Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
The basal ganglia are a group of interconnected subcortical structures that comprise the dorsal striatum (caudate nucleus and putamen); the ventral striatum (nucleus accumbens); the external and internal segments of the globus pallidus (GPe and GPi, respectively); the subthalamic nucleus (STN); the substantia nigra, which includes the substantia nigra pars reticulata (SNr) and substantia nigra pars compacta (SNc); and the ventral tegmental area (VTA). The striatum and STN are the main entry points for extrinsic glutamatergic inputs from the cerebral cortex and thalamus. The basal ganglia outflow arises predominantly from the GPi and SNr and is directed toward frontal areas of the cerebral cortex (via the thalamus), the lateral habenula, and various brainstem structures.
The basal ganglia are part of the neural circuits that involve the cerebral cortex and thalamus. According to the functions of the cortical regions from which they originate, three main functionally segregated loops can be identified: sensorimotor, limbic, and associative/cognitive. In each of these circuits, functionally related cortical areas project topographically to specific areas of the striatum. This segregation of cortical information is maintained at all levels of other basal ganglia nuclei and in the basal ganglia–receiving regions of the thalamus. Functional interactions between these loops may occur at the cortical level. The extent of functional segregation is reduced in Parkinson disease.
Cortical glutamatergic inputs to the striatum originate from most functional areas of the cerebral cortex, while the thalamostriatal glutamatergic projections arise mainly from the caudal intralaminar thalamic nuclei (the centromedian and parafascicular nuclei, CM/PF), but also with significant contributions from the rostral intralaminar, midline, associative, and motor-related nuclei. The thalamostriatal system mediates communication between the cerebellum and the basal ganglia.
At the synaptic level, the major targets of corticostriatal and a significant subset of thalamostriatal terminals are the dendritic spines of spiny projection neurons (SPNs), the main GABAergic striatal output cells. However, thalamic inputs from the CM/PF contact predominantly the dendritic shafts of the SPNs and different subtypes of striatal cholinergic and GABAergic interneurons.
Striatal SPNs are divided into two groups based on their dopamine receptor expression and main basal ganglia projection targets. The “direct pathway” SPNs project directly to the basal ganglia output nuclei, GPi, and SNr, and they express preferentially D 1 dopamine receptors, whereas the “indirect pathway” SPNs project mainly to the GPe and express D 2 dopamine receptors. An imbalance of activity between these two pathways is a cardinal feature of the basal ganglia pathophysiology in Parkinson disease and related movement disorders.
The striatum receives a massive innervation from the ventral midbrain dopaminergic cell groups, which play a critical regulatory role over cortical glutamatergic transmission on SPNs through presynaptic and postsynaptic mechanisms. Dopamine neurons process and transmit to the striatum complex and heterogeneous information related to movement, reward-related stimuli and aversive events. Additional significant inputs to the striatum originate from the GPe, hypothalamus, subthalamic nucleus, raphe, and pedunculopontine nucleus (PPN).
Along with the striatum, the STN is considered an input nucleus of the basal ganglia. As for the corticostriatal system, the corticosubthalamic projection arises from different motor, associative, and limbic cortical regions that innervate functionally segregated regions of the STN in primates. In turn, the STN sends glutamatergic excitatory projections to the globus pallidus, the substantia nigra, the pedunculopontine nucleus, and the pontine nuclei, the latter being considered as a route through which basal ganglia information can reach the cerebellum.
The GPi and the SNr are the output nuclei of the basal ganglia. Both nuclei receive functionally segregated inputs from the striatum (through direct and indirect routes), and in turn send GABAergic projections to their thalamic and brainstem target structures. Through their thalamic projections to the ventral motor nuclei and the CM/PF complex, cortical information can be sent back to frontal cortical regions via segregated basal ganglia–thalamocortical loops or to specific striatal regions via basal ganglia–thalamostriatal projections. The brainstem targets of the basal ganglia output include the superior colliculus, the pedunculopontine nucleus, the lateral habenula, and the parvicellular reticular formation.
Through its connections with most basal ganglia nuclei, the thalamus, and various lower brainstem structures, the pedunculopontine nucleus is in a strategic location to mediate widespread basal ganglia influences in the CNS, while bypassing the conventional thalamocortical system. The lateral habenula is considered as an interface between the limbic system and the basal ganglia because it receives reward-related information from the GPi. The basal ganglia–collicular projection is important in the control of saccadic eye movements and visual orientation.
Disruption of communication by degenerative neuronal loss or by neurochemical changes in various nodes of the basal ganglia–thalamocortical loops leads to the development of motor and nonmotor signs and symptoms of a large number of neurological and psychiatric disorders (including Parkinson disease, Huntington chorea, Tourette syndrome, dystonia, attention-deficit disorders, and obsessive-compulsive disorder).
The basal ganglia are a group of subcortical brain nuclei originally named based on their location at the base of the cerebral hemispheres. As a crucial part of the extrapyramidal motor system, these nuclei are involved in the control of voluntary movements, but they also participate in high-order cognitive and limbic functions so that basal ganglia diseases result in complex motor and neuropsychiatric disturbances, including Parkinson disease (PD), Huntington disease, dystonia, hemiballism, Tourette syndrome, attention-deficit disorders, and obsessive-compulsive disorder.
The basal ganglia structures include the caudate nucleus and putamen, which make up the dorsal striatum, and the nucleus accumbens and olfactory tubercle, which form the ventral striatum. In rodents, the dorsal striatum is composed of a single mass of gray matter called the caudate-putamen complex. Other basal ganglia nuclei include the pallidum, which, in primates, consists of two parts: the internal and external segments of the globus pallidus (GPi and GPe, respectively). In rodents, the homologue of the GPi is the entopeduncular nucleus (EPN) and that of the GPe is the globus pallidus (GP). The complex composed of the putamen and globus pallidus is called the lenticular nucleus. The subthalamic nucleus (STN), a small almond-shaped nucleus located laterally just below the thalamus at the junction between the diencephalon and midbrain, is another key basal ganglia structure often referred to as the “pacemaker” or “driving force” of the basal ganglia. The fact that the STN is the prime target of surgical therapy for PD heavily supports its critical role in regulating basal ganglia function in normal and pathologic conditions.
Finally, another major component of the basal ganglia network is the substantia nigra, located at the base of the mesencephalon. The substantia nigra is divided into the pars compacta (SNc), which consists of dopaminergic neurons, and the pars reticulata (SNr), largely composed of GABAergic projection neurons. Other neighboring cellular groups related to the dopaminergic SNc include the ventral tegmental area (VTA) along the midline and the more caudal retrorubral field (RRF), which lies along the ventrolateral edge of the upper brainstem. The basic circuit of the basal ganglia involves information originating from the entire cortical mantle and thalamus sent to the striatum and STN. Once this information has been processed, it is transmitted to frontal cortical regions or the brainstem through the GPi and SNr, commonly known as the basal ganglia output nuclei ( Fig. 102.1 ).
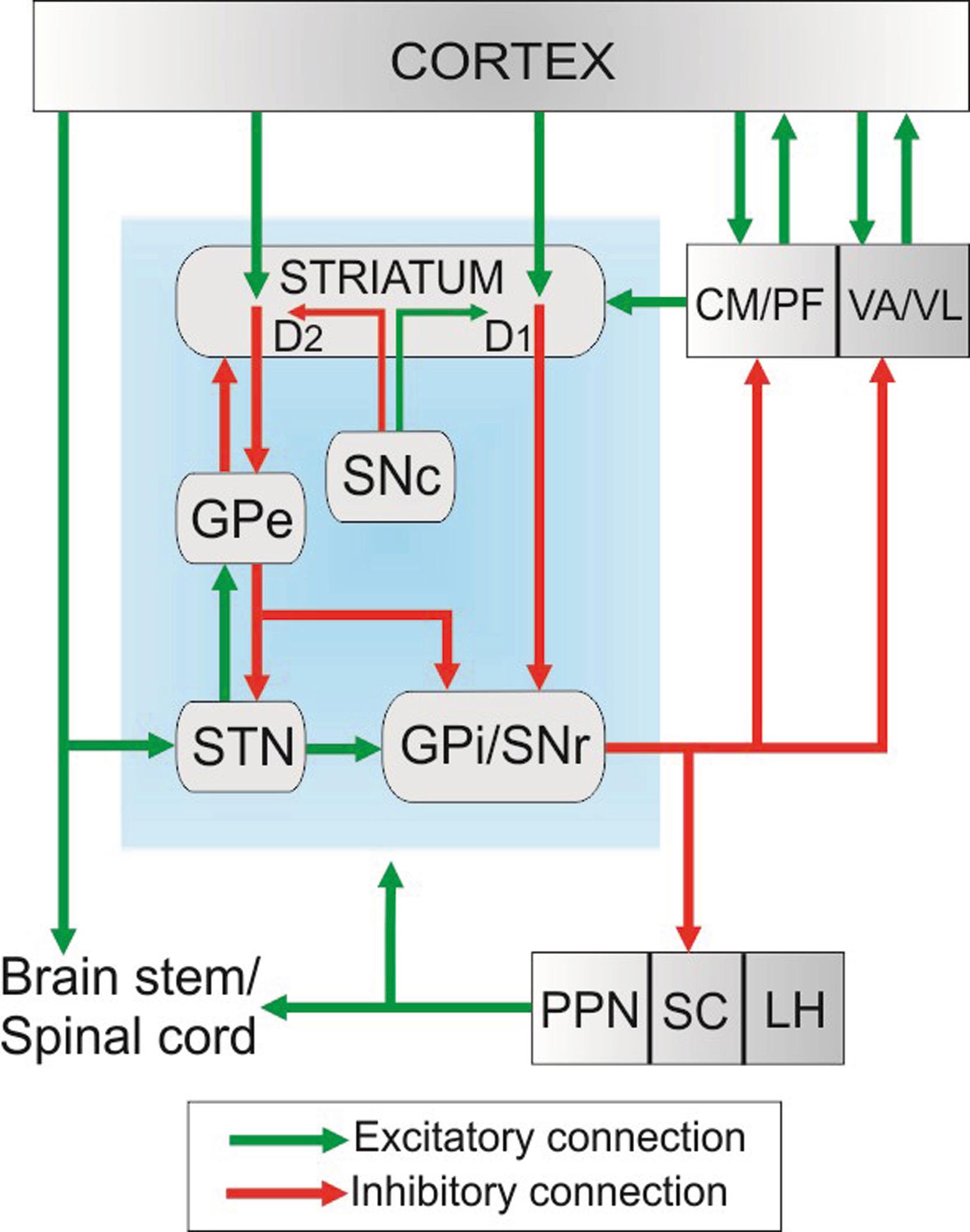
Although traditionally the basal ganglia–thalamocortical loops were considered to be separate from the cerebello–thalamocortical connections, studies over the past decade have provided evidence for different communication streams between the basal ganglia and the cerebellum (see “The Thalamostriatal System: A Potential Route of Cerebellar Outflow to the Striatum?” later in this chapter). ,
The striatum is considered the main input structure of the basal ganglia. Based on its location along the dorsoventral extent of the telencephalon, the striatum is divided into dorsal and ventral components. These striatal regions are functionally different and process segregated information from the cerebral cortex. The main cortical input to the dorsal striatum originates from associative and sensorimotor areas, whereas the ventral striatum is predominantly innervated by limbic cortical regions.
The striatal neuropil is further compartmentalized into two distinct territories called the patch (or striosomes ) and the extrastriosomal matrix. These two striatal compartments receive distinct afferent projections and display a significant degree of neurochemical heterogeneity. Despite a clear understanding of the anatomic organization of these compartments, their contributions to striatal function still remain poorly understood. However, there is evidence that imbalanced activity between the patch and matrix compartments may underlie some aspects of several basal ganglia–related disorders. Recent studies have indicated the importance of striosomal striatal neurons in cognitive and limbic behaviors.
GABAergic spiny projection neurons (SPNs) account for the vast majority of the total striatal cell population (about 97% in rodents, but this proportion might be smaller in primates ). Based on their projection target and their neuropeptide and relative dopamine receptor expression, SPNs can be divided into two major subtypes: the “direct pathway” neurons preferentially express substance P, dynorphin, and D 1 dopamine receptors, and they project directly to the GPi and SNr, whereas the “indirect pathway” neurons preferentially express enkephalin and D 2 dopamine receptors and target mainly the GPe (see “The Direct and Indirect Pathways of the Basal Ganglia” later in this chapter). , Both neuronal populations look morphologically similar, but recent rodent studies have shown subtle anatomic and functional differences that make D 2 striatopallidal SPNs more excitable than D 1 striatonigral neurons. The two neuronal populations are intermingled throughout the whole striatum (except in a caudal region adjacent to the GPe) that comprises exclusively D 1 striatonigral neurons. Recent optogenetic studies have shown that selective activation of direct versus indirect SPNs induce opposite effects on motor behaviors such that direct SPN stimulation increases locomotion, while the opposite is true for indirect SPN activation. , However, the basal ganglia regulation of complex self-paced motor programs involves coordinated activation of both direct and indirect SPNs.
SPNs give rise to a rich plexus of intrinsic axon collaterals within the vicinity of the parent cell bodies that contact neighboring projection neurons. Although this local connectivity was originally considered the main substrate for lateral inhibition in the striatum, electrophysiologic data have demonstrated that these connections are sparse and distal, and consequently poorly influential on the activity of striatal projection neurons. These connections are specifically organized and unidirectional between pairs of D 1 - or D 2 -containing neurons or from D 2 - to D 1 -positive projection neurons, while connections from D 1 - to D 2 -positive neurons are rare. The strength of these intrinsic connections is regulated by dopamine, thereby significantly altered in rodent models of PD. , Importantly, striatal cholinergic interneurons (see later) are a major target of GABAergic collaterals from SPNs.
Besides SPNs, interneurons account for the rest of the neuronal population in the striatum. Striatal interneurons represent about 2% to 3% of the total striatal neuronal population in rats, whereas in monkeys they likely account for a much larger proportion of all striatal neurons. , , The cholinergic interneurons, which probably correspond to the “tonically active” neurons physiologically identified in the rat and monkey striatum, receive strong synaptic inputs from GABAergic axon collaterals of striatofugal neurons and from GABAergic interneurons in rats and monkeys, and they play a key role in reward-related learning and saliency discrimination. , Cholinergic interneurons are connected to each other through GABAergic interneurons, thus providing a mechanism for their widespread recurrent inhibition via nicotinic excitation. , They receive robust excitatory glutamatergic inputs from the caudal intralaminar nuclei of the thalamus, , , but limited (although functionally relevant) direct innervation from the cerebral cortex. They are key mediators of dopamine-dependent striatal plasticity in normal and pathologic conditions.
The GABA/parvalbumin interneurons are known as the “fast-spiking interneurons.” They are electrotonically coupled through gap junctions, and they control spike timing in projection neurons through axosomatic GABAergic synapses, thereby providing the substrate for fast-forward intrastriatal inhibition of projection neurons in response to cortical activation , , , (but see Berke ). Their physiologic properties differ between striatal regions. Loss of striatal parvalbumin-containing interneurons has been reported in Tourette syndrome and Huntington disease.
The GABA/nitric oxide synthase/neuropeptide Y/somatostatin interneurons are categorized physiologically as “persistent and low-threshold spike (LTS)” neurons. , , , , These cells induce large inhibitory currents in projection neurons and release nitric oxide to modulate plasticity at glutamatergic synapses. , In addition, because somatostatin actions entrain projection neurons into the rhythms generated by some interneurons, they are capable of modifying the processing and output of the striatum.
The medium GABA/calretinin interneurons represent the largest population of striatal interneurons in humans and other primates, , but they are less abundant in rodents. These neurons display physiologic characteristics similar to the persistent and low-threshold spike neurons, and exert powerful monosynaptic inhibition of MSNs. , A population of large GABA/calretinin interneurons that coexpress choline acetyltransferase has been identified in the monkey and human striatum, but not in rodents. , Three segregated populations of calretinin-positive interneurons have recently been described in the rodent and monkey striatum based on their differential expression in secratogogin, specificity protein 8 (SP8), and/or LIM homeobox protein 7 (Lhx7). ,
Tyrosine hydroxylase (TH)–positive GABAergic interneurons have been described in the striatum in normal conditions and after dopamine depletion in animal models of parkinsonism and humans with PD. These small aspiny neurons receive scarce synaptic input and coexpress various markers of catecholaminergic neurons besides TH, the rate-limiting enzyme in dopamine synthesis and the most common marker to identify dopaminergic neurons; however, they do not release dopamine. , , , Their intrastriatal effects are mediated through GABAergic synapses. , Their density significantly increases after dopamine depletion , , , , , and after administration of glial-derived neurotrophic factor in the striatum.
In transgenic mice in which the green fluorescent protein (GFP) marker is selectively expressed in neurons that contain TH, the number of TH-expressing neurons is notoriously higher than in the striatum of normal rodents (perhaps caused by a greater sensitivity of the GFP labeling compared with traditional TH immunohistochemistry). In these animals, various subtypes of TH-expressing striatal interneurons with specific electrophysiologic properties have been described as playing an important role in striatal information processing, even in normal conditions. After dopaminergic depletion, these TH-expressing striatal neurons present changes in their electrophysiologic and morphologic properties, which could be additional compensatory mechanisms to the loss of dopamine. ,
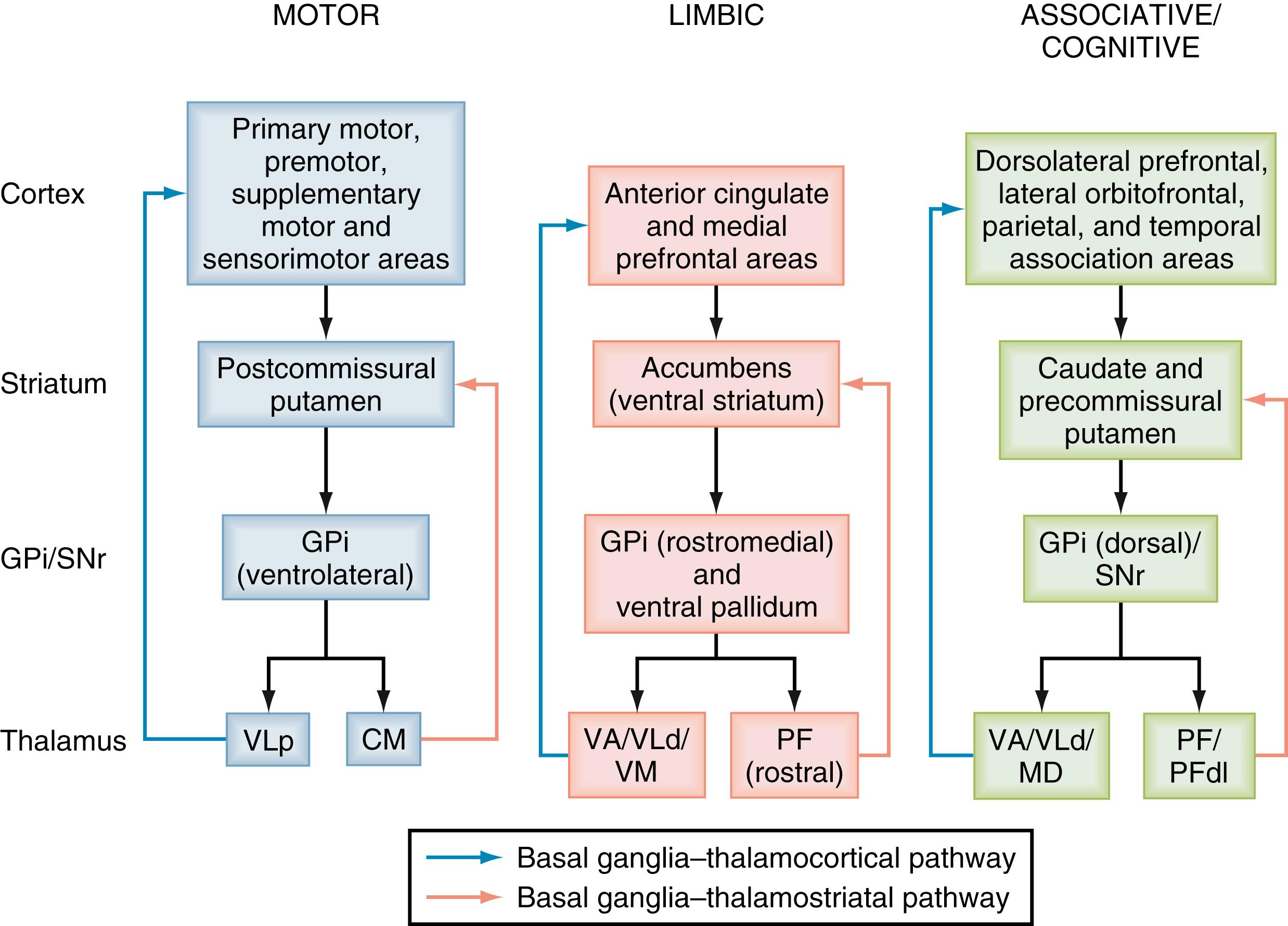
The cerebral cortex is the main source of glutamatergic inputs to the striatum. Corticostriatal projections originate from all cortical areas and display a highly topographic pattern of distribution in the striatum, which leads to functionally segregated pathways through the basal ganglia circuits ( Fig. 102.2 ). The anatomic organization of the corticostriatal system has been extensively studied in rats and monkeys, and more recently in humans via functional imaging approaches. These findings led to a basic scheme of functional connectivity between the cerebral cortex and striatum. The somatosensory, motor, and premotor cortices innervate the postcommissural region of the putamen somatotopically in a bandlike pattern; the associative cortical areas from the frontal, parietal, and temporal lobes project to the caudate nucleus and the precommissural putamen; and the limbic cortices, the amygdala, and the hippocampus terminate preferentially in the ventral striatum (see Fig. 102.2 ). , Functionally related associative or sensorimotor cortical inputs from areas connected through corticocortical projections either overlap or remain segregated in the striatum. Differential cortical inputs innervate the striosomes and the extrastriosomal matrix; such striosomes receive most of their cortical inputs from limbic-related cortices, while the matrix is mainly targeted by associative and sensorimotor cortical afferents.
In rodents, corticostriatal neurons are divided into two distinct subsets: the superficial “intratelencephalic neurons,” which project solely to the striatum and the contralateral cerebral cortex,
and the deep “pyramidal tract” neurons, which send their main axonal projections to the brainstem and spinal cord with collaterals to the striatum. Although anatomic studies showed that direct and indirect pathway SPNs are differentially targeted by these two cortical inputs, , the two populations of neurons respond to activation of inputs from both pathways or solely from intratelencephalic neurons. , There is evidence for extensive axonal arborization of pyramidal tract corticostriatal neurons throughout the brain in rodents, but this may not be the case in primates. ,
The dendritic spines of SPNs and the GABA/parvalbumin (PV)–containing interneurons are the main targets of corticostriatal projections. , Through these connections, cortical afferents can have a dual action on striatal projection neurons: a direct excitatory effect or an indirect feed-forward inhibitory effect through activation of GABA/PV interneurons. , , The cortical drive of feed-forward inhibition from GABA/PV interneurons may contribute to the imbalance of activity between the two populations of striatal projection neurons in the rat model of PD, although this concept has been challenged by electrophysiologic evidence for the lack of correlation between cortical information flowing to GABA/PV neurons and projection neurons. ,
Although its functional significance is poorly understood, the thalamostriatal system is another major source of glutamatergic projections to the striatum. Recent evidence that neurosurgical interventions targeted to the caudal intralaminar nuclei, the main sources of thalamostriatal projections, alleviate some symptoms of PD and Tourette syndrome has generated significant interest in the thalamostriatal systems. Two major sources of thalamostriatal projections have been recognized: the caudal intralaminar nuclei and other thalamic nuclei.
In primates, the centromedian (CM) and parafascicular (PF) nuclei give rise to projections that largely terminate in different functional regions of the striatum (see Fig. 102.2 ). The medial part of the CM nucleus projects to the sensorimotor postcommissural sensorimotor putamen, whereas the PF nucleus predominantly innervates the associative caudate nucleus and the limbic ventral striatum. , , The dorsolateral PF nucleus selectively projects to the precommissural putamen. CM/PF neurons send only sparse projections to the deep layers of the cerebral cortex. At the synaptic level, CM and PF inputs preferentially target the dendritic shafts of striatal projection neurons. CM terminals also innervate cholinergic neurons and other types of striatal interneurons. In agreement with this anatomic evidence, projections from the CM/PF complex tightly regulate the electrophysiologic activity and release of neurotransmitters from cholinergic interneurons, , and are required for the sensory responses of the tonically active (likely cholinergic) neurons that are acquired through sensorimotor learning in monkeys. In recent years, the thalamostriatal projection from the CM/PF complex has been recognized as a key regulator of cognitive processes related to attention, goal-directed actions, and behavioral flexibility. , , Combined with neuropathologic evidence of severe degeneration of the CM/PF complex in PD, these findings raise the possibility that thalamostriatal degeneration may contribute to the development of cognitive impairments in PD. , ,
The CM/PF complex is not the only source of thalamostriatal projections. Albeit more limited, most thalamic nuclei also contribute to a topographically and functionally organized striatal innervation. , , , However, there are several important anatomic differences in the pattern of striatal innervation between the thalamostriatal projections from the CM/PF versus other thalamic nuclei (reviewed by Galvan and Smith in references 147 and 153). For instance, at the synaptic level, CM/PF terminals form synapses predominantly with the dendritic shafts of MSNs and interneurons, while projections from other thalamic nuclei, including the rostral intralaminar, midline, and relay thalamic nuclei, almost exclusively target dendritic spines. , , , , The degree of axon collateralization to the striatum from CM/PF neurons compared to other thalamic cells is significantly different in contrast to neurons in most thalamic nuclei that provide major inputs to specific cortical regions, and only a modest diffuse projection to the striatum. , CM/PF neurons give rise to extensive, topographically organized, striatal projections with more modest inputs to the cortex. , , Thalamostriatal projections from single CM/PF neurons are much more focused and give rise to a significantly larger number of terminals than do individual thalamostriatal axons from non-CM/PF neurons. , , The functional significance of these anatomic differences between striatal inputs from CM/PF and non-CM/PF thalamic nuclei still remains poorly understood, but the use of optogenetics and other approaches that facilitate experimental modulation of specific neuronal pathways should help address this issue. , , ,
Characterization of the connectivity of the corticostriatal and thalamostriatal terminals has been greatly advanced by the demonstration that vesicular glutamate transporters 1 or 2 (vGluT1 or vGluT2) are selective markers of corticostriatal and thalamostriatal glutamatergic terminals, respectively. More than 95% of vGluT1 terminals contact spine heads, whereas only 50% to 60% of vGluT2 terminals do so in monkeys, a pattern that does not change in parkinsonism. , In rats, there is a significant difference in the microcircuitry of vGluT2 terminals between the patch and matrix striatal compartments such that most axodendritic vGluT2 synapses are found in the matrix, consistent with the idea that the CM/PF complex, which projects exclusively to the striatal matrix, is the main source of this synaptic input. , , , In contrast with vGluT2, the pattern of synaptic innervation of corticostriatal vGluT1-positive terminals does not differ between patch/striosome and matrix compartments. The potential significance of this compartmental segregation of CM/PF inputs to the striatum is underscored by the evidence that imbalance between the patch/striosome and matrix compartments may be involved in various basal ganglia disorders.
For many years, the circuits to and from the cortex to the basal ganglia and the cerebellum were considered to flow independently through largely separated thalamic nuclei. However, recent findings show reciprocal communication between the cerebellum and the basal ganglia. These studies, based on the use of transneuronal transport of rabies virus, indicate that the deep cerebellar nuclei, the main outputs of cerebellar processing, connect with the striatum through the thalamus. , In turn, the basal ganglia connect with the cerebellum by a disynaptic pathway that involves the STN and the pontine nucleus. In both cases, the projections involve both motor and nonmotor domains, suggesting that the basal ganglia and cerebellum constitute a complex network encompassing several behavioral modalities. ,
The functional relevance of these connections is just starting to emerge. For instance, evidence from rodent studies indicate that some forms of dystonia could be mediated by thalamic interactions between the cerebellum and basal ganglia.
Electrical stimulation of the CM nucleus, in vivo, induces complex excitatory and inhibitory electrophysiologic responses in striatal projection neurons and cholinergic interneurons. , In vitro studies in rodent brain slices have shown that corticostriatal and thalamostriatal glutamatergic synapses display different functional properties, as do synapses established by terminals from CM/PF versus non-CM/PF thalamic nuclei. , , Thus the ratio of NMDA/non-NMDA glutamatergic receptors is higher at thalamic than cortical synapses, , an observation that extends earlier neurochemical studies in adult rats. There is also evidence that the thalamostriatal system gates corticostriatal signaling via activation of striatal cholinergic interneurons, and that this functional interaction might be altered in a mouse model of dystonia. , One of the main limitations of these in vitro experiments was that thalamostriatal projections from CM/PF or non-CM/PF could not be activated separately. The use of optogenetic methods will help overcome this issue. For instance, recent optogenetic studies showed significant differences in the synaptic properties of thalamostriatal projections from PF versus the centrolateral nucleus or between different subsets of PF thalamostriatal neurons. , , , ,
Our knowledge about the roles of the thalamostriatal systems in the functional circuitry of the basal ganglia remains limited. Recent findings have suggested that CM/PF neurons are active during attention-demanding tasks, which is consistent with the original concept that this nuclear group is part of the ascending “reticular activating system” that regulates arousal and attention. More recent studies in nonhuman primates have shown that CM/PF neurons most likely supply striatal neurons, particularly cholinergic interneurons with information that has attentional value, which serve to detect salient events and signal the need to change a behavioral response. , Stimulation of rostral intralaminar nuclei results in complex alterations in cognitive processing, most likely through regulation of cortical and striatal activity. , Patients with infarcts that involve the CM/PF complex display cognitive dysfunction related to attention, semantic retrieval, and memory processes. Recent rodent studies also support a role of the PF-striatal system in cognition through regulation of striatal cholinergic interneurons. , , The function of other thalamostriatal systems is less studied, but the available evidence suggest that they act as a positive reinforcer of specific populations of striatal neurons involved in performing a selected cortically driven behavior. ,
Postmortem studies of patients’ brains affected with progressive supranuclear palsy, Huntington disease, or PD revealed as much as a 50% loss of cells in the CM/PF nuclei. , Loss of CM/PF neurons was also seen in a monkey model of PD that was chronically treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). In both patients and MPTP-treated monkeys, CM/PF degeneration is an early event that likely precedes the development of parkinsonian motor signs. , Although the thalamic degeneration in both PD patients and MPTP-treated monkeys affects mostly the CM/PF complex, , , neuronal loss was also observed in the paratenial, cucullar, and central lateral nuclei in PD patients. Combined with the behavioral data discussed in the previous section, these neuropathologic observations raise the possibility that thalamostriatal degeneration may contribute to the development of early cognitive impairments in PD and other neurodegenerative disorders. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here