Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Arterial or venous thromboembolism is a major cause of morbidity and mortality. Arterial thrombosis is the most common cause of acute myocardial infarction, ischemic stroke, and limb gangrene, whereas deep vein thrombosis can lead to pulmonary embolism, which can be fatal, and to post-thrombotic syndrome. Most arterial thrombi are superimposed on disrupted atherosclerotic plaque because plaque rupture exposes thrombogenic material in the plaque core to the blood. This material then triggers platelet aggregation and fibrin formation, which results in the generation of a platelet-rich thrombus that can temporarily or permanently occlude blood flow (see [CR] ). In contrast to arterial thrombi, venous thrombi rarely form at sites of obvious vascular disruption. Although they can develop after surgical trauma to veins or secondary to indwelling central venous catheters, venous thrombi usually originate in the valve cusps of the deep veins of the calf or in the muscular sinuses, where they are triggered by stasis. Sluggish blood flow in these veins reduces the oxygen supply to the avascular valve cusps. Endothelial cells lining the valve cusps become activated and express adhesion molecules on their surface. These adhesion molecules tether tissue factor–bearing leukocytes and micro particles to the surface of activated endothelial cells, where the tissue factor triggers coagulation (see Chapter 121 ). Local thrombus formation is exacerbated by reduced clearance of activated clotting factors because of impaired blood flow. If the calf vein thrombi extend into more proximal veins of the leg, thrombus fragments can dislodge, travel to the lungs, and produce a pulmonary embolism.
Arterial and venous thrombi are composed of platelets and fibrin, but the proportions differ. Arterial thrombi are rich in platelets because of the high shear in the injured arteries. In contrast, venous thrombi, which form under low-shear conditions, contain relatively few platelets and are composed predominantly of fibrin and trapped red cells. Because of the predominance of platelets, arterial thrombi appear white, whereas venous thrombi are red in color, reflecting the trapped red cells.
Antithrombotic drugs are used for the prevention and treatment of thrombosis. Targeting the components of thrombi, these agents include (1) antiplatelet drugs, which inhibit platelets; (2) anticoagulants, which attenuate coagulation; and (3) fibrinolytic agents, which induce fibrin degradation ( Fig. 143.1 ). With the predominance of platelets in arterial thrombi, strategies to inhibit or treat arterial thrombosis are focused mainly on antiplatelet agents, although in the acute setting they often include anticoagulants and fibrinolytic agents. The observations that the addition of low-dose rivaroxaban, an oral factor Xa inhibitor, to dual-antiplatelet therapy reduces recurrent ischemic events in patients with acute coronary syndrome, and its addition to aspirin reduces the risk of major adverse coronary and limb events in patients with coronary or peripheral artery disease, highlight the potential usefulness of anticoagulants on top of antiplatelet agents for secondary prevention (see Chapter 145 and 146 ).
Anticoagulants are the mainstay of prevention and treatment of venous thromboembolism because fibrin is the predominant component of venous thrombi. Antiplatelet drugs are less effective than anticoagulants in this setting because of the limited platelet content of venous thrombi. Fibrinolytic therapy is used in selected patients with venous thromboembolism. For example, patients with severe pulmonary embolism as evidenced by hypotension benefit from systemic or catheter-directed fibrinolytic therapy. Catheter-directed fibrinolytic therapy also can be used as an adjunct to anticoagulants for the treatment of patients with deep vein thrombosis involving the iliac and femoral veins.
This chapter is focused on antithrombotic agents. In addition to describing antiplatelet, anticoagulant, and fibrinolytic drugs that are in current use, new agents in advanced stages of development also are discussed.
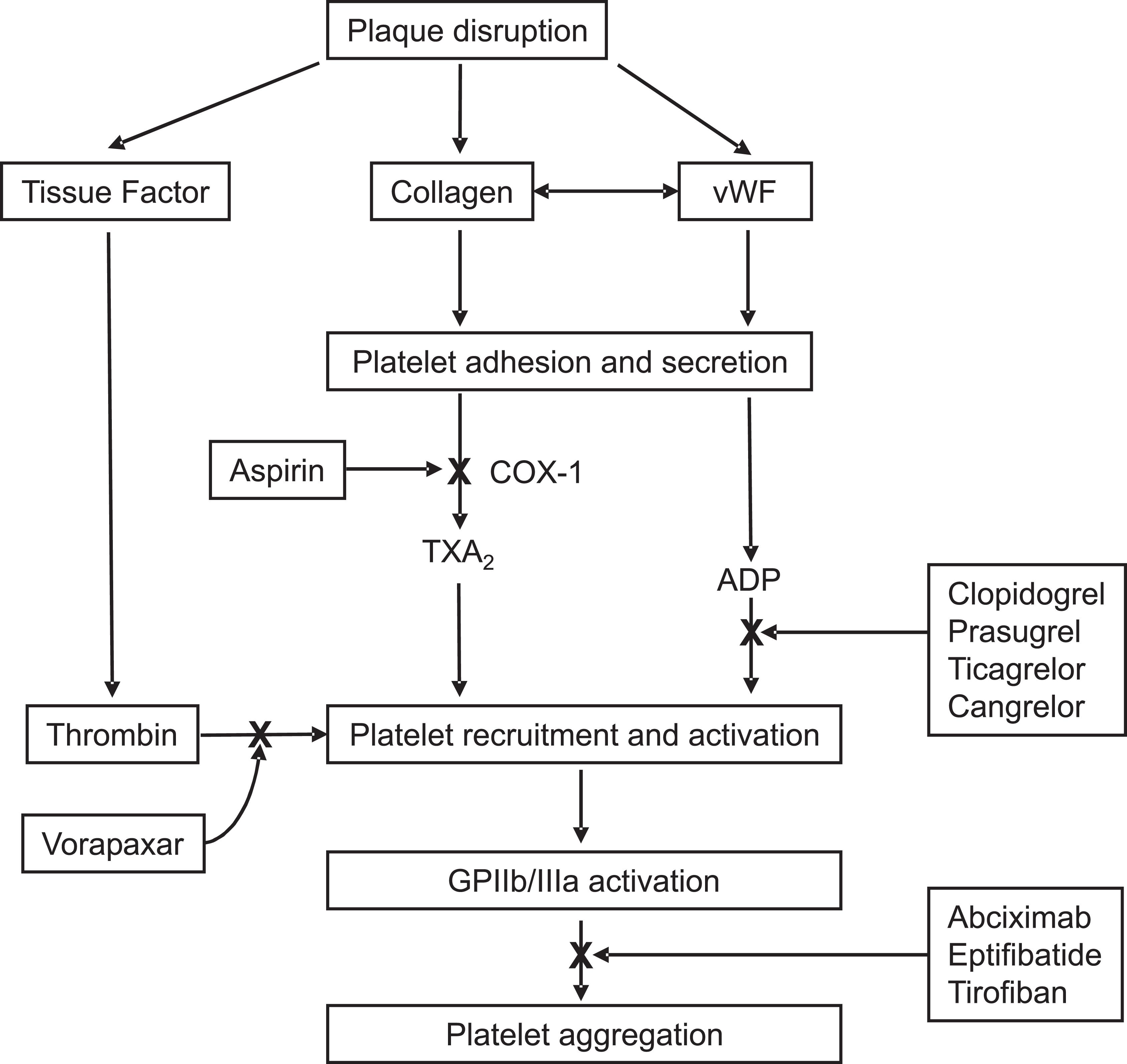
In healthy vasculature, circulating platelets are maintained in an inactive state by nitric oxide (NO) and prostacyclin released by endothelial cells lining the blood vessels (see Chapter 121 ). In addition, endothelial cells also express CD39—an ecto-ADPase—on their surface, which degrades adenosine diphosphate (ADP) released from activated platelets. When the vessel wall is damaged, the release of these substances is impaired and the subendothelial matrix is exposed. Platelets adhere to exposed collagen and to von Willebrand factor via glycoprotein (GP) VI and GPIb–IX, respectively; receptors are constitutively expressed on the platelet surface (see Chapter 124 ). Adherent platelets undergo a change in shape, secrete ADP from their dense granules, and synthesize and release thromboxane A 2 . Released ADP and thromboxane A 2 , which are platelet agonists, activate ambient platelets and recruit them to the site of vascular injury.
Disruption of the vessel wall also exposes tissue factor–expressing cells to the blood. Tissue factor initiates coagulation (see Chapter 125 ). Activated platelets potentiate coagulation by binding clotting factors and supporting the assembly of activation complexes that enhance thrombin generation. In addition to converting fibrinogen to fibrin, thrombin amplifies its own generation and serves as a potent platelet agonist, thereby recruiting additional platelets to the site of injury.
When platelets are activated, GPIIb/IIIa (α IIb β 3 ), the most abundant receptor on the platelet surface, undergoes a conformational change that enables it to ligate fibrinogen (see Chapter 124 ). Divalent fibrinogen molecules bridge adjacent platelets together to form platelet aggregates. Fibrin strands, generated through the action of thrombin, then weave these aggregates together to form a platelet–fibrin mesh.
Antiplatelet drugs target various steps in this process ( Fig. 143.2 ). The commonly used drugs include aspirin, thienopyridines (clopidogrel and prasugrel), ticagrelor, cangrelor, dipyridamole, GPIIb/IIIa antagonists, and vorapaxar. Each is briefly described in the following.
The most widely used antiplatelet agent is aspirin. As a cheap and effective antiplatelet drug, aspirin serves as the foundation of most antiplatelet strategies.
Aspirin produces its antithrombotic effect by irreversibly acetylating and inhibiting platelet cyclooxygenase-1 (COX-1), a critical enzyme in the biosynthesis of thromboxane A 2 (see Fig. 143.2 ). At high doses (about 1 g/day), aspirin also inhibits COX-2, an inducible COX isoform found in endothelial cells and inflammatory cells. In endothelial cells, COX-2 initiates the synthesis of prostacyclin, a potent vasodilator, and inhibitor of platelet aggregation.
Aspirin is widely used for secondary prevention of cardiovascular events in patients with coronary artery disease, cerebrovascular disease, or peripheral vascular disease. Compared with placebo, aspirin produces about a 25% reduction in the risk of cardiovascular death, myocardial infarction, or stroke in these patients. Aspirin is of limited benefit for primary prevention in subjects without established cardiovascular disease. If used for primary prevention, aspirin should be restricted for those at high risk of cardiovascular events.
Aspirin is usually administered at doses of 75 to 325 mg once daily. There is no evidence that higher-dose aspirin is more effective than lower aspirin doses, and some analyses suggest reduced efficacy with higher doses. Because the side effects of aspirin are dose-related, daily aspirin doses of 75 to 150 mg are recommended for most indications. When rapid platelet inhibition is required, an initial aspirin dose of at least 160 mg should be given.
The most common side effects are gastrointestinal and range from dyspepsia to erosive gastritis or peptic ulcers with associated bleeding. These side effects are, at least to some extent, dose related. The use of enteric-coated or buffered aspirin in place of plain aspirin does not eliminate the prostaglandin-mediated gastrointestinal side effects. The risk of major bleeding with aspirin ranges from 1% to 3% per year and is higher when aspirin is used in conjunction with anticoagulants, such as warfarin. When dual antiplatelet therapy is used, low-dose aspirin should be given (75 to 100 mg daily). Eradication of Helicobacter pylori infection and concomitant administration of proton pump inhibitors may reduce the risk of upper gastrointestinal bleeding, particularly in patients with a history of peptic ulcer disease.
Aspirin should not be administered to patients with aspirin allergy characterized by bronchospasm. This problem occurs in about 0.3% of the general population and is more common in those with chronic urticaria, asthma, nasal polyps, or chronic rhinitis. Hepatic and renal toxicity is observed with aspirin overdose.
The term aspirin resistance has been used to describe both clinical and laboratory phenomena. Clinical aspirin resistance is defined as the failure of aspirin to protect patients from ischemic vascular events. This is not a helpful definition, because it is made after a breakthrough adverse cardiovascular event occurs. Furthermore, it is not realistic to expect aspirin, which blocks only thromboxane A 2 –induced platelet activation, to prevent all vascular events.
Aspirin resistance also has been described biochemically as a failure of the drug to produce its expected inhibitory effects on tests of platelet function, such as thromboxane A 2 synthesis or arachidonic acid–induced platelet aggregation. Poor adherence is a common cause of apparent aspirin resistance (see Chapter 128 ). Even in those taking the drug, however, the tests used for the diagnosis of aspirin resistance are not well standardized. Furthermore, there is no definitive evidence that these tests identify patients at risk of recurrent vascular events, or that resistance can be reversed either by giving higher doses of aspirin or by adding other antiplatelet drugs. Until such information is available, testing for aspirin resistance remains a research tool.
P2Y 12 is the major ADP receptor on platelets. Agents that inhibit P2Y 12 include oral agents such as the thienopyridines and ticagrelor, and cangrelor, which is a parenteral inhibitor.
The thienopyridines include clopidogrel and prasugrel, as well as ticlopidine, which is rarely used.
The thienopyridines are structurally related drugs that selectively inhibit ADP-induced platelet aggregation by irreversibly blocking P2Y 12 (see Fig. 143.2 ). Clopidogrel and prasugrel are prodrugs that must be metabolized by the hepatic cytochrome P450 (CYP) enzyme system to acquire activity. Consequently, their onset of action is delayed unless loading doses are given. The metabolic activation of prasugrel is more efficient than that of clopidogrel. Consequently, prasugrel produces a more rapid, greater, and more uniform P2Y 12 blockade than clopidogrel.
When compared with aspirin in patients with recent ischemic stroke, myocardial infarction, or peripheral arterial disease, clopidogrel reduced the risk of cardiovascular death, myocardial infarction, and stroke by 8.7%. Therefore, clopidogrel is slightly more effective than aspirin, but it is more expensive. Clopidogrel and aspirin are often combined to capitalize on their capacity to block complementary pathways of platelet activation. For example, dual antiplatelet therapy with the combination of aspirin plus clopidogrel is recommended after stent implantation in the coronary arteries for management of acute coronary syndrome (see Chapter 145 ) or in the arteries of the leg for management of acute limb ischemia (see Chapter 146 ).
The combination of clopidogrel and aspirin also is effective in patients with unstable angina. Thus in 12,562 such patients, the risk of cardiovascular death, myocardial infarction, or stroke was 9.3% in those randomized to the combination of clopidogrel and aspirin and 11.4% in those given aspirin alone, a 20% relative risk reduction. However, combining clopidogrel with aspirin increases the risk of major bleeding to about 2% per year. This bleeding risk persists even if the daily dose of aspirin is 100 mg or less. Therefore, the combination of clopidogrel and aspirin should be used only when there is a clear benefit. For example, this combination has not proven to be superior to clopidogrel alone in patients with acute ischemic stroke or to aspirin alone for primary prevention in those at risk for cardiovascular events.
When compared with clopidogrel in 13,608 patients with acute coronary syndromes undergoing percutaneous coronary intervention (PCI), prasugrel reduced the combined rate of cardiovascular death, myocardial infarction, or stroke from 12.1% to 9.9%, a decrease driven mainly by a reduction in nonfatal myocardial infarction. However, prasugrel increased the rate of major bleeding and fatal bleeding, particularly in patients over the age of 65 years, in those weighing less than 60 kg, and in those with a history of stroke. Therefore, prasugrel should not be used in patients with these characteristics. Prasugrel was compared with clopidogrel in 7243 aspirin-treated patients under the age of 75 years with unstable angina or myocardial infarction without ST-segment elevation. At a median follow-up of 17 months, the primary endpoint, the composite of cardiovascular death, myocardial infarction, and stroke had occurred in 13.9% of the prasugrel group and in 16% of the clopidogrel group, a difference that was not statistically significant. Rates of major bleeding and intracranial bleeding were similar with the two treatment regimens. Therefore, prasugrel has no advantage over clopidogrel in medically managed patients with acute coronary syndrome.
Clopidogrel is given once daily at a dose of 75 mg. Because its onset of action is delayed for several days, loading doses of clopidogrel are given when rapid ADP receptor blockade is desired. For example, patients undergoing coronary artery stenting are often given a loading dose of 600 mg, which produces inhibition of ADP-induced platelet aggregation within 6 hours. Prasugrel is given as a loading dose of 60 mg followed by 10 mg once daily. Patients older than 75 years or weighing less than 60 kg should receive a daily prasugrel dose of 5 mg.
Gastrointestinal and hematologic side effects, other than bleeding, are rare with clopidogrel and prasugrel.
There is considerable between-patient variability in the capacity of clopidogrel to inhibit ADP-induced platelet aggregation. Reduced responsiveness to clopidogrel is termed clopidogrel resistance and platelets from patients with this phenomenon exhibit high reactivity to ADP. Reduced responsiveness to clopidogrel reflects, at least in part, genetic polymorphisms in CYP2C19, which is critical for metabolic activation of clopidogrel in the liver. Loss-of-function CYP2C19*2 and CYP2C19*3 alleles are found in 2% of Whites, 4% of Blacks, and 14% of Asians, and the levels of the active metabolite of clopidogrel are reported to be up to 33% lower in carriers of either of these alleles than in those with healthy alleles. Patients with clopidogrel resistance are reported to have a higher risk of ischemic complications after PCI, including myocardial infarction and stent thrombosis. Based on these findings, some experts recommend pharmacogenetic testing and point-of-care assessment of platelet reactivity to predict or assess clopidogrel responsiveness. However, attempts to manage such patients with higher doses of clopidogrel or with additional antiplatelet drugs have not resulted in improved outcomes. Instead, patients with clopidogrel resistance may benefit from a switch to prasugrel, which produces more uniform inhibition of ADP-induced platelet aggregation, or to ticagrelor.
An orally active agent belonging to the cyclopentyl-triazolopyrimidine class, ticagrelor acts as a direct inhibitor of P2Y 12 .
Ticagrelor reversibly binds to P2Y 12 at a location distinct from the ADP binding site and blocks ADP-mediated receptor activation in a noncompetitive fashion, likely through an allosteric mechanism. Because it does not require metabolic activation, ticagrelor has a more rapid onset of action than clopidogrel or prasugrel.
When compared with clopidogrel in 18,624 patients with acute coronary syndromes, ticagrelor reduced the rate of cardiovascular death, myocardial infarction, or stroke from 11.7% to 9.8%. All-cause mortality was also reduced with ticagrelor compared with clopidogrel (4.5% and 5.9%, respectively; P < .001), but ticagrelor produced more major bleeding not related to bypass surgery (2.8% and 2.2%, respectively). An invasive strategy was planned for 72% of the patients entered in the trial. In this subset, the primary composite endpoint occurred in 9% of patients randomized to ticagrelor and in 10.7% of those given clopidogrel, a 16% relative risk reduction. Rates of major bleeding were similar with ticagrelor and clopidogrel (11.6% and 16.5%, respectively; P = .88). Therefore ticagrelor also was superior to clopidogrel in patients undergoing coronary interventions.
If cost is not an issue and patients are compliant with twice-daily dosing, ticagrelor is a reasonable alternative to clopidogrel in patients with acute coronary syndrome, regardless of whether they are managed medically or undergo PCI with stent implantation. For these indications, ticagrelor should be given for at least 1 year. It also is reasonable to use ticagrelor in place of clopidogrel in patients with clopidogrel resistance or in those who develop in-stent thrombosis despite clopidogrel therapy.
Ticagrelor is given as a 180-mg oral loading dose followed by 90 mg twice daily. The dose does not need adjustment in patients with renal impairment, but caution is needed in patients with hepatic impairment or in those receiving potent inhibitors or inducers of CYP3A4 because ticagrelor is metabolized in the liver via CYP3A4. Ticagrelor is usually administered in conjunction with aspirin; the daily aspirin dose should not exceed 100 mg.
In addition to bleeding, the most common side effects of ticagrelor include dyspnea, which can develop in up to 15% of patients, and bradyarrhythmia. The dyspnea, which tends to occur soon after initiating ticagrelor, is usually self-limited and mild in intensity but can be persistent and may necessitate drug discontinuation in some patients. Although the exact mechanism responsible for these side effects is unknown, they may be adenosine-mediated because ticagrelor inhibits its reuptake.
Cangrelor is the only available parenteral inhibitor of P2Y 12 .
Cangrelor is a rapidly acting reversible inhibitor of P2Y 12 . It has an immediate onset of action after intravenous administration, a half-life of 3 to 5 minutes, and an offset of action within 1 hour.
Cangrelor is licensed for use in patients undergoing PCI, in whom it produces rapid ADP receptor blockade in those who have not received pretreatment with clopidogrel, prasugrel, or ticagrelor and are not receiving a GPIIb/IIIa inhibitor.
Cangrelor is administered as a 30 μg/kg intravenous bolus before PCI followed by an infusion of 4 μg/kg/min for at least 2 hours or for the duration of PCI, whichever is longer. When transitioning to oral P2Y 12 inhibitor therapy, ticagrelor can be given at a loading dose of 180 mg at any time during the cangrelor infusion or immediately after discontinuation. In contrast, loading doses of prasugrel or clopidogrel (60 and 600 mg, respectively) should be given only after cangrelor is stopped because cangrelor blocks the interaction of their active metabolites with P2Y 12 .
The major side effect of cangrelor is bleeding.
Dipyridamole is a relatively weak antiplatelet agent on its own. An extended-release formulation of dipyridamole combined with low-dose aspirin, a preparation known as Aggrenox, is used for the prevention of stroke in patients with transient ischemic attacks.
By inhibiting phosphodiesterase, dipyridamole blocks the breakdown of cyclic AMP (cAMP). Increased levels of cAMP reduce intracellular calcium and inhibit platelet activation. Dipyridamole also blocks the uptake of adenosine by platelets and other cells. This produces a further increase in local cAMP levels because the platelet adenosine A 2 receptor is coupled to adenylate cyclase ( Fig. 143.3 ).
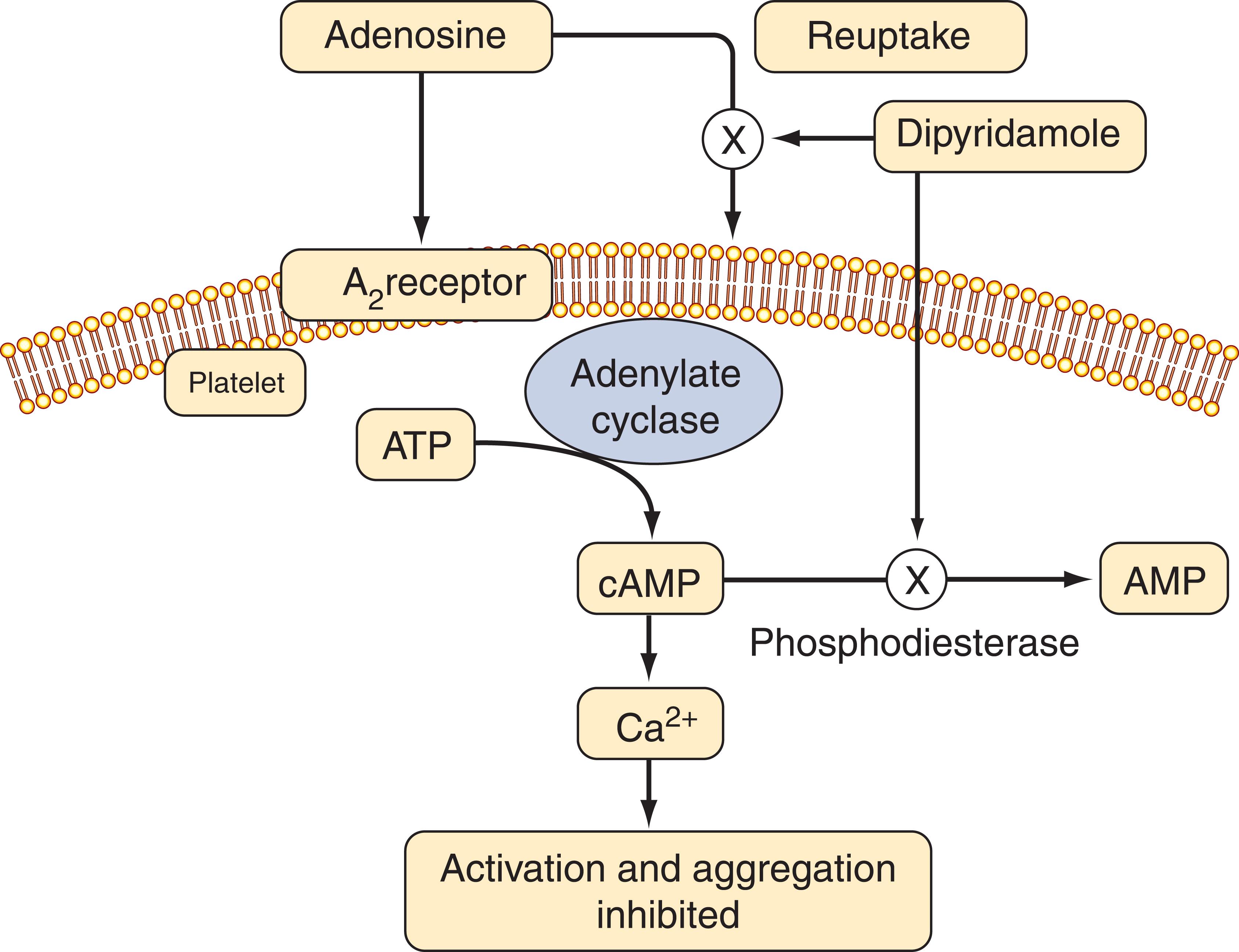
Dipyridamole plus aspirin was compared with aspirin or dipyridamole alone, or with placebo, in patients with an ischemic stroke or transient ischemic attack. The combination reduced the risk of stroke by 22.1% compared with aspirin alone and by 24.4% compared with dipyridamole alone. In a second trial, researchers compared dipyridamole plus aspirin with aspirin alone for secondary prevention in patients with ischemic stroke. Vascular death, stroke, or myocardial infarction occurred in 13% of patients given combination therapy and in 16% of those treated with aspirin alone. When Aggrenox was compared with clopidogrel, however, there was no difference in efficacy, and there was more intracranial bleeding with Aggrenox. Although Aggrenox is used for stroke prevention, because of its vasodilatory effects and the paucity of data supporting the use of dipyridamole in patients with symptomatic coronary artery disease, Aggrenox should not be used for stroke prevention in such patients.
Aggrenox is given twice daily. Each capsule contains 200 mg of extended-release dipyridamole and 25 mg of aspirin.
Because dipyridamole has vasodilatory effects, it must be used with caution in patients with coronary artery disease. Gastrointestinal complaints, headache, facial flushing, dizziness, and hypotension also can occur. These symptoms often subside with continued use of the drug.
As a class, parenteral GPIIb/IIIa receptor antagonists have an established niche in patients with acute coronary syndromes. The three agents in this class are abciximab, eptifibatide, and tirofiban.
A member of the integrin family of adhesion receptors, GPIIb/IIIa is found on the surface of platelets and megakaryocytes. With about 40,000 to 80,000 copies per platelet, GPIIb/IIIa is the most abundant receptor (see Chapter 124 ). Consisting of a noncovalently linked heterodimer, GPIIb/IIIa is inactive on resting platelets. When platelets are activated, inside-outside signal transduction pathways trigger a conformational activation of the receptor. Once activated, GPIIb/IIIa binds adhesive molecules, such as fibrinogen and, under high shear conditions, von Willebrand factor. Binding is mediated by Arg-Gly-Asp (RGD) sequences found on fibrinogen and von Willebrand factor, as well as by the Lys-Gly-Asp (KGD) sequence located within a unique dodecapeptide domain on the γ-chains of fibrinogen. Once bound, fibrinogen and/or von Willebrand factor bridge adjacent platelets together to induce platelet aggregation.
Although abciximab, eptifibatide, and tirofiban all target the GPIIb/IIIa receptor, they are structurally and pharmacologically distinct ( Table 143.1 ). Abciximab is a Fab fragment of a humanized murine monoclonal antibody directed against the activated form of GPIIb/IIIa. Abciximab binds to the activated receptor with high affinity and blocks the binding of adhesive molecules. In contrast to abciximab, eptifibatide, and tirofiban are synthetic small molecules. Eptifibatide is a cyclic heptapeptide that binds GPIIb/IIIa because it incorporates the KGD motif, whereas tirofiban is a nonpeptidic tyrosine derivative that acts as an RGD mimetic. Abciximab has a long half-life and can be detected on the surface of platelets for up to 2 weeks. Eptifibatide and tirofiban have shorter half-lives.
| Feature | GPIIb/IIIa Antagonists | ||
|---|---|---|---|
| Generic name | Abciximab | Eptifibatide | Tirofiban |
| Trade name | ReoPro | Integrilin | Aggrastat |
| Description | Fab fragment of humanized mouse monoclonal antibody | Cyclical KGD-containing heptapeptide | Nonpeptidic RGD mimetic |
| Specific for GPIIb/IIIa | No | Yes | Yes |
| Plasma half-life | Short (min) | Long (2.5 h) | Long (2.0 h) |
| Platelet-bound half-life | Long (days) | Short | Short |
| Renal clearance | No | Yes | Yes |
| Dosing | 0.25 mg/kg bolus followed by a 12-h infusion of 10 μg/min | Two 180 μg/kg boluses given 10 min apart | 25 μg/kg boluses followed by an 18-h infusion of 0.15 μg/kg/min |
| Adjustment for renal impairment | No | Yes | Yes |
In addition to targeting the GPIIb/IIIa receptor, abciximab also inhibits the closely related α v β 3 receptor, which binds vitronectin, and α M β 2 , a leukocyte integrin. In contrast, eptifibatide and tirofiban are specific for GPIIb/IIIa. Inhibition of α v β 3 and α M β 2 may endow abciximab with antiinflammatory and/or antiproliferative properties that extend beyond platelet inhibition.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here