Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Total ankle replacement (TAR) is increasingly recommended as a treatment option in patients with end-stage ankle osteoarthritis. Ankle arthrodesis can no longer be accepted as the sole gold standard procedure for end-stage ankle arthritis. Between 2005 and 2014, diagnosis-adjusted rates of TAR in the USA Medicare population increased 182% (7.6 to 21.5 per 1000 ankle arthritis diagnoses) while ankle fusion rated decreased 32% (34.5 to 32.3 per 1000 ankle arthritis diagnoses).
The high congruency of the ankle joint and the complex dynamic nature of the ankle’s axis of rotation have important implications in the design of total ankle prostheses. Incongruent surfaces and minimally constrained designs in early TARs led to high local stresses and increased polyethylene wear. The early use of fully congruous surfaces was expected to provide better resistance to wear as a result of better pressure distribution, but an inadequate restoration of three-plane rotation and glide led to loosening and early failure. The introduction of mobile-bearing implants was intended to solve this dilemma by combining congruence with minimally constrained components to enable the soft tissues to control physiological motion at the joint ( Fig. 23-1 ).
The success of uncemented, metal-backed TAR in the 1990s has led to a resurgence in innovation, refinement in implant design, and expansion of study of all aspects of the procedure and its outcomes. Evolution of three-component designs tends toward replication of ankle anatomy to further minimize stress experiences by the implants. Modern two-component designs restore compatible function of the ankle’s ligaments and articular surfaces, but with slighstly nonanatomical shapes of the articular surfaces ( Fig. 23-2 ). Highly cross-linked polyethylene (HXLPE) is used in some designs, bone-ingrowth surfaces have progressed, both anterior and lateral approaches to the ankle may be considered, and newer three-dimensional (3D) printed custom patient-specific guides and implants have become available. These designs, reflecting ankle kinematics and varied fixation approaches, hope to achieve outcomes and longevity similar to knee and hip replacement. Whether these hopes are warranted are not yet fully known; however, intermediate-term results with the newer approaches appear to justify the optimism.
Despite advances in implant technology, both short- and long-term success still relies on prudent patient selection and understanding lessons learned about the procedure. Minimizing complications depends on refined indications, meticulous handling of soft tissues, less dissection for lower-profile implants, and better instrumentation. Complications should be identified and addressed early to maximize chances of implant retention. Knowledge of expected functional and patient-reported outcomes helps guide an informed discussion with patients considering TAR. Surgeons should respect the learning curve associated with TAR to maximize chances of success. Further independent studies with greater numbers of patients and longer follow-up periods are necessary to understand which factors affect improved outcomes.
The first report of an artificial implant in the ankle was a 40-year follow-up on a Vitallium talar resurfacing in 1962 through a transfibular approach who had excellent and long-lasting clinical success. The first series of ankle replacements, reported by Lord and Marotte, used a total hip prosthesis with the femoral stem inserted in the tibia and the acetabular cup implanted into the calcaneus. The high failure rate led the authors to recommend ankle-specific implants would be necessary for success given the complexity of ankle motion.
Successful innovation in total hip and knee replacement in the 1970s led to a period of experimentation in total ankle implant design. Competing themes of constraint, congruency, and geometry led to at least 17 devices that serve as the base of our current implant design knowledge. A number of outstanding review articles detail the history and evolution of these initial attempts.
When evaluating historical and current implant designs, a variety of implant- and procedure-specific characteristics should be considered to help gauge innovation and define advances against what has been tried and failed ( Table 23-1 ). As failures of first-generation designs lead to success of second- and third-generations, we see the decisions converge and the variations narrow.
| Procedure | Bearing | Bone Interface | |
|---|---|---|---|
| Type |
|
|
Fixation strategy: cemented, bone ingrowth |
| Material | Amount of bone resected |
|
|
| Construct | Joints resurfaced superior talus medial gutter lateral gutter subtalar talonavicular |
|
|
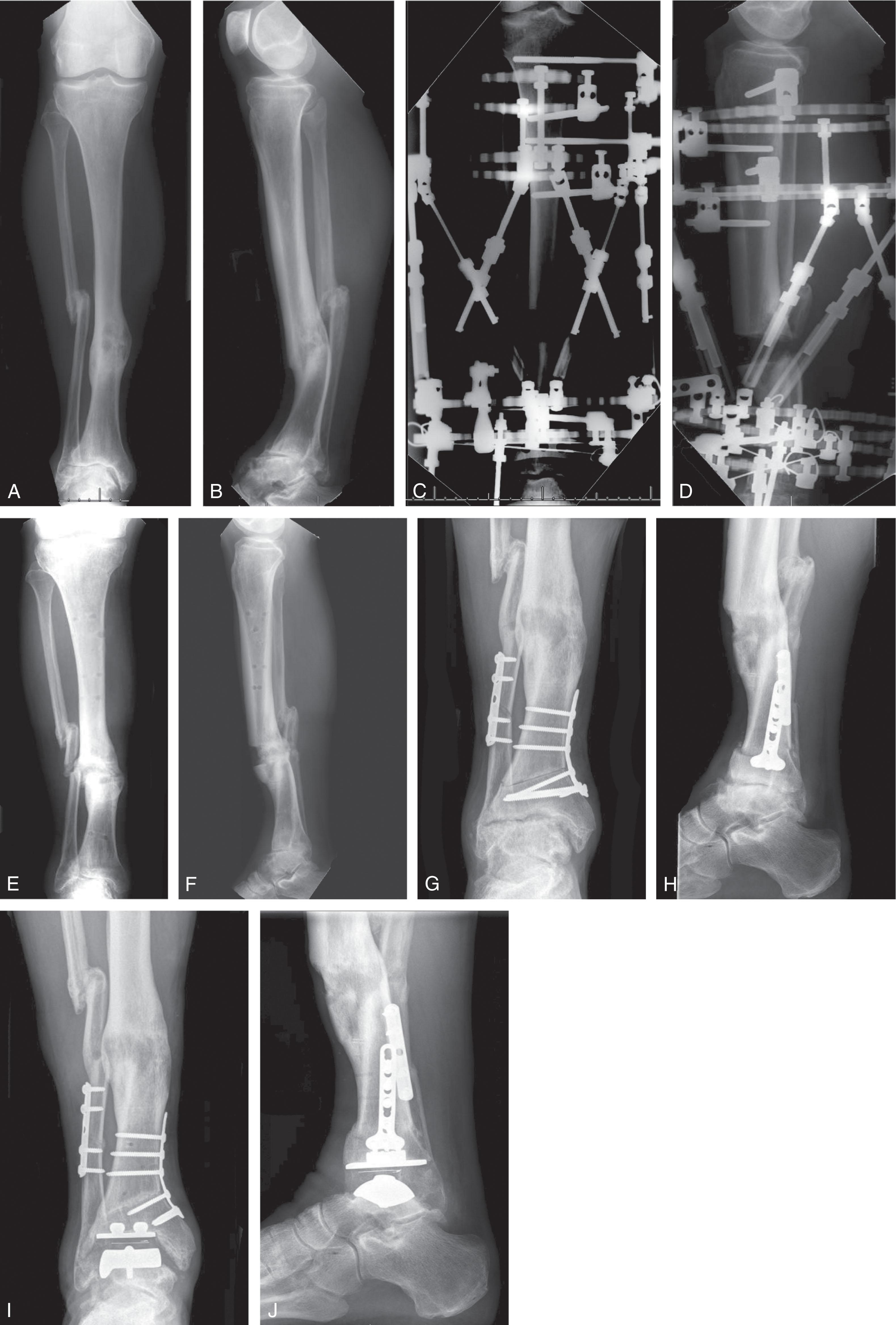
First-generation TAR designs used in the 1970s and early 1980s are characterized by use of bone cement for fixation, which was the standard for hip and knee arthroplasty fixation at the time ( Table 23-2 ). Most were two-component prostheses using a concave polyethylene block in the tibia and a convex metal talus component, though some reversed the relationship favoring the thicker talar component be made of polyethylene ( Fig. 23-3 ). Cobalt chrome alloys were typically used for the metal components. Both constrained and unconstrained designs were advanced.
| Implant | Year Introduced | Description | Results and Observations |
|---|---|---|---|
| Preliminary Implants | |||
| Muir | 1962 | Custom Vitallium talar resurfacing | A machinist with posttraumatic arthritis underwent talar resurfacing through a lateral transfibular approach with an implant that mirrored femoral head resurfacing implants of that time. After 40 years he had AOFAS score of 85, no pain, 35° plantarflexion, and finished his career. |
| Lord and Marotte | 1970 | Modified total hip stem and acetabular cup | The stem is inverted in the tibia and the acetabular cup is implanted in the calcaneus. In 25 ankles, 7 were satisfied and 12 failed. The authors suggest ankle-specific implants will be necessary to match the complexity of ankle motion. |
| First-Generation TAR (Two-Component, Cemented)—Comparison Studies | |||
| Constrained vs. unconstrained | 1974 |
|
The rate of radiolucency was 88% and implant loosening 10% at mean 1.3 year follow-up. Constrained implants tended to loosen while unconstrained implants tended to subluxate. Clinical results and functional improvement were poor. |
| TAR vs. ankle fusion | 1986 | 25 TAR: 11 Oregon, 6 TPR, 5 Smith, 1 Newton, 1 Mayo, 1 Buchholz; 18 ankle fusions | At mean 3.8 year follow-up, complications were similar including infection (16% TAR, 28% fusion), loosening (20%), and nonunion (33%). TARs did better in rheumatoid than posttraumatic ankles. |
| First vs. second generation TAR | Cemented TPR, cementless STAR | Two comparison studies including 27 cemented TPR and 26 cementless hydroxyapatite-coated STAR confirm greater periprosthetic lucencies (TPR 53%–67% vs. STAR 0%–16%) and failure (TPR 19% vs. STAR 0%). The TPR had 12-year survival of 87% while the STAR had 6-year survival of 100% for metal components and 94% including polyethylene revision. | |
| First-Generation TAR (Two-Component, Cemented)—Constrained Designs—Case Series | |||
| Imperial College of London Hospital (ICLH) TAR | 1972 | Concave polyethylene tibia and convex cylindrical metal talus | In 75 ankles, 70% reported acceptable results with overall high complication rate, especially delayed wound healing. In 41 ankles reviewed at 5.5 years, only 13 had satisfactory results, and 13 were converted to fusion. In 18 ankles at 3 years, there were 11 cases of loosening or lucent regions >2 mm. Other report include in vitro wear analysis and history of the design evolution. |
|
1973 | Semi-constrained, lateral approach | In 15 ankles with 10-year follow-up, 11 early and 32 late complications required conversion to arthrodesis in most patients. |
| Mayo TAR | 1974 | Constrained (Mayo I) and semi-constrained (Mayo II) designs with polyethylene tibia and metal talus | Gait analysis showed TAR improved load bearing and restored ankle range of motion but cadence remained diminished. In 102 ankles at 1.9 years, over 90% reported pain relief and 55% improved motion, but 54% of ankles with posttraumatic arthritis and 23% of ankles with rheumatoid arthritis had complications resulting in 7 revisions and 10 fusions. In 204 ankles at 9 (2–17) years, 5-, 10-, and 15-year survivorship was 79%, 65%, and 61%, respectively, with young age an independent risk for failure. In 15 rheumatoid ankles (14 Mayo TAR) at 3.3 years, patients had pain relief and improved ROM, but 73% had radiolucency and 40% subsided ; by 5.6 (2–10) years clinical scores deteriorated, 93% had talar subsidence, and 80% had tibial subsidence. |
| New Jersey Cylindrical Replacement | 1974 | Cylindrical metal tibia and polyethylene talus | Although the clinical results with the first-generation of the New Jersey Cylindrical Replacement design were not satisfactory, the authors identified numerous problems regarding the design of TARs that were addressed in later generation TAR. |
| Conaxial (Beck-Steffee) TAR | 1975 | Concave polyethylene tibia and convex metal talus | In 30 total ankles reviewed at 10.8 years, early complications included 39% wound-healing problems, 22% fractures, and 14% impingement. At 2 years, 27% showed signs of loosening or subsidence, and at 10 years, 90% showed loosening. |
| Oregon TAR | 1975 | Single axis | In 71 ankles at 6.5 years, reoperation was necessary in 19 including 11 fusions and 5 revisions. |
| TNK (first generation and early second generation) | 1975 (first generation) 1980 (second generation) | Metal on polyethylene (first generation) and ceramic on polyethylene (second generation) | In 30 metal and 12 ceramic cemented implants, poor midterm results (27% of 33 satisfactory at 8 years) were found. |
| Thompson-Parkridge-Richard (TPR) | 1976 | Semi-constrained polyethylene tibia with concave surface and side rails | In 23 ankles followed at 5 years, only 69% of patients were satisfied with their results, 52% had radiolucent lines, and 2 required revision. |
| Scandinavian Total Ankle Replacement (early STAR) | 1981 | Cylindrical metal tibia and polyethylene talus | In 28 ankles, 7 failed resulting in survivorship of 70% at 12 years and excellent but slowly declining improvement in clinical outcome scores. Comparison of rheumatoid and osteoarthritic ankles shows similar 14-year survival of 73%–75%. |
| First-Generation TAR (Two-Component, Cemented)—Unconstrained Designs—Case Series | |||
| (Richard) Smith TAR | 1972 | Ball and socket (spherocentric) with metal tibial socket and polyethylene talar ball | In 18 ankles at 7 years, 7 developed loosening of the tibia resulting in 3 revisions and one fusion. Delayed wound healing occurred in 7 patients. Most patients had substantial pain relief and 16 were satisfied with the procedure. In 21 ankles at 25–30 months, there were 5 failures but improved pain and function in the nonfailures. |
| Newton TAR | 1973 | Cylindrical polyethylene tibia and spherical metal talus | In 50 ankles with minimum 1 year follow-up, 74% of posttraumatic patients had radiographic lucent lines and nearly 50% failed. Incongruity between the tibial and talar components was felt to lead to accelerate polyethylene wear. |
| Irvine (Howmedica) TAR | 1975 | Metal-backed polyethylene tibia and metal talus with a toroidal shape to match the medial and lateral talar radii of curvature | The morphology of 32 ankles was measured to create a talus meant to match normal anatomic axes of rotation. In 25 Irvine ankles and 4 Smith ankles, pain relief and functional improvement was seen at 0.8 years, with 2 malalignment and 2 failures noted. Failure was felt to be from unacceptable loading of surrounding soft tissue due to the unconstrained design. |
| Bath-Wessex TAR | 1984 | Polyethylene tibia and metal talus | In 69 ankles with rheumatoid arthritis, there was 83% 5-year survivorship decreasing to 66% at 10 years, and all showed signs of loosening, resulting in 12 fusions and 6 revisions. |
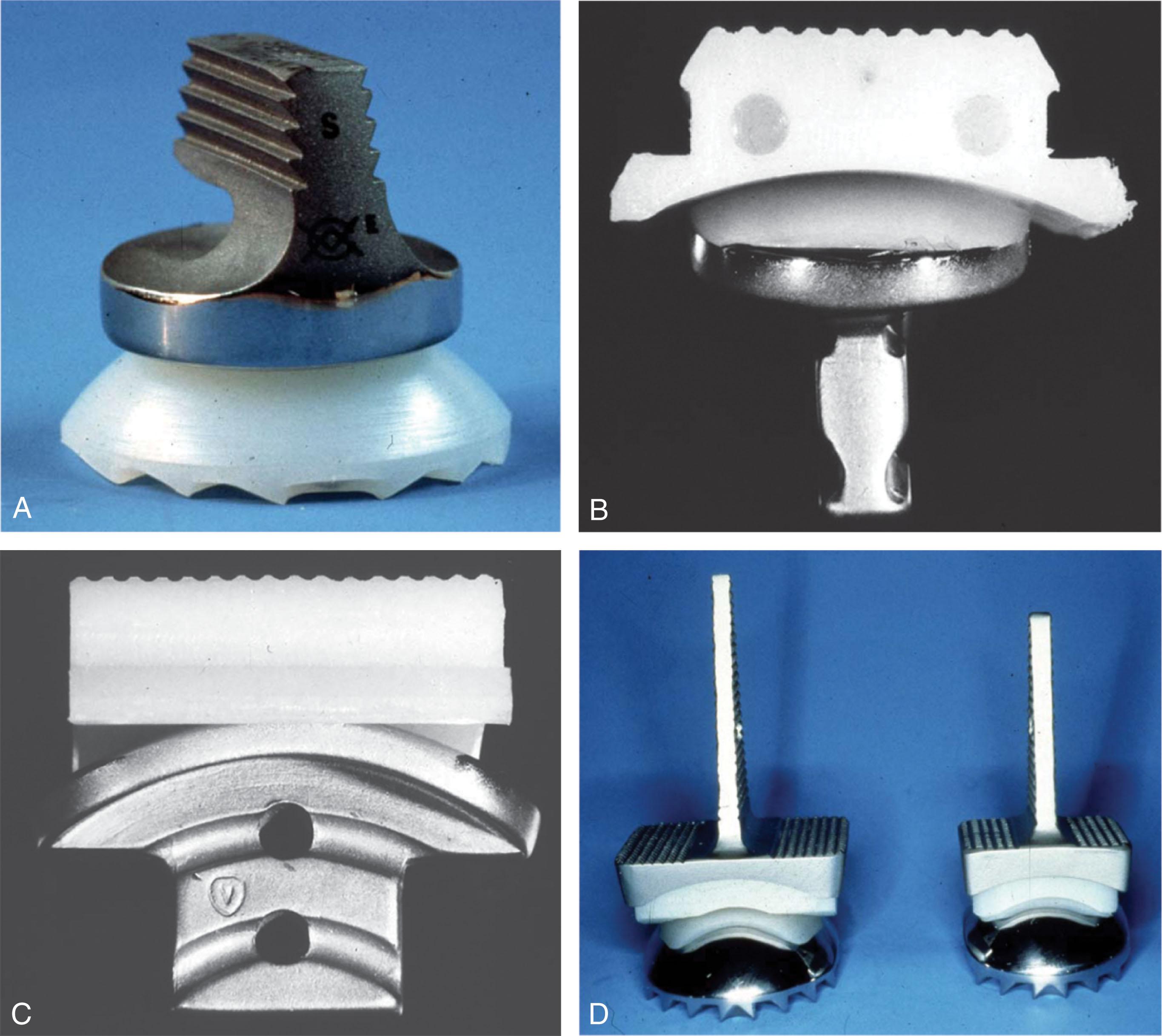
However, first-generation TARs were recognized to have unacceptably high complication rates, including aseptic loosening on both tibial and talar sites, wide osteolysis with cyst development, subsidence, and mechanical failure of prosthesis components (see Table 23-2 ). Most clinical series from that period included 20 to 40 patients followed for an average of 5 years or less yet showed rates of radiographic loosening from 22% to 75%. First-generation TAR designs also experienced a high incidence of wound-healing problems. These problems led most authors of the time to recommend ankle fusion as the primary treatment option for ankle osteoarthritis.
Unconstrained designs suffered from premature loosening and unacceptable loading of surrounding soft tissue. The loosening was perhaps related to excessive polyethylene wear debris resulting from their incongruent design placing greater pressure on a smaller surface area. Size mismatch between components meant to promote a physiologic range of motion as well as axial rotation may have promoted shear forces, which also results in polyethylene wear, especially in patients with preexisting chronic ligamentous instability.
Constrained designs failed by premature loosening, subsidence, or by dislocation of the components. It was later recognized that the constrained nature of the implant and the lack of recognition of the 3D nature of ankle motion transferred excessive forces to the cement-bone interface. In addition, cement fixation required greater bone resection to accommodate the volume of cement, placing the implant in bone with reduced mechanical quality, especially in the tibia. Modern techniques of cement preparation were not in use at the time, which we now know may affect bone-implant interface stability as well.
Failure analysis of first-generation total ankle arthroplasties showed that only significant improvements in prosthetic design incorporating more conservative bone-sparing cuts, a change of fixation (elimination of cement), and greater recognition of natural ankle kinematics would change arthroplasty outcomes, making this procedure a valuable treatment option in patients with end-stage ankle osteoarthritis. During the first-generation period, designers recognized that designs more closely matching the ankle’s natural motion may reduce the problems seen with highly constrained or unconstrained designs. Average talar morphology was mapped in an effort to recreate natural bony and soft tissue constraints.
In the early 1980s, new designs with unique innovations were introduced to address the issues of too much and too little constraint, as well as issues with poor bone quality and cement use ( Table 23-3 ). New biologic interfaces with porous coatings and/or the addition of hydroxyapatite were incorporated into second-generation designs, making them more biocompatible as demonstrated by increased periarticular bone mineral density as the implant becomes integrated. While these implants were successful as measured by improved survival compared with first-generation implants, ankle fusion remained the choice of most surgeons for end-stage ankle arthritis during this period.
| Implant | Year Introduced | Description | Results and Observations |
|---|---|---|---|
| Two-Component ‡ Metal or Ceramic on Polyethylene—Case Series | |||
|
1984 | Semi-constrained, requires syndesmosis fusion | In 100 ankles, the designer’s series had 95% survival at 5 years and 85% survival in 132 ankles at 10 years. Other reports show only 80% 5-year survival, 67% 6-year survival, and 70% 9-year survival. Patients report excellent pain relief and improved AOFAS scores, though radiolucency was seen in 76%–85% and implant migration were seen in 45%. Failure of syndesmosis fusion is associated with implant failure. Explant studies show wear debris–induced osteolysis, perhaps related to edge loading, could contribute to component loosening or polyethylene fracture. |
| TNK |
|
Ceramic on polyethylene (second generation) and ceramic with a hydroxyapatite-coated beaded alumina surface on polyethylene (third generation) | Initial comparison of metal or ceramic on polyethylene implants shows uncemented (67% of 30 satisfactory at 4 years) outperforms cemented (27% of 33 satisfactory at 8 years), though unequal follow-up and mixed implant types may bias results. First- and second-generation TNK implants have high rates of loosening and subsidence (100% at 17 years, 77% at 13 years respectively). In 70 third-generation implants at 5 years, 3 (4%) failed, and the remainder had improved pain and functional scores, though osteoarthritic patients may do better than rheumatoid patients in some but not all series. 73 Additional hydroxyapatite coating in 16 rheumatoid ankles at 2 years still resulted in half of ankles having radiolucency but component migration was not seen. |
| Three-Component ¶ Metal on Polyethylene—Case Series | |||
| Low Contact Stress (LCS)Buechel-Pappas Mark I & Mark II (BP) |
|
Unconstrained semi-congruent, stemmed tibia. The Mark I design had a shallow sulcus in the talus while the later Mark II had a deeper sulcus. | This redesign of the New Jersey TAR is the first reported mobile-bearing three-component design. The designer’s series of 40 ankles with the Mark I design followed an average 12 years had 70% good to excellent results, 25° range of motion, and 20 year survivorship of 74%. The Mark II design in 75 ankles had a 12-year survivorship of 92%. Short-term results have been independently confirmed with 88%–97% survivorship at 5 years, 89% at 8 years, and 86%–90% at 10 years, but high rate of painful impingement and intraoperative fracture. In 101 ankles, functional outcome scores were moderate to poor by 16 years. |
| Scandinavian Total Ankle Replacement (STAR) |
|
|
A prospective comparison of 158 uncemented STAR and 66 ankle fusion at 2 years found TAR improved Buechel-Pappas score more than fusion (40.5 ± 15.1 vs. 26.3 ± 17.1 * ) but they had comparable decrease in VAS (51.8 vs. 44.6, NS). In 68 ankles at 3.7 years, 67% were graded good to excellent and AOFAS improved from 25 to 84 * , but 13% required revision, 63% developed heterotopic bone, and 4% developed osteolysis. In 51 uncemented, HA-coated ankles, 5-year survival was 70%, with better results as the surgeon gained experience. In 52 ankles, 5- and 8-year survival were 90% and 84%, with improved AOFAS scores. In 200 consecutive uncemented STAR, 5-year survival was 93% and 10-year survival 80%. In 84 STAR, 10-year survival was 90% and 15-year survival 73%. In 77 ankles, 10-year survival was 71% and 46% at 14 years, with failures primarily related to loosening, subsidence, osteolysis, and polyethylene fracture. In 34 STAR, 15-year survival was 63.6% (95% CI, 30.8%–89.0%). In 200 STAR, 16-year survival was 76% (95% CI 64%–88%). In 50 STAR, 19-year survival of all metal components was 75% (95% CI 53%–88%) and survival including mobile-bearing polyethylene was 55% (34%–71%). Issues somewhat unique to the congruent mobile bearing design include edge-loading and polyethylene fracture. |
| Ramses | 1989 | Semi-constrained cemented (1989), uncemented (2000), tibial pegs | In 69 ankles the design group reports 88% survival at 10–14 years. However, New Zealand registry data of 11 ankles shows 2 failures (18%) at 18 months. A mixed series including 66 Ramses, 8 STAR, and 36 LCS implants with 3-year follow-up found pain improvement in 70% of cases but no increase in range of motion. |
† Uncemented, implants have surface modifications meant to improve bony ingrowth, though they may not be approved for uncemented use in the United States.
‡ Two-component, two metal or ceramic components with polyethylene-bearing surface fixed to the tibial component.
¶ Three-component, two metal components with an interposed polyethylene bearing that has mobile articulations with both metal components. AOFAS , American Orthopaedic Foot & Ankle Society hindfoot scale; HA , hydroxyapatite.
Examples of second-generation TARs include the Agility, Buechel-Pappas, and Scandinavian Total Ankle Replacement (STAR) ( Fig. 23-4 ). The Agility prosthesis used a semi-constrained two-component design. A metal tibial implant affixes to an ultra-high molecular weight polyethylene (UHMWPE) insert, creating a non-mobile tibial component that allows some triplane movement between the tibia and talus. To address issues with tibial bone quality and cement use, the implant relies on fusion of the tibiofibular syndesmosis, creating a broader area for implantation, and porous coated surfaces for bony ingrowth. The Low Contact Stress (LCS) and Beuchal-Pappas implants similarly used a semi-constrained design and porous coated metallic surfaces, but included a freely mobile polyethylene insert that allows motion at both the tibial and talar bearing surface (three-component design). This allows multiplanar motion with the hope that these stresses are not transferred to the bone-metal interface. The STAR evolved from a two- to three-component design with a flat interface between the tibia and upper polyethylene and a congruent cylindrical interface between the lower polyethylene and the talus. Porous surfaces and hydroxyapatite coating were used promote bony ingrowth.
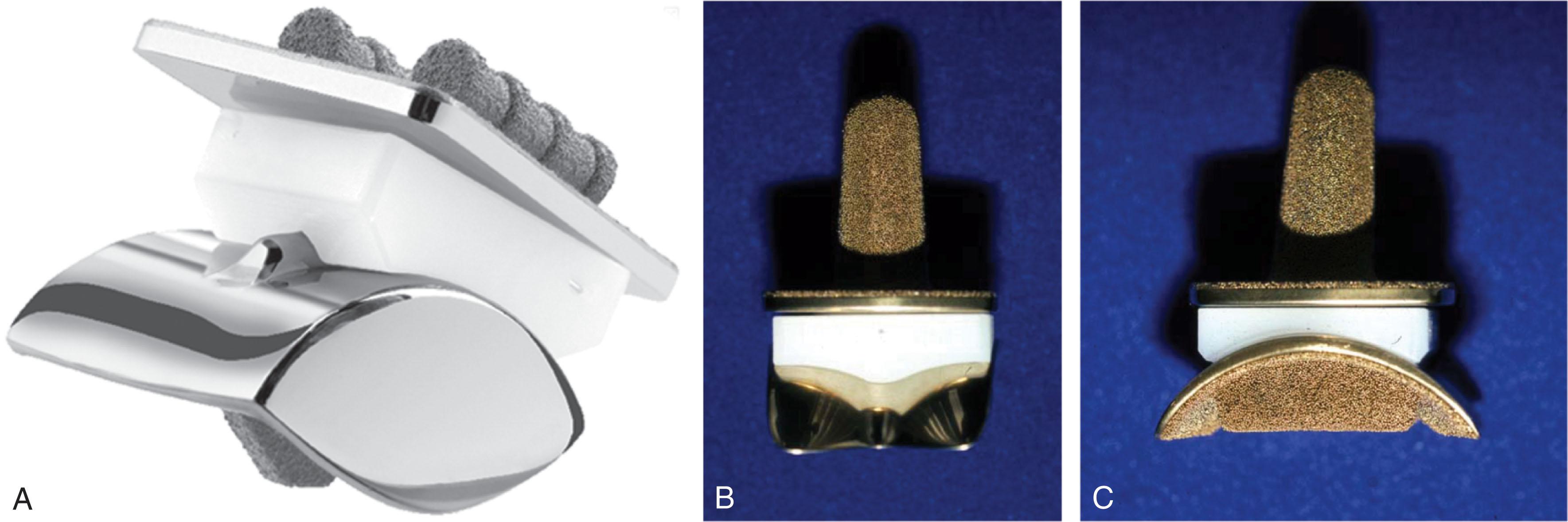
A large number of implants were introduced over a short period based on the success of the Beuchal-Pappas and STAR designs ( Table 23-4 ). In Europe, these were typically introduced with three components, while peculiarities of the United States Food and Drug Administration (FDA) approval process led to a number of two-component designs that appear very similar to their three-component inspirations other than the polyethylene bearing being locked to the tibial implant. The FDA also requires cementation for most implants in the United States, though the ingrowth surfaces of these modern implants allow for uncemented use. The exception in the United States is the STAR, which is the only approved three-component design and the only one approved for uncemented use.
| Implant | Year Introduced | Description | Results and Observations |
|---|---|---|---|
| Three-Component ¶ Metal on Polyethylene—Case Series | |||
| Alphahorn | 1996 | Unconstrained, semi-congruent with stemmed tibia and talar resurfacing | |
| Salto | 1997 | Unconstrained, semi-congruent, keel/peg for tibial fixation and anatomically inspired talus with cage | In 98 ankles followed prospectively by the implant designer, 10-year survival was 85% with failures attributed to osteolysis, polyethylene failure, or intractable pain. The surgery-free survival at 10 years was 65%, demonstrating a high rate of secondary surgeries. In 55 consecutive ankles, 5-year survival was 93.3%. In 60 ankles with posttraumatic arthritis followed 4.9 (1.8–7.6) years, 85% were still implanted, 20% had symptomatic bone cysts, and 42% had secondary surgeries. |
| Ankle Evolutive System (AES) | 1999 | Unconstrained, semi-congruent, modular stem tibia, talar resurfacing, HA coated | Initial studies with few patients and short-term follow-up found generally good results with high rate of revision and malleolar fracture. In 93 ankles, 5-year survival was 90%, and in 38 ankles 6-year survival was 95% but 24% had radiological osteolysis. This was confirmed in 50 prospectively followed ankles at minimum 2-year that showed tibia/implant interface cysts greater than 5 mm in 62% of cases and talar/implant interface cysts in 43%, in 77% of 18 ankles at 3 years, and in 79% of 36 ankles at 2 years. Examination of the contents of osteolysis cavities were consistent with foreign body reaction and were three times as likely after the implant changed to a hydroxyapatite on titanium ingrowth surface. In a prospective study of 50 ankles, 10-year survival was 68% and AOFAS scores decreased over the study period. High rates of osteolysis and early failure led to withdrawal of this implant from use. |
| HINTEGRA | 2000 | Unconstrained, congruent with conically shaped railed talus, peg fixation with optional screw augmentation | In 122 ankles at 1–2 years, clinical results show 82% good to excellent outcomes. In 722 ankles, 5- and 10-year survival was 94% and 84%, respectively. In 50 ankles at 3.8 years, AOFAS increased from 43.5 to 83.8, ROM increased from 23.3° to 28.3°, but osteolysis was noted in 24 (48%) of ankles, and 14 year survival was 90%. |
| Mobility | 2002 | Unconstrained, semi-congruent with short tibial stem and talar resurfacing | In 240 ankles at 2.7 years, the developers report 8% reoperation and 2% failure follow-up. AOFAS score improved 48 ± 18 to 84 ± 12. * , In 100 ankles 4-year survival was 94% with osteolysis seen in 15% of ankles. Overall, there are similar outcomes to HINTEGRA. |
| Bologna-Oxford (BOX) | 2003 | Congruent, spherical convex tibia and talus with large sagittal radius of curvature | Design based on finite element analysis modeling of stereophotogrammetric analysis of cadaveric ankle motion that respects ligament-guided motion. A multicenter prospective trial of 158 ankles followed mean 1.4 years found 1.3% revision and 4.4% additional procedures. In 62 ankles, 3.5-year survivorship was 92% with 78% patient satisfaction, but radiolucency was noted in 16 (26%) tibia and 4 (6%) tali. In 51 patients at 4 years, AOFAS scores increased from 38.5 to 79.0 with 97% survival. In 34 ankles at 4.8 years, only one required revision (97% survival), VAS improved from 7.5 to 3.1, * and all MOXFQ subscales improved. In 75 ankles with 6.5 ± 1.1 year follow-up, AOFAS scores (37 ± 5 to 78 ± 8), dorsiflexion (1 ± 2° to 18 ± 7°), and plantarflexion (12 ± 4° to 18 ± 7°) all improved ( P <0.05), and 97.3% remained implanted. |
| Ceramic Coated Implant (CCI) | 2003 | Congruent with short tibial fin and pegged talus resurfacing, with central sulcus | Twenty-eight (19%) of 151 CCI implants from the Swedish Total Joint Registry required revision, primarily for component loosening. In 58 ankles at 1.8 years, 12 (21%) underwent component revision including polyethylene exchange, and radiolucencies are noted to be increasing with duration of follow-up. |
| TARIC | 2006 | Congruent, with tibial and talar fins and talar sulcus | |
| German Ankle System | 2007 | Congruent, spherical convex tibia wit pegs and conical talus with side rails | Simulated motion using a 6 degree-of-freedom robotic arm in seven paired cadavers, compared with the HINTEGRA TAR showed the German Ankle System had smaller increases in forces and torques compared with the HINTEGRA prosthesis relative to native, noninstrumented ankles, suggesting this implant may show improved long-term wear and reduced aseptic loosening. |
| Zenith | 2007 | Unconstrained semi-congruent design with stemmed tibia and talar resurfacing with surface coating to promote bony ingrowth | A comparison between 19 rheumatoid and 45 osteoarthritic ankles replaced with the Zenith TAR found no difference in perioperative revision rates, complications, or functional outcomes at 3 years with the numbers available. |
| Rebalance | 2011 | Unconstrained semi-congruent design with tibial stem | In 267 TAR, 6-year survival was 88.3%, with 90% of patients very satisfied or satisfied. |
| Vantage | 2017 | Three- or two-component, semi-constrained, tibial with small cage fixation, and cylindrical talar-bone interface, pegged | In 49 ankles after three-component Vantage TAR followed 12 months, AOFAS improved 42.12 ± 2.42 to 96.02 ± 0.82 * and VAS improved 6.70 ± 0.28 to 0.26 ± 0.12. * ROM improved from 22.55 ± 1.51° to 45.43 ± 1.56°. * Two cases (4%) showed a radiolucency and one (2%) a tibial periprosthetic cyst. |
| Two-Component ‡ Metal on Polyethylene—Case Series | |||
| ESKA | 1990 | Medial or lateral approach with osteotomy. Tibial pegs | In the developer’s series, 17 of 20 (85%) survived 5–10 years and 8 of 12 (67%) survived 10–15 years, with improved pain and function scores. |
|
2005 |
|
In 194 TAR, VAS improved from 70 ± 21 to 14 ± 20 * and AOFAS improved from 40 ± 17 to 78 ± 14, * with survival of 94% (mixed INBONE I and II) at 3.7 (2.2–5.5) years, 90.6% (INBONE I) at 5.9 (4–9.4) years, and greater for INBONE II (97.4%) compared with INBONE I (94%) at 2 years. In 121 ankles with 2.4 (1–5.8) years follow-up, survival was 95%. In 15 INBONE II followed 7.0 (5–9.4) years, 1 (6.7%) required revision and 4 (27%) required additional procedures. |
| Salto Talaris | 2006 | Semi-constrained, large keel tibia with barrel | In 78 ankles followed for 5.2 (3.4–8.2) years, survivorship was 97.5%. Total ROM increased from 35.5° to 39.9°. * Lucency was evident in 25 (31%) of ankles. In 72 ankles at 6.8 (5–9.6) years, survivorship was 95.8%. VAS (70 ± 23 to 12 ± 21 * ) and AFOAS (43 ± 15 to 79 ± 14 * ) both improved. In 87 ankles followed 7.1 (5–12) years, survivorship was 97.6% and reoperation rate 21.2%. The main reoperation was debridement of bony impingement. Osteolytic cysts developed in 18 (21%) of ankles. |
| Eclipse | 2007 | Medial or lateral approach with malleolar osteotomy | |
| Trabecular Metal (TM) | 2012 | Semiconstrained, lateral approach with fibular osteotomy, tantalum porous–coated cylindrical bone interface, conical talar component, highly cross-linked polyethylene insert | In the developer’s series of 55 ankles followed 2.2 (2–3.3) years, there were 3 revision (5.5%) and 10 reoperations (18.2%). All fibula healed. VAS decreased from 7.9 ± 1.3 to 0.8 ± 1.2 * and total ROM increased from 22.9 ± 12.7° to 40.2 ± 1.8°. * , In 104 ankles followed 3.6 (1.4–6) years, overall pain score improved and 51 (50%) reported no pain. There were 3 (2.9%) revision and 38 reoperation in 31 (29.8%) ankles. In 73 ankles followed 2.6 ± 0.7 years there were 2 major and 14 minor (21.9% overall) complications. In 89 ankles at 3.5 ± 2.0 years, AOFAS improved (33.8 ± 14.3 to 88.5 ± 6.6 * ) and VAS improved (80.5 ± 17.0 to 14.1 ± 9.2 * ), with one revision for infection. |
| Infinity | 2013 | Semi-constrained, pegged | In 159 ankles followed 1.7 (1–3.1) years, 6 (5%) required revision for tibial component loosening, and 8 (7.4%) of 108 with follow-up imaging had global lucency around the tibial component concerning for loosening. In 67 ankles followed 3.0 (2.3–3.9) years, 2 (3%) underwent revision for aseptic loosening and 6 (9%) had an additional procedure postoperatively. FFI (43.5 ± 4.9 to 21.8 ± 2.7 * ) and AOS (59.1 ± 4.0 to 24.4 ± 4.9 * ) improved. In 55 ankles followed 1.8 (1–3.6) years, 1 (1.8%) required revision and 5 (9.1%) required reoperation. Periprosthetic lucency was noted in 19 (34.5%) ankles, though with short follow-up the significance is indeterminate. In a comparison series, 20 Infinity TAR was found to have achieved better coronal and sagittal alignment than the preceding 20 Zenith TAR. Patient-specific cutting guides may also improve implant alignment. |
| Cadence | 2016 | Semi-constrained, pegged | In 32 ankles with mean 2.0 (1–2.8) year follow-up, 5 (15.6%) had intraoperative medial malleolus fracture, 2 (6.3%) required revision for early aseptic loosening, and 6 (18.8%) required an additional operation. |
| Axiom | 2020 | Semi-constrained, keel tibia and pegged talus | Design based on new analysis of tibiotalar multiplanar motion. |
| Apex 3D | 2020 | Semi-constrained, pegged, 3D printed | |
† Uncemented, implants have surface modifications meant to improve bony ingrowth, though they may not be approved for uncemented use in the United States.
‡ Two-component, two metal components with polyethylene bearing surface fixed to the tibial component.
¶ Three-component, two metal components with an interposed polyethylene bearing that has mobile articulations with both metal components. AOFAS , American Orthopaedic Foot & Ankle Society hindfoot scale; AOS , Ankle Osteoarthritis Scale; FFI , Foot Function Index; HA , hydroxyapatite; ROM , range of motion; VAS , Visual Analog Scale for pain.
While overall appearance and philosophy of these implants can be traced back to the BP and STAR designs, refinements continued, including further emphasis on natural ankle kinematics, varied surgical approaches, and a variety of bone fixation strategies ( Fig. 23-5 ). A greater understanding of soft-tissue balancing and deformity correction expanded the indications for reliable success. Short stems, modular stems, and a return to the lateral approach as well as introduction of revision implants give surgeons additional options to individualize treatment approaches. Intermediate- and long-term outcomes provide surgeons and patients alike confidence in the reliability of their treatment choices. However, the lack of direct comparison between implant designs and the relatively short follow-up for many newer designs leave choices regarding the ideal implant design inconclusive. Surgeons should be comfortable with multiple implant designs to allow for individualized decisions based on each patient’s ankle anatomy.

Intractable pain related to end-stage ankle arthritis with a desire to retain or restore gait parameters is the main indication for TAR. Patients with stiff or auto-fused ankles that have satisfactorily accommodated to their stiff joint are typically pleased with the pain relief from fusion, while patients whose gait alterations are predominantly antalgic in nature may benefit the most from the motion ankle replacement maintains.
Prior trauma, including fractures and sprains, are the most likely etiology leading to ankle replacement since the ankle joint is rarely affected by primary osteoarthritis. Other common indications for TAR are systemic inflammatory conditions such as rheumatoid arthritis, and secondary causes of osteoarthritis such as hemophilia, hemochromatosis, and gout.
Patients with bilateral ankle osteoarthritis do well with both ankle replacement and bilateral ankle fusion, though bilateral tibiotalocalcaneal (TTC) or pantalar fusion may do slightly worse. Patients with bilateral involvement have a greater chance of having primary or nontraumatic secondary etiology of their arthritis and have baseline lower SF-36 physical component scores before surgery, suggesting some of the perception that these patients do not do as well with bilateral fusions that may be related to their whole body disease burden. Patients opting for bilateral TAR can have two separate surgeries or simultaneous bilateral replacement, resulting in significant pain relief and functional improvement comparable to the results achieved with a unilateral procedure. Patients considered for simultaneous bilateral TAR should be informed about a prolonged initial recovery period and the challenge of bilateral walking casts or boots.
Recent data suggest gait alterations after ankle fusion are not further worsened by the addition of ipsilateral hindfoot fusion. Despite this, the concern for over-stiffening patients with prior subtalar or triple with the addition of a tibiotalar fusion leads many surgeons to recommend TAR in this situation. Fortunately, the clinical outcome of TAR when combined with hindfoot fusion is comparable to that of ankle replacement alone.
With increased use, an emerging indication for TAR is salvage of failed primary TAR. A major concern in revision arthroplasty of the ankle can be anchorage of prosthesis components in deficient bone stock to ensure long-term prosthesis stability. After removal of the prosthesis component and careful debridement, if the residual bone stock is not sufficient, ankle fusion may be a better option, though patient satisfaction is not universally high.
Another special indication for total ankle arthroplasty is the salvage of painful nonunion or malunion of prior ankle fusion. Conversion of a fused ankle to TAR is a technically demanding procedure that should be performed only if remaining bone stock is sufficient and soft tissue conditions are not too compromised (especially in patients with previous long-standing ankle arthrodesis). The conversion ankle arthroplasty should be limited only to experienced surgeons with adequate experience in primary TAR.
The contraindications for TAR may be divided into two major groups: absolute and relative contraindications ( Table 23-5 ).
| Absolute | Relative |
|---|---|
| Active acute or chronic infection | Modifiable medical risk factors including uncontrolled diabetes or hypertension |
| Avascular necrosis (that cannot be bypassed or excised) | Active smoking |
| Neuroarthropathy | Severe osteoporosis |
| Significant polyneuropathy | Malalignment beyond the scope of the surgeon’s experience |
| Unmanageable joint laxity | Multiple prior surgeries with poor soft tissue |
| Unmanageable malalignment | Unrealistic patient goals (high impact activity) |
| Severe metal allergy |
Active infection or concern for active infection is a clear absolute contraindication to TAR. If doubt exists, thorough work-up including laboratory and imaging studies, aspiration, and possibly open biopsy should be obtained until an answer is reached. Although some authors suggest that prior ankle infection is a contraindication for TAR, comfort with replacement in this setting has grown, In a series of 22 TAR after prior native joint infection, no postoperative infections or wound-healing issues were found at over 2 year follow-up. Replacement after prior infected total ankle has been less successful. Myerson found revision after treatment of acutely infected TAR was only successful in three of seven (43%) attempts.
Avascular necrosis of the talus has been identified as an absolute contraindication for TAR based on concerns about implant subsidence and loosening, though no studies specifically address the issue. In patients with avascular necrosis of more than one third of the talus, or in whom the area of avascular bone cannot be excised or bypassed with a standard prostheses, a revision talar component or a custom-made talar prosthesis can be considered in addition to fusion. Small series without long-term outcomes suggest TAR after revascularization of avascular necrosis of the talar body or subtalar fusion may be considered as well.
Patients with neuromuscular disorders, neuroarthropathy (e.g., Charcot arthropathy of the midfoot and/or hindfoot), massive joint laxity, or diabetes with clinically significant distal polyneuropathy should be excluded for concerns about excess or unexpected forces placed on the implant and surrounding bone and soft tissue leading to premature failure.
Patients with preoperative intraarticular deformity and/or lower-leg malalignment that cannot be surgically corrected or accommodated should not be considered for TAR. Early series found preoperative deformity greater than 10 to 15 degrees, particularly incongruent deformity, predisposes to postoperative edge loading of the polyethylene in mobile bearing three-component designs leading some surgeons to recommend avoiding TAR in these patients. However, as experience with soft tissue and bony realignment procedures grew, indications for allowable preoperative deformity have expanded. Results have confirmed that preoperative deformity should not be an absolute contraindication, as long as additional realignment procedures can correct the deformity. For example, Kim et al found no difference in VAS, AOFAS score, or revision rate for 23 ankles with greater than 10 degrees varus deformity compared with 22 neutrally aligned ankles. Consideration for correction before the implantation of the prosthesis, during the TAR procedure, or after TAR as a two-stage procedure have all been advocated. We found no increased risk of wound-healing complications with additional incisions at the time of TAR, suggesting concurrent correction is safe, though we did not specifically consider the effects of additional procedures on factors such as talar blood supply. However, the techniques for soft tissue and bony realignment in the setting of TAR can be challenging and should not be taken on until the surgeon feels a mastery of more straightforward cases.
The relative contraindications for this procedure include significantly reduced bone quality often resulting from severe osteoporosis, immunosuppressive therapy, or long-term steroids therapy. Smoking is another relative contraindication because it is associated with higher risk of perioperative complications, including wound breakdown. Patients with expectation to participate in high-demand physical activities should be informed about possible premature mechanical wear and a potentially higher rate of aseptic loosening. Multiple prior surgeries are a risk factor for infection, though the preponderance of posttraumatic arthritis in the ankle makes it difficult to avoid TAR in patients who underwent prior surgery.
Modifiable medical risk factors, particularly uncontrolled diabetes, should be corrected prior to surgery to minimize risk of wound-healing issues and premature failure but not risk of hospital readmission. Choi et al found higher rates of delayed wound healing and lower postoperative AOFAS scores in uncontrolled diabetics in a study of 130 nondiabetic, 25 controlled diabetic, and 18 uncontrolled diabetic unilateral TARs with 4.6 (2–9) year follow-up. Furthermore, implant survival at 5 years favors the nondiabetic group 88.4% vs. 79.0% ( p < 0.05). In contrast, emergency room evaluation or readmission within 90 days of 1024 TAR was not found to be related to any preoperative risk factors or comorbidities with the numbers available for review.
Finally, and perhaps most importantly, the patient and the surgeon must have realistic expectations of this surgery. Meeting a patient’s expectations of surgery correlates strongly with their satisfaction after hip and knee replacement. The high satisfaction of rheumatoid osteoarthritis patients after TAR likely reflects the low demands and low expectations of this patient cohort. On the other hand, the expectations of patients with posttraumatic ankle osteoarthritis are generally higher than those of patients with other etiologies. In particular, predictions regarding postoperative improvement in range of motion should be made carefully; as with current implants, postoperative improvement in ankle range of motion is relatively small (generally <10°) and therefore should not be one of the expected benefits from this procedure. Aligning expectations regarding hospitalization versus care at home are equally important to discuss before surgery so preparations for an uneventful recovery can be made.
Previous medical reports and imaging data are analyzed, paying particular attention to prior surgery reports and comorbid medical conditions. The patient’s history is assessed to address their current level of pain and disability, including limitations in activities of daily living and recreation. Previous surgeries should be noted to plan a safe surgical approach to the ankle. The exact type of previously implanted hardware should be identified to ensure any impeding hardware can be removed without difficulty during TAR. Patients with any of the aforementioned absolute contraindications are excluded. Patients with modifiable medical comorbidities should be referred for consultation in internal medicine, cardiology, endocrinology, vascular surgery, or neurology before undertaking TAR.
The routine physical examination starts with careful inspection of the foot and ankle while sitting, standing, and walking, allowing dynamic comparison of changes at weight bearing versus non–weight bearing, and findings should be compared with the contralateral limb. Ankle alignment, including whole leg, knee, and supramalleolar deformities, and the position of the heel in relation to the longitudinal axis of the lower leg, is assessed with the patient standing. However, clinical assessment of the ankle and hindfoot alignment should be interpreted carefully because it does not always correlate with radiographic and pedobarographic findings. Skin and soft tissue are carefully inspected, with special attention given to previous surgical scars as well as evidence of arterial or venous insufficiency.
Range of motion of the tibiotalar and hindfoot joints is determined, preferably by goniometer, which correlates well with radiographic measurements, though intertester reliability is low. The rigidity versus correctability of identified deformity, compensatory mid- or forefoot deformities, as well as associated ligament laxity should be examined.
Neurovascular evaluation as well as lower limb muscle function should be evaluated, noting weakness or atrophy, which is common in patients with end-stage osteoarthritis.
Radiographic examination of affected ankles includes recent weight-bearing orthogonal views of the ankle. The standing position displays the joints in their functional, loaded state and helps standardize the radiographic technique, resulting in a more reliable comparison between pre- and postoperative radiographs. Standing foot, hindfoot alignment, and standing long-leg alignment (hip to ankle) radiographs are also recommended ( Fig. 23-6 ). Hip to ankle alignment films are important to avoid missing significant deviation from overall limb alignment if just the lower portion of the tibia is visualized. All coexisting degenerative changes in the adjacent joints as well as any deformities above or below the ankle should be identified.
Concomitant deformities in osteoarthritic ankles may occur at multiple levels. Deformity of the knee from osteoarthritis or in the tibial shaft from prior trauma is common in patients with ankle arthritis and may require correction prior to TAR to avoid weight bearing on a misaligned ankle prosthesis. Supramalleolar ankle alignment is measured in coronal and sagittal planes between the tibial anatomic axis and the distal tibial joint line. The average medial distal tibial angle has been measured to be 92.4 ± 3.1 degrees (range, 88–100°) in a radiographic study and 93.3 ± 3.2 degrees (range, 88–100°) in a cadaver study. Values may vary by up to 3 degrees dependent on radiograph technique, so methods should be standardized within a clinic. We suggest using a measurement bisecting the tibial shaft on a long leg AP view of the tibia with the beam centered as close to the ankle as possible. The average anterior distal tibial angle has been measured to be 84.0 ± 3.0 degrees (range, 76–92°).
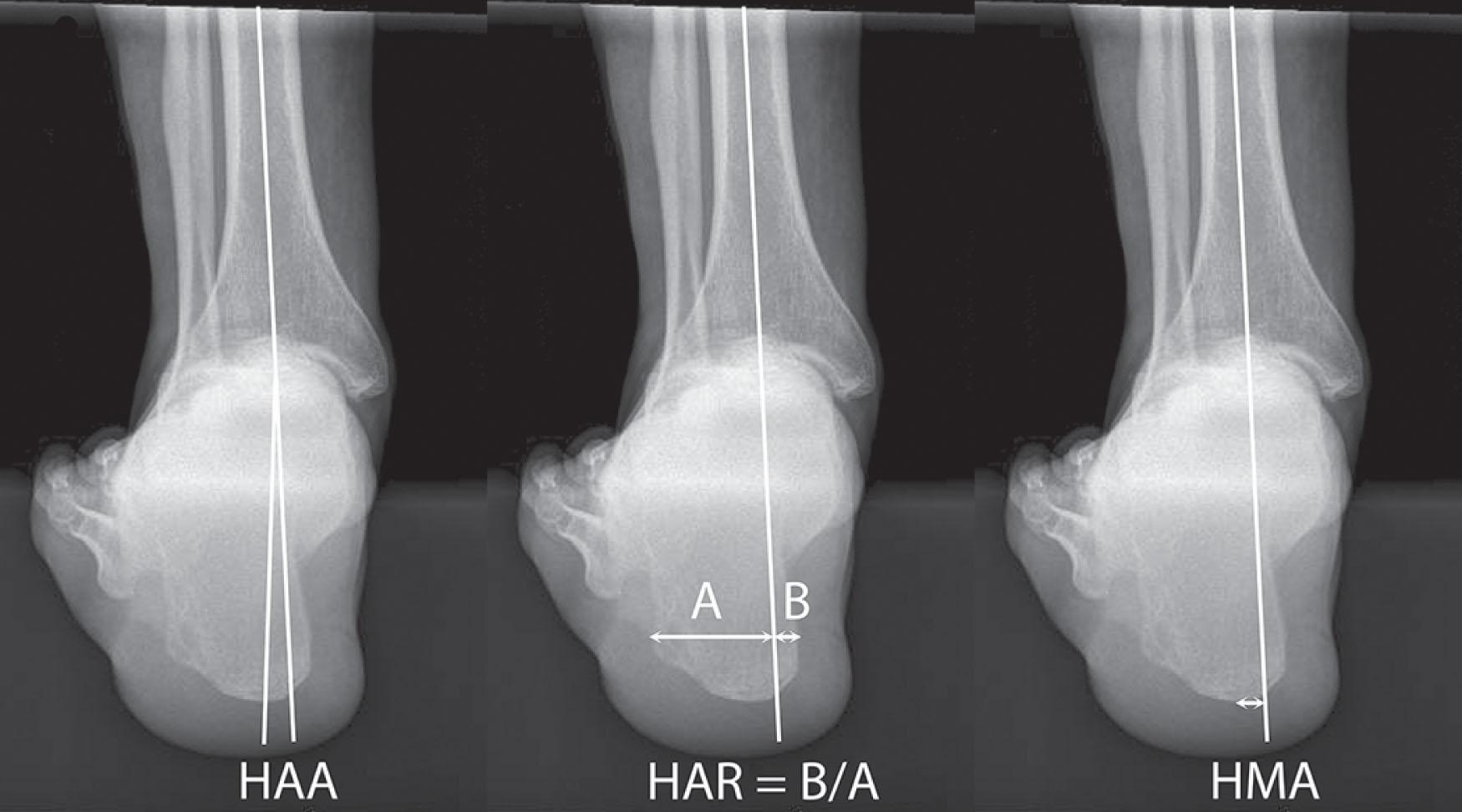
The inframalleolar ankle alignment is measured using the hindfoot alignment view, which has been shown to outperform visual inspection alone when assessing the heel position in relation to the longitudinal axis of the lower leg. The hindfoot alignment view requires a special platform that allows the beam to make a 20-degree angle to the floor, the cassette perpendicular to the beam, and the weight-bearing foot placed with its medial border parallel to the radiographic beam. Measurements are then made including hindfoot alignment angle (mean 4.07 ± 3.48° valgus), hindfoot alignment ratio (mean 0.21 ± 0.15°), and hindfoot moment arm (mean 6.12 ± 5.22° lateral) ( Fig. 23-7 ). Hindfoot alignment measured using this view correlates with pedobarographic parameters and SF-36 physical function scores after TAR and ankle fusion. Similar measurements made from non–weight-bearing 3D imaging modalities do not substitute for this view because of the lack of weight-bearing stress on the hindfoot joints.
A computed tomography (CT) scan may be helpful to assess periarticular bony defects and quantify bone mineral density, which predicts intra- and postoperative periprosthetic fracture risk. In patients with degenerative changes of the adjacent joint, single photon emission computed tomography with standard CT (SPECT-CT) may help to evaluate the morphologic changes and their biologic activities. Preoperative magnetic resonance imaging (MRI) may help to assess the concomitant ligamental injuries, pathologic changes of tendons, and avascular necrosis of osseous structures.
A learning curve is the period of time during which one becomes proficient at a new task. In the surgical setting, it designates the increase in complications, technical errors, and subpar outcomes seen during an initial number of a particular type of surgery. Numerous clinical studies have demonstrated a steep learning curve associated with performing TAR, leading some authors to suggest the procedure should only be done by experienced foot and ankle surgeons.
Many studies have confirmed the TAR learning curve by comparing complication rates for an early group and a later group of patients. Myerson and Mroczek compared the first and second 25 Agility TAR performed by the same surgeon and found minor wound complications (24% vs. 8%), intraoperative fractures (20% vs. 8%), and nerve or tendon lacerations (16% vs. 0%) all improved with surgeon experience. Similar findings have been confirmed for fractures, major revisions, and malpositioning in the Agility TAR, for minor perioperative complications (decreased from 60% to 20% of cases) using the HINTEGRA TAR, and for technical errors (decreased from 25% to 8%) using the Salto Talaris TAR. Others have not shown a statistical learning curve effect, perhaps due to prior experience with similar implants.
The learning curve effect may be seen in reduced complication rates by surgeons with greater experience compared with less experienced surgeons. Examining 4800 TAR from the United States Healthcare Cost and Utilization Project Nationwide Inpatient Sample (NIS) from 2003 to 2009, high volume surgeons (90th percentile = 21 cases/year) had decreased overall complication rate (OR 0.5, P <0.05), medial malleolar fracture rate (OR 0.1, P <0.05), and length of hospital admission (-0.9 days, P <0.05).
A learning curve effect may also be demonstrated by the inferior implant survival rates reported by surgeons not involved in a particular implant’s design. In one such study, survivorship of 86% at 3 years was reported for the SALTO TAR performed at centers with fewer than 10 TAR per year. This was similar to other reports of survivorship but was lower than the survivorship reported by the implant’s designers of 95% to 98% at 6 years. One explanation is that the designers spend so much time focused on the intricacies of the implant that they are able to bypass some of the learning curve’s negative effect while low-volume surgeons are continually in the learning-curve phase. Alternatively, the designer’s reputational and financial biases may unknowingly influence outcomes.
The generalizability of the learning curve phenomenon has been demonstrated in multicenter, joint registry, and systematic review studies. Zhao et al reported a systematic review of 73 studies and found the inexperienced surgeons had over five times the risk of intraoperative complications compared with experienced surgeons or implant designers (OR 5.43, P <0.05). Haskell and Mann reported a multicenter study with similar design to the single surgeon studies, showing a 3.1 relative risk of complication during the learning curve period, and a reduction in complication rate in 9 of 10 surgeons after the learning curve. The Swedish Joint Arthroplasty Registry data reported three surgeons who had performed more than 40 ankle replacements had their aggregate 5-year survival increased from 70% for the first 90 cases, to 86% for the following 132 cases ( P = 0.01).
The learning curve effect may bias outcomes in unrelated study parameters and should be considered when analyzing longitudinal comparisons. Carlsson analyzed survivorship of a consecutive STAR prostheses in two groups based on hydroxyapatite coating: 51 STAR with single coating followed by 58 STAR with double coating. While overall the double coating had greater survival, the latter half of the single coating group had similar outcome to the subsequent double coating group, suggesting surgeon learning curve during the initial half of the single coating group may have played a larger role than implant surface on survival.
The number of cases needed to reach a learning curve plateau can be studied using the moving average method. Using a three-component implant requires 11 to 28 cases to stabilize intraoperative complications, postoperative outcomes, and radiographic alignment parameters. Transition from the anterior to the lateral approach leads to a new period of learning curve with over 20 cases required to stabilized outcomes such as VAS and AOFAS scores as well as radiographic alignment parameters.
Efforts should be made to minimize the rate of complications during a surgeon’s learning curve including emphasis on adequate training and education. Unfortunately, the type of TAR training a surgeon receives, including observing an expert, taking a training course, or completing a fellowship, was not found to affect complication rates during the learning curve period, though numbers may have been too small to reveal a difference. It is uncertain how much a surgeon’s experience using a prosthesis with similar design may reduce the learning curve when transitioning to a new prosthesis.
More informed preoperative planning and the emergence of improved instrumentation should reduce the variability to which initial use is particularly susceptible. Among the improvements seen in the current TAR generation as compared with the previous TAR generation are far better cutting guides and the use of a greater range of implant sizes. The introduction of patient-specific instrumentation based on preoperative CT scans has demonstrated reliability and accuracy in placing TAR components and may be a way to shorten the learning curve for new surgeons. To date, however, studies have involved experienced ankle arthroplasty surgeons and have not specifically addressed the learning curve of this technology. Caution should be applied to relying on this technology to bypass a typical learning curve for new surgeons.
Perhaps the best way to avoid unnecessary complications during a surgeon’s first 10 to 20 TAR is to select patients at lowest risk for complication. A nonsmoking patient older than 60 years with low physical demands and no medical comorbidities is ideal. The lower extremity should be neutrally aligned and the ankle flexible without deformity or instability. The surrounding bone should be of good quality and surrounding soft tissue with minimal scarring (ideally no prior operations). The foot should have intact strength, sensation, and arterial inflow. Increased body mass index (BMI) is not a contraindication to TAR. Finally, the patient should have reasonable expectations about the typical outcomes of TAR including pain relief, range of motion, and activity level.
![]() General or regional anesthesia, or a combination of the two, can be used for TAR. Augmenting the anesthetic with a single shot or continuous popliteal catheter and single shot saphenous nerve block helps minimize intraoperative anesthetic requirement and postoperative pain.
General or regional anesthesia, or a combination of the two, can be used for TAR. Augmenting the anesthetic with a single shot or continuous popliteal catheter and single shot saphenous nerve block helps minimize intraoperative anesthetic requirement and postoperative pain.
The patient is positioned supine with the feet on the edge of the table ( Fig. 23-8 ). A bump is placed under the ipsilateral hip until a strictly upward position of the lower extremity is obtained. We recommend placing blankets or a foam pad under the leg to elevate the ankle, making lateral fluoroscopy easier. A small bump may be used intraoperatively under the calf to allow the heel to float free, thereby avoiding inadvertent anterior ankle translation. A padded thigh tourniquet is applied.
The author recommends administration of preoperative intravenous antibiotics and tranexamic acid. Data to supports tranexamic acid are mixed, but its use is generally considered safe.
If significant concomitant deformity has to be corrected, the unaffected lower extremity may be also draped to allow intraoperative comparison.
The surgical site undergoes sterile preparation and draping; an impervious skin drape may be used.
After exsanguination, the thigh tourniquet is inflated.
A standard anterior ankle approach is used ( Fig. 23-9 ). An anterior longitudinal incision (8–15 cm) is made between the tibialis anterior (TA) and extensor hallucis tendon (EHL) tendons. A modification anteromedial extensile approach may be considered in higher risk patients.
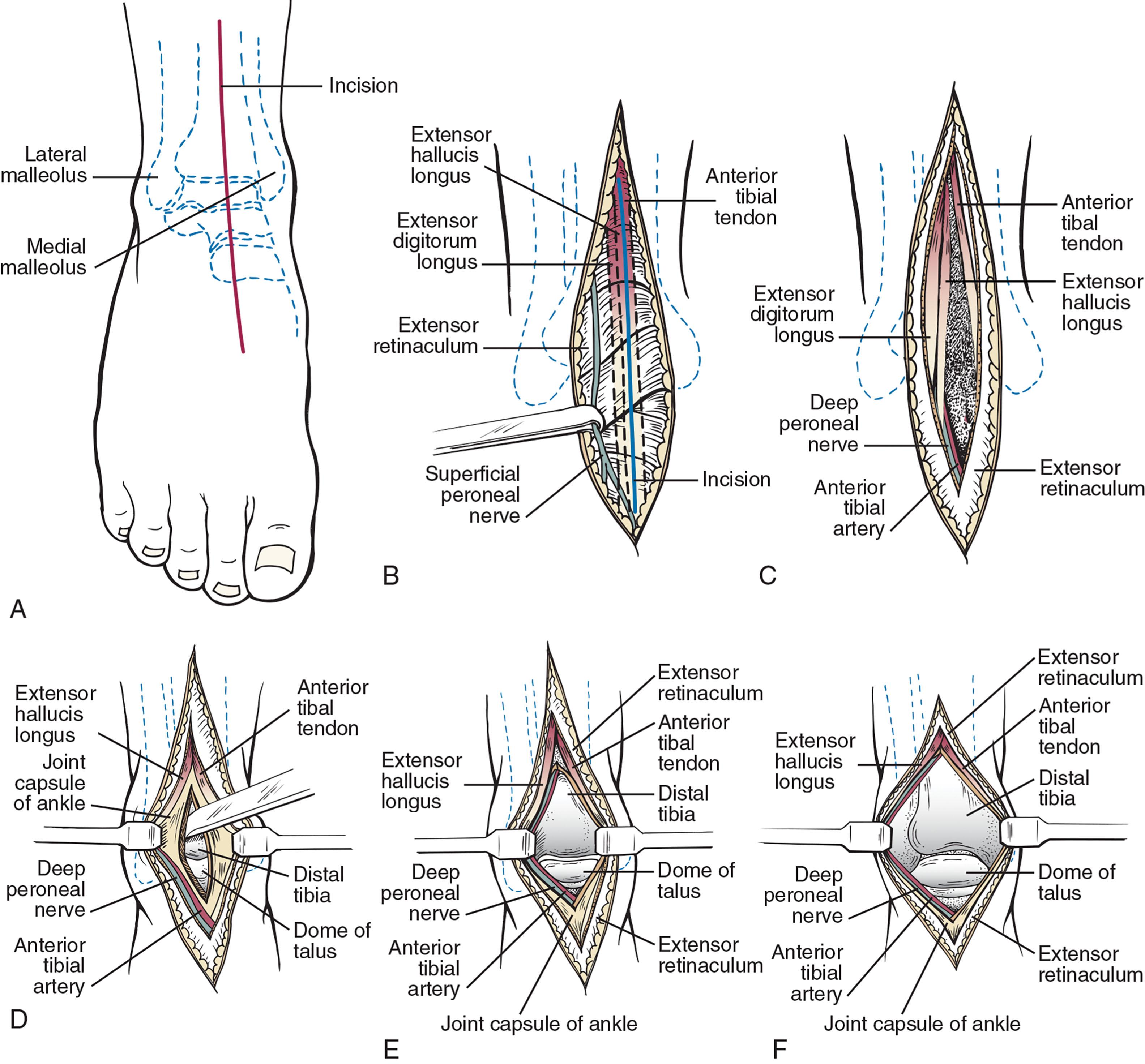
The incision is initially taken through the skin only. The medial fascicles of the superficial peroneal nerve's medial branch are identified and dissected free into the foot. These are retracted laterally. Occasionally, the authors intentionally transect a fine fascicle, coursing medially across the neck of the talus to gain more exposure. It is important to tell the patient postoperatively they may have areas of numbness along the dorsal medial aspect of the foot.
After the superficial peroneal nerve has been carefully retracted, the anterior tibial tendon is identified. The author opens the extensor retinaculum between the TA and EHL tendon sheaths and dissects the interval between the muscles and tendons down to the joint capsule. The retinaculum is the thickening of the deep fascia above the ankle, running from tibia to fibula.
The deep neurovascular bundle is gently teased free and retracted laterally with the EHL muscle and tendon. The neurovascular bundle is located behind the extensor hallucis longus or between the extensor hallucis longus and the extensor digitorum longus. It is frequently easiest to begin this dissection at the proximal extent of the incision between the TA and EHL muscle bellies, then bluntly dissect distally while protecting the bundle. It is protected by the lateral retractor throughout the case. Medially traversing small veins may be safely cauterized. The dissection is deepened to the level of the joint capsule. There is frequently a transversely directed vein immediately anterior to the ankle joint overlying the capsule.
The capsule is incised longitudinally incorporating the distal tibial periosteum with the hope this can be closed later. It is elevated medially and laterally to the level of the malleoli, allowing visualization of the ankle gutters. Elevation of the capsule from the dorsal talar neck may be required as well. Care must be taken to do this gently in those at risk for bone injury.
Nontraumatic hand-held retraction is recommended to avoid skin edge necrosis. A deep blunt retractor used strategically at critical times for exposure may protect the tissues as well. If a self-retaining retractor is applied to control the soft tissue envelope, it must be relieved when not necessary and placed deep to avoid skin necrosis.
After the joint is sufficiently exposed, osteophytes are removed on the tibia and the talar neck retaining the original bone cortex. Some patient specific cutting jigs require the osteophytes to be left in place for accurate positioning.
At this point, implantation is performed by following the specific guidelines of the manufacturer ( Fig. 23-10 ).
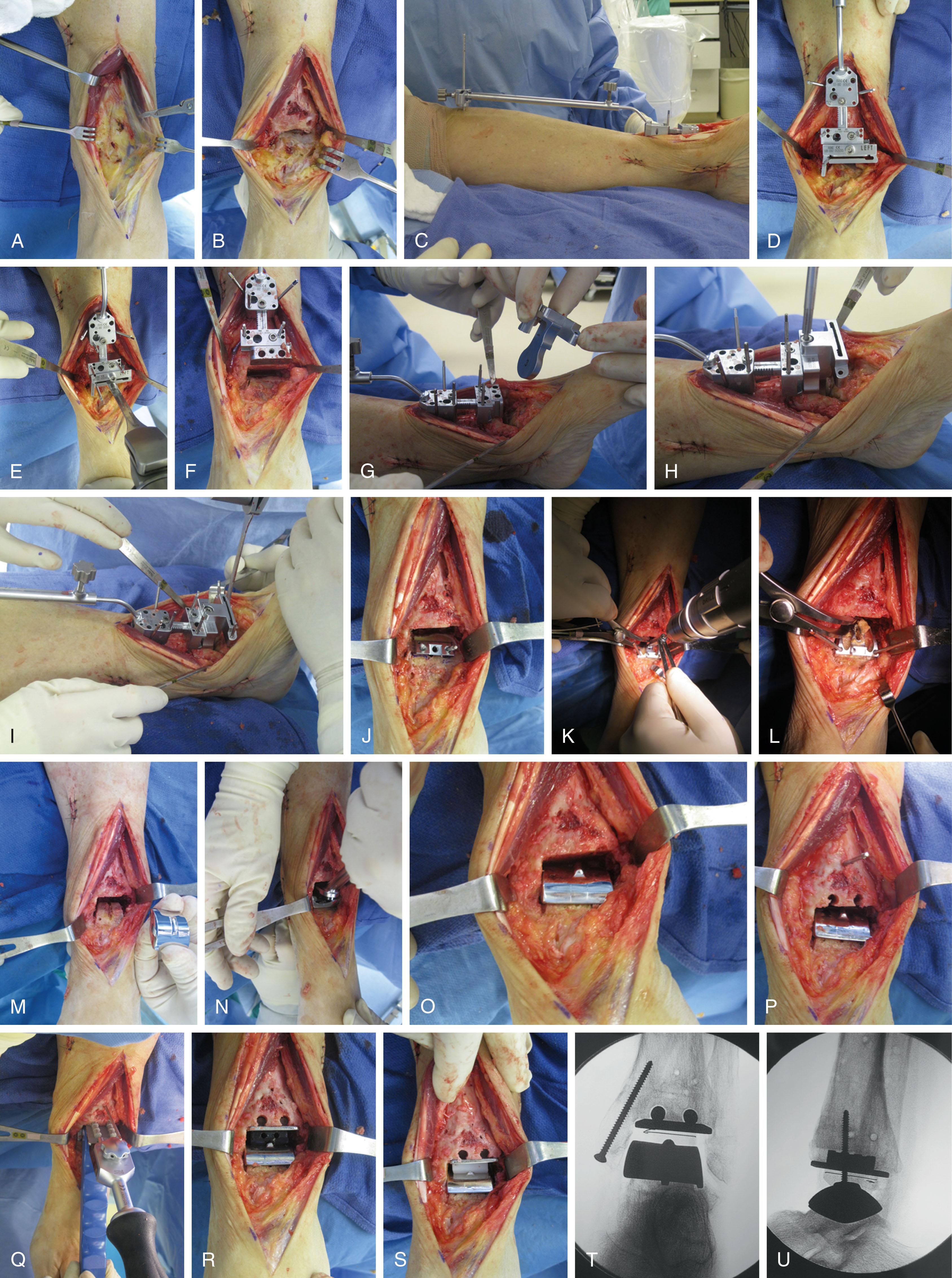
Every effort must be made to avoid injury to the “shoulder” region of the medial malleolus because this can cause intraoperative malleolar fracture, a common intraoperative complication of TAR. Avoid over-retracting the medial soft tissue, and do not allow the medial retractor to fall into the medial gutter after bone cuts have been made.
Because the lateral malleolus in some patients is relatively more posterior, it can be inadvertently cut with approaches from front to back. Rarely, this can block placement of the implants and a modest resection will be required.
After implantation, all distractors are removed, and the stability and motion of the ankle is checked. Additional soft tissue balancing is performed when required. The position of prosthesis components is checked and documented using fluoroscopy.
Wound closure is performed sequentially in layers, starting with the periosteum, then the extensor retinaculum, subcutaneous tissue, and skin. Be sure there is a good retinacular closure, which may help protect against deep infection if wound-healing issues occur. Drainage with suction may be used but is generally not necessary.
Soft wound dressing is used to avoid any pressure so as not to compromise wound healing. A splint is used to keep the foot in a neutral position.
Elevation for the first 2 to 7 days is critical to reduce swelling for wound healing. Some surgeons permit early motion. The author recommends immobilization for 2 weeks in the splint to allow wound healing and low-dose aspiring for deep venous thrombosis prophylaxis in patients without a history of deep venous thrombosis, pulmonary embolism, or current antithrombotic use, though prophylaxis for deep venous thrombosis in patients without preexisting risk factors is not universally required.
![]() General or regional anesthesia can be used for TAR.
General or regional anesthesia can be used for TAR.
The patient is placed supine on a radiolucent table with a bump placed under the ipsilateral hip to internally rotate the foot.
A pneumatic thigh tourniquet is applied.
The surgical site is prepared in a sterile manner; in addition, a skin drape may be used.
After exsanguination, the thigh tourniquet is inflated.
A midline incision of the fibula of about 20 cm is used, with full exposure of the lateral distal fibula ( Fig. 23-11 ).
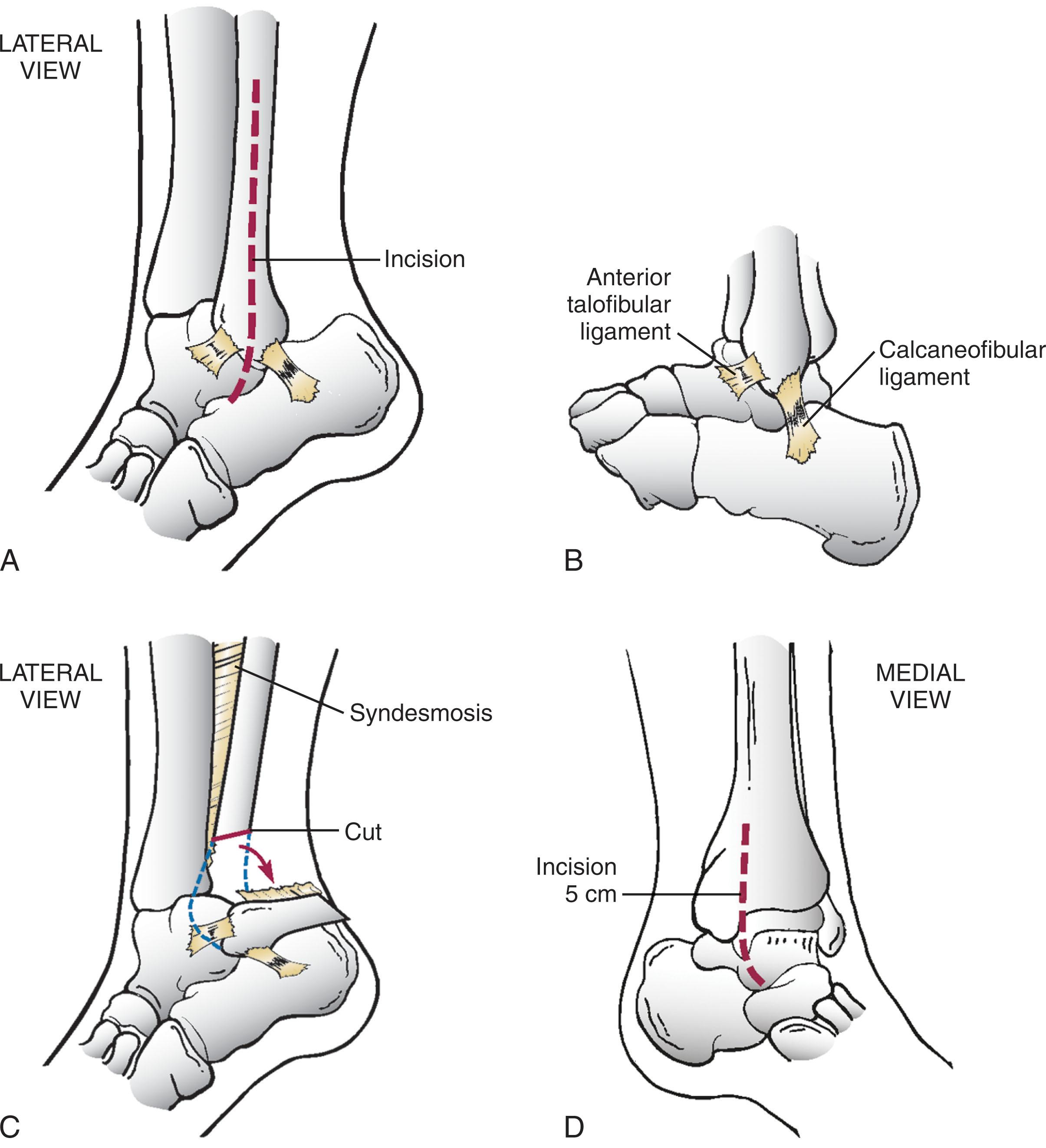
The anterior talofibular ligament (ATFL) is identified and transected for the exposure and is repaired at the end of the procedure.
An oblique osteotomy of the distal fibula is then performed, ending 10 to 15 mm above the joint line. A long oblique osteotomy may reduce wound healing and hardware complications when fixed with interfragmentary screws.
The tibiofibular ligaments are released from the distal fibular segment to allow the segment to be reflected and pinned to the calcaneus while maintaining the calcaneofibular ligament (CFL) and posterior talofibular ligament (PTFL) attachments to the fibula.
A secondary exposure may be performed over the medial gutter to allow osteophytes to be cleared, to aid in alignment of the foot into the bone preparation system, and to allow for retractors to protect the medial malleolus during bone resection.
At this point, implantation is performed by following the specific guidelines of the manufacturer.
After the ankle is implanted, the fibula is reapproximated and fixed with plates and screws. The transected ligaments are repaired.
The position of the prosthesis components is checked and documented using fluoroscopy.
Wound closure is performed sequentially in layers. Drainage with suction may be used but is generally not necessary.
Soft wound dressing is used to avoid any pressure so as not to compromise wound healing. A splint is used to keep the foot in a neutral position.
Elevation for the first 2 days is critical.
The goal of TAR in the setting of angular deformity is to achieve a plantigrade foot with a neutrally aligned and stable ankle prosthesis. This can generally be achieved, as long as all malalignments and unstable soft tissues can be corrected sufficiently by additional procedures. When that cannot safely be achieved, ankle fusion should be considered to avoid excess joint contact pressures that may lead to premature polyethylene failure. The level of evidence to recommend various techniques are case series and expert opinion. Much of our understanding is extrapolated from managing similar deformities in patients without ankle arthritis. A general rule regarding deformity correction is to start proximally and work distally. This allows the more distal segments to be assessed in their new position after proximal realignment is complete.
The sequence and timing of adjuvant procedures is similarly based on case series and expert opinion. Adjuvant procedures may be performed concurrent with TAR or as staged procedures prior to or following TAR. How much realignment and ligament reconstruction can be safely performed concurrently with TAR is a matter of surgeon preference and experience. Additional incisions at the time of TAR do not lead to increased risk of infection or wound-healing problems. However, this does not account for potential injury to blood supply to the distal tibia and talus. Subtalar fusion with TAR did not lead to increased short-term complications, revision, or subsidence in a case-control study of 33 mixed implant TAR plus subtalar fusion compared to 33 demographically matched TAR alone. Also, experience with the techniques employed is crucial to avoiding excessive total surgical time. Certain procedures such as supramalleolar osteotomy and triple arthrodesis lend themselves to a two-stage approach, though concurrent surgery is not contraindicated. Other procedures such as ligament repair and malleolar osteotomy may benefit from being done concurrently with TAR to allow ligament balancing to match the final position of the implants. When done concurrently, the timing of hindfoot realignment relative to TAR implantation is a matter of surgeon preference as well and comes down to what the surgeon feels most comfortable with to attain a plantigrade foot.
The author recommends staging larger procedures that risk affecting the blood supply to the periarticular bone, or where failure may affect the decision to proceed with TAR versus fusion. Supramalleolar osteotomies are typically medially based opening or closing wedge and done 4 to 6 weeks prior to TAR to allow some bone and soft tissue healing to occur. Isolated subtalar or talonavicular joint fusions are done concurrently, taking advantage of the bone graft available after TAR bone cuts, while concern over devascularizing the talus leads triple arthrodesis to be done prior to TAR. Subtalar fusion technique is modified to avoid disrupting the sinus tarsi with joint preparation or fixation. In 81 patients 2 years after TAR plus subtalar or subtalar plus talonavicular fusion, 44% of patients with screws penetrating the sinus taris developed talar subsidence compared with 4% without sinus tarsi penetration ( P <0.05). Sinus tarsi penetration was more common when screws were placed from dorsal to plantar. Most soft tissue procedures are done concurrently with TAR, as they typically require a step-wise series of releases that is best assessed as cutting blocks are placed and the implants are trialed. If circumferential or extensive releases are required, it is reasonable to stage TAR and even pin the talus in a neutral position to help soft tissue heal in the appropriate position. Achilles lengthening, calcaneal osteotomy, and first metatarsal osteotomy are done concurrently when necessary.
As with all deformities around the ankle, clinical and radiographic examination of the entire lower extremity, including weight-bearing ankle and foot x-rays, long-leg alignment views and heel alignment views should be made before formulating a surgical plan to address the deformity. Stress views can help determine correctability of deformities visible on static radiographs. Deformities are divided into those arising proximal to the ankle joint, those at the level of the joint (talar tilt), and those associated with deformity below the ankle joint. Deformities proximal to the joint are further divided into remote or supramalleolar deformities. Examples of remote proximal deformities include genu varum, genu valgum, and tibial diaphyseal malunion. Supramalleolar deformities may be congenital, posttraumatic, or acquired secondary to erosion of the tibial plafond joint surface.
Deformities at the joint level manifest as titling of the talus in the ankle mortise. In these cases, careful scrutiny for deformity in the bones and joints above and below the ankle as well as ligament competency around the ankle should be made. Intraarticular deformities frequently involve erosive changes of the tibial plafond, malleolar dysplasia, and osteophytes that may make reduction difficult.
Deformities distal to the joint, such as pes planovalgus or cavovarus, may leave the ankle joint unaffected or may be associated with ankle instability and talar tilt. Manual correction of the ankle and hindfoot to a neutral position helps differentiate between revealed residual hindfoot deformity and a neutral hindfoot where the deformity is focused at the tibiotalar joint. The foot should be examined focusing on compensatory varus or valgus deformity.
Ankle alignment may also result from a combination of these deformities in an accentuating and compensatory fashion. The Z-type deformity with a talar tilt and oppositely directed hindfoot deformity requires careful inspection of the suppleness of the hindfoot since correcting the talus with a rigid Z-deformity will only worsen the hindfoot position. In these cases, either an extensive reconstruction or adjustment of the talar cuts to accept the hindfoot position with the foot in a plantigrade position may be chosen.
![]() Sources of deformity above the ankle must be recognized and addressed. Generally, deformity resulting in ankle malalignment greater than 10 degrees should be considered for realignment though prospective evidence to guide a discrete cutoff is lacking.
Sources of deformity above the ankle must be recognized and addressed. Generally, deformity resulting in ankle malalignment greater than 10 degrees should be considered for realignment though prospective evidence to guide a discrete cutoff is lacking.
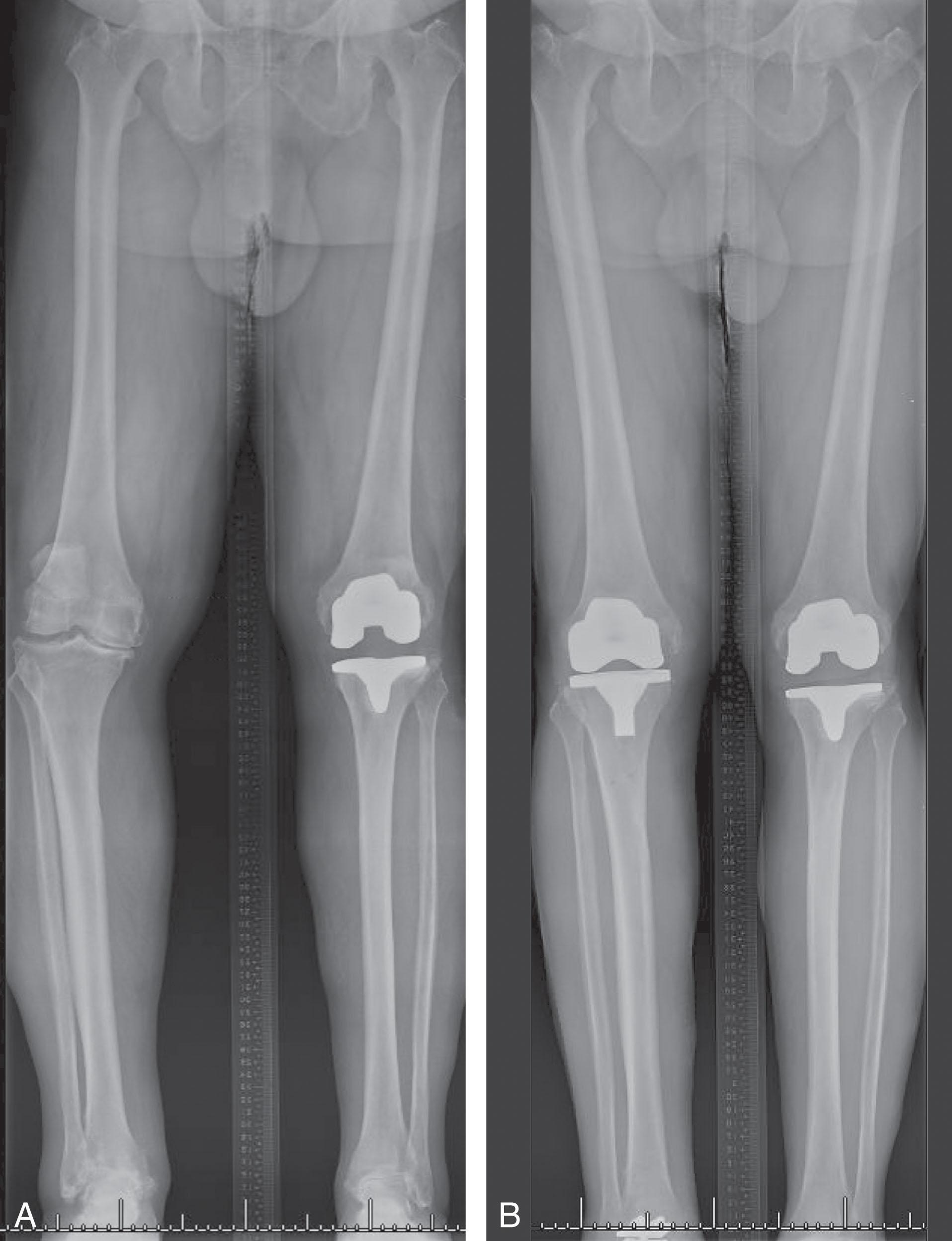
Knee arthritis resulting in genu varum or valgum may require total knee arthroplasty prior to TAR ( Fig. 23-12 ). Knee alignment is measured at the medial angle created by a line along the joint surface intersecting a mid-diaphyseal line, called the medial proximal tibial angle relative to the anatomic axis, which is typically approximately 87 degrees. Knee alignment is not related to hindfoot alignment, meaning genu varum or valgum both can coexist with hindfoot varus, neutral hindfoot alignment, or hindfoot valgus. Correction of deformity about the knee with total knee arthroplasty typically corrects about half of the associated hindfoot deformity. Total knee replacement (TKR) is recommended prior to TAR when symptomatic arthritis coexists so any alignment correction at the knee is appreciated at the time of TAR. When the knee is angulated but less symptomatic, thorough discussion with the patient and knee arthroplasty surgeon about the timing options should be had before a surgical plan is made.
Posttraumatic tibial deformity may require osteotomy and realignment ( Fig. 23-13 , ![]() ). These deformities are often missed if only ankle radiographs are seen. For example, an ankle with intraarticular valgus tilt may be in compensation for a mid-tibial varus deformity rather than a posterior tibial tendon (PTT) dysfunction. The deformity is assessed at the intersection of a mid-diaphyseal line drawn in the segment above and below the deformity. This intersection point is called the center of rotational angulation (CORA), which is the point around which rotation should occur to achieve anatomic alignment after correction. Surgical considerations include acute correction or distraction osteogenesis techniques. Axial rotational deformities should be addressed at the same time. Alternatively, some smaller tibial shaft deformities may be addressed with supramalleolar osteotomy accepting some residual translation of the weight-bearing axis, or by adjustment of the TAR bone cuts ( Fig. 23-14 ).
). These deformities are often missed if only ankle radiographs are seen. For example, an ankle with intraarticular valgus tilt may be in compensation for a mid-tibial varus deformity rather than a posterior tibial tendon (PTT) dysfunction. The deformity is assessed at the intersection of a mid-diaphyseal line drawn in the segment above and below the deformity. This intersection point is called the center of rotational angulation (CORA), which is the point around which rotation should occur to achieve anatomic alignment after correction. Surgical considerations include acute correction or distraction osteogenesis techniques. Axial rotational deformities should be addressed at the same time. Alternatively, some smaller tibial shaft deformities may be addressed with supramalleolar osteotomy accepting some residual translation of the weight-bearing axis, or by adjustment of the TAR bone cuts ( Fig. 23-14 ).
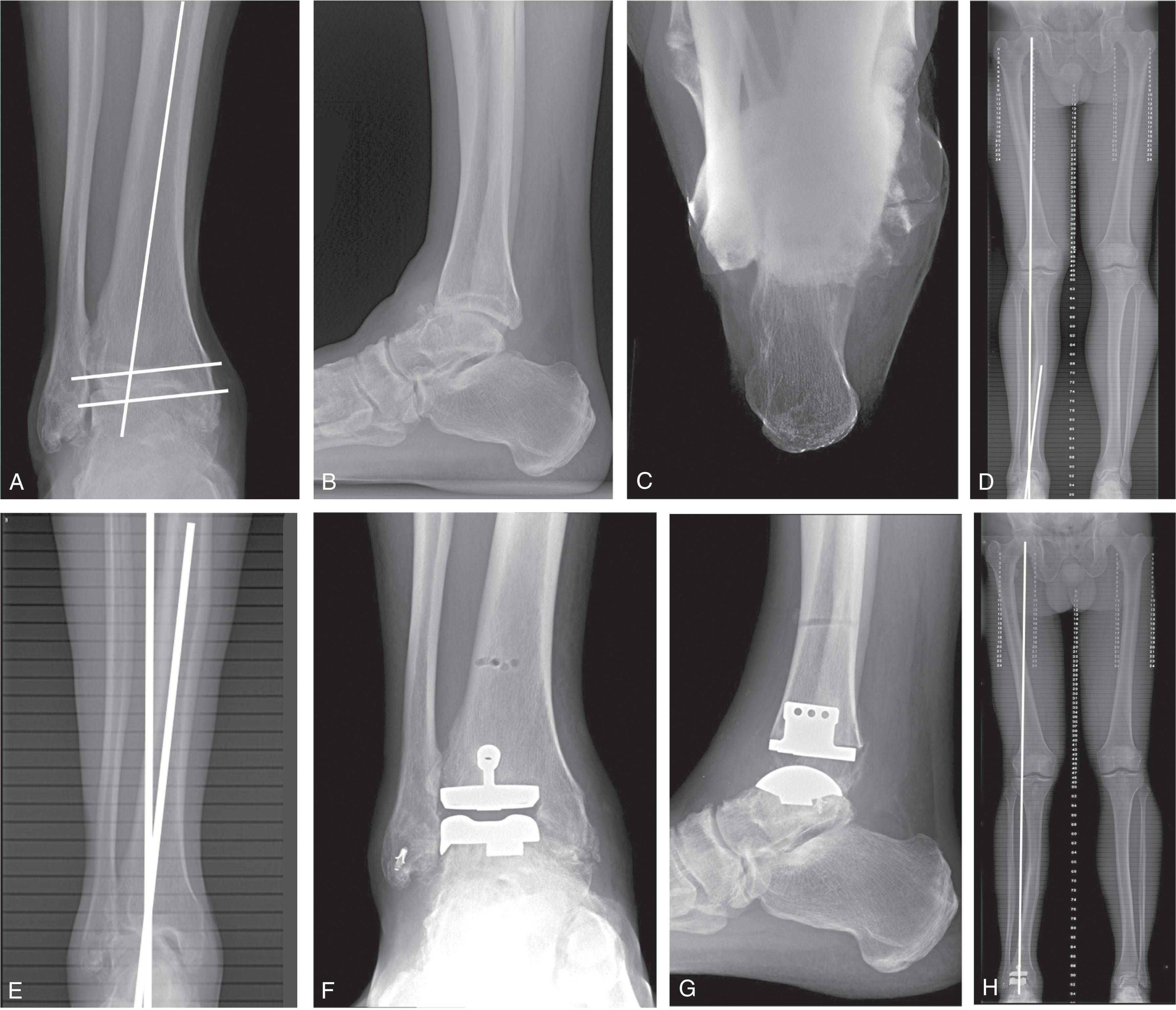
Periarticular tibial deformity may require supramalleolar osteotomy if the deformity cannot be managed with the total ankle bone cuts. Coronal plane supramalleolar deformity less than 10 degrees is frequently corrected with the tibial cut, with or without medial malleolar osteotomy. Supramalleolar deformities of 10 degrees or greater should be considered for supramalleolar osteotomy, particularly if the inframalleolar axis is abnormal or hindfoot alignment accentuates the deformity. Sagittal plane supramalleolar deformity is frequently associated with malunited posterior malleolar fractures and typically can be corrected with the TAR tibial bone cut ( Fig. 23-15 ). Adjusting the sagittal distal tibial articular angle can help realign talar translation associated with malaligned distal tibial sagittal alignment. Multilevel deformities, such as mid-tibial deformity associated with knee or ankle periarticular deformity, are also possible and may require complex or staged deformity correction techniques.
Supramalleolar osteotomy techniques are described in Chapter 22. Briefly, they are typically medial or laterally based and may consist of a closing-wedge or opening-wedge osteotomy. A dome-shaped supramalleolar osteotomy may be performed from the anterior incision as well. With long-stemmed, modular implants, the tibial stem may be used to augment osteotomy fixation. If the osteotomy is done concurrently with TAR, bone from the tibial and talar cuts may be used to fill opening wedge defects. Alternatively, staging TAR after the supramalleolar osteotomy but within the period of protected weight bearing after the osteotomy allows adequate bone and soft tissue healing to begin but does not change the total period of convalescence.
Case series describing deformity correction proximal to the ankle show high union rates and improvement in clinical outcomes. In 26 TAR with associated tibia or fibula osteotomies, all of the isolated tibial (9) or fibular (5) osteotomies healed, and 11 (92%) of 12 combined osteotomies healed uneventfully. Staged limb deformity correction followed by TAR improves overall alignment as well as pain and functional outcomes.
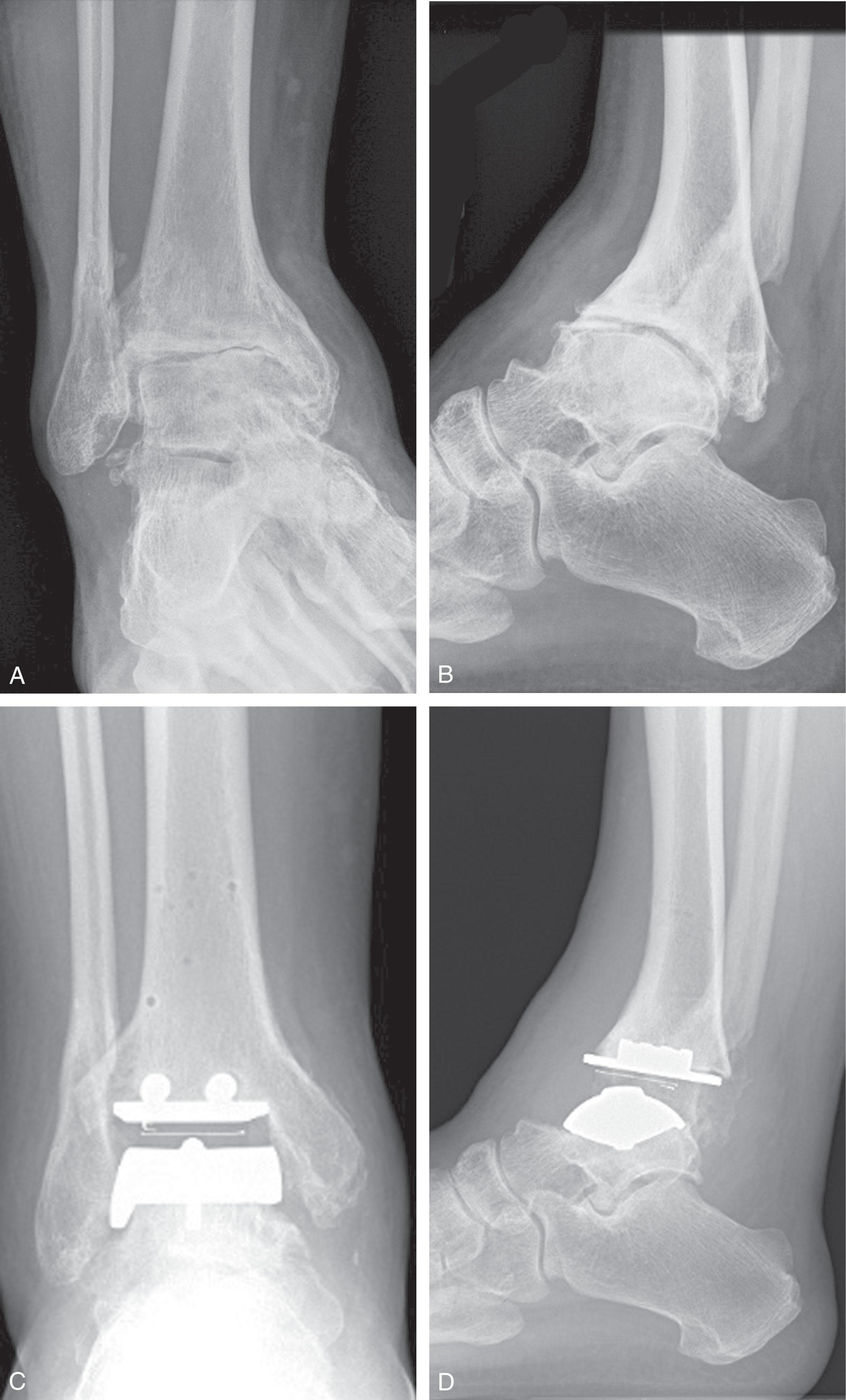
Ankle arthritis with valgus tilt tends to be supple and associated with varying degrees of instability of the medial ankle structures. There is frequently an associated hindfoot planovalgus deformity, such as in stage IV posterior tibia tendon dysfunction ( Fig. 23-16 ). In these cases, the hindfoot deformity drives the ankle deformity; as the deltoid ligament becomes progressively incompetent, tibiotalar tilt worsens and the deformity becomes incongruent. Other valgus deformities originate at the ankle joint or periarticular bone. For instance, chronic deltoid insufficiency from sprains may lead to valgus tilt and lateral plafond erosion. In either case, compensatory forefoot deformities should be identified and deltoid competence assessed as part of formulating a treatment plan.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here