Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thoracic surgery in children is performed for a wide variety of congenital, neoplastic, infectious, and traumatic lesions ( Box 32.1 ). The patient may be a few hours old with a congenital cystic adenomatoid malformation (CCAM, also commonly referred to as congenital pulmonary airway malformation, or CPAM) and life-threatening respiratory distress or an adolescent with an asymptomatic mediastinal tumor. Regardless of age or disease, four principles are common to all patients undergoing general anesthesia for thoracic surgery, as follows:
Preoperative evaluation and preparation can minimize intraoperative problems and improve the safety of the anesthetic.
The anesthesiologist must be aware of potential intraoperative problems.
Modern monitoring techniques have increased safety with regard to anesthetic management.
Surgical approaches and techniques are constantly changing as surgeons make efforts to refine minimally invasive procedures.
Empyema
Chest wall deformities
Chest wall masses
Lung abscess
Bronchiectasis
Lobar emphysema
Tumor (primary or metastatic)
Pulmonary sequestration
Congenital adenomatoid malformation
Congenital cysts of the lung
Bronchogenic cysts
Esophageal lesions
Mediastinal masses
Scoliosis
A thorough preoperative evaluation is essential in caring for the pediatric patient scheduled for thoracic surgery. Appropriate imaging and laboratory studies should be performed preoperatively according to the lesion involved. Guidelines for fasting, choice of premedication, and preparation of the operating room are used as for other infants and children scheduled for major surgery. After induction of anesthesia, placement of an IV catheter, and tracheal intubation, arterial catheterization should be performed for most patients undergoing thoracotomy and for those with severe lung disease having thoracoscopic surgery. This facilitates monitoring of arterial blood pressure during manipulation of the lungs and mediastinum, as well as monitoring of arterial blood gas tensions during single-lung ventilation (SLV). For thoracoscopic procedures of relatively short duration in patients without severe lung disease, the insertion of an arterial catheter is not required ( ). Placement of a central venous catheter is generally not indicated if peripheral IV access is adequate for projected fluid and blood administration.
Intravenous anesthetic agents have little effect on hypoxic pulmonary vasoconstriction (HPV), but HPV is attenuated by inhaled anesthetic agents ( ). Inhaled anesthetic agents are commonly administered in 100% O 2 during maintenance of anesthesia. Isoflurane may be preferred because there is less attenuation of HPV than with other inhaled agents, although this has not been studied in children ( ). Nitrous oxide is avoided. Use of IV opioids may facilitate a decrease in the concentration of inhaled anesthetics used and thereby limit impairment of HPV. Alternatively, total IV anesthesia may be used with a variety of agents. The combination of general anesthesia with regional anesthesia and postoperative analgesia is particularly desirable for thoracotomy, but it may also be beneficial for thoracoscopic procedures, especially when thoracostomy tube drainage, a source of significant postoperative pain, is used after surgery. A variety of regional anesthetic techniques have been described for intraoperative anesthesia and postoperative analgesia, including intercostal and paravertebral blocks, intrapleural infusions, erector spinae blocks, and epidural anesthesia (see Chapter 24 : Regional Anesthesia; ).
In awake patients, except for young infants, ventilation is normally distributed preferentially to dependent regions of the lung, so that there is a gradient of increasing ventilation from the most nondependent to the most dependent lung segments. Of note, in awake infants (until 18 months of age), ventilation is usually distributed preferentially to the nondependent regions (see Chapter 3 : Respiratory Physiology). Because of gravitational effects, perfusion normally follows a similar distribution, with increased blood flow to dependent lung segments; therefore ventilation and perfusion are normally well matched. However, controlled ventilation under general anesthesia with decreased functional residual capacity (FRC) and absent diaphragmatic contractions results in a reverse distribution of ventilation (see Chapter 3 : Respiratory Physiology). During thoracic surgery, these and other factors act to increase ventilation/perfusion (V/Q) mismatch. Compression of the dependent lung in the lateral decubitus position may cause atelectasis. Surgical retraction, SLV, or both result in collapse of the operative lung. Hypoxic pulmonary vasoconstriction, which acts to divert blood flow away from underventilated lung regions, thereby minimizing V/Q mismatch, may be diminished by the use of inhaled anesthetic agents and other vasodilating drugs. These factors apply similarly to infants, children, and adults. The overall effect of the lateral decubitus position on V/Q mismatch, however, differs in infants compared with older children and adults (see Fig. 32.1 ).
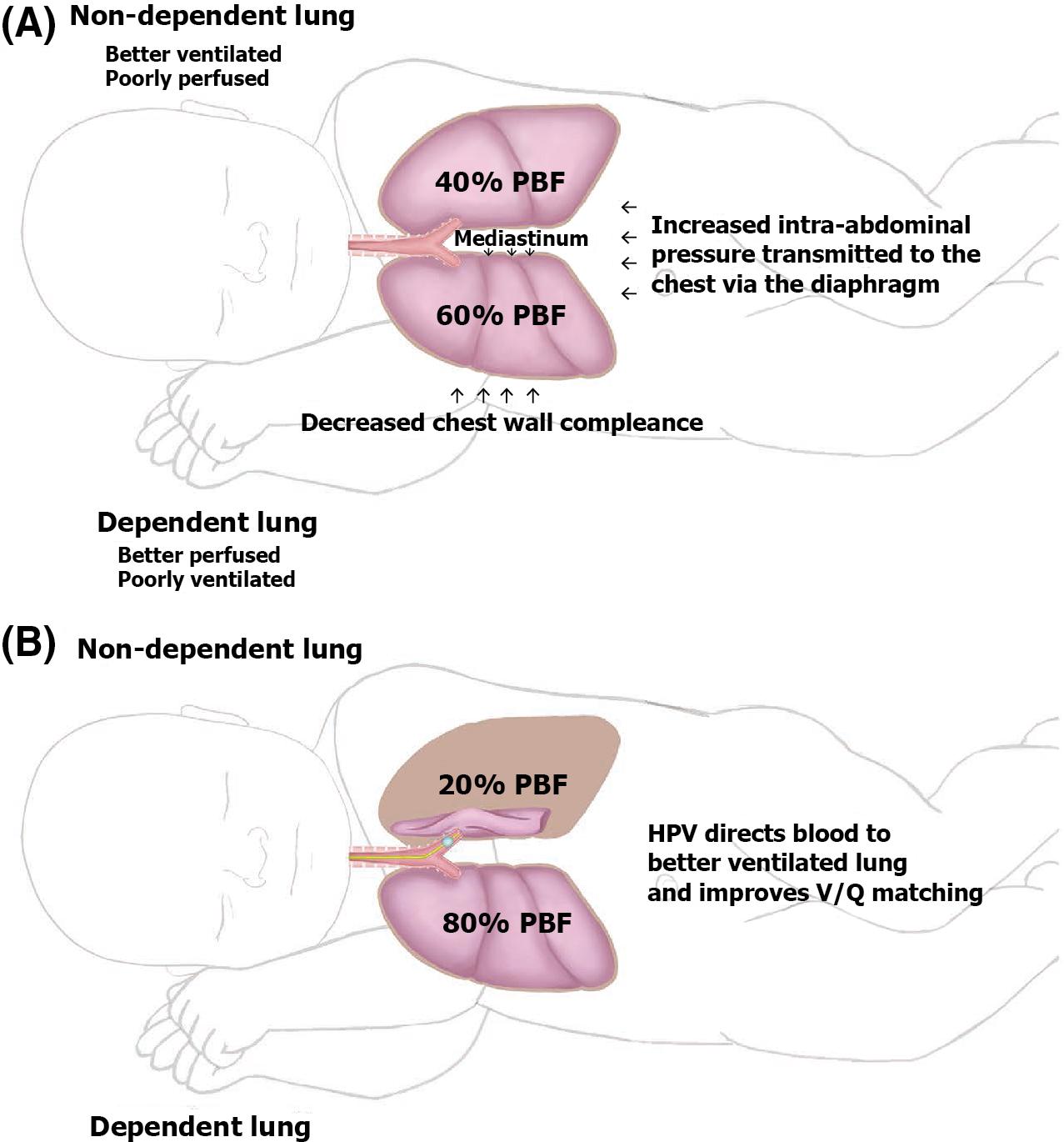
In adults with unilateral lung disease, oxygenation is optimal when the patient is placed in the lateral decubitus position with the healthy lung dependent (down) and the diseased lung nondependent (up) ( ; ). Presumably, this is related to an increase in blood flow to the dependent, healthy lung and a decrease in blood flow to the nondependent, diseased lung as a result of the hydrostatic pressure (i.e., gravitational) gradient between the two lungs. This phenomenon promotes V/Q matching in the adult patient undergoing thoracic surgery in the lateral decubitus position.
In infants with unilateral lung disease, however, oxygenation is better with the healthy lung up ( ). Several factors account for this discrepancy between adults and infants. Infants have a soft, easily compressible rib cage that cannot fully support the underlying lung. Functional residual capacity, especially in the lower lung, is closer to or at residual volume, making airway closure likely to occur in the dependent lung even during tidal breathing ( ) (see Chapter 3 : Respiratory Physiology).
Finally, the infant’s increased oxygen requirement, coupled with a small functional residual capacity, predisposes the patient to hypoxemia. Infants normally consume 6 to 8 mL of O 2 /kg per minute, compared with a normal O 2 consumption in adults of 2 to 3 mL/kg per minute ( ). For these reasons, infants are at increased risk for significant oxygen desaturation during surgery in the lateral decubitus position.
Perioperative ventilation strategies for lung protection during single-lung ventilation are inconclusive. Although have shown that lung protection ventilation decreased postoperative pulmonary complications compared with conventional ventilation in children, the lack of positive end-expiratory pressure (PEEP) in the control group may have influenced their findings (see Minimizing Injury section).
During the past two decades, the use of video-assisted thoracoscopic surgery has dramatically increased in both adults and children (see through ). As with laparoscopy, reported advantages of thoracoscopy include smaller chest incisions, reduced postoperative pain, more rapid postoperative recovery, fewer musculoskeletal sequelae, and better cosmetic outcome ( ; ; ).
Thoracoscopic surgery is being used extensively for pleural debridement in patients with empyema, lung biopsy, and wedge resections for interstitial lung disease, mediastinal masses, and metastatic lesions. More extensive pulmonary resections, including segmentectomy and lobectomy, have been performed for lung abscess, bullous disease, sequestrations, lobar emphysema, CCAM, and neoplasms. Thoracoscopic procedures used in infants and children are listed in Box 32.2 .
Anterior spinal fusion
Aortopexy
Biopsy
Abscess
Interstitial lung disease
Mass
Cyst excision
Decortication or debridement of empyema
Diaphragmatic plication
Diaphragmatic hernia repair
Drainage
Abscess
Cyst
Esophageal atresia repair
Exploration
Infection
Mass
Trauma
Foregut duplication resection
Hiatal hernia repair
Lobectomy
Mediastinal mass excision
Patent ductus arteriosus ligation
Segmentectomy
Sequestration resection
Sympathectomy
Tracheoesophageal fistula ligation
Thymectomy
Thoracic duct ligation
Thoracoscopy can be performed while both lungs are being ventilated using CO 2 insufflation and a retractor to displace lung tissue in the operative field. However, SLV is extremely desirable during thoracoscopy, because lung deflation improves visualization of thoracic contents and may reduce lung injury caused by the retractors ( ).
Pectus excavatum (funnel chest) ( Fig. 32.2 ) and the less common pectus carinatum (pigeon breast) deformities are congenital abnormalities of the sternum, ribs, and costal cartilages. These deformities are usually minimal at birth but progress with age. A higher incidence of both deformities occurs in children with Marfan syndrome or congenital heart disease and in families in which other children have the defect ( ; ). These children often appear asymptomatic but occasionally have cardiac or pulmonary abnormalities related to the deformity ( ). Patients with pectus excavatum generally present with normal or modestly reduced forced vital capacity and total lung capacity and, in severe cases, V/Q mismatch. The heart is displaced to the left and compressed, lending to arrhythmias, right-axis deviation on electrocardiogram, a functional murmur, and reduced stroke volume—most noticeable in the standing position and during exercise, explaining the mild exercise intolerance experienced by some patients. The cardiac and pulmonary abnormalities are in most instances benign and may worsen as the child ages but may be improved by surgical repair. There also is an increased incidence of mitral valve prolapse in patients with pectus deformities ( ).

Preoperative assessment focuses on exercise tolerance and other signs of cardiopulmonary compromise, such as lung infections. Laboratory evaluation includes a chest radiograph; pulmonary function tests, arterial blood gases, and electrocardiogram are added only if there is clinical evidence of significant underlying disease. Echocardiography is now commonly performed to detect the presence of mitral valve prolapse. Patients are often emotionally distressed by the appearance of a chest deformity and may benefit from preoperative counseling and, if needed, premedication. A Haller index is calculated from preoperative CT scans and measures the ratio of the horizontal diameter inside of the rib cage and the anteroposterior diameter between the vertebrae and sternum. A normal Haller index is approximately 2.5. Values greater than 3.2 are considered severe and may be considered for surgical repair ( ).
Classic operative repair involves extrapleural excision of the sternocostal cartilages and mobilization of the sternum and ribs (the Ravitch procedure). The Ravitch procedure is generally reserved for the more severe cases of pectus excavatum. For the majority of patients, a minimally invasive technique (i.e., the Nuss procedure) is used in which the costal cartilages are preserved and the sternum is elevated with a bar used to correct the deformity. Under direct vision and through a thorascope, a transmediastinal tunnel is created and a prebent bar is passed behind the sternum with the convex side down. The bar is then rotated 180 degrees to elevate the sternum ( Fig. 32.3 ) ( ). Borowitz and colleagues showed that static pulmonary function and ventilatory response to exercise were normal both before and after surgery, thereby suggesting that placement of the bar does not result in an increased chest wall restriction ( ). In addition, Lawson and colleagues noted that the surgical repair of the pectus excavatum after the Nuss procedure had a positive impact on the patient’s physical and emotional well-being ( ). Complications of this minimally invasive approach include atelectasis, subcutaneous emphysema, pericardial and pleural effusions, myocardial perforation, diaphragmatic perforation, and dislocation of the stabilizing bar ( ; ; ; ; ; ; ). Postoperative pain after the Nuss procedure is significant.
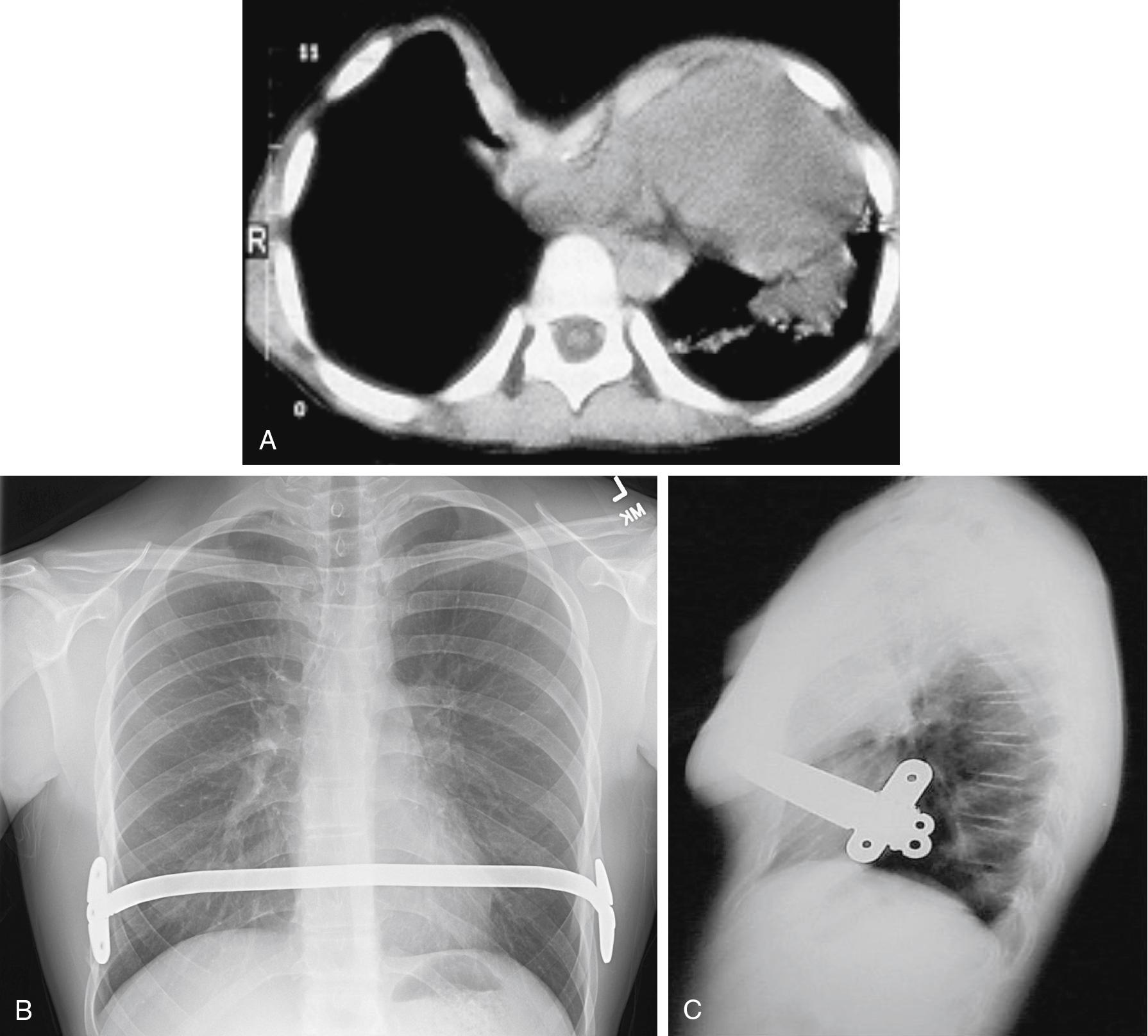
The most common complications of operative repair are pneumothorax, flail chest, and postoperative atelectasis; blood loss is usually minimal to moderate. Standard intraoperative monitoring is used; arterial catheterization is needed only if there is a specific indication. General anesthesia with controlled ventilation is the method of choice, with no agents specifically indicated or contraindicated because of the operation itself. Oxygen by face mask is administered in the recovery room, but may not be needed after the patient fully awakens.
Postoperative pain management is a challenge in these patients. Though the procedure is “minimally invasive,” the postoperative pain is intense and generally lasts days to weeks. Although patient-controlled analgesia is commonly used for postoperative analgesia, intercostal nerve blocks, paravertebral blocks, erector spinae blocks, and thoracic epidural analgesia have become increasingly popular for children undergoing pectus repair ( ; ; ; ; ; ; ; ). A thoracic epidural catheter provides more reliable analgesia to the operative area than a lumbar epidural that has been threaded a great distance. However, thoracic epidural catheters are not as easy to insert as lumbar catheters, and many clinicians are not comfortable with their routine use. Although a technique using electrocardiographic guidance and insertion from the caudal space has been described, it is not widely used ( ). An additional issue with thoracic catheters is the safety of their insertion under general anesthesia ( ; ). Although some children allow insertion before induction ( ), many younger children are not likely to remain cooperative for the procedure, mandating insertion after induction ( ; Birmingham et al. 2003). Moreover, several centers have actively and successfully used thoracic epidural techniques in anesthetized children for thoracic and cardiac procedures without complications related to insertion after induction ( ; Birmingham et al. 2003). Solutions of local anesthetic alone, local anesthetic with fentanyl, and fentanyl alone have been used successfully, including in the patient-controlled mode for appropriately mature children ( ; Birmingham et al. 2003) (see Chapter 24 : Regional Anesthesia). Thoracic epidural analgesia for 2 to 3 days, followed by oral opioid and nonsteroidal antiinflammatory drug therapy, is a frequently used technique as reported in a web-based survey sent to representatives from pediatric hospitals in the United States, Europe, Asia, and Australia. Thoracic epidurals were used for analgesic management in 91% of the institutions ( ). Though used less frequently in patients undergoing the Nuss procedure, reported on the equal analgesic efficiency of paravertebral catheters and epidural catheters.
Thoracotomy in the infant or child may be indicated for congenital abnormalities (cysts), tumors (mediastinal teratomas), trauma (gunshot wounds), or infective lesions (bronchiectasis). Subsegmental resection is used for biopsy and removal of metastatic tumors, whereas lobectomy is most commonly used for removal of congenital anomalies and extensive tumor metastasis. Pneumonectomy in children is done for various tumors, congenital abnormalities, and inflammatory lesions such as bronchiectasis. Lung resection may be performed via thoracoscopy in selected centers, though open resection remains the standard. Perioperative management differs dramatically depending on both the indication for surgery and the surgical approach.
If a space-occupying lesion is present, the patient is examined for signs of decreased cardiac output, diminished lung volume and reserve, and airway compression ( ). History focuses not only on general exercise tolerance but also on signs of intermittent airway obstruction (stridor, cyanosis, or wheezing). Physical examination includes checking for a shift in the trachea, asymmetric chest movement, wheezing, and any signs of respiratory distress. Laboratory assessment should include a chest radiograph, but additional studies such as angiography or computed tomography often provide more exact data about vascular or airway compression and compromise. It is crucial to determine the extent of airway compression and physiologic compromise, because impairment may worsen with induction of anesthesia as sympathetic and muscular tones are reduced.
If the intrathoracic lesion is a primary or metastatic tumor, the history should focus on previous treatment ( ), especially chemotherapy and radiation. Special attention is given to anthracycline (cardiac toxicity), bleomycin (pulmonary toxicity), and steroid (adrenal suppression) therapy. If there is any question about functional disability caused by this treatment, consultation with the child’s oncologist is useful. Anemia, thrombocytopenia, and malnutrition are common in these patients and should be evaluated before surgery ( ). A special consideration is the immunocompromised patient with an unknown pulmonary infiltrate. This is usually assumed to be an opportunistic infection, but because it may represent metastasis, a biopsy is occasionally requested. These patients may be in poor general condition and may require postoperative ventilatory support, especially if they had only marginal compensation before surgery ( ; ).
General assessment of the child starts with vital signs and overall appearance. Because children tolerate the loss of large amounts of usable lung tissue without obvious distress, the appearance of dyspnea or diminished exercise tolerance is an ominous sign. The history in older children focuses on complaints of dyspnea, cyanosis, wheezing, coughing, and weight loss. Infants often show less specific signs, such as poor feeding, irritability, choking, or change in sleep habits. If the child has had previous surgery, the perioperative course should be examined. The chest is inspected for asymmetric expansion and use of accessory muscles and then is auscultated for wheezes, rales, rhonchi, and absent breath sounds in both the supine and sitting positions. Physical assessment of the cardiovascular system concentrates on the presence of a gallop, murmurs, arrhythmias, and adequate peripheral pulses.
Preparation for surgery starts with a discussion of the proposed anesthetic with the parents and, if appropriate, with the child. The anesthetic plan is discussed, including monitoring, possible complications, and potential for postoperative ventilation. It is best to delay surgery until any infection or bronchospasm has been brought under optimal medical control with antibiotics, chest physiotherapy, and bronchodilators, as needed. It may be difficult or impossible to eradicate infections or bronchospasm completely in destructive lesions such as bronchiectasis. If this is the case, it is acceptable to proceed after reasonable medical therapy has optimized the patient’s status so that no further improvement is anticipated.
Standard monitoring is performed as well as monitoring of arterial blood pressure and blood gas tensions via an indwelling arterial catheter. Central venous monitoring is used less commonly but can be helpful for guiding extensive volume replacement. Urinary drainage is a consideration for particularly long procedures.
Positioning of the patient has often been used to minimize spillage of lung contents from the operative to non-operative lung because double-lumen tubes (DLTs) are impractical in smaller patients ( ). Suction through the endotracheal tube (ETT) may not be adequate to control the large quantities of purulent material freed during surgical manipulation. The supine and lateral positions are the most commonly used. Positioning can cause significant ventilatory changes in children. FRC decreases during general anesthesia ( ), and it decreases dramatically once the pleura is opened ( ). The practical problems of dislodgment of the ETT with movement and adequate padding in these positions are especially important in children. Open-celled foam with adhesive backing (Reston; 3M, St. Paul, MN) can be applied to the thorax, pelvic rim, and other pressure points to minimize the effects of positioning. Also, the tube position must be rechecked each time the patient is moved.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here