Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Warfarin has historically been the most commonly used oral anticoagulant. It is a vitamin K antagonist and blocks γ-carboxylation of a series of glutamic acid residues during the synthesis of factors II, VII, IX, and X, and proteins C and S. The resulting decreased functionality of the coagulation factors leads to prolongation of prothrombin time (PT), which is monitored as the international normalized ratio, and partial thromboplastin time.
Heparin binds to antithrombin and increases its ability to inhibit thrombin, factor Xa, and, to a lesser extent, other serine protease coagulation factors. Thus, heparin is considered to be an “indirect” thrombin inhibitor. The anticoagulant effect of heparin is commonly monitored by prolongation of partial thromboplastin time, with actual quantitation of heparin anticoagulant activity being assayed as anti–factor Xa activity. Where necessary to monitor low-molecular-weight heparin or fondaparinux, this is done by measuring its anti–factor Xa activity.
Heparin-induced thrombocytopenia (HIT) is a complication of heparin therapy in which antibodies develop against complexes of heparin and platelet factor 4 (PF4). The antibody/PF4/heparin complexes bind to platelets through the Fcγ receptor and cause platelet activation, leading to arterial and/or venous thrombosis. Both immunologic and functional assays are employed in the evaluation of possible HIT.
Target-specific oral anticoagulants directly inhibit either thrombin or factor Xa. These anticoagulant agents do not typically need to be monitored, but they have variable effects on the PT and activated partial thromboplastin time (APTT). The oral thrombin inhibitor dabigatran etexilate can be assayed by a dilute thrombin time or an ecarin clot time, and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban can be assayed by anti–factor Xa assays.
Antiplatelet therapy is used primarily for the prevention of recurrent stroke or myocardial infarction. The laboratory can monitor the resulting reactivity of platelets from treated patients and, in some instances, can detect apparent resistance to aspirin or other therapeutic agents.
We gratefully acknowledge the contribution of Louis M. Fink, Richard A. Marlar, and Jonathan L. Miller, who authored this chapter in previous editions.
Antithrombotic therapy, which includes therapies targeting platelets as well as the coagulation system, is used to prevent and treat thromboembolic disease. Antiplatelet agents are most frequently used to prevent stroke and myocardial infarction, but they are also used in patients with peripheral arterial disease and may have a modest benefit in preventing recurrent venous thromboembolism (VTE). Anticoagulant agents are most frequently used to prevent and treat VTE, to prevent stroke and peripheral arterial embolism in patients with atrial fibrillation, and to prevent thromboembolic complications in patients with prosthetic heart valves. The clinical laboratory plays a critical role in the evaluation and management of patients on these therapies.
Warfarin is the most commonly prescribed of the oral vitamin K antagonists. It is a racemic mixture of the stereoisomers S-warfarin and R-warfarin, with S-warfarin being the more potent in producing an anticoagulant effect. Warfarin inhibits vitamin K reductase (vitamin K 2,3-epoxide reductase [VKOR]) and blocks regeneration of the active form of vitamin K, which is necessary for γ-carboxylation of specific glutamate residues within the amino-terminal Gla domains of factors II, VII, IX, and X and proteins C and S ( Fig. 43.1 ). With the decreasing extent of γ-carboxylation of glutamic acid within these domains, the affected proteins are unable to bind into the highly ordered protein/Ca ++ /phospholipid membrane complexes that are essential for normal hemostatic activity ( ). Consequently, those laboratory tests that are dependent on the proper function of these factors become more abnormal with increasing antagonism of vitamin K.
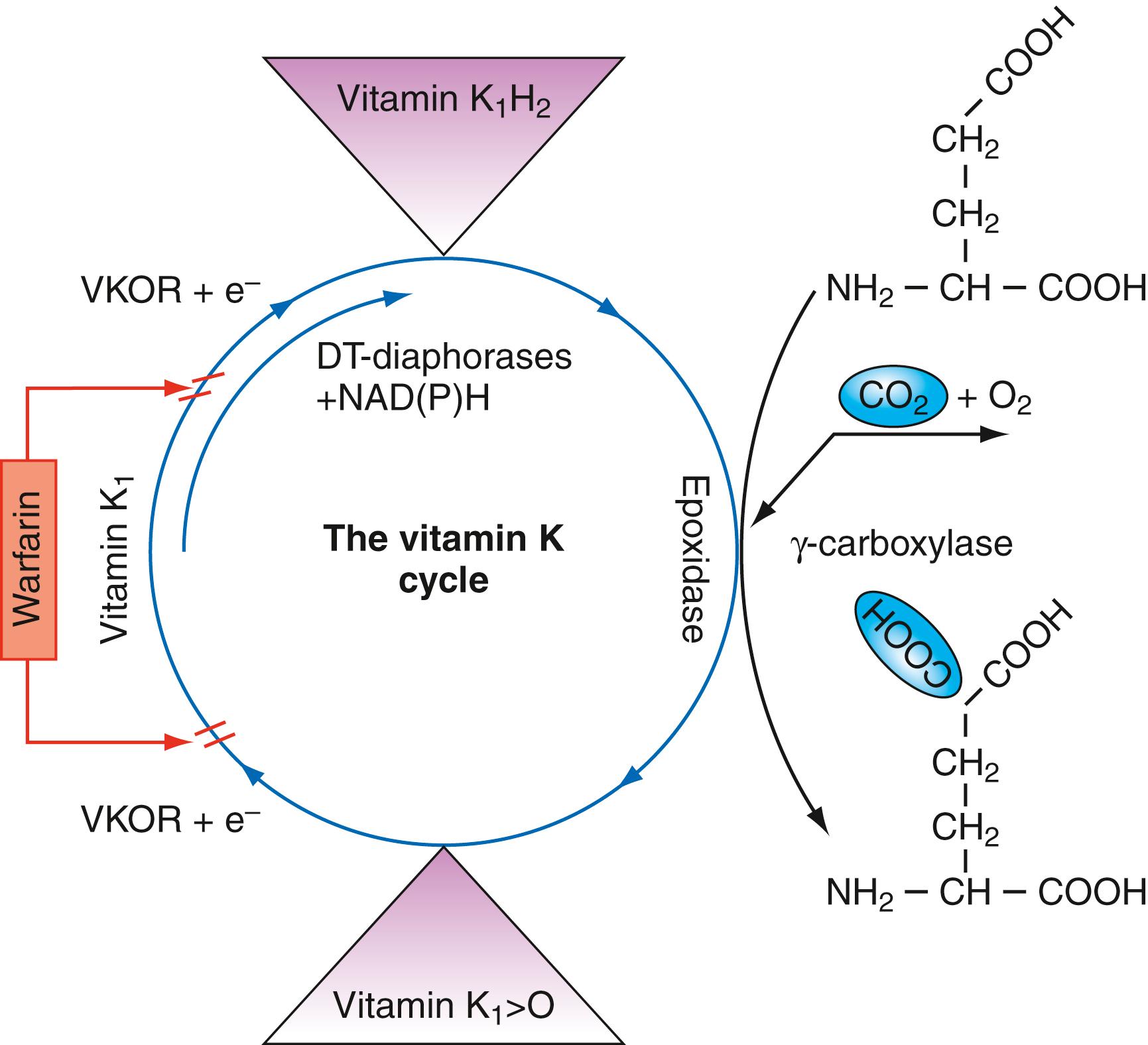
The degree of anticoagulation with warfarin is typically assessed by measuring prothrombin time (PT), although activated partial thromboplastin time (APTT) is also typically prolonged. Thrombin time (TT) is unaffected by warfarin therapy, because fibrinogen synthesis is not vitamin K dependent. Prolongation of PT is determined by decreases in the activity of three of the vitamin K–dependent coagulation factors—specifically, factors VII, X, and II. Initial prolongation of PT will primarily be due to the effect on factor VII, which has the shortest half-life (4–6 hours). Therapeutic efficacy depends on decreased levels of factors X and II, however, which have longer half-lives (24–48 hours and 48–72 hours, respectively). The different half-lives of the vitamin K–dependent factors is why 4 to 6 days of therapy with warfarin is generally recommended before assuming that laboratory monitoring actually reflects a steady-state level of anticoagulation. For clinical situations that require a more rapid onset of effective anticoagulation (e.g., treatment of acute VTE), heparin or low-molecular-weight heparin (LMWH) is administered concomitantly with warfarin until steady-state efficacy of the warfarin is confirmed by laboratory testing. Because warfarin also interferes with the γ-carboxylation of proteins C and S, which are part of the natural anticoagulant system, there are certain clinical settings where initiation of warfarin can be associated with a paradoxical prothrombotic state, such as in patients with protein C deficiency ( ), protein S deficiency ( ), or acute heparin-induced thrombocytopenia (HIT) ( ).
PT is performed by adding a source of phospholipid and tissue factor (referred to as thromboplastin ), and calcium, to citrated plasma. The time to clot formation is then measured by mechanical or optical methods. Individual thromboplastin reagents can vary substantially in their sensitivity to the decreased levels of the vitamin K–dependent factors, however. This variable sensitivity is also affected by the instrument system used to measure PT ( ). To correct for these differences, the international normalized ratio (INR) was developed in order to improve standardization of PT reporting worldwide ( ). To calculate an INR, the logarithms of PT results obtained on normal individuals and patients on warfarin using a primary international reference thromboplastin are plotted against the logarithms of PT results obtained using the testing laboratory’s thromboplastin reagent ( Fig. 43.2 ). The resulting slope of this plot is the international sensitivity index (ISI) for that particular thromboplastin reagent and instrumentation combination ( ; ; ; ; ). The PT ratio is determined by dividing the patient PT result by the geometric mean normal PT for the local laboratory (determined by using a population of normal individuals with the same reagent/instrumentation). This PT ratio is then raised to the power of the ISI to determine the INR according to the following equation:
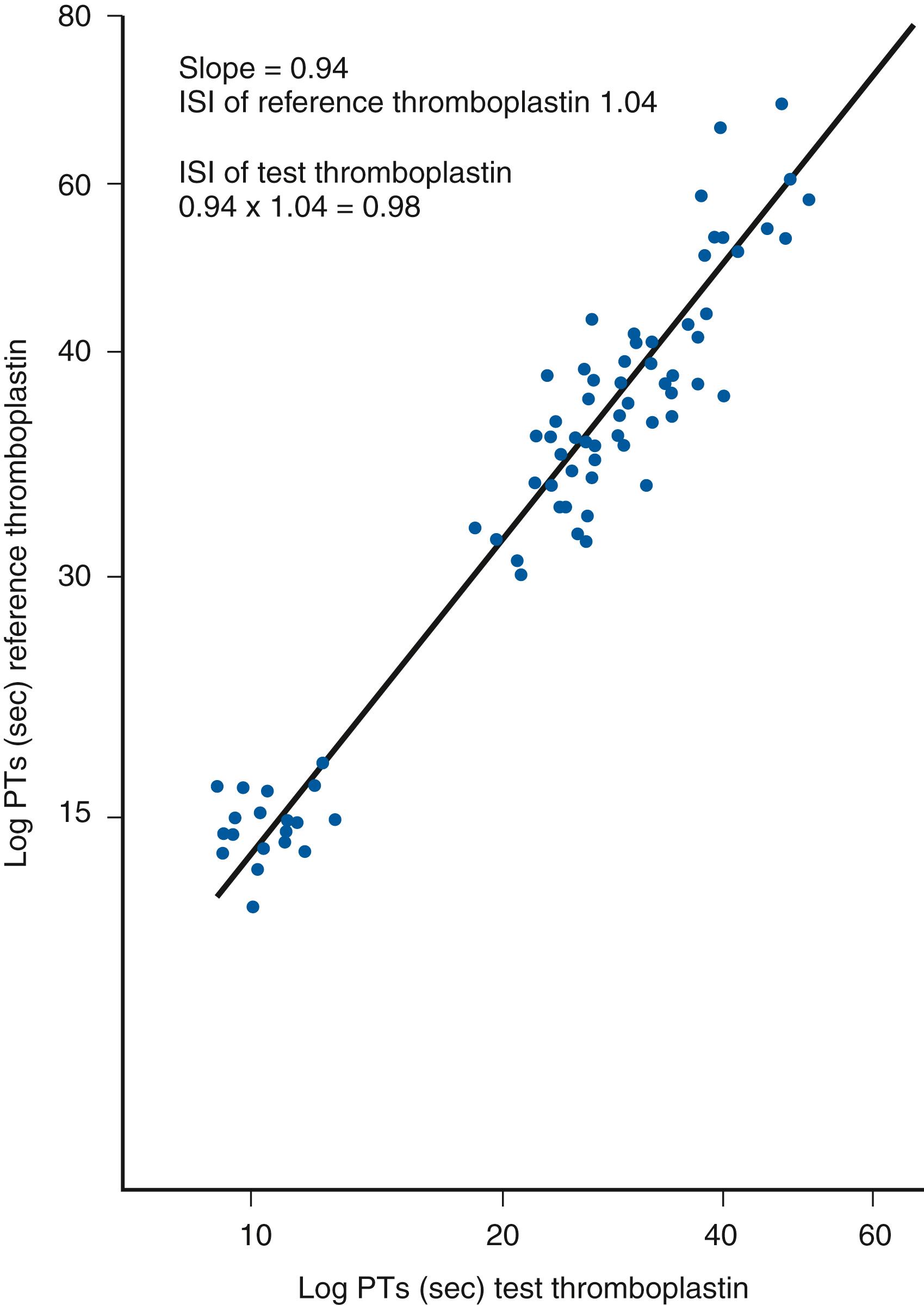
The INR seeks to represent the PT ratio that would be obtained if the international reference thromboplastin had actually been used to test the patient. Although the concept of generating the INR sounds straightforward, actually obtaining an accurate INR result can be difficult in practice ( ; ).
Because of the importance of accurate monitoring of anticoagulant therapy with the vitamin K antagonists, several additional points regarding the ISI and the INR need to be taken into consideration. First, the ISI must be correctly specified not only for each type of PT reagent but also for each new lot of thromboplastin in addition to specifying the coagulation instrument model being used in a given laboratory. Second, in plasma samples from patients being treated with warfarin and a second anticoagulant (e.g., heparin in the acute management of VTE), the prolonged PT will reflect the combined effect of both anticoagulant agents. Heparin can either be removed or neutralized to more specifically assess the anticoagulant contribution of the warfarin alone, as described later. An analogous strategy does not exist for the removal or neutralization of the parenteral direct thrombin inhibitors argatroban and bivalirudin, which are used in the treatment of patients with HIT (see later discussion). Transitioning the patient to warfarin can be difficult because of the effect that the parenteral direct thrombin inhibitors have on PT and the INR ( ; ). Third, although most lupus anticoagulants exert a more pronounced effect on APTT than on PT, the presence of a lupus anticoagulant in some patients may render the PT inaccurate as a means of monitoring warfarin therapy. This may depend on the reagents used ( ). In such instances, a direct chromogenic assay for factor X (rather than a clotting assay) may aid in monitoring of anticoagulant intensity ( ). Finally, thromboplastins are prepared from a variety of sources, spanning recombinant human tissue factor and nonhuman-derived materials. In some cases, thromboplastin originating from a nonhuman source may contribute to an abnormally prolonged PT (and, thus, an increased INR) that would not have been obtained had a human thromboplastin been used instead. This phenomenon has been reported in patients with factor VII Padua, who have a nonpathogenic polymorphism in their factor VII molecule that supports normal hemostatic interactions with human tissue factor but diminished interaction with tissue factor of various animal origins, notably rabbit ( ; ).
The plasma half-life of warfarin is 20 to 60 hours, with a mean of 40 hours. The maximum effect of a dose lasts for up to 48 hours after administration. S-warfarin is metabolized by CYP2C9, and R-warfarin is metabolized by the CYP1A2 and CYP3A4 enzymes. Genetic polymorphisms in CYP2C9 lead to an alteration in the pharmacokinetics of warfarin ( ; ). In addition, mutations in genes encoding for VKORC1, which generates the reduced form of vitamin K necessary for γ-carboxylation, have been identified to lead to variable sensitivity to inhibition by warfarin, affecting pharmacokinetics and dosing ( ). Specific allelic combinations of CYP2C9 and VKORC1 are associated with a wide range of warfarin sensitivities ( Table 43.1 ). Allelic frequencies and their relationships to race and therapeutic warfarin dose are shown in Table 43.2 . Although observational studies suggested potential benefits from genotype-guided dosing of vitamin K antagonists ( ; ), three large prospective randomized clinical trials demonstrated that pharmacogenetic-based dosing resulted in either no ( ; ) or minimal ( ) improvement in the time that the INR was in the therapeutic range for the first 4 to 12 weeks of therapy. Whether CYP2C9 or VKORC1 genotype data will be beneficial in other aspects of warfarin management needs to be determined.
| GENOTYPE COMBINATION | ||||
|---|---|---|---|---|
| Warfarin Sensitivity | VKORC1 | CYP2C9 | Prevalence N (%) | Clinical Considerations |
| Very high | A/A G/A |
∗1/∗3, ∗2/∗2, ∗2/∗3, ∗3/∗3 ∗3/∗3 |
23 (2.6%) | May need a lower dose and more frequent INR monitoring |
| High | A/A G/A G/G |
∗1/∗2 ∗2/∗3 ∗3/∗3 |
36 (4.0%) | Lower dose and frequent INR monitoring |
| Moderate | A/A G/A G/G |
∗1/∗1 ∗1/∗2, ∗1/∗3, ∗2/∗2 ∗2/∗3 |
238 (26.6%) | Lower dose and frequent INR monitoring |
| Mild | G/G | ∗1/∗2, ∗1/∗3, ∗2/∗2 | 109 (12.2%) | Frequent INR monitoring |
| Normal | G/A | ∗1/∗1 | 262 (29.2%) | Standard INR monitoring |
| Less than normal | G/G | ∗1/∗1 | 228 (25.4%) | May require higher dose to maintain optimal INR |
∗ Genotype is defined by the combination of measured allelic variations in CYP2C9 and VKORC1. Phenotype is the expected warfarin sensitivity based on the genotype.
| SNP Alleles | Gene Location | White (N = 838) | African American (N = 153) | Other or Mixed Race (N = 24) | Univariate Association with Dose in Total Population, Per Allele (95% CI) |
|---|---|---|---|---|---|
| CYP2C9∗2 (C > T) | 3608C > T | 13.1% | 5.2% | 10.4% | –17% (–22% to –12%) |
| CYP2C9∗3 (A > C) | 42614A > C | 6.0% | 1.0% | 4.2% | –30% (–36% to –24%) |
| CYP2C9∗5 (C > G) | 42619C > G | 0% | 1.3% | 2.1% | NS |
| VKORC1 861 C > A | –4451C > A | 36.0% | 8.8% | 26.1% | 14% (9% to 18%) |
| VKORC1 3673 G > A | –1639G > A | 36.6% | 9.5% | 41.7% | –29% (–31% to 26%) |
| VKORC1 5808 T > G | IVS1 + 324T > G | 25.1% | 4.6% | 8.3% | 25% (29% to 22%) |
| VKORC1 6853 G > C | IVS2 + 124G > C | 37.2% | 24.3% | 41.7% | –37% (–42% to –32%) |
| VKORC1 9041 G > A | 626 G > A | 39.4% | 51.3% | 41.7% | 18% (14% to 23%) |
| F2 Thr165Met | 494 C > T | 13.3% | 1.3% | 30.0% | –5.8% (–11.4% to 0.0%) |
∗ Frequency values are reported for the variant allele at each SNP. CYP2C9 gene locations are taken from http://www.cypalleles.ki.se/cyp2c9.htm .
True genetic resistance to warfarin therapy, characterized by exceptionally high doses of warfarin (e.g., >100 mg/day) to achieve a target therapeutic range, is uncommon ( ). In instances in which inappropriate responses to warfarin are marked and persistent, the metabolites can be measured using high-performance liquid chromatography ( ). These measurements can also be used to confirm medication compliance. More commonly, drugs that affect S-warfarin metabolism may markedly alter the potency of warfarin treatment ( Box 43.1 ). Some drugs or herbal medications induce CYP2C9 and cause warfarin to be metabolized faster, necessitating an increased dose to achieve the same anticoagulant effect. Other drugs interfere with the metabolism of warfarin and, thus, enhance the anticoagulant effect. In fact, several hundred compounds are known to alter the metabolism of warfarin ( ). Antibiotics may interfere with the metabolism of warfarin or they may suppress the gut flora, which supplies a significant amount of vitamin K in humans. Rifampin, for example, may dramatically increase the metabolism of warfarin, thereby necessitating very high doses of warfarin to achieve anticoagulation ( ). Foods, particularly green leafy vegetables rich in vitamin K, may make anticoagulation with warfarin more difficult. Different warfarin products may vary in potency so that switching to, or between, different generic formulations may require more frequent monitoring and dosage adjustments ( ).
Warfarin is considered an agent with a narrow therapeutic index; a target INR has been established for most clinical indications in which it is used. For patients with atrial fibrillation and for the prevention and treatment of VTE, a target INR of 2.5 (range, 2–3) is recommended ( ; ). For patients with mechanical aortic valves (bileaflet and newer-generation tilting disk), the recommended target INR is also 2.5 (range, 2–3). The recommended target INR for patients with mechanical mitral valves and patients with older mechanical aortic valves (e.g., the caged-ball valve) is higher, at 3.0 (range, 2.5–3.5) ( ). The INR is regularly monitored to keep patients on warfarin therapy within the target range to prevent thrombotic complications but minimize the risk for potential hemorrhagic complications. The most important factor influencing the risk of bleeding is the intensity of anticoagulant therapy, represented by the target INR ( ). Other variables include a prior history of bleeding, advanced age, serious comorbid conditions such as renal insufficiency or liver disease, and the use of concomitant therapies, particularly antiplatelet agents ( ).
Efforts to improve the control of warfarin therapy have led to several advances, including the formation of anticoagulation clinics dedicated to the management of warfarin therapy and the development of point-of-care monitors that use capillary blood obtained by a fingerstick to provide immediate feedback with the INR result. These monitors measure a thromboplastin-mediated clotting time using a capillary whole-blood or nonanticoagulated venous whole-blood sample, converting the result to a PT and/or an INR ( ). Results obtained with different point-of-care PT instruments and laboratory-based instruments may not be comparable ( ). Indeed, even the patient specimen itself will vary, as the point-of-care monitor will use whole blood as opposed to the clinical laboratory, which will routinely use a citrated plasma sample. Quality assurance and quality control plans should be in place to oversee point-of-care INR testing ( ). In general, markedly out-of-range INR measurements obtained with a point-of-care monitor should be confirmed by analyzing a sample of citrated plasma in the clinical laboratory ( ).
Brodifacoum is a long-acting vitamin K antagonist, with a prolonged terminal half-life of 16 to 36 days ( ). Brodifacoum belongs to a class of long-acting anticoagulant rodenticides (LAARs) that were originally developed in the 1970s to overcome warfarin resistance in rodent populations ( ). Poisoning by LAARs has historically occurred after accidental/intentional ingestion or after occupational exposure ( ). In 2018, a multistate outbreak of LAAR poisoning was reported in >300 patients due to brodifacoum-tainted synthetic cannabinoids ( ). Clinically, affected patients present with a bleeding diathesis, most commonly with gross hematuria, oral mucosal bleeding, epistaxis, and gastrointestinal bleeding, although deaths have been reported ( ). On laboratory testing, patients have a prolonged INR (mean INR = 15) and select deficiency of vitamin K–dependent proteins (factors X, VII, IX, and II). Patients can undergo confirmatory anticoagulant testing, usually by quantifying LAAR levels using high-performance liquid chromatography, where brodifacoum and/or additional vitamin K antagonists will be detected (examples include difenacoum, bromadiolone, warfarin, and chlorophacinone). Initial management of brodifacoum and LAAR toxicity is focused on controlling bleeding and correcting the coagulopathy. All patients should receive oral vitamin K 1 . If the patient is actively bleeding and rapid correction of INR is required, intravenous vitamin K 1 should be administered as well. For patients with major bleeding, 4-factor prothrombin complex concentrate or fresh frozen plasma should be considered as first-line therapy. There is no consensus on vitamin K 1 dosing; however, patients should receive at least twice-daily dosing titrated to clinical signs of bleeding and laboratory test results ( ). Once bleeding has resolved and the PT/INR have significantly shortened, the patient can be transitioned to oral vitamin K 1 ( ). Because brodifacoum is fat soluble and has a high volume of distribution, prolonged maintenance treatment with vitamin K 1 is necessary. Again, there is no consensus on duration of therapy; however, in a review of LAAR poisoning, median duration of vitamin K 1 treatment was 140 days, up to 730 days ( ). During this time, monitoring of PT/INR is considered sufficient, as serum brodifacoum levels do not necessarily correlate with tissue levels ( ). Vitamin K 1 can be discontinued when it is no longer needed to maintain a normal PT/INR ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here