Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work was supported by the American Association under grant number 18POST33990293 (BAB) and the National Institutes of Health under grant numbers P01HL051971 (BAB, JPG), P20GM104357 (BAB, JPG), R01HL136684 (JPG), R01HL137791 (EMG), R01HL123527 (EMG), T32HL105324 (JPG), and U54GM115428 (JPG).
Editors’ comment: Animal models were not mentioned in Chesley’s initial edition, and it is not clear that he acknowledged that they could be useful to develop as surrogates for preeclampsia pathophysiology. Early strategies used uterine ischemia to produce hypertension and proteinuria; this approach was perfected in rodents by the authors of this chapter. In this model, reduction of the uteroplacental perfusion pressure (RUPP) in pregnant rats is achieved by surgically constricting the aorta and both uterine artery arcades. Using this approach, many investigators have generated a plethora of data that have improved our understanding of preeclampsia pathophysiology. This chapter also explores pharmacological interventions to improve preeclampsia pathophysiology in this and other animal models, as well as describes inbred strains and genetically manipulated rodent models to further the identification of mechanisms and novel potential therapeutic targets for clinical preeclampsia interventions. As a result, animal models have become an integral part of research both to understand and treat this syndrome.
Preeclampsia (PE), a life-threating multiorgan syndrome unique to pregnancy, is estimated to affect 3%–5% of all pregnancies worldwide. Despite its position as the leading cause of maternal death and major contributor to maternal and perinatal morbidity, incomplete knowledge of pathophysiological mechanisms underlying this syndrome remains one of the major obstacles to developing specific prevention and/or treatment strategies. Unfortunately, there is no effective drug treatment to delay the progression of PE, thus allowing for further fetal development, and current management therapies have significant limitations. At present, the only effective treatment for PE is early delivery (removal of the placenta). A better understanding of pathophysiological mechanisms of PE and the discovery of novel approaches for the treatment of this disease are major unmet needs in the field.
A close interaction between basic research involving animal models and clinical research in humans is of critical importance for the understanding of pathophysiological mechanisms of PE and the identification of therapeutic targets. On the one hand, the discovery of potential biomarkers and pathogenic factors that associate with the progression of PE can only be achieved in human studies. On the other hand, experimental results obtained from clinical studies are often correlative and unable to establish cause and effect relationships. Moreover, the relative importance of factors postulated to be involved in the pathophysiology of this syndrome cannot be discerned in human studies. Despite their limitations that will be discussed later in chapter, studies in animals have a number of advantages. Proof-of-concept studies can be conducted in animal models. In addition, studies in animal models allow investigators to examine whether certain circulating factors found in women with PE can indeed lead to hypertension and other manifestations of this disease. In this brief review, we report on progress made using some of the more widely studied animal models that were described in our previous chapter in the third edition of Chesley. We also provide a summary of recently developed animal models that have been employed to investigate novel pathophysiological mechanisms that are thought to be involved in PE. These mechanisms and the animal models used to investigate them include placental ischemia (reduced uterine perfusion pressure), impaired angiogenesis (chronic sFlt-1 administration), animal models for study of maternal immune activation in preeclampsia, and recently identified genetic mouse models that examine specific pathogenic pathways.
Experimental induction of chronic uteroplacental ischemia appears to be a promising and widely used animal model to study potential mechanisms of PE. PE develops during pregnancy and remits after delivery, implicating the placenta as a central culprit in the disease. As discussed in a recent comprehensive review, an initiating event in the clinical manifestations of PE is postulated to be reduced placental perfusion that leads to widespread dysfunction of the maternal vascular endothelium. Uterine artery resistance is markedly increased in PE as a result of impaired spiral artery remodeling. Indeed, the most reliable predictors of early-onset PE are the combination of angiogenic balance and uterine artery Doppler indices. Moreover, overexpression of HIF-alpha proteins in the placenta of women destined to develop preeclampsia likely contributes to the dysregulation of numerous genes that perturbs placental function leading to the elaboration of various proteins deleterious to the endothelium during late gestation.
Reduction in uteroplacental blood flow in a variety of species leads to a maternal cardiovascular phenotype that closely resembles PE in women. While numerous laboratories have utilized the chronic reduced uteroplacental perfusion pressure (RUPP) rat model, placental ischemic models of PE have also been applied in mice, dogs, rabbits, and nonhuman primate models. , In early studies of the relationship between placental blood flow and PE, Ogden et al. observed that partially occluding the infrarenal aorta of pregnant but not nonpregnant dogs resulted in hypertension. In subsequent studies, the bilateral ligation of the uteroovarian arteries and placement of nonconstrictive bands around the uterine arteries resulted in hypertension in dogs only after they achieved pregnancy. The animals uniformly developed hypertension and proteinuria. The hypertensive state persisted until the postpartum period, at which time blood pressures returned to normal. These findings were confirmed by studies where precise constriction of the aorta below the dog's renal arteries resulted in hypertension and proteinuria. Losonczy et al. constricted the aortas of rabbits and demonstrated elevated blood pressure in chronically instrumented pregnant animals. These studies demonstrated elevated arterial pressures accompanied by increased peripheral resistance, a feature commonly observed in preeclamptic women. The relationship between reduced uteroplacental perfusion and hypertension during pregnancy has also been demonstrated in subhuman primates. Cavanagh and coworkers studied pregnant baboons with ligated uteroovarian arteries and bands around the uterine arteries. , Females bred after the surgery exhibited higher blood pressures than nonpregnant or sham-operated control pregnant animals and were also reported to manifest glomerular endothelial swelling. An aortic constriction model was also reported in rhesus monkeys to study the effects of reduced uteroplacental perfusion from early pregnancy through delivery. In this latter model, the degree of aortic constriction was precisely controlled, and progressive hypertension, proteinuria, and glomerular endotheliosis developed.
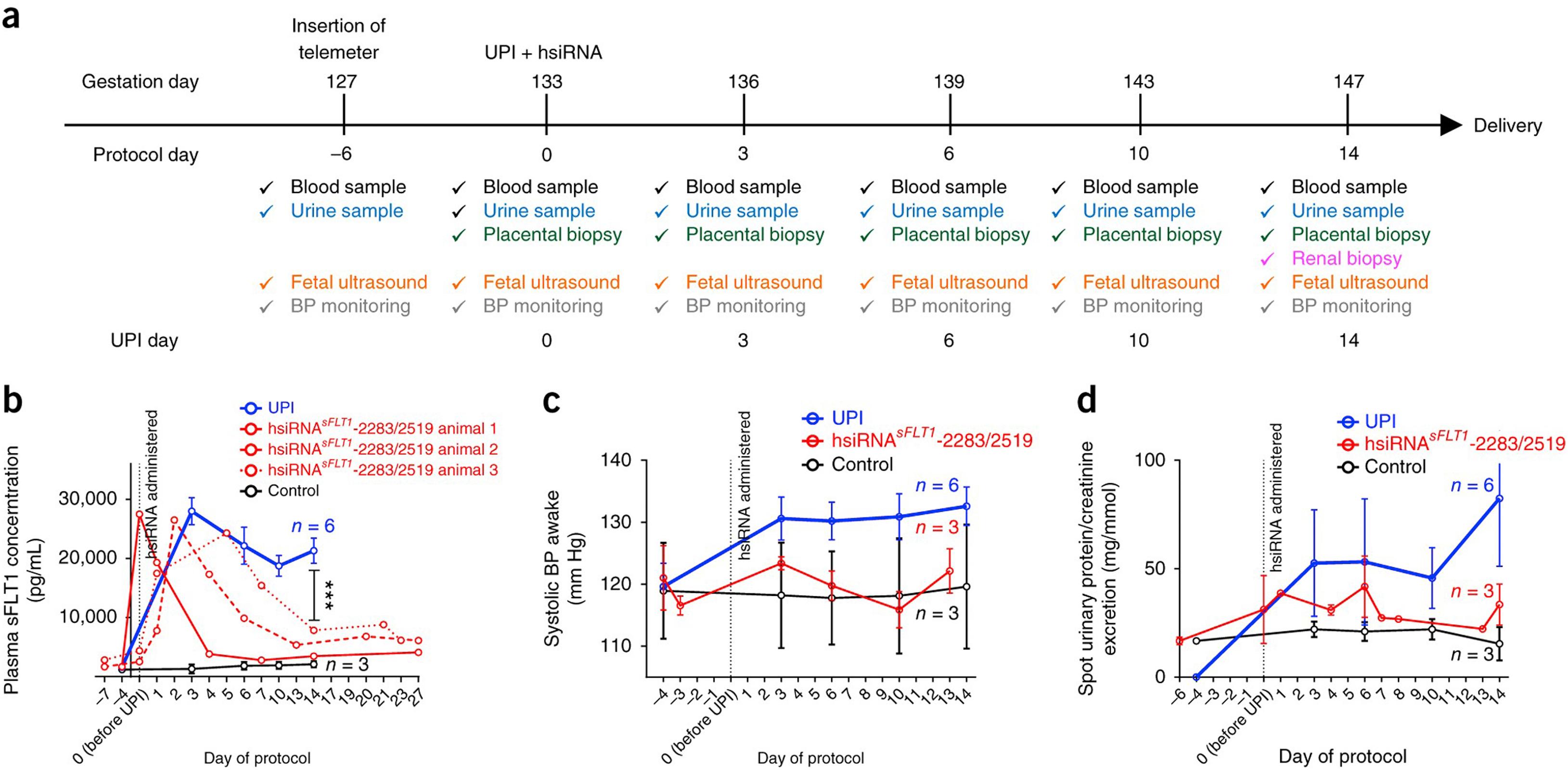
Makris et al. also characterized an uteroplacental (UPI) model in pregnant baboons equipped with radiotelemeters for recording of blood pressure by selective ligation of one uterine artery resulting in a 40% decrease in uteroplacental blood flow. Hypertension, proteinuria, and increased production of antiangiogenic markers were reported in the pregnant baboons compared to control animals who underwent a sham procedure. Turanova and colleagues also recently reported that RNAi modulation of placental sFlt-1 significantly attenuated the blood pressure and and circulating levels of sFlt-1 in placental ischemia in baboons ( Fig. 20.1 ).
The chronic RUPP rat model was extensively characterized by our laboratory and others to examine potential pathophysiological mechanisms that mediate cardiovascular and endothelial dysfunction in response to placental ischemia. Indeed, a PUBMED search indicates over 125 published studies from laboratories across the world have utilized the RUPP model. The RUPP surgical procedure is typically performed in timed-pregnant Sprague-Dawley rats (gestation day 14), but this approach has also been successfully performed in other rat strains, including Wistar rats. Uterine perfusion pressure in the gravid rat is reduced by slipping a silver constriction clip around the aorta below the renal arteries, immediately above the iliac bifurcation where the uterine arteries lie. We have found this procedure to reduce uterine perfusion pressure by ~40%. Because compensation of blood flow to the placenta occurs in pregnant rats through an adaptive increase in ovarian blood flow, branches of both the right and left ovarian arteries that supply the uterus are also clipped and positioned so as not to compromise ovarian blood flow. The timing of the vessel constriction is an important consideration. During a series of pilot studies to determine the appropriate clip size and the ideal gestational time for reducing uterine perfusion pressure, it was found that placement of the clips prior to day 14 of gestation in the rat resulted in significant fetal death. However, placement of clips on gestational day 14 to reduce placental perfusion produced a consistent blood pressure effect and minimized fetal reabsorption.
The physiological response to placental ischemia is normally assessed 5–6 days after constriction of the vessels that feed the uteroplacental unit. Uterine and placental blood flow at day 19 of pregnancy is decreased by approximately 40% in response to RUPP. Using in vivo, longitudinal photoacoustic imaging methods, Lawrence and colleagues determined that the RUPP placenta is hypoxic and that this hypoxia is maintained through late gestation. Associated with the decrease in placental blood flow is a 20–25 mmHg increase of arterial pressure by day 19 of pregnancy as compared to sham operated pregnant rats. In contrast, RUPP in virgin rats results in no significant effect on arterial pressure relative to control (unoperated) virgin rats. The hypertension in RUPP rats is associated with increases in total peripheral resistance and decreased cardiac output, systemic hemodynamic characteristics consistent with preeclampsia in women. , , Glomerular filtration rate and to a lesser extent renal blood flow decrease in the RUPP model relative to normal pregnancy at day 19. Although quite variable, an increase in urinary protein excretion has also been observed in the RUPP model when compared with normal pregnancy. The reason for the variability is unknown but may be due to the short time frame of exposure to placental ischemia. However, it is important to note that proteinuria is also quite variable in women with PE.
Endothelial dysfunction, reduced vascular nitric oxide production, and increased reactive oxygen species and lectin-like oxidized LDL-1 receptor expression (LOX-1, a specific receptor for oxidized LDL) are all characteristics of PE in women and are also present in the RUPP rat. , , , Enhanced LOX-1 expression in RUPP rats is thought to impair vascular endothelial function through the downstream actions of increased superoxide production and decreased NO-mediated vasodilation. Vascular endothelin-1 production is elevated in RUPP rats and chronic administration of selective endothelin type A receptor antagonist markedly attenuates the blood pressure response to placental ischemia.
Angiogenic imbalance observed in PE women is also present in RUPP rats. Placental ischemia in rats increases placental expression of hypoxia-inducible factor (HIF)-1α and immunoreactive sFlt1 in the placenta and plasma (29, 30). sEndoglin levels are also elevated in the RUPP model. , Moreover, RUPP is associated with decreases in plasma concentrations of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) . Interestingly, restoration of VEGF or PlGF via chronic infusion of exogenous VEGF or PlGF normalizes blood pressure in RUPP rats. ,
Fetal growth restriction (FGR), another feature of human preeclampsia, is also evident as pup weights are decreased in RUPP rats relative to controls. Alexander and colleagues have performed a series of studies utilizing the FGR rat offspring from the RUPP model to examine the mechanisms underlying the relationship between birthweight and cardiovascular diseases later in life. Thus, FGR offspring from RUPP rats is an important model to examine mechanisms of fetal programming of cardiovascular disease.
The RUPP model has also been used to examine the mechanisms whereby ischemia initiates a cascade of events resulting in placental inflammation and subsequent systemic inflammation and hypertension in the mother. , , RUPP rats have increased placental expression and circulating levels of prohypertensive cytokines (TNF-α, IL-6, IL-17, etc.) and immune cell counts (T and B lymphocytes), as well as increases in the production of the agonistic autoantibody to the angiotensin type 1 receptor (AT1-AA), and imbalance in T helper and T regulatory cells. , , Activation of the complement system also occurs in RUPP rats, as detected in the circulation by decreased complement component 3 and increased complement activation product C3a.
The RUPP model was also used to examine cerebrovascular response to placental ischemia. Cerebrovascular disturbances are now part of the diagnostic criteria for PE when accompanied by new-onset hypertension following the 20th week of gestation. The cerebrovascular disturbances in women with PE are associated with blood–brain barrier (BBB) disruption, cerebral edema, and impaired cerebral blood flow regulation. Placental ischemia in the RUPP model leads to increased BBB permeability and cerebral edema. Impaired cerebral blood flow regulation and vascular myogenic reactivity in RUPP rats were particularly evident at higher perfusion pressures. The RUPP model also has a shorter latency to the onset of drug-induced seizures and is associated with increased concentrations of proinflammatory cytokines in the cerebrospinal fluid. These findings were consistent with a study utilizing the RUPP rat coupled with a high cholesterol diet in which the threshold for seizures was reduced compared to normal pregnant rats. Importantly, magnesium sulfate has the capability of reducing placental ischemia-induced increases in proinflammatory cytokines in the cerebrospinal fluid . While these studies demonstrated that the RUPP model can be utilized to study mechanisms of cerebrovascular abnormalities, it is still not known what factors are responsible for BBB disruption, cerebral edema, and impaired cerebral blood flow following placental ischemia.
More recent studies have used the RUPP model to study mechanisms of peripartum and postpartum cardiac dysfunction in PE. There is growing evidence that PE is associated with peripartum and postpartum myocardial fibrosis and heart failure. Despite the high incidence of serious morbidity and mortality due to postpartum heart failure after a preeclamptic pregnancy, there is a substantial gap in our knowledge regarding the cardiac dysfunction during PE. Recent studies from our laboratory indicate that the RUPP model has many features of cardiac dysfunction seen in preeclamptic women. Importantly, the RUPP model demonstrates reduced myocardial function (ejection fraction and global longitudinal strain) as seen in preeclamptic women. Furthermore, biomarkers consistent with cardiac fibrosis including cardiac troponin, increased collagen mRNA expression, markers of pathological hypertrophy including increased brain natriuretic peptide (BNP), myosin heavy chain (MHC) α/β, and atrial natriuretic peptide (ANP) levels are present in RUPP rats , . We also have data showing that reduced ejection fraction and fractional shortening persists up to 8 weeks postpartum in the RUPP model despite blood pressure returning to normal by this time. Thus, the RUPP model may also be useful tool to provide a better understanding of the mechanisms underlying cardiac dysfunction that occurs during preeclampsia and in the postpartum period.
In summary, the RUPP model involves mechanical constriction of vessels that feed the uteroplacental unit as a means to reduce blood flow to the placenta. As a result, the RUPP rat displays a similar phenotype to PE, where placental ischemia is also thought to play a central role. The RUPP model of placental ischemia is associated with endothelial dysfunction, angiogenic imbalance, decreased NO production, enhanced ET-1 production, immune activation, and cardiac and cerebrovascular dysfunction; all features seen in women with PE ( Table 20.1 ). A major strength of the RUPP model is that investigators have been able to assess the functional and quantitative role for each of the systems in mediating the hypertension in the RUPP model by utilizing pharmacological agents to disrupt their actions. The RUPP model has also been used by numerous laboratories as a valuable tool to investigate potential therapeutic targets for the treatment of preeclampsia. A limitation of this model, however, is that it is not useful for studying the mechanisms involved in the abnormal spiral artery remodeling proposed to underlie placental ischemia in women with PE.
| Features | Preeclampsia | RUPP Rat |
|---|---|---|
| Mean arterial pressure | ↑ | ↑ |
| Total peripheral resistance | ↑ | ↑ |
| Circulating sFlt-1 | ↑ | ↑ |
| Circulating free PIGF | ↓ | ↓ |
| Circulating free VEGF | ↓ | ↓ |
| Oxidative stress | ↑ | ↑ |
| Lox-1 | ↑ | ↑ |
| AT1-AA | ↑ | ↑ |
| Prohypertensive cytokines | ↑ | ↑ |
| T And B lymphocytes | ↑ | ↑ |
| Endothelin-1 | ↑ | ↑ |
| Nitric oxide | ↓ | ↓ |
| Renal plasma flow | ↓ | ↓ |
| Glomerular filtration rate | ↓ | ↓ |
| Proteinuria | ↑/↔ | ↑/↔ |
| GLS | ↓ | ↓ |
| Ejection fraction | ↓ | ↓ |
| Cerebral blood flow regulation | ↓ | ↓ |
| Cerebral edema | ↑ | ↑ |
| BBB permeability | ↑ | ↑ |
| IUGR | ↑ | ↑ |
| Cardiac output | ↓ | ↓ |
Perhaps no single factor has been as extensively studied in PE than the antiangiogenic protein soluble Flt-1 (sFlt-1). sFlt-1 is a secreted form of the VEGF receptor Flt-1, which through alternative splicing has no transmembrane or intracellular domain. After secretion, the secreted recognition domain can still interact with free VEGF and importantly, also PlGF, effectively acting as a sink for the sequestration of VEGF and PlGF and thereby decreasing VEGF signaling. While elevated circulating levels of sFlt-1 are observed in normal pregnancies, numerous groups have reported excess circulating sFlt-1 in PE, often correlating with the disease severity. Tellingly, sFlt-1 expression and secretion seem to be upregulated by hypoxia through an HIF-1α-dependent mechanism in both placental trophoblasts and human placental villous explants. ,
Due to this intense interest, several different approaches have been used to simulate this imbalance between sFlt-1 and VEGF in animal models. Viral ectopic expression of sFlt-1 in pregnant rats leads to a PE-like state by causing hypertension, glomerular endotheliosis, and proteinuria. sFlt-1 has also been infused directly into both pregnant mice and rats with similar phenotypic results, including hypertension and fetal growth restriction. , Interestingly, the hypertensive effects of these models of sFlt-1 excess have proven treatable with ectopic administration of either recombinant VEGF or endothelin-1 receptor A antagonists, similar to results from placental ischemia models. , , Coupled with reports demonstrating therapeutic effects of administering VEGF in both placental ischemia induced and genetic models of PE, this supports the idea that the imbalance between sFlt-1 and VEGF observed in PE patients is a major driver of the human disease state.
Besides sFlt-1, another major antiangiogenic factor, soluble endoglin (sEng), has been noted to be dysregulated in PE. Similarly to sFlt-1, sEng is a soluble receptor for transforming growth factor β (TGF-β). Also like sFlt-1, sEng levels are increased normally during pregnancy, but more dramatically so during PE. Venkatesha et al. utilized an adenoviral vector to cause overproduction of sEng in pregnant rats, finding that it caused hypertension, intrauterine growth restriction, and focal endotheliosis. Furthermore, when coadministering adenoviral vectors to express both sEng and sFlt-1, the maternal syndrome appeared much more serious, demonstrating hemolysis, elevated liver enzymes, and low platelets (HELLP). This of course is reminiscent of HELLP syndrome and represents a potential model for this specific presentation. Follow-up studies directly infusing the two proteins together have supported these initial observations.
These data suggest a major pathogenic role of sFlt-1 in preeclampsia. Various techniques have been suggested for targeting the sFlt-1 axis in PE, from extracorporeal removal of sFlt-1 by apheresis to administration of either native or chimeric VEGF family members among others. , , These animal models of angiogenic imbalance during pregnancy could be powerful tools for not only future discovery research into the mechanisms of preeclampsia, but also as a screening tool for determining preclinical efficacy of new therapeutic agents.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here