Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The American Burn Association curates an extensive range of statistics submitted by participating burn centers in addition to information derived from federal and state registries. The National Burn Repository is a comprehensive data set that depicts thermal injury patterns within the United States (reflecting aggregate data gathered between 2005 and 2016) that has been used to guide the creation of evidence-based clinical protocols, quality improvement efforts, effective resource allocation, and multidomain research efforts ( ).
Pediatric burns account for almost a quarter of historical patient encounters, and burn-related trauma remains a significant cause of accidental death in individuals younger than 18 years (exceeded only by vehicular trauma and drowning). Presentation patterns have remained relatively stable: scald injuries are most prevalent in children less than 5 years of age, whereas flame burns affect the majority of older children and teenagers ( ). Knowledge of the mechanism of injury may provide insight into expected systemic manifestations, and the clinician should always retain a high index of suspicion for child abuse when the injury pattern is inconsistent with age-related physical capabilities or the parental history changes with repeat inquiries. Additional indicators of nonaccidental burn trauma include clear demarcations (a “glove and stocking” distribution involving the hands and/or feet), concurrent fractures or evidence of old bruising, and a deliberate delay in seeking appropriate medical attention. See Chapter 40 (Anesthesia for Pediatric Trauma). In children less than 2 years of age, almost 20% of thermal injuries were the subject of social services investigations, and a significant subset of those patients were ultimately discharged into foster care ( ).
Expected survival associated with severe burn trauma in the United States has continued to improve, and these outcomes are attributed to a number of organizational and clinical principles: (1) prompt access to specialty medical care; (2) better understanding of the pathophysiology of inhalation injury and the need to mitigate barotrauma; (3) improved infection control practices; (4) aggressive nutritional support; (5) early burn wound excision and grafting; and (6) the attenuation of the hypermetabolic response ( ). A minority of pediatric patients will require transfer to a regional burn center, and the highly specialized care delivered in such facilities has limited the mortality from major burn trauma to approximately 3% ( ). Reconstructive surgery efforts have improved overall functionality and mitigated the significant psychosocial trauma experienced by pediatric burn survivors as measured by several behavioral tools ( ; ).
Burn injuries incurred through thermal, chemical, electrical, or radiologic sources grossly impair the protective functions of the skin, and physical examination surveys the depth, size, and anatomic location of these injuries to further characterize the wound severity, guide early treatment interventions, and create prognostic estimates. Serial assessment is an essential aspect of burn care; regardless of the mechanism of injury, burn wounds have a propensity to be dynamic in nature, with subsequent evolution and extension of tissue damage to adjacent and/or deeper structures ( ).
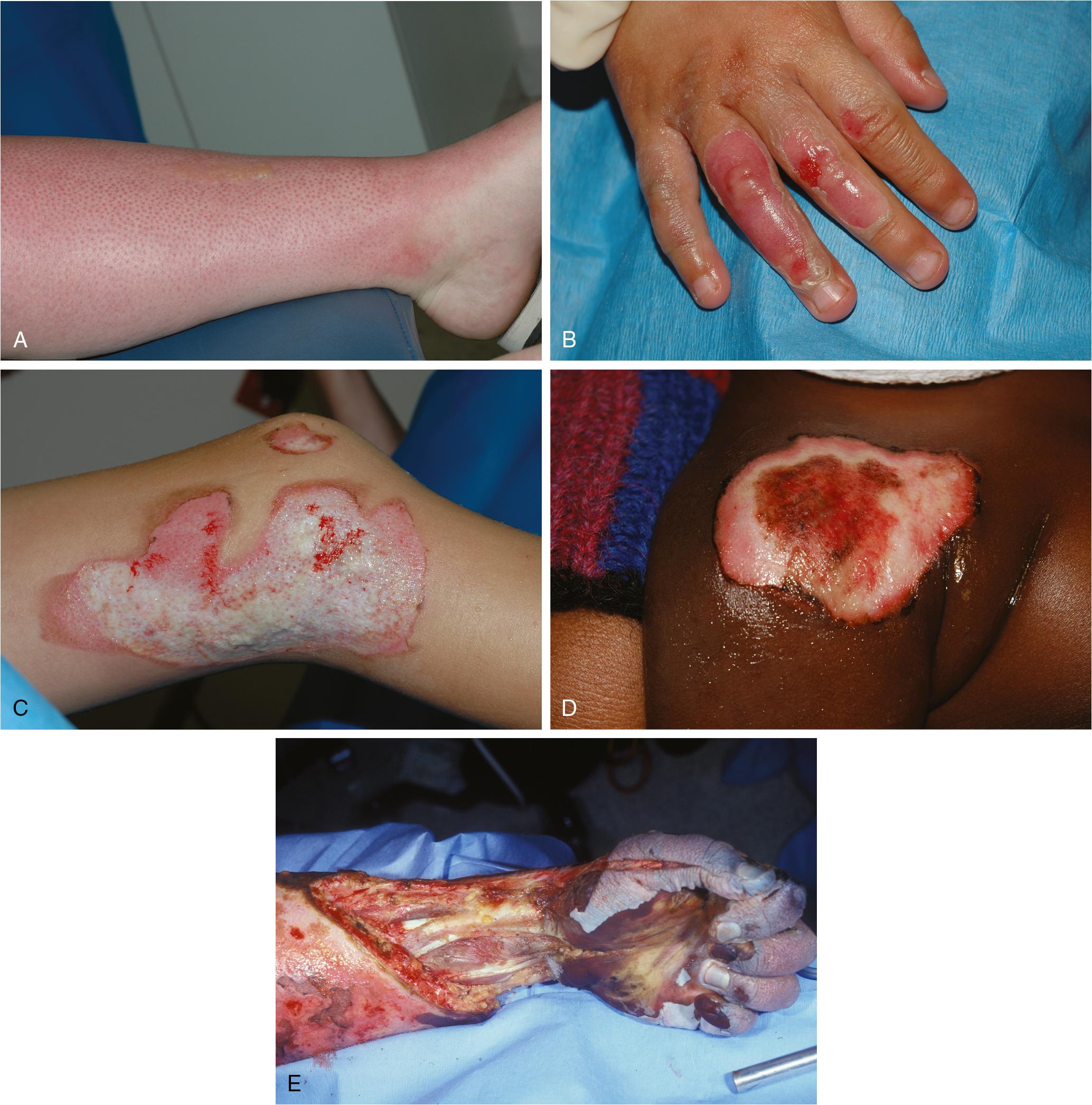
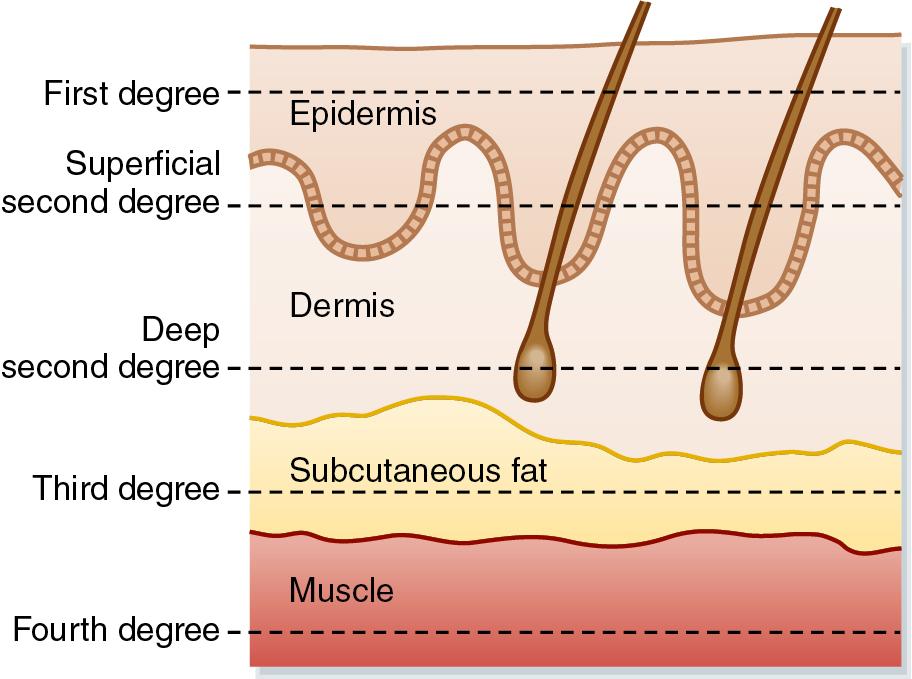
The traditional classification of burn depth (first through fourth degree) has been replaced by the designation of superficial, superficial partial-thickness, deep partial-thickness, and full-thickness injury categories, a system that better aligns with the need for surgical excision ( Table 41.1 ). Superficial burns (the most common of which is sunburn) are limited to the epidermis and present with erythema, mild pain, and possibly minor blistering ( Fig. 41.1 A). These injuries usually heal without scarring, pigmentation changes, or residual contractures. Partial-thickness burns encompass the entire epidermis and variable portions of underlying dermal structures and are further delineated by the superficial or deep descriptors. A superficial partial-thickness burn poses minimal risk of scar formation because the dermal structures (nail beds, hair follicles, sebaceous glands, and nerves) remain grossly intact, allowing for injuries to heal within 2 weeks ( Fig. 41.1 B). In contrast, deep partial-thickness injuries disrupt the dermal matrix and subsequent regeneration may be affected by significant scar formation. Extensive deep partial-thickness wounds may result in sufficient nerve damage that paradoxically blunts local pain responses. Partial-thickness burns will typically require some measure of excision and grafting in order to promote effective healing and limit infection ( Fig. 41.1 C). Full-thickness burns cause deep tissue destruction, and the necrotic debris adheres to the dermal matrix remnant as a thick, waxy layer of eschar ( Fig. 41.1 D). Prompt surgical intervention is needed to reestablish normal barrier functions and prevent infectious sequelae ( ). Even with early excision and grafting, hypertrophic scarring may persist years after the original injury. Fourth-degree burns are full-thickness burns that extend beyond the fascia, involve the bone, present with obvious muscle mass loss or the disruption of major joint capsule integrity ( Fig. 41.1 E), and are often a precursor to surgical amputation due to expected nonviability.
| First degree | Superficial | Epidermal layer only Mild pain No scarring Barrier functions preserved (Sunburn) |
| Second degree | Superficial/partial thickness | Epidermal layer with varying degree of dermal extension Wet appearance Hyperemic Edematous Blistering Painful Heals in 7–10 days Scar formation is uncommon (Scald) |
| Deep/partial thickness | Dry May appear red or pale Moderate-severe blistering Less pain (nerve damage) May advance to full-thickness injury Heals in 2–8 weeks Probable scar formation without surgical therapy (Flame/chemical exposure) |
|
| Third degree | Full thickness | Epidermal and complete dermal layer involvement Dry Waxy white or leathery appearance Painless (nerve destruction) Evolving wound site Excision and grafting required (Prolonged flame contact) |
| Fourth degree | Full thickness | Epidermal/dermal loss Fascia violation down to tendon or bone Muscle necrosis (Electrical injury) |
It must be emphasized that all burn wounds are dynamic injuries necessitating well-documented, serial assessments of size and depth to guide ongoing fluid management goals, nutritional requirements, and surgical interventions. The progression of dermal impairment can be unpredictable with caustic chemical exposures and may even be influenced by clinician experience and subjectivity. Several methods have been developed to minimize these potential variances and the potential for suboptimal care plans. These methods include applied thermography, vital dye fluorescence, video angiography, and laser Doppler techniques ( ; ; ).
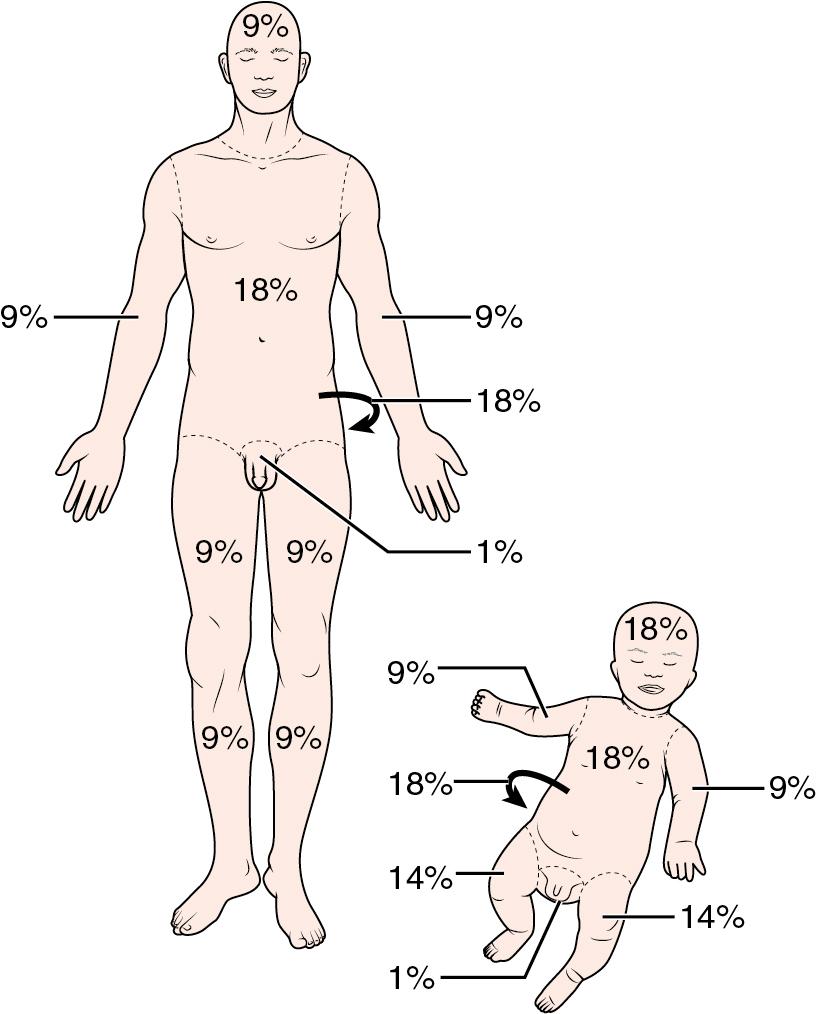
Total body surface area (TBSA) is an important parameter of burn wound classification, and several tools have been successfully employed to standardize serial assessments ( ; ). The “rule of nines” heuristic provides a rapid estimate of burn injuries to facilitate prehospital management and identify criteria for transfer to a regional burn center, but it is of limited use for smaller children ( ) ( Box 41.1 ). Pediatric burn patients have previously been evaluated using the palmar method, in which the size of the patient’s palm represents the equivalent of 1% TBSA ( ; ). The Lund–Browder diagram and its variants have become the most common assessment tool because they address the variations of normal integument distribution observed across a wide age spectrum ( Fig. 41.2 ). This instrument has been further modified to allow for the expected alterations in body proportions of the morbidly obese ( ). Complex burn wounds often present with highly irregular distributions across multiple body segments that may introduce additional inaccuracies to surface area assessments. Other techniques have been pioneered to improve the precision and reproducibility of clinician assessments, and include a flexible single-use nomogram that can be applied directly to the patient as well as advanced graphics software which creates a 3D virtual display ( ; ; ). Widespread adoption of these more advanced assessment tools has been influenced by their perceived ease of use and cost limitations.
Partial-thickness burns greater than 10% total body surface area (TBSA).
Burns that involve the face, hands, feet, genitalia, perineum, or major joints.
Third-degree (full-thickness) burns in any age group.
Electrical burns, including lightning injury.
Chemical burns.
Inhalation injury.
Burn injury in patients with preexisting medical disorders that could complicate management, prolong recovery, or affect mortality.
Any patients with burns and concomitant trauma (such as fractures) in which the burn injury poses the greatest risk of morbidity or mortality. In such cases, if the trauma poses the greater immediate risk, the patient may be stabilized initially in a trauma center before being transferred to a burn center. Physician judgment will be necessary in such situations and should be in concert with the regional medical control plan and triage protocols.
Burned children in hospitals without qualified personnel or equipment for the care of children.
Burn injury in patients who will require special social, emotional, or rehabilitative intervention. For specific patient questions, consult with your local/regional burn center.
Burns are further classified by their overall severity (minor, moderate, or major) as defined by the American Burn Association and American College of Surgeons Committee on Trauma ( Table 41.2 ). These parameters may serve as useful indicators of the anticipated degree of physiologic derangement but do not necessarily reflect expectant morbidity and mortality. Importantly, major burn wounds may be classified as such despite a relatively small surface area presentation if the wound mechanism was caused by an electrical source, is associated with concurrent inhalation injury, or has the capacity for significant functional impairment (as seen with hand, foot, or facial involvement).
| Minor | Superficial burns <15% TBSA |
| Moderate | Superficial burns = 15%–25% TBSA Superficial burns = 10%–20% TBSA in children Full-thickness burns <10% TBSA and burns not involving eyes, ears, face, hands, feet, or perineum |
| Major | Partial-thickness burns >25% TBSA Full-thickness burns >10% TBSA Concomitant inhalation injury Electrical burns Any complicated burn injury (i.e., patients with comorbid conditions; patients with burns to the eyes, ears, face, hands, feet, or perineum) Burns involving the face, eyes, ears, hands, feet, or perineum that may result in functional or cosmetic impairment |
An evidence-based, multidisciplinary approach directed by a pediatric specialist is the most effective way to minimize mortality and improve long-term functional outcomes among pediatric burn trauma victims ( ; ). The spectrum of burn-related physiologic abnormalities is wide. This spectrum includes but is not limited to (1) loss of thermal insulation and antimicrobial barriers, (2) distortions of airway anatomy and pulmonary insufficiency, (3) fluctuations of intravascular volumes and the need for individualized fluid replacement, (4) disruptive effects of hypermetabolism and elevated nutritional needs, (5) underlying septicemia, (6) prolonged inflammatory response to systemic trauma, and (7) altered responses to common anesthetic agents. Patients with significant and complex burn injuries will benefit from the capabilities of a regional burn unit to deal with these physiologic derangements, and the American Burn Association has forwarded selection criteria to facilitate referrals ( Table 41.3 ).
| Early resuscitation phase (0–48 hours) | Upper airway compromise Persistent bronchospasm Conducting airway obstruction Impaired ciliary clearance Decreased lung and chest wall compliance |
| Late resuscitation phase (48+ hours) | Surfactant loss Increased dead space Increased closing volume Decreased functional residual capacity Tracheobronchitis ARDS/pulmonary edema/pneumonia |
The epidermis provides an effective barrier to heat loss, evaporation, and infection, whereas the dermis and its supporting neurovascular structures provide elasticity, flexibility, and the mechanism for epithelial regeneration. Burn trauma causes localized tissue coagulation and microvascular reactions that can propagate injury extension ( ). Skin integrity loss leaves the patient at high risk for subsequent systemic infection and hypothermia. Infants and young children are particularly vulnerable due to their increased surface area to body mass ratio. Deliberate control of ambient temperature and humidity is needed to limit heat loss and lessen caloric expenditure from shivering.
Burn wound severity correlates to the contact temperature and duration of exposure. Burns typically produce three zones of heterogeneous tissue damage radiating from the epicenter of maximal tissue destruction ( ). The zones of stasis and hyperemia represent affected tissues that are potentially salvageable with supportive care and have the most impact upon the final TBSA measurement ( ) ( Fig. 41.3 ). During the first 24 to 48 hours, systemic hypotension, acidosis, and developing sepsis may exacerbate and extend the injury from reactive edema, impaired microcirculation, and perfusion deficits. Serial examination is the most prudent method to address the variability of burn wound progression. Early detection of nonviable areas with subsequent surgical excision and grafting can decrease morbidity and mortality ( ).
Inhalational injury refers to the pulmonary derangements of mucosal exposure to the toxic products of combustion and the systemic response to severe thermal trauma. Pathophysiological changes affect both the upper and the lower airways, contributing to significant restrictions of total airflow, clearance mechanisms, and gas exchange. The relationship between inhalation injury and increased mortality has been well established ( Table 41.3 ) ( ; ).
Direct-heat injury via superheated gas inhalation and toxic smoke exposure may cause gross edema of the tongue, epiglottis, and aryepiglottic folds. Macroglossia, micrognathia, and tonsillar hypertrophy are common findings in previously healthy children. These features coupled with an inhalational injury may further compromise an airway ( ). Reductions in the conducting airway cross-sectional area produce increased resistance and turbulent flow patterns that are poorly tolerated by infants and young children, who already have smaller airway diameters, elevated baseline minute ventilation, increased oxygen consumption, and limited reserve of the major respiratory muscle groups. Large-volume intravenous fluid administration may accelerate the effects of oropharyngeal edema and convert a marginal airway into a severely compromised one with anatomic distortions that make successful direct laryngoscopy attempts very challenging or impossible. Avoidance of the “lost airway scenario” in the pediatric burn patient is an early critical step during initial management. The clinician should be guided by frequent serial airway evaluations in order to avoid a potential airway catastrophe. Early, definitive control of the airway is preferable in patients with suspected airway burns, and this principle applies equally to other burn modalities in which the local oropharyngeal architecture may be severely compromised (e.g., ingestion of caustic agents such as lye).
Lower airway injury is a clinical diagnosis supported by the presenting history and serial examination. Chest radiographs and bedside pulmonary function tests may remain relatively normal until infectious complications arise, and these studies often underestimate the severity of lung damage ( ). Fiberoptic bronchoscopy and bronchoalveolar lavage have been used to identify the presence of carbonaceous debris and mucosal sloughing, but a negative examination does not necessarily exclude significant injury in symptomatic patients. Technetium scanning has been explored as a diagnostic tool to detect lung injury in those patients with pulmonary signs and symptoms that are not supported by abnormal chest radiographs or pulmonary function test (PFT) values ( ), but this technique has not seen widespread adoption.
A host of poisonous aerosols (carbon monoxide, hydrogen cyanide, various aldehydes, hydrogen chloride, and other aromatic hydrocarbons such as benzene) may be released in large quantities during combustion and are the toxic catalysts that contribute to inhalation injury. Even a brief exposure may result in acute endobronchial changes with impairment of mucociliary clearance and the subsequent accumulation of cellular debris, secretions, and bacteria ( ). Endobronchial sloughing and edema contribute to small airway narrowing while surfactant loss causes widespread atelectasis. Leukocytes aggregate in the affected lung tissue and release potent inflammatory mediators in addition to other chemotaxins, which further increase endothelial permeability ( ). Dyspnea, tachypnea, diffuse rhonchi, and bronchospasm are among the clinical manifestations of evolving pulmonary insufficiency and impaired gas exchange induced by aerosolized irritants ( Table 41.4 ). The cumulative effects of impaired airway clearance and inflammation quickly predispose the patient to barotrauma, significant ventilation/perfusion mismatch, and pneumonia. Lower airway injuries also increase systemic fluid requirements, and while fluid restriction protocols may minimize undesirable pulmonary edema, they may also produce systemic hypoperfusion states that are poorly tolerated ( ).
| System | Early: Hours to Days | Late: Days to Months |
| Cardiovascular | ↓ CO from myocardial depression (TNF-α) ↓ Circulating blood volume ↑ SVR ↑ PVR |
↑CO/CI ↑ Heart rate |
| Pulmonary | ↓ Mucociliary function ↓ FRC ↓ Pulmonary compliance ↓ Chest wall compliance Acute respiratory distress |
Bronchopneumonia Bronchiectasis Airway reactivity Tracheal stenosis Restricted chest wall expansion |
| Renal | ↓ GFR caused by:
|
↑ GFR caused by ↑ CO Tubular dysfunction |
| Hepatic and gastrointestinal | ↑ ALT/AST ↓ Synthetic function ↑ Abdominal compartment syndrome Reduced mucosal pH and bowel bacterial translocation |
|
| Hematopoietic | ↓ Red blood cell mass, anemia Thrombocytopenia ↑ Fibrin split products Coagulopathies |
Thrombocytosis Coagulopathies Transfusion reactions Transfusion-related infection |
| Neurologic | ↑ Cerebral edema Encephalopathy Seizures ↑ ICP |
Encephalopathy Seizures ICU disorientation ↓ Cutaneous sensation ↑ Chronic pain |
| Skin | ↑ Thermal, fluid loss ↑ Electrolyte loss |
Contractures and scarring |
| Metabolic | ↓ Ionized calcium | ↑ Oxygen consumption ↑ CO 2 production ↓ Ionized calcium |
| Pharmacokinetics | Altered volume of distribution Altered protein binding Altered pharmacokinetics Altered pharmacodynamics |
↑ Opioid/sedative tolerance Enzyme induction Altered receptor function Drug interactions |
Acute respiratory distress syndrome (ARDS) is not an uncommon development in patients with major burn injuries. Pulmonary toilet is needed to clear endobronchial debris, purulent exudates, mucous plugs, and residual particulates ( ). Some studies have reported that the administration of aerosolized heparin and mucolytics may reduce cast formation and attenuate respiratory failure ( ). Lung-protective ventilation strategies address persistent hypoxia and evolving ARDS by employing low tidal volume patterns augmented by the application of positive end-expiratory pressure and permissive hypercarbia (clinical tolerance of elevated Pa co 2 while maintaining pH >7.25 and SpO 2 >90%) and aim to preserve tissue oxygenation in the setting of severe pulmonary dysfunction ( ; ). High-frequency percussive ventilation and other advanced ventilation modalities have been prescribed, but outcomes data for these techniques remain unclear ( ; ; ).
Pneumonia is a frequent consequence in burn patients who experience prolonged intubation intervals, and its presentation is often complicated by the generalized immunosuppression associated with severe burn trauma. It is typically characterized by fever, mucopurulent secretions, and lobar consolidation depicted in chest radiographs. Bronchoalveolar lavage and brushings may support the diagnosis ( ; ). Antibiotic therapies should be reviewed and edited to optimize desirable bacteriocidal effects and limit the potential development of microbial resistance.
A persistent need for a definitive airway raises the possibility of tracheostomy placement for pediatric patients, although tracheostomy is not without risk ( ). The stability offered by a surgical airway is highly desirable when the treatment plan requires multiple transports to/from the operating room or frequent patient repositioning for wound care. Limiting dead-space ventilation and airway resistance, improved pulmonary toilet, and facilitation of the weaning process are also potential benefits ( ). Despite these obvious advantages, surgical airways in younger children may be associated with the development of structural abnormalities such as subglottic stenosis or tracheoesophageal fistula. The routine use of this technique lacks broad consensus among clinicians, and any care plan should review all pertinent pros and cons specific to the patient’s individual circumstances ( ).
The clinician should always maintain a high index of suspicion for carbon monoxide (CO) intoxication, because this common by-product of incomplete combustion found almost universally among house and industrial fires produces a life-threatening, functional anemia secondary to its strong binding affinity for heme species. Although CO reversibly binds to hemoglobin, it does so at approximately 200 times the strength of oxygen. Only a brief exposure is necessary to produce clinical symptoms, and a person exposed to 1% CO vapor attains measured carboxyhemoglobin (COHb) levels of 30% within 2 minutes. Severe CO poisoning may occur even without evidence of overt burn trauma, and delayed diagnosis contributes to significant morbidity and mortality after exposure ( ; ). The half-life of CO in a patient breathing room air may exceed 200 minutes, but this time can be reduced by more than half with the application of 100% inspired oxygen delivered via nonrebreathing face mask ( ).
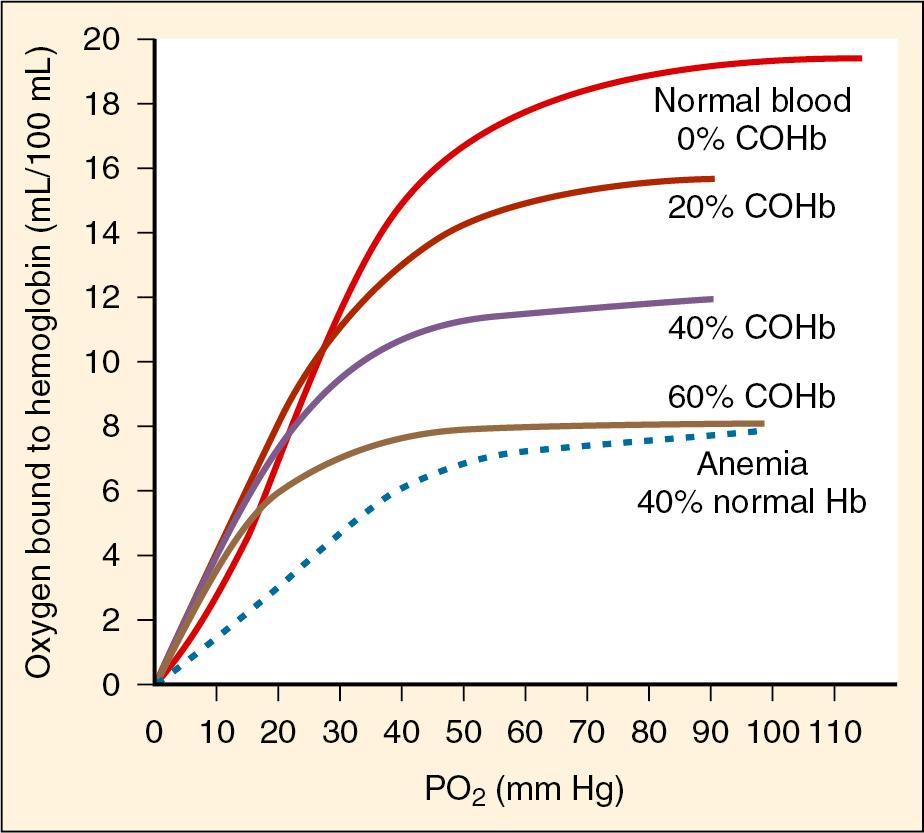
CO binding of hemoglobin and cytochrome P450 produces a leftward shift of the oxygen–hemoglobin dissociation curve and subsequently interferes with erythrocyte transport and oxygen use within the mitochondria ( Fig. 41.4 ). CO-induced alterations of oxygen delivery and cellular usage have the greatest impact on those organ systems with the highest baseline oxygen requirements (i.e., the brain and heart). Ambient levels of 100 parts per million are sufficient to produce acute neurologic symptoms (e.g., agitation, dizziness, lethargy, and seizures) in addition to chest pain, dyspnea, and noncardiogenic pulmonary edema. In animal models, CO has been observed to have direct myocardial depressant properties that are independent from the effects of global hypoxemia ( ). The classically described cherry-red appearance of patients with CO toxicity is rare and should actually be considered a late sign associated with high mortality (“when you’re cherry red, you’re dead”). Most patients present with a degree of pallor that reflects the functional anemia previously described.
Standard oximetry devices will overestimate oxygen saturation in the patient with acute CO intoxication, thereby masking profound hypoxemia ( ). Arterial sampling of Pa o 2 is also misleading because this technique quantifies plasma levels of dissolved oxygen rather than actual hemoglobin saturation. COHb may be accurately assayed with blood samples or measured directly with a noninvasive cooximeter (Masimo Rad-57, Masimo Corporation; Irvine, CA) that measures and compares multiple light wavelengths to identify COHb and methemoglobin levels ( ).
Neuropsychiatric effects of CO poisoning may appear days to weeks after the initial exposure, and although most patients eventually achieve complete clinical recovery, radiographic evidence of CO-induced changes persist for far longer. A smaller subset of patients may develop long-term sequelae that include chronic headaches, memory disturbances, learning disabilities, and residual motor dysfunction. Some studies suggest that CO induces brain lipid peroxidation and leukocyte-mediated inflammation, which culminate in white matter demyelination or focal necrosis ( ). The specific risk for long-term neurologic effects remains unclear, although some clinicians have advocated the use of hyperbaric oxygen therapy (HBOT) to limit such changes. The evidence supporting the empirical use of HBOT is unclear, possibly because some of the neurocognitive symptoms may be attributed to other factors ( ; ).
Cyanide inactivates cytochrome oxidase and impairs mitochondrial oxidative phosphorylation, even in the presence of adequate oxygen, creating a life-threatening condition in which normal aerobic metabolism is converted to an anaerobic state ( ). The clinician will observe supranormal venous oxygen levels in the presence of a metabolic lactic acidosis that does not resolve with oxygen therapy. The patient displays many neurologic and cardiovascular symptoms similar to those observed with CO poisoning and in fact may require treatment for both types of exposure. Once identified, prompt intervention includes the administration of agents that scavenge intracellular cyanide and promote its conversion to nontoxic metabolites. Sodium thiosulfate donates a sulfur group to cyanide and increases its conversion rate to thiocyanate, which then undergoes renal elimination ( ). Nitrites (e.g., amyl nitrite and sodium nitrite) induce methemoglobin, which then interacts with cyanide to release it from cytochrome-oxidase binding sites. Nitrites should be used with caution in children because concurrent methemoglobin and carboxyhemoglobinemias significantly reduce oxygen-carrying capacity ( ). Hydroxocobalamin combines with cyanide to form nontoxic cyanocobalamin (vitamin B 12 ), which then undergoes renal elimination ( ).
Acute dermal necrosis from burn trauma is characterized by protein denaturation, loss of elasticity, and tense contractures of the dermal remnant. Full-thickness burns often develop a noncompliant eschar, and depending on the degree of abdominal and thoracic involvement, the patient may experience a severe compromise of diaphragmatic and chest wall expansion that quickly progresses to respiratory failure ( ). Large-volume fluid resuscitation protocols predictably aggravate existing edema in which elevated intraabdominal and intrathoracic pressures compromise venous return, with a subsequent reduction of cardiac output and systemic hypotension. Eschar involving the upper and lower extremities may produce profound neurovascular insults, rendering the limbs nonviable ( ). Abdominal, thoracic, and extremity escharotomies are performed to relieve compartment syndromes and to restore chest wall compliance and adequate extremity perfusion.
Pediatric patients with severe burn trauma experience a biphasic cardiovascular response: the early phase is characterized by marked reductions in cardiac output from intravascular volume loss and mediator-induced myocardial depression; the later phase involves a persistent catecholamine surge that marks the well-documented hypermetabolic phase and multifactorial cardiac dysfunction. Some studies suggest that burn-related cardiac stress and cardiovascular derangements may continue for months after the inciting injury and may lead to increased morbidity observed in pediatric burn patients ( ).
The hypotension commonly seen in pediatric burn patients during the acute phase is the result of volume deficits and diminished cardiac output. This condition is exacerbated by systemic inflammatory response syndrome (SIRS), a complex milieu of cytokines, chemokines, and proinflammatory mediators that alter capillary permeability, depress myocardial contractility, and impair the normal function of many other organ systems ( Table 41.4 ).
Tumor necrosis factor-α (TNF-α) from cardiac myocytes has been demonstrated to contribute to progressive cardiac contractile dysfunction in several models of trauma, thermal injury, and sepsis ( ; ). TNF-α is an inflammatory mediator that normally modulates the antimicrobial and metabolic response to tissue injury, but in the context of burn trauma, this mediator recruits additional neurohumoral agents that exacerbate organ injury and initiate cellular apoptosis. Interleukins (IL-1β, IL-6, and others) also possess negative inotropic effects and may act synergistically with TNF-α to perpetuate postburn myocardial cardiac depression ( ). Prolonged hypotension treated with volume replacement may result in reperfusion injury and the formation of oxidized free radicals and lipid peroxidation by-products, which may exacerbate the tissue damage occurring during the low-flow state ( ). Free radical generation is accompanied by impaired antioxidant mechanisms and the upregulation of inducible nitric oxide synthetase (iNOS). This cascade promotes peripheral vasodilation, enhanced NF-κβ release, and the release of additional reactive tissue injury mediators such as peroxynitrite ( ). Leukotrienes, platelet activation factor, thromboxane A2, and complement are other factors that potentially modulate burn-induced inflammatory responses.
Burn shock is the clinical summation of severe intravascular fluid deficits and cellular ischemia that presents as decreased cardiac output, impaired contractility, and hypoperfusion of organ systems. There are also significant alterations of endothelial permeability, resulting in pronounced fluid translocation and generalized edema formation ( ). Transcellular electrolyte shifts and the loss of circulating plasma proteins further impair tissue perfusion. Pediatric burn patients possess limited compensatory responses to systemic hypotension, and vasoactive infusions (e.g., dopamine or norepinephrine) may be needed to achieve adequate blood pressure and cardiac output. Aggressive initial volume resuscitation with isotonic crystalloid will not correct the myocardial dysfunction. Animal data suggests that hypertonic dextran may correct this dysfunction, but it must be performed early (within 4 to 6 hours) ( ). Resuscitation guidelines have matured to address the individual patient’s clinical needs, where frequent reassessment and serial lab analysis drive goal-oriented interventions to restore organ perfusion and tissue oxygenation.
Acute renal insufficiency occurs in the setting of hypoperfusion, intravascular depletion, myoglobin-induced tubular damage, and mechanical obstruction secondary to cast formation by the products of hemolysis ( ) ( Table 41.4 ). Hyperglycemia that results from the stress response may contribute to an osmotic diuresis that exacerbates intravascular volume depletion. Altered responses to plasma renin and aldosterone further contribute to renal derangements ( ). Efforts to maintain urine output within a recommended range of 1 to 1.5 mL/kg per hour should be confirmed with serial urimeter measurements that demonstrate ongoing evidence of adequate fluid resuscitation.
Alterations of creatinine clearance and glomerular filtration rates have implications for antibiotic plasma concentrations, and individual dosing schedules should be adjusted to achieve therapeutic levels rather than arbitrary time frames. Significant interpatient differences concerning the pharmacodynamics and pharmacokinetics of commonly used antibiotics have been reported ( ; ). Antibiotics with nephrotoxic properties are likely to exacerbate renal dysfunction.
Alterations in extracellular water and macromolecules affect electrolyte composition, and rapid changes of serum sodium, potassium, and calcium concentrations are associated with increased morbidity; serial monitoring and correction of these alterations is an essential component of acute burn management. Patients with thermal injuries release elevated levels of atrial natriuretic peptide (ANP), which may mitigate the adverse effects of low-flow states by improving renal blood flow and urine output ( ).
Burn-related hepatic dysfunction is a significant contributor to morbidity and mortality ( Table 41.4 ). Hepatic blood flow is compromised in the early burn phase secondary to blood loss, large-volume fluid shifts, and compensatory vasoconstriction. Sepsis may exacerbate hepatic derangements. Liver dysfunction is likely modulated by cytokines IL-1β and TNF-α, both of which are potent inflammatory mediators ( ). Elevations of serum transaminases, reduced protein synthesis (e.g., albumin, transferrin, and retinol-binding protein), and focal hypertrophy are all clinical markers of evolving hepatic dysfunction ( ).
Restoration of hepatic blood flow precedes an extended phase of hypermetabolism and initiates a catabolic cascade with adverse systemic effects. Acute-phase protein production (α1 acid glycoprotein) occurs at the expense of albumin synthesis, and subsequent changes in the protein-binding capacity may result in unpredictable drug plasma levels. The acute-phase process may continue for months, prolonging systemic organ dysfunction and delaying efforts to restore lean body mass ( ). The duration and severity of hepatic dysfunction are variable and ultimately dependent on the ability to restore the patient’s hepatic function to an anabolic state; this process requires successful wound closure, infection control, and adequate nutritional support.
The severity of burn trauma correlates directly with the anticipated degree of inflammatory and hypermetabolic responses, and these physiologic derangements are associated with increased mortality for patients who have experienced more than 60% TBSA burn ( ). Endogenous catecholamines and other stress hormones (e.g., glucagon and glucocorticoids) act synergistically to produce metabolic changes: a hyperdynamic circulation, sharply elevated basal energy expenditure, and the catabolism of skeletal muscle proteins.
The catabolic phase that follows severe thermal injury includes accelerated gluconeogenesis, glucose intolerance, lipolysis, glycogen mobilization, and insulin resistance. Increased amino acid oxidation causes urea formation and nitrogen loss. These processes contribute to delayed wound healing, muscle wasting, and long-term rehabilitative failure if not adequately treated. Several studies have examined the effects of β-adrenergic blockade, human growth hormone, insulin, synthetic testosterone analogs, and nutrient-specific enteral formulations to restore anabolic balance ( ; ; ; ; ).
Gastroduodenal ulceration is a common complication of extensive burns that can be prevented with the early administration of proton-pump inhibitors, H2-receptor antagonists, and neutralizing agents such as sucralfate. Acid prophylaxis is an important adjunctive therapy, because thermal injury is strongly associated with disruptions of the mucosal intestinal barrier. Mucosal gap junction changes seen in severe burns provide a vector for bacterial translocation and systemic absorption of endotoxins; these two factors contribute to an increased risk for sepsis ( ). Enteral feeding is superior to intravenous caloric administration as a means of preserving gastrointestinal motility and mucosal barrier integrity and limiting the potential for enterogenic infection ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here