Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Diffuse lung diseases are often detected and initially evaluated on chest radiographs (CXR). A CXR can provide valuable clues regarding pulmonary pathology such as the lung volume, distribution, and characterization of abnormalities. Radiographs assess lung volumes and distribution of disease. Whereas low volumes suggest the presence of a restrictive defect such as pulmonary fibrosis, large lung volumes suggest hyperinflation or obstructive lung disease. Ancillary findings such as lymphadenopathy, pleural effusions, and pleural plaques can assist in determining the cause of the pulmonary abnormality. Serial radiographs allow assessment of the acuity of abnormalities; those that persist for more than 4 weeks often indicate a chronic etiology. Findings on a CXR are often nonspecific, and necessitate further evaluation with thin-section chest computed tomography (CT) or high-resolution CT (HRCT).
High-resolution CT is a noninvasive cross-sectional examination of the whole lung. The distribution and characteristics of HRCT findings can indicate which group of differential diagnoses to consider or point to a specific disease without the need for biopsy. HRCT can suggest further tests for a definitive diagnosis or, if tissue sampling is required, can direct the site of biopsy. After diagnosis, follow-up HRCT scans monitor disease activity, the response to treatment, or the development of complications. Evolution of abnormalities over time may have prognostic implications.
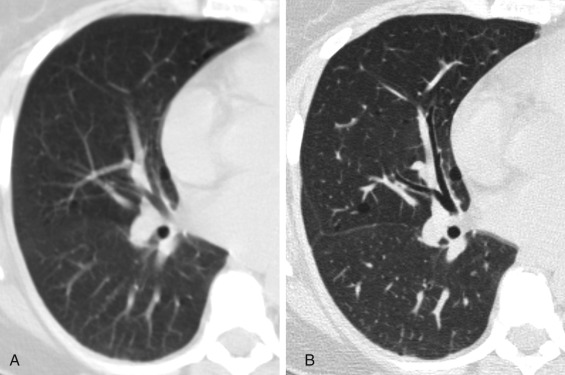
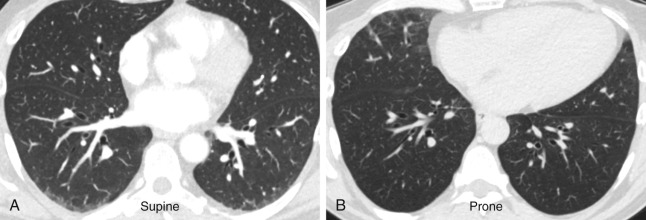
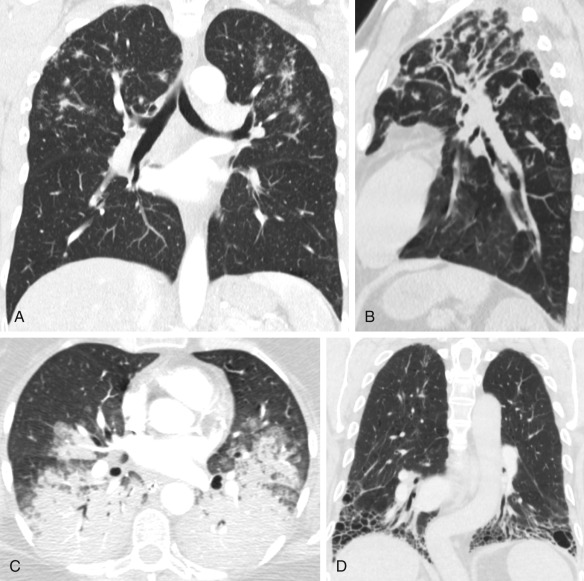
The protocol for an HRCT scan is described in detail in Chapter 1 . The principle of HRCT is acquisition of thin transverse sections (1–1.5-mm thickness) with a high spatial resolution technique and lung kernel algorithms to visualize the lung parenchyma ( Fig. 17.1 ). Multiplanar reformatting in the coronal and sagittal planes facilitates evaluation of the distribution of parenchymal abnormalities. Maximum-intensity projection (MIP) and minimum-intensity projection (MinIP) images aid detection of multiple nodules and decreased attenuation, respectively. Prone images are routinely included in the HRCT protocol to differentiate early reticular abnormalities in the dependent peripheral portions of the lower lobes that persist from gravity-dependent density that resolves on prone imaging ( Figs. 17.2 and 17.3 ).

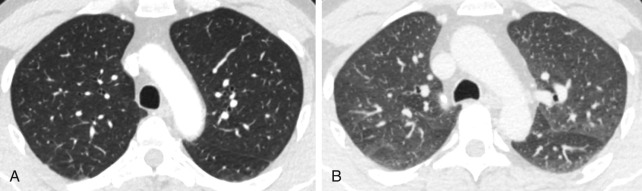
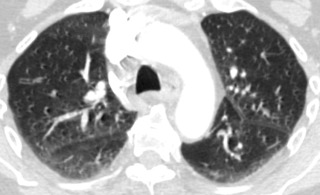
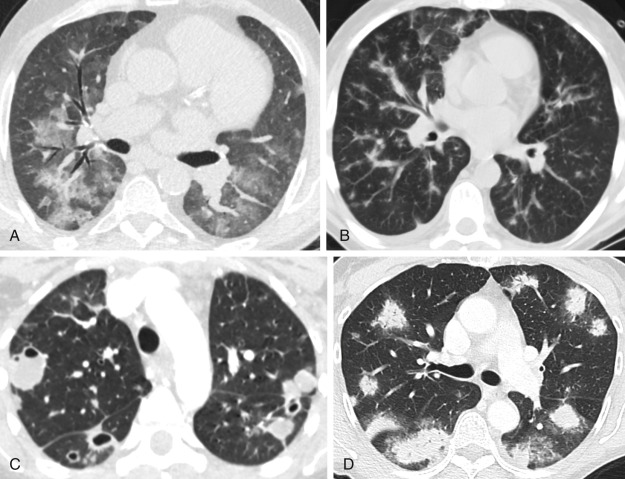
Expiratory HRCT images are performed either dynamically (during expiration) or on static images (after end-expiration) ( Fig. 17.4 ). The attenuation of the lung parenchyma on expiratory images is compared with inspiratory images at equivalent levels in the thorax. During normal expiration, the lung parenchyma decreases in volume and increases in attenuation. Areas of air trapping can be easily visualized on expiratory HRCT images as regions that remain similar in volume and attenuation when compared with the inspiratory images. Adequate expiration is confirmed if the posterior membrane of the trachea and main bronchi becomes flat or concave. It is important to consider normal expiration as the cause for increased parenchymal attenuation before suggesting a diagnosis of lung pathology. Respiratory motion can result in artifacts that mimic bronchiectasis, pulmonary embolism, and pulmonary nodules ( Fig. 17.5 ).
Many lung diseases involve the lung parenchyma, interstitium, or both. The key to diagnosis is recognition of the distribution of disease in relation to various anatomic regions ( Table 17.1 ). Craniocaudal distribution refers to the preference of a disease process for the upper, mid, or lower zones ( Fig. 17.6 ). Inhalational diseases, including smoking-related lung disease, have a predilection for the upper zones. This is because of the relative paucity of lymphatic drainage in the upper zones clearing inhaled pathogens. Hematogenous diseases, such as miliary metastases, have a predilection for the lower zones, where blood flow is greatest. Axial distribution refers to distribution on a single transverse image; certain diseases are central in distribution, and others predominantly involve the peripheral third of the lung ( Fig. 17.7 ). A dependent distribution of disease involves the posterior portion of the lung in supine patients and anterior portion in the prone position. Most importantly, thin-section CT permits evaluation of the distribution of disease at the level of the secondary pulmonary lobule (SPL), a key step in the accurate assessment and diagnosis of diffuse lung disease.
| Distribution | Disease |
|---|---|
| Upper zone | Pulmonary Langerhans cell histiocytosis |
| Emphysema | |
| Respiratory bronchiolitis | |
| Cystic fibrosis | |
| Hypersensitivity pneumonitis | |
| Sarcoidosis | |
| Reactivation tuberculosis | |
| Pneumoconioses (silicosis, coal worker's pneumoconiosis, berylliosis) | |
| Ankylosing spondylitis, neurofibromatosis 1 | |
| Lower zone | Hematogenous metastases |
| Aspiration | |
| Usual interstitial pneumonitis | |
| Nonspecific interstitial pneumonitis | |
| Asbestosis | |
| Central | Pulmonary edema |
| Pulmonary hemorrhage | |
| Pneumocystis pneumonia | |
| Lymphoma | |
| Kaposi sarcoma | |
| Peripheral | Organizing pneumonia |
| Chronic eosinophilic pneumonia | |
| Aspiration pneumonia | |
| Pulmonary infarction | |
| Septic emboli | |
| Sarcoidosis | |
| Adenocarcinoma | |
| Lymphoma | |
| IgG4-related lung disease | |
| Dependent | Aspiration |
| Diffuse alveolar damage (noncardiogenic pulmonary edema or acute lung injury) |
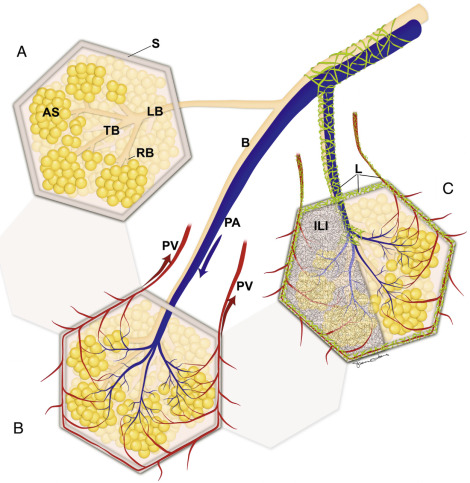
The SPL is the functional unit of lung where gaseous exchange occurs. The SPL contains bronchioles, branches of the pulmonary artery and veins, lymphatics, and interstitial tissue. The SPL measures approximately 1 to 2.5 cm in diameter, is roughly polyhedral in shape, and is outlined by an interlobular septum ( Fig. 17.8 ). The structures in the center of the SPL, the centrilobular structures, consist of a lobular bronchiole and pulmonary artery that course and divide together. The pulmonary artery forms a dot approximately 0.5 to 1 mm in diameter, visible on HRCT in the center of the SPL. The wall of the bronchiole is 0.05 to 0.15 mm and beyond the resolution of HRCT to be normally visible unless thickened or fluid filled. Lobular bronchioles divide into terminal bronchioles, the most distal conducting airways that then divide into several respiratory bronchioles in the center of the SPL. Respiratory bronchioles open into several alveolar ducts to form an acinus. The 10 to 15 acini in each SPL participate in gas exchange and surround the centrilobular bronchovascular structures. Deoxygenated blood in the pulmonary artery passes through the rich capillary network surrounding the acini, where gas exchange occurs. The SPL is outlined by a connective tissue septum, the interlobular septum, within which course pulmonary veins and pulmonary lymphatics. Interlobular septa measure 0.1 cm and are often identified on HRCT by the pulmonary veins that course through the septa and measure 0.5 cm in diameter. The capillary network connects the centrilobular structures to the septal pulmonary veins that carry oxygenated blood back to the heart .
The lung interstitium forms a support system of connective tissue surrounding the lung parenchyma. The interstitium is divided into a central, peripheral, and intralobular interstitium. The central (axial) interstitium extends from the hila into the lung parenchyma, enveloping the bronchovascular bundles like a sheath and dividing as the bronchovascular bundles branch and arborize. The axial interstitium eventually reaches and terminates in the center of the SPL, surrounding the centrilobular pulmonary artery and bronchiole. The peripheral interstitium forms a subpleural cloak around the lung and extends along the fissures and forms the interlobular septa that outline the SPL. Between the central, centrilobular interstitium and the peripheral interlobular interstitium runs a fine meshwork of interstitial lines, the intralobular interstitium, that supports the structures within the SPL. The septa are most easily identified on CT in the subpleural region, along the fissures, and at the lung apices and bases.
Lymphatics are present in the interstitium. They course along the bronchovascular bundles surrounding the bronchiole and pulmonary artery that supply each lobule and within the interlobular septa. Lymphatic obstruction from tumor or edema can result in thickening of the bronchovascular interstitium and interlobular septa. Because the interlobular septa are best developed in the subpleural region and along the fissures, these areas are often involved with perilymphatic diseases, but centrilobular diseases involve structures in the center of the SPL; do not extend to the pleura, fissures, or interlobular septa; and are located approximately 5 to 10 mm from those surfaces. This distinction is helpful in localizing the site of abnormality on HRCT and directing the differential diagnosis.
Most abnormalities identified on thin-section CT can be categorized into one of four main patterns: nodular, reticular, increased, or decreased attenuation. The predominant pattern as well as its anatomic location are extremely helpful in determining the imaging differential diagnosis. Diseases may show a craniocaudal and axial distribution as well as a predisposition to particular areas at the level of the SPL.
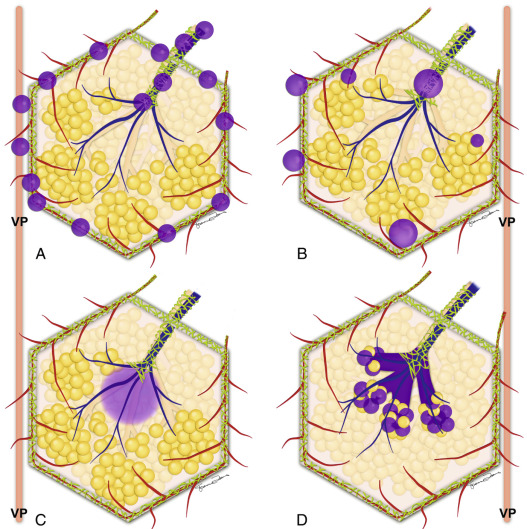
A nodular pattern consists of multiple well-defined rounded soft tissue or ground-glass nodules. The nodules usually measure between 2 and 10 mm in diameter, and their distribution can be related to the structures of the SPL ( Fig. 17.9 ).
| Type | Causes |
|---|---|
| Perilymphatic | Sarcoidosis |
| Lymphangitic carcinomatosis, lymphoma | |
| Silicosis, coal worker's pneumoconiosis | |
| Rare | Berylliosis |
| Amyloidosis | |
| Lymphocytic interstitial pneumonia | |
| Random | Metastases |
| Miliary infection: tuberculosis, fungal | |
| Centrilobular: nonbranching | Respiratory bronchiolitis |
| Hypersensitivity pneumonitis | |
| Pulmonary hemorrhage | |
| Infection | |
| Pulmonary edema | |
| PLCH | |
| Rare | Follicular bronchiolitis |
| Pulmonary arterial hypertension | |
| Invasive mucinous adenocarcinoma | |
| Organizing pneumonia | |
| Capillary hemangioendotheliosis | |
| Metastatic calcification, talcosis | |
| Pneumoconiosis (coal worker's pneumoconiosis, silicosis) | |
| Centrilobular: branching (tree-in-bud) | Infection: bacterial, mycobacterial, viral, fungal, ABPA, panbronchiolitis |
| Aspiration | |
| Bronchiectasis, cystic fibrosis | |
| Rare | Invasive adenocarcinoma |
| Follicular bronchiolitis | |
| Organizing pneumonia |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here