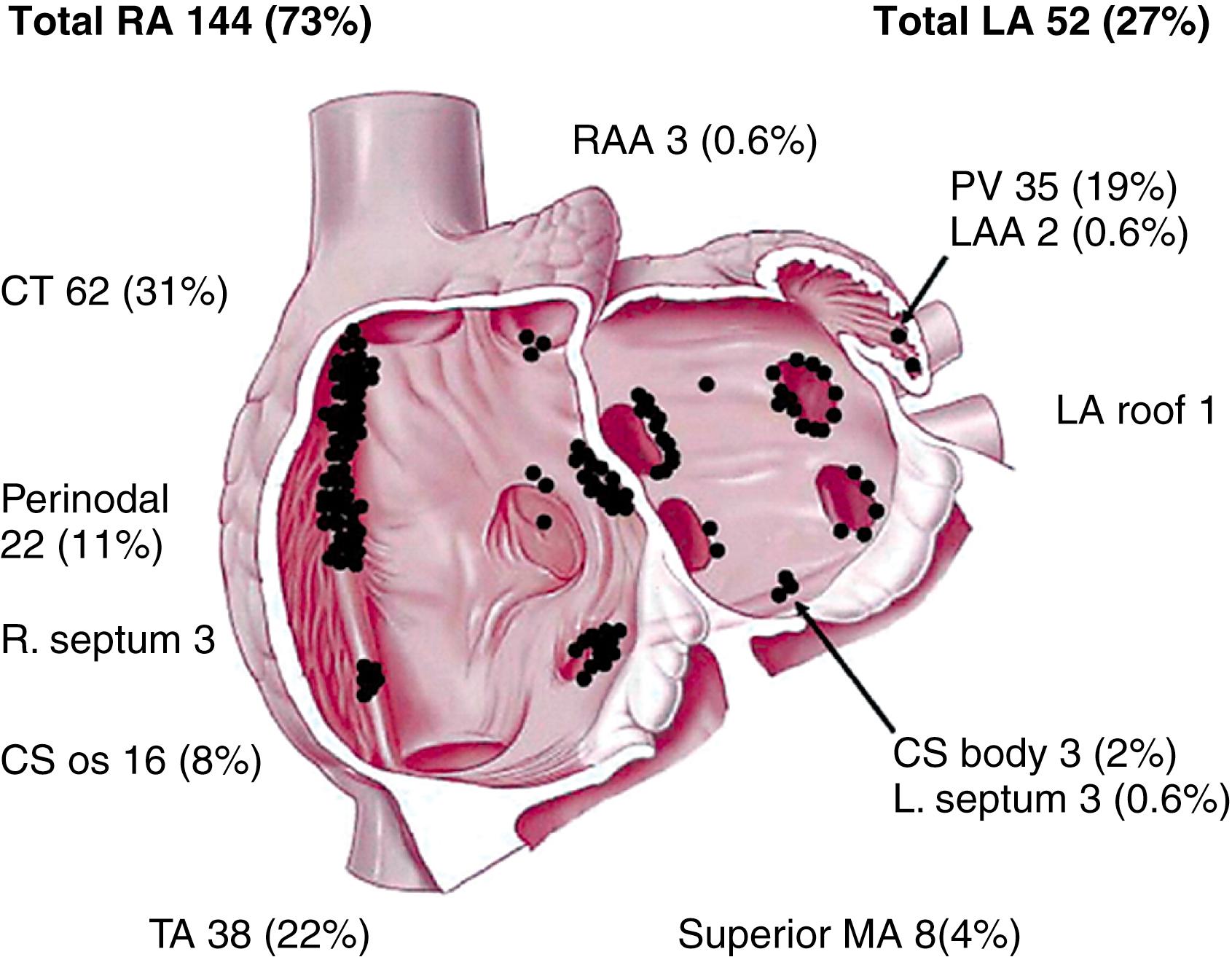
AT foci have a characteristic anatomic distribution. The predominant areas of origin of focal AT include the crista terminalis, near or inside the pulmonary veins (PVs; superior veins more commonly), around the AV annuli, around or inside the coronary sinus, and in the para-Hisian region (atrial septum and Koch’s triangle) (see Fig. 71.2 ). Less common sites of origin include the atrial appendages and the superior vena cava (SVC), atria adjacent to the noncoronary sinus of Valsalva (NCSOV), and aortic mitral continuity region.
The 12-lead ECG typically shows a long R-P tachycardia with a 1:1 AV relation, but 2:1 AV conduction and variable AV conduction is not uncommon exposing the fact that the AV node is not involved in the AT. In patients who have not undergone previous procedures, a distinct isoelectric interval between the P waves (atrial diastole) is suggestive of a focal arrhythmia and is consistent with a total atrial activation time that is generally shorter than the A-A interval on intracardiac mapping. Hence, the isoelectric interval represents an electrically silent isoelectric period without any atrial depolarization.
Characterization of the mechanism of AT based on electrocardiographic findings is fraught with limitations. Long isoelectric intervals between P waves are not uncommon in macroreentrant ATs when the zone of slow conduction occupies a disproportionate temporal fraction of the TCL while occupying a minute spatial component of the tachycardia circuit. This can cause an erroneous assumption of a tachycardia being focal when it is indeed macroreentrant.
There is extensive literature on using the P wave morphology recorded on the 12-lead ECG to identify the site of the atrial focus. The literature on P wave morphology dates back to early studies of differential pacing during electrophysiology studies and during open-heart surgery. Multiple algorithms have been developed and refined over the years to localize the site of origin of AT.
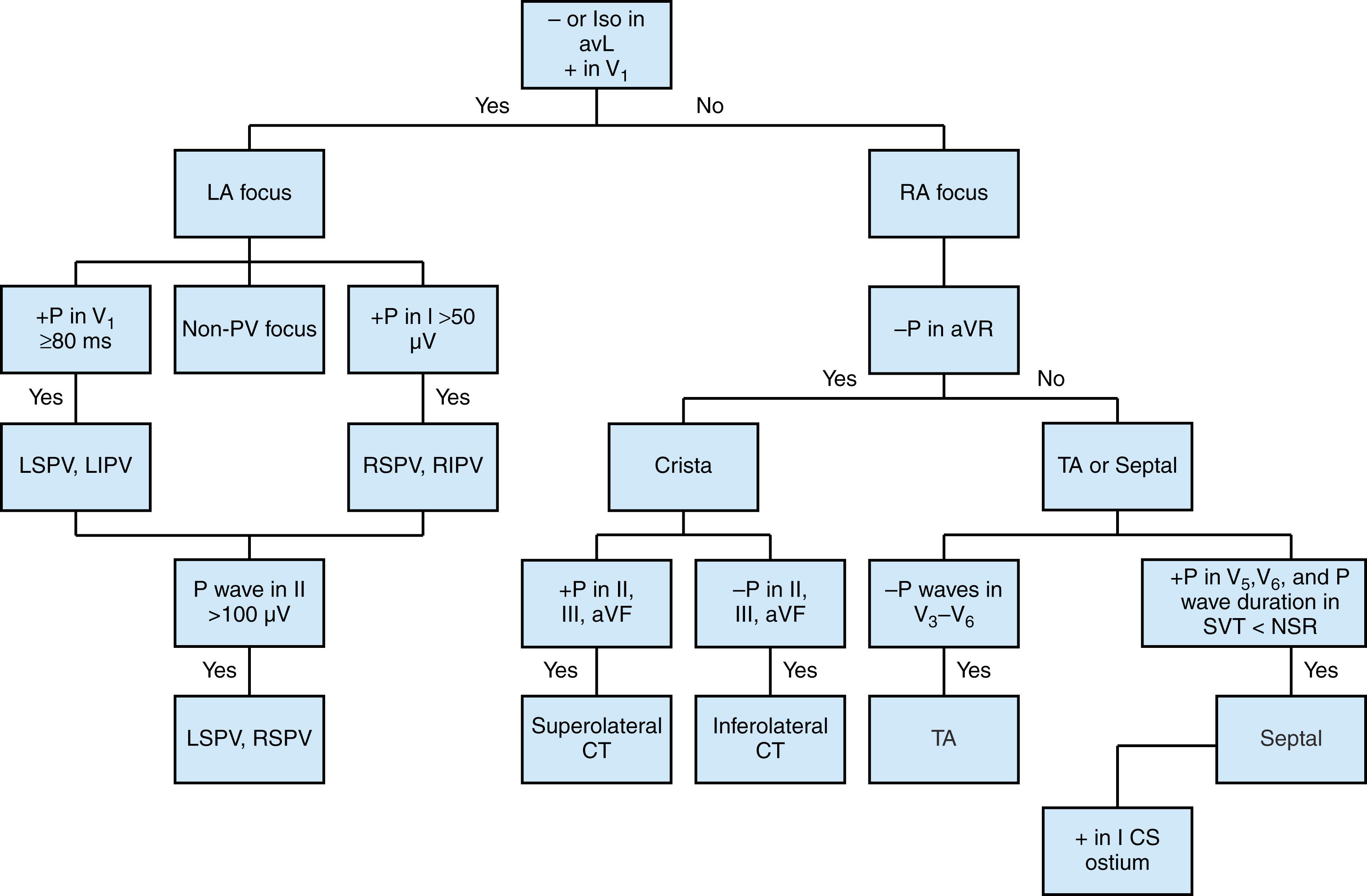
A positive or negative/positive P wave in V 1 had 100% sensitivity and 81% specificity for left atrial origin. Right atrial ATs are characterized by a negative P wave in aVR, negative or biphasic (positive/negative, i.e., an initial positive component followed by a negative component) P wave morphology in V 1 , and positive deflection in aVL. A positive P wave in lead V 1 is suggestive of left atrial focal tachycardia. ATs of inferior and coronary sinus origin have negative P waves in the inferior leads. Superior origins, such as the high crista terminalis or right superior pulmonary vein (RSPV), produce positive P waves in the inferior lead. P wave morphology in left ATs is usually positive in V 1 and negative in aVL. These general rules are not absolute by any means, and there are significant overlaps. P wave morphology is discussed further in the specific tachycardia sections that follow.
Fig. 71.3 shows an algorithm that is derived from distilling the results of various studies. , Although limitations exist for identifying the source of AT by electrocardiography, we feel that this algorithm offers a starting point for localization. The limitations of the ECG for deducing the origin of focal AT are caused by several factors ( Box 71.1 ). The P wave is often obscured as a result of overlap with the T or R wave. The body surface ECG has low fidelity for differentiating the source of initial atrial activation (spatial resolution ≤17 mm with pace mapping). Anatomic and pathologic variability of the atria, differences in preferential conduction, atrial scar or prior ablation(s), variable body habitus, and variable ECG lead placement in routine clinical settings reduce the relation between p wave morphology and site of activation.
Obscuring of the P wave by QRS complex or T wave and inability to identify an “unencumbered” P wave.
P wave morphology is in large part dependent on left atrial and interatrial septal activation. Preferential routes of interatrial conduction causing discordant P wave morphologies despite tachycardia origins being anatomically very close to one another.
Less than 2 cm spatial resolution of surface electrocardiography in localizing P wave origin.
Varying routes of preferential interatrial conduction dependent on atrial cycle length such that the same site can give rise to varying P wave morphologies.
Reentrant tachycardia in “scarred” atria can generate electrocardiograms that resemble focal atrial tachycardia if the zone of slow conduction occupies a very significant portion of the cycle length within a very small anatomic space.
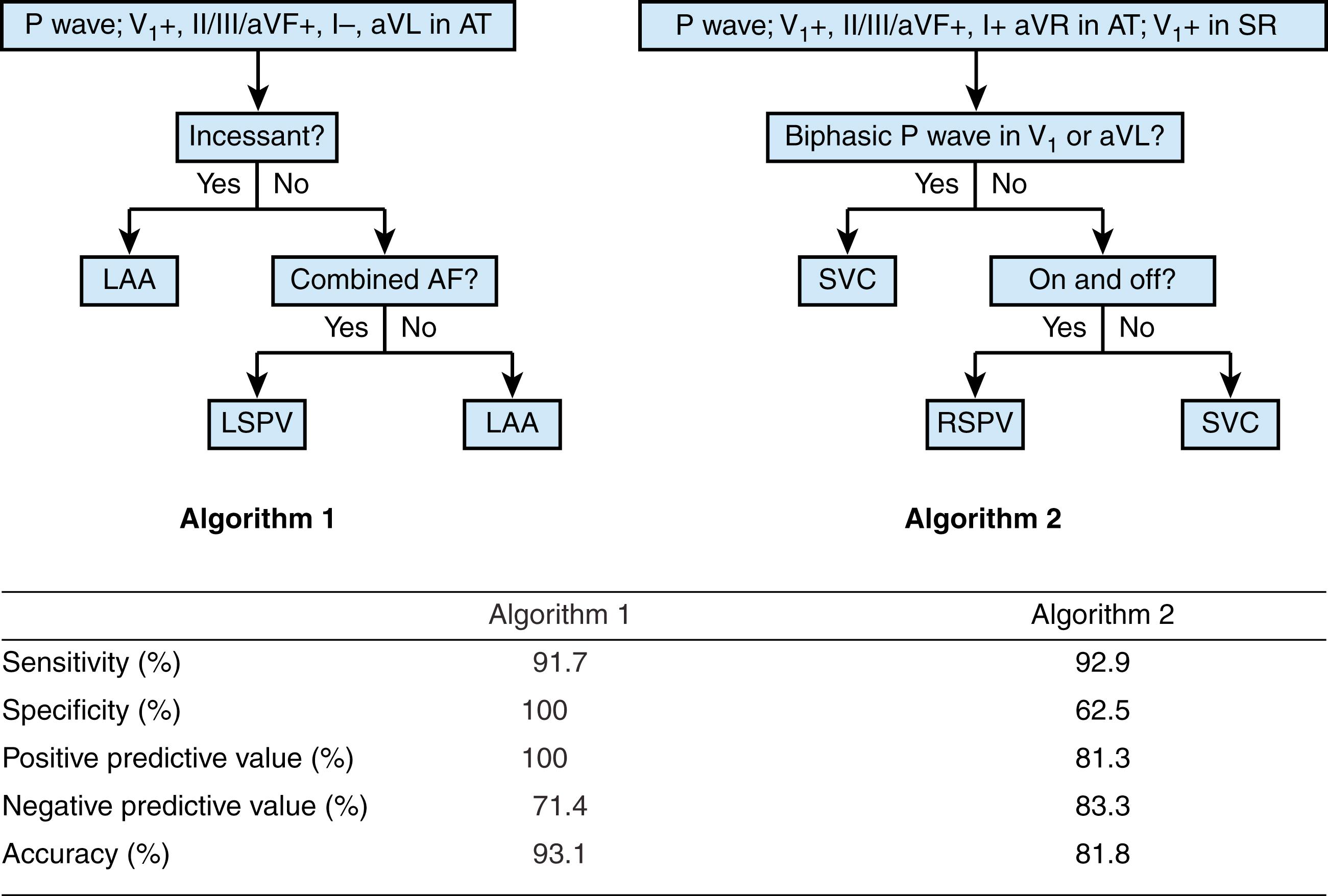
Algorithms are limited in their inability to differentiate sites of origin that are adjacent to each other (e.g., RSPV vs. SVC). Uhm and colleagues have combined clinical features, including Holter findings, to further elucidate the origin of ATs and differentiate adjacent sites of origin. Their algorithm was developed by analyzing data from 194 patients. This algorithm is depicted in Fig. 71.4 .
Advances in electrocardiographic imaging (ECGI) using body surface electrograms or a combination of body surface electrograms with chest imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) provide new hope for enhancing the accuracy of noninvasive localization of the site of origin of focal ATs. , Body surface potential mapping incorporates a much larger number of electrodes but does not provide anatomic information. The development of ECGI (see Chapter 68 ) is an advance in high-resolution noninvasive mapping that combines body surface electrodes and heart-torso geometrical information to produce detailed electroanatomic maps of the epicardial surface through application of inverse solution mathematical algorithms. This technique has permitted accurate localization of focal, microreentrant, and macroreentrant ATs.
The differential diagnosis of focal AT includes AV nodal reentrant tachycardia (AVNRT) (see Chapter 72 ) and orthodromic reciprocating tachycardias (see Chapter 74 ). These can be systematically excluded by invasive (see Chapter 70 ) and noninvasive analysis. A distillation of the various methods by which the diagnosis of AT can be achieved is detailed in Box 71.2 .
Gradual onset and offset, which is typical for automatic mechanisms of tachycardia, thus suggesting AT
Palpitations, especially felt in the neck and throat, suspicious for AVNRT because of simultaneous atrial and ventricular contraction
Inferiorly directed P wave axis excludes AVNRT
AV block (more P waves than QRS) excludes ORT
Spontaneous changes in PR and RP intervals, but fixed PP intervals, suggest AT
Bundle branch block during tachycardia does not affect PP intervals during AT
Reproducible termination of tachycardia with a P wave excludes AT
Reproducible termination of tachycardia with a PVC that does not conduct to the atrium excludes AT
The first P wave that initiates AT is similar to P wave morphology of AT, which suggests automatic AT
The first P wave that initiates the AT is dissimilar to the P wave morphology of AT, suggesting a PAC initiating microreentry or triggered activity as the mechanism of the AT
Induction of tachycardia with ventricular pacing is more likely with ORT or atypical AVNRT rather than AT
VA conduction is mandatory for induction of AT with ventricular pacing
When induced with ventricular pacing a V-A-A-V pattern of induction is typically observed
When VA block CL > TCL, ORT is less likely as the mechanism of tachycardia
Atrial activation sequence during ventricular pacing is unlikely to be identical to atrial activation during AT
High to low right activation sequence is incompatible with any form of AVNRT
AV block during tachycardia excludes ORT
Changes in HV interval during tachycardia (bundle branch block) does not affect TCL in AT or AVNRT
If oscillations in tachycardia CL are noted, then AA intervals predict HH and VV intervals predict AT
When TCL is fixed but VA intervals are not, AT is the likely mechanism
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here