Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypertrophic cardiomyopathy (HCM) is a primary disease of cardiac muscle characterized by a hypertrophied, nondilated left ventricle unassociated with other cardiac diseases that can reasonably account for the magnitude of hypertrophy present. The distribution of left ventricular hypertrophy (LVH), the hallmark of HCM, occurs in many patterns within the ventricle. , Relatively uncommon, apical HCM is a unique subtype of HCM in which the LVH involves predominantly the apex. Particularly common in Asia, this variant has been reported in up to 41% of HCM patients in China, , up to 30% in Japan, and up to 38% in Korea. In non-Asian populations, it has been observed in 1% to 14% , ( Table 63.1 ). However, most of the epidemiologic data are derived from studies conducted in the early 1990s, and the exact prevalence of apical HCM in non-Asians may be underestimated because of diagnostic unawareness and difficulty making the diagnosis on transthoracic echocardiography (TTE), the most frequently used diagnostic modality, when the diagnosis is not suspected. The basis for the differences in the phenotypic expression of apical HCM between Asians and non-Asians is unknown.
| Authors | Year | Total HCM | Apical HCM | % | |
|---|---|---|---|---|---|
| Japan | Sakamoto | 2001 | — | 126 | 15 |
| Japan | Kitaoka et al | 2003 | 100 | 15 | 15 |
| Japan | Nasermoaddeli et al | 2007 | 1605 | 532 | 33 |
| Japan | Kubo et al | 2009 | 264 | 80 | 30 |
| China | Ho et al | 2004 | 118 | 49 | 41 |
| China | Yan et al | 2012 | 1320 | 208 | 16 |
| Taiwan | Lee et al | 2006 | 163 | 40 | 25 |
| Korea | Moon et al | 2011 | 1204 | 454 | 38 |
| Louie and Maron | 1987 | 965 | 23 | 2 | |
| Klues et al | 1995 | 600 | 7 | 1 | |
| Eriksson et al | 2002 | — | 105 | 7 | |
| Kitaoka et al | 2003 | 361 | 10 | 3 | |
| Gruner et al | 2011 | 429 | 61 | 14 | |
| Klarich et al | 2013 | 2662 | 210 | 7.9 |
Sakamoto and colleagues, in 1976, first described symmetrical apical hypertrophy based on the appearance of the left ventricle on M-mode echo in patients with giant negative T waves in the precordial leads ( Fig. 63.1 ). Yamaguchi and associates described its configuration on end-diastolic ventriculography in the right anterior oblique projection. This configuration was nicknamed “spade-like” because the LV cavity in this projection resembled the “spade” in playing cards.
In contrast to most other forms of HCM, apical HCM was initially thought to be a benign condition, especially in Asians, in whom cardiovascular morbidity was reportedly rare. , , , However, early studies included relatively small sample sizes, and in recent years, especially in Western populations, this condition has been reported to be less benign than previously thought. Recent case reports have described an association of apical HCMs with ventricular arrhythmias, sudden death, and apical aneurysms. , , , , , Despite these complications, apical HCM is still generally characterized by a benign clinical course with a favorable long-term prognosis.
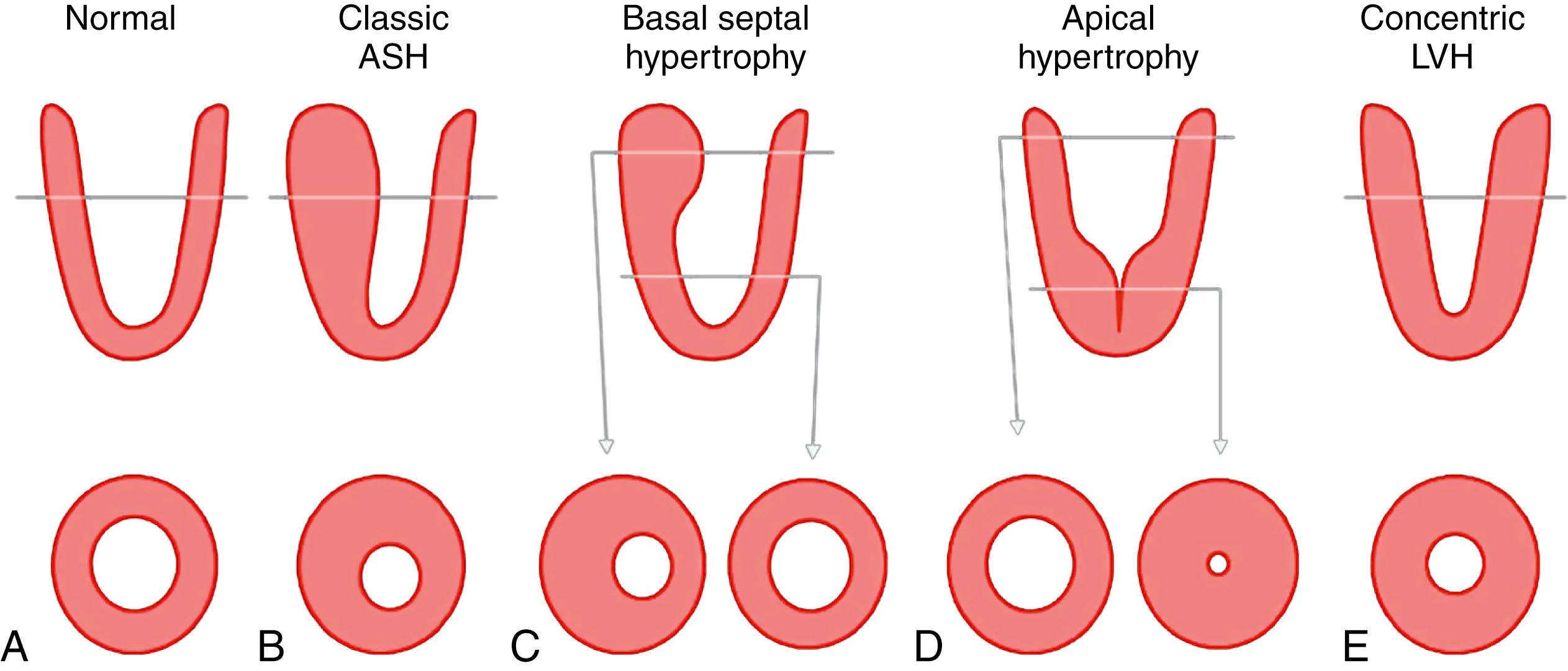
The criteria for the diagnosis of apical HCM include the demonstration of asymmetric LVH confined to the most distal portion of the left ventricular (LV) apex below the papillary muscles, usually circumferential, with an apical wall thickness at least 15 mm and a ratio of maximal apical to posterior wall thickness of 1.5 or greater at end-diastole , ( Box 63.1 and Fig. 63.2 ). This morphology can be identified by echocardiography, ventriculography, computed tomography, and cardiac magnetic resonance imaging (CMRI). The most readily available of these, noncontrast echocardiography, typically provides an appreciation of this unusual distribution of ventricular hypertrophy. , , , ,
Hypertrophy predominantly confined to the left ventricular (LV) apex
Maximal wall thickness within apical segments
Hypertrophy may extend from the apex to the level of the papillary muscle
Absence of significant hypertrophy at the basal level
Hypertrophy may be present in the basal septum or other basal segment if there is no evidence of LV outflow tract obstruction
Wall thickness ≥15 mm and ratio of apical to posterior wall thickness ≥1.5
The performance of TTE in apical HCM requires careful attention to the technical aspects of the examination. The parasternal long-axis view, which is critical to the diagnosis of “classic” asymmetric septal HCM, is inadequate for examining the LV apex. Apical HCM is best visualized from the apical four-chamber view and apical short-axis views. Identification of apical HCM requires good endocardial definition to demonstrate the characteristic features of this disorder, thickened apical walls and obliteration of the apical cavity during systole. Apical HCM may escape echocardiographic detection because the thickness of the LV wall is not significantly increased at the basal and papillary muscle levels. The parasternal long-axis and short-axis views may appear normal. Moreover, the likelihood of overlooking this condition is increased in the face of inadequate image quality, especially when a low-frequency transducer is used for scanning. Several maneuvers can be used to identify an apical HCM when it is not apparent on routine clinical examination ( Box 63.2 ). The first is to use relatively shallow focal depths and high-frequency transducers. Second, color flow Doppler imaging from the apex, at a relatively low Nyquist limit, can demonstrate the blood pool–tissue boundary and therefore the narrowing of the LV cavity at the apex. Ultimately, contrast echocardiography, using transpulmonary agents to opacify the left ventricle, may confirm the presence of apical HCM. , ,
Carefully aligned (nonforeshortened) apical images
Use of apical short-axis views
Use of color Doppler focused on the left ventricular apex
Use of high-frequency transducers with focusing at the apex
Intravenous contrast echocardiography
With good opacification of the LV cavity with contrast, the true extent of hypertrophy can be easily appreciated and the abnormal contour of the LV cavity documented. Because the hypertrophy is confined to the apex, dynamic LV outflow tract obstruction and systolic anterior motion (SAM) of the anterior mitral leaflet are absent. Although echocardiography usually identifies the presence of apical HCM, it can still be overlooked, particularly if adequate images of the apex are not obtained. In patients with a clinical suspicion of HCM (e.g., “giant” precordial T-wave inversions) and suboptimal echocardiographic images, the use of contrast echocardiography as mentioned, or CMRI, should be considered , , ( Figs. 63.3 and 63.4 ).
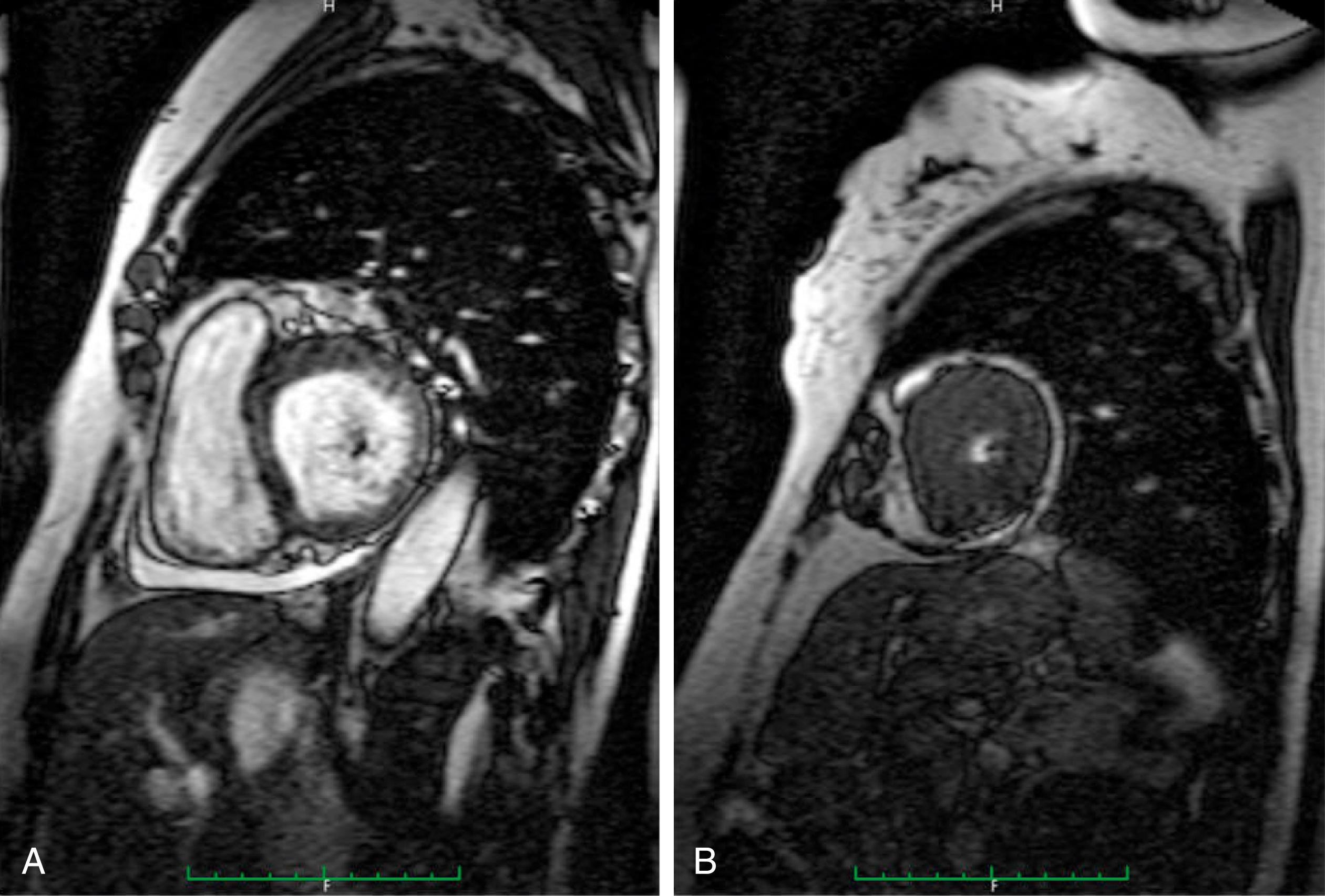
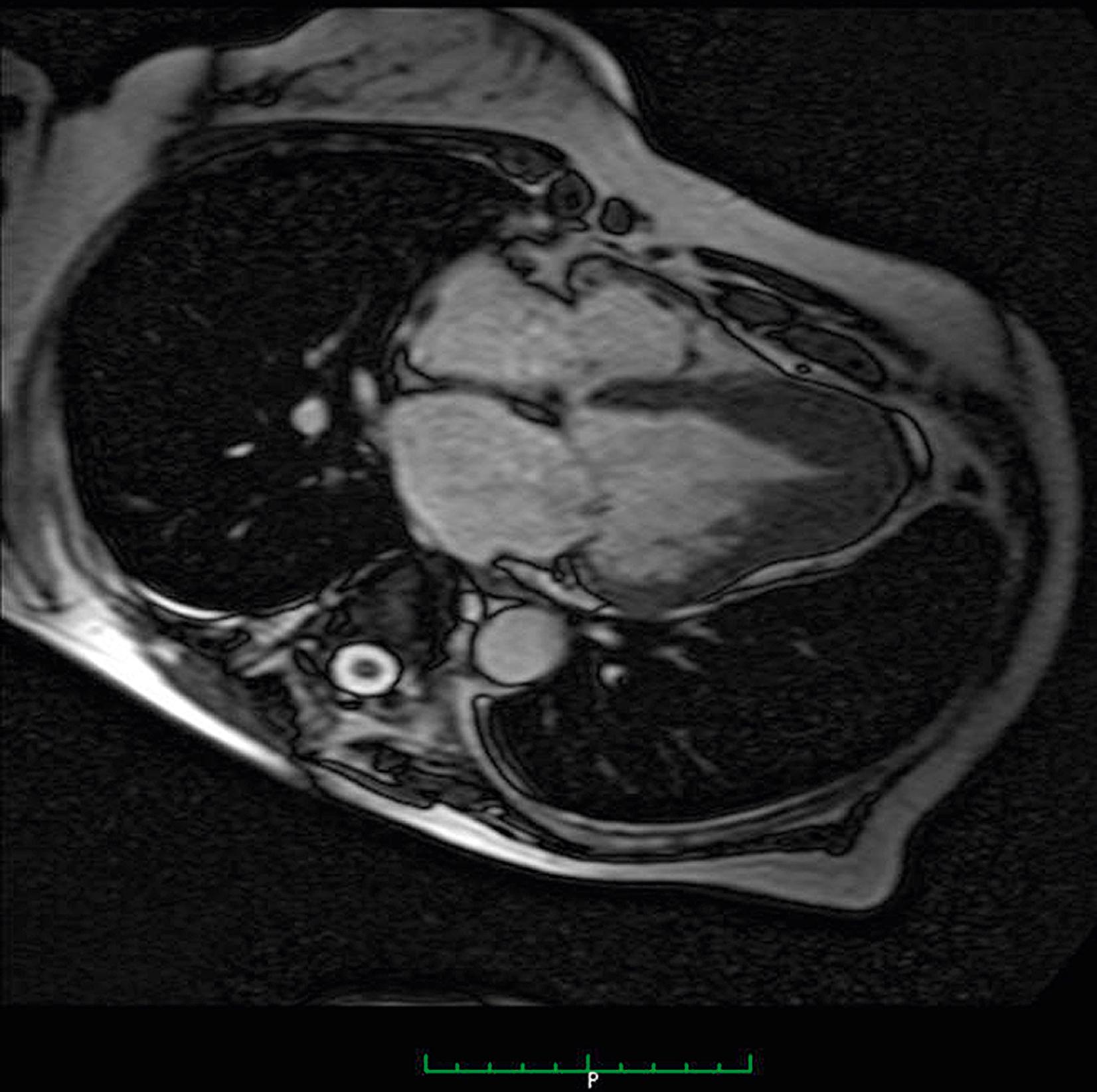
In fact, there have been reports of cases where MRI has detected apical HCM unrecognized by echocardiography. CMRI provides a large field of view and high blood-to-myocardium contrast, which is ideal for consistent and accurate imaging of the apex. In addition, CMRI can identify the presence of apical aneurysms. Transesophageal echocardiography (TEE) has also been used to diagnose apical HCM when standard TTE has been suboptimal. Finally, sarcomere protein gene mutations have been found in up to 47% of patients with apical HCM ( Table 63.2 ). , , Therefore, genetic testing may also be useful for evaluating patients with suspected apical HCM by helping to confirm the diagnosis in borderline cases, exclude disease in others, and identify family members who are carriers with a negative phenotype and may develop the disease later in life. ,
| Arad et al. | Binder et al. | Gruner et al. | |
|---|---|---|---|
| Probands with apical HCM ( n ) | 15 | 37 | 61 |
| Patients with a positive genotype (%) | 47 | 30 | 13 |
| Patients with a positive family history for HCM (%) | 40 | 22 | 16 |
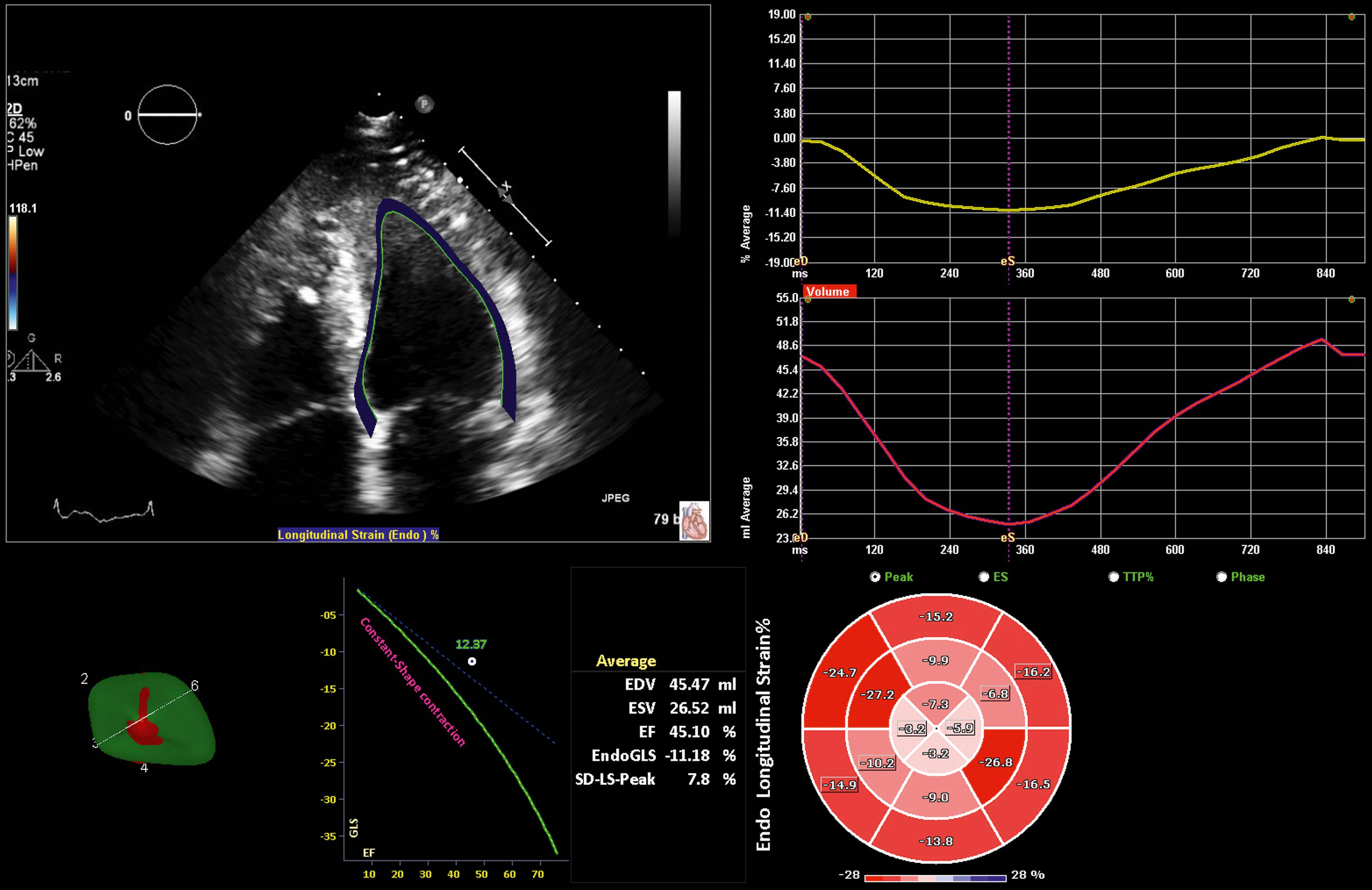
Apical longitudinal strain is typically decreased as illustrated in Fig. 63.5 . Paradoxical apical longitudinal strain (systolic lengthening) also has been described in apical HCM despite an apparently normal apical wall motion on conventional TEE.
Apical HCM must be differentiated from apical cavity obliteration caused by thrombus, tumor, noncompaction cardiomyopathy, or hypereosinophilic syndrome. Moreover, a false-positive finding may result from hypertrophied papillary muscles or a foreshortened apical four-chamber view that produces the appearance of increased apical wall thickness because of an oblique cut ( Box 63.3 ). Diastolic dysfunction is typically present and leads to increased filling pressures and left atrial enlargement and is central to several of the clinical manifestations of apical HCM, including dyspnea, exercise intolerance, and pulmonary edema.
Hypertrophied papillary muscles
Noncompaction cardiomyopathy
Apical thrombus
Left ventricular tumor
Hypereosinophilic syndrome
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here