Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
advanced glycation end-product
antigen-presenting cell
calcitonin gene-related peptide
Golgi-SER-lysosome
high density
hepatic stellate cell
intercellular adhesion molecule-1
liver-associated lymphocyte
low density
lymphocyte associated antigen-1
large granular lymphocyte
lipopolysaccharide
myeloid-derived suppressor cell
natural killer
neuronal nitric oxide syndrome
neuropeptide Y
prostaglandin
rough endoplasmic reticulum
stromal-derived factor
smooth endoplasmic reticulum
smooth muscle actin
somatostatin
substance P
transforming growth factor β
toll-like receptor
tumor necrosis factor α
regulatory T cell
thromboxane A 2
vascular cell adhesion molecule-1
vasoactive intestinal peptide
The liver is the largest organ in the human body. With the exception of the daily secretion of several liters of bile, there are no “moving parts” in the liver. This deceptive anatomic simplicity belies the extraordinary complexity of the biosynthetic and biodegradative pathways within the liver, serving as major elements of systemic metabolic and physiologic homeostasis ( Table 1-1 ). In the process, the liver generates enough metabolic heat to be a prime source of core homeostatic temperature maintenance.
| Cell Type | Function |
|---|---|
| Hepatocytes |
|
| Cholangiocytes | Secretion of bicarbonate-rich fluid into bile |
| Sinusoidal endothelial cells |
|
| Kupffer cells | Phagocytosis of particulate matter in sinusoidal blood Phagocytosis of apoptotic and necrotic hepatocellular debris Clearance of circulating microorganisms and endotoxin Immunoregulatory function Regulatory input into hepatic stellate cell, hepatocyte, and sinusoidal endothelial cell function |
| Hepatic stellate cells | Storage of vitamin A Control of microvascular tone of the sinusoid Production of extracellular matrix Regulatory input into hepatic regeneration Immunoregulatory function |
| Fibrocytes (portal tract) | Production of extracellular matrix |
The mature liver lies mainly in the right hypochondriac and epigastric regions of the abdominal cavity, below the diaphragm. The liver is attached to the diaphragm and protected by the ribs. In adults, the healthy liver weighs approximately 1400 g to 1600 g and extends along the midclavicular line from the right fifth intercostal space to just inferior to the costal margin. The anterior border of the liver then extends medially and crosses the midline just inferior to the xiphoid process. A small portion of the organ projects across the midline and lies in the upper left abdominal quadrant.
The liver is incompletely separated into lobes that are covered on their external surfaces by a thin connective tissue capsule. On the basis of external surface features, the liver is divided into right and left lobes by the falciform ligament, which is a peritoneal fold connecting the liver to the anterior abdominal wall and the diaphragm ( Fig. 1-1 ). The right lobe is further subdivided inferiorly and posteriorly into two smaller lobes—the caudate and quadrate lobes.
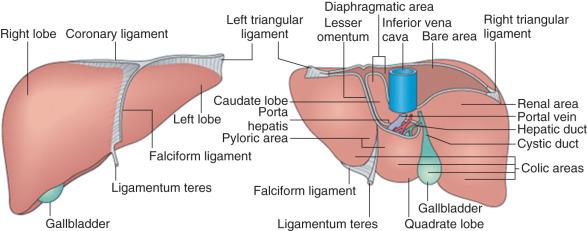
The macroscopic functional divisions of the liver, however, are defined on the basis of vascular anatomy ( Fig. 1-2 ). First is a plane that passes through the gallbladder and inferior vena cava, to the right of the midplane of the abdomen, that defines the halves of the liver supplied by the right and left branches of the portal vein and hepatic artery, together with biliary drainage into the right and left hepatic ducts. As a result, the quadrate lobe and a large portion of the caudate lobe are located to the right of the groove of the inferior vena cava but belong functionally to the left hemiliver. Further functional subdivision of the liver into eight segments having independent vascular and biliary supplies enables surgeons to resect segments of the liver while maintaining relative hemostasis.
The liver is encapsulated by a thin connective tissue layer (Glisson's capsule) consisting mostly of regularly arranged type I collagen fibers, scattered type III fibers, fibroblasts, mast cells, and small blood vessels and containing sensory nerves. On the convex liver surface facing the abdominal cavity this connective tissue layer is covered by the simple squamous mesothelial cells of the peritoneal serosal lining. At the superior site of attachment of the falciform ligament to the liver, the two leaves of the ligament separate to form a large convex area devoid of peritoneum, the bare area, on the superior surface of the liver, directly apposed to the diaphragm. The right and left leaves of the falciform ligament then merge with reflections of the parietal peritoneum extending from the diaphragm forming, respectively, the triangular ligament over the left dome of the liver and the coronary ligament over the right dome of the liver. The posterior aspect of the liver is covered by peritoneal serosa, with reflections at the groove of the inferior vena cava into which the multiple hepatic veins empty.
The dual blood supply of the liver enters the organ at its hilus (porta hepatis) accompanied by the hepatic bile duct, lymphatics, and nerves. Approximately 80% of the blood entering the liver is poorly oxygenated and is supplied by the portal vein. This is the venous blood flowing from the intestines, pancreas, and spleen; venous blood from the gallbladder either drains into a cystic vein, which empties into the right branch of the portal vein, or via small veins in the gallbladder bed directly into the parenchyma of the liver. The remaining 20% of the hepatic blood supply is well oxygenated and delivered by the hepatic artery.
The development of the liver has been extensively described, and is illustrated in Fig. 1-3 . Briefly, the liver primordium appears in human embryos during the third week of gestation as an endodermal bud from the ventral foregut just cranial to the yolk sac. This bud becomes the hepatic diverticulum as it enlarges, elongates in a cranio-ventral fashion, and develops a cavity contiguous with the foregut. The hepatic diverticulum consists of three portions: (1) the hepatic portion forms the hepatic corpus, including parenchymal cells and the elements of the portal tree; (2) the cystic portion forms the gallbladder; and (3) the most ventral portion forms the head of the pancreas. The hepatic portion grows into the septum transversum—a plate of mesenchyme that incompletely separates the thoracic and peritoneal cavities. During the fourth week of development, buds of epithelial cells extend from the hepatic diverticulum into the mesenchyme of the septum transversum as thick multicellular anastomosing cords. They become interspersed within the developing anastomotic network of capillaries arising from the symmetrically arranged vitelline veins returning from the abdominal cavity of the embryo, thus beginning to establish the close relationship of the hepatic parenchymal cells to the sinusoids. Blood draining from the parenchymal sinusoidal plexus within the nascent liver passes through the symmetric right and left hepatocardiac channels, to enter the sinus venosus.
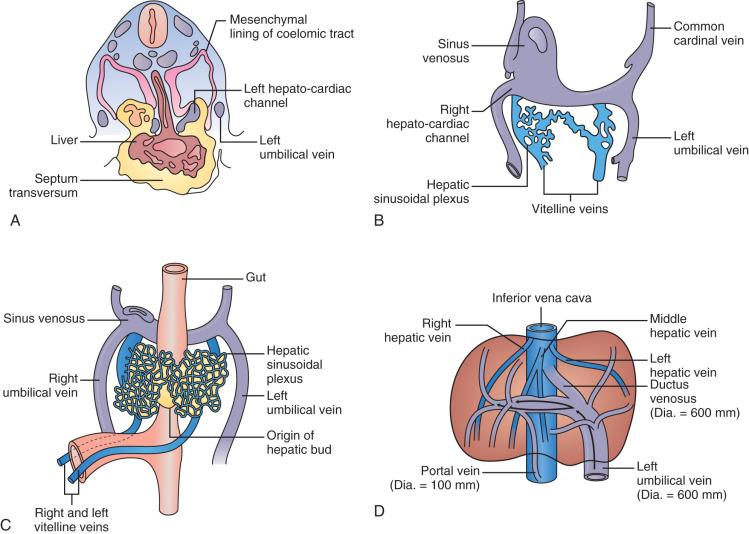
This embryonic vascular pattern gives way to the definitive fetal vascular pattern by the seventh week: the paired vitelline veins unite to form a single portal vein, which divides into right and left branches upon entering the liver, and arterial sprouts from the hepatic arterial branch of the celiac axis course along the intrahepatic portal tree and become the organizing elements for formation of the intrahepatic biliary tree. Within the parenchyma, the anastomotic pattern of both multicellular cords of parenchymal cells and sinusoids persists until several years after birth, by which time cords consisting of two or more parenchymal cells bounded on several sides by sinusoids have become plates consisting of single parenchymal cells bounded on at least two sides by sinusoids, particularly in the centrilobular region.
Between the sixth week and birth the fetal liver serves as a hematopoietic organ and as the primary site for fetal blood formation until the third trimester, when most hematopoietic sites disappear as the bone marrow develops. Throughout the third trimester and well through childhood, the liver microanatomy matures from the hilum outwards, with the final maturation of the parenchyma not being completed until the adolescent years.
Fundamental understanding of liver function begins with knowledge of microscopic architecture ( Fig. 1-4 , Table 1-2 ). Portal tracts are the distribution network for the portal vein and hepatic arterial systems, and in turn the effluent collection network for the biliary tree. The parenchyma is home to hepatocytes, which account for 60% of the total cell population and 80% of the volume of the liver and are organized in an anastomosing system of plates that traverse the distance from the portal tract to the terminal hepatic venule. Between the plates of hepatocytes are the vascular spaces, termed sinusoids . This is a unique vascular bed, with large-bore fenestrated vascular channels that lack a basement membrane and allow free exchange of circulating macromolecules with hepatocytes ( Fig. 1-5 ). So-called nonparenchymal cells lining the sinusoid include: sinusoidal endothelial cells, perisinusoidal hepatic stellate cells, and intraluminal Kupffer cells. Lastly, blood flowing through the sinusoids exits the liver through the branches of the hepatic venous system, the smallest of which is the terminal hepatic vein (also termed the central vein, as will be discussed).
| Anatomic Compartment | Structural Element | Cell Types |
|---|---|---|
| Portal tract | Portal vein Hepatic artery Bile duct Peribiliary glands (larger portal tracts only) |
Endothelial cells, smooth muscle cells Endothelial cells, smooth muscle cells Cholangiocytes Glandular epithelial cells |
| Bile ductule Lymphatics Nerves |
Cholangiocytes Endothelial cells Terminal twigs of autonomic nervous system |
|
| Canals of Hering (at the parenchymal-portal tract interface) Portal tract mesenchyme |
Cholangiocyte-hepatocyte channels Fibroblasts |
|
| Parenchyma | Liver cell plates Sinusoids |
|
| Terminal hepatic vein | Terminal hepatic vein | Endothelial cells |
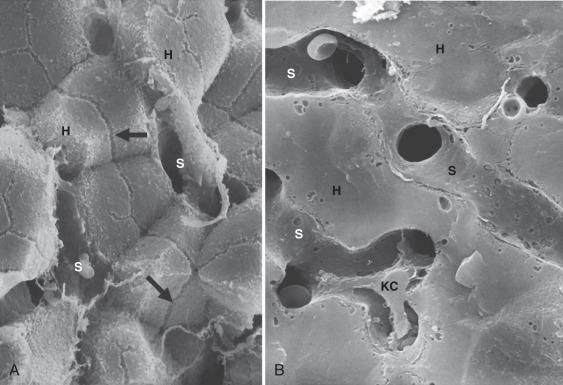
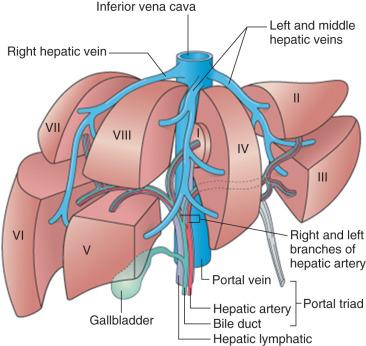
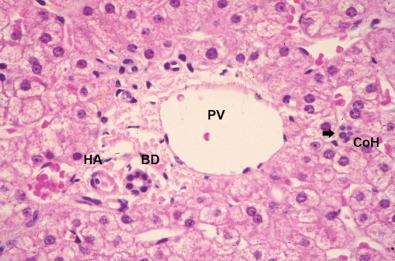
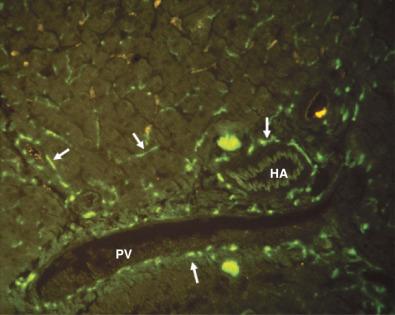
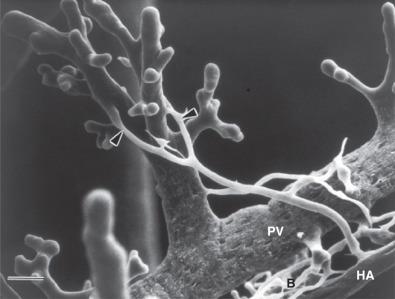
Both the portal vein and the hepatic artery, together with efferent autonomic nerves, enter the liver at the hilum. The hilum is also the site where bile ducts and lymphatics exit the liver. Branches of the hepatic artery, portal vein, bile duct, and lymphatic vessels ramify together in portal tracts through the liver parenchyma ( Fig. 1-6 ). Portal tracts are sometimes referred to as portal triads owing to their three main elements (portal vein, hepatic artery, bile duct, Fig. 1-7 ). However, portal tract is the preferred terminology, because there is often a multiplicity of hepatic artery–bile duct pairs within any given portal tract. The lymphatic channels within portal tracts are usually collapsed and inconspicuous, as are the autonomic nerves, unless the latter are highlighted by special techniques ( Fig. 1-8 ).
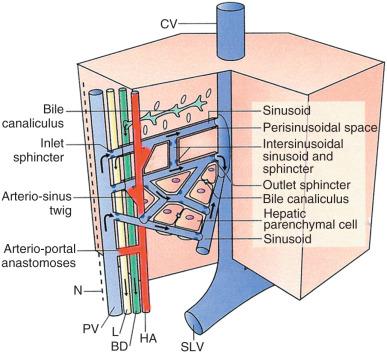
After repeated bifurcation, terminal branches of the portal veins and hepatic arteries supply blood to the sinusoids, extending roughly perpendicularly into the watershed regions between neighboring portal tracts ( Fig. 1-9 ). Branches of hepatic arteries also supply the peribiliary plexus of capillaries nourishing the bile ducts. These capillary plexuses then drain into sinusoids (via arteriosinus twigs, Fig. 1-10 ), or occasionally into portal venules (arterioportal anastomoses). Because all these vessels have independently contractile periarteriolar sphincters, the sinusoids receive a varying mixture of portal venous and hepatic arterial blood. After flowing through the sinusoids, blood is collected in small branches of hepatic veins (terminal hepatic veins, see Fig. 1-9 ). These veins course independently of the portal tracts and drain via the major hepatic veins, which emerge to join the inferior vena cava through separate orifices from the major liver lobes.
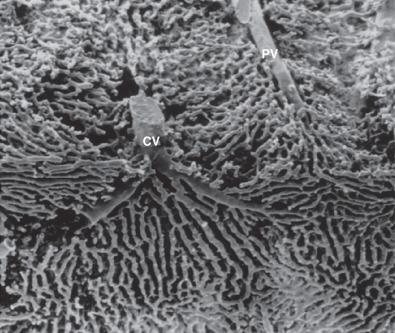
Lymphatic fluid originates from retrograde fluid flow in the space of Disse and is thereby collected into blind-ended lymphatic capillaries in the connective tissue spaces within the portal tracts. The actual connection between the proximal end of the space of Disse at the portal tract–parenchymal interface and lymphatic channels is attributed to a proposed space of Mall, the existence of which has never been proven. The fluid contained in these lymphatics moves toward the hepatic hilus and eventually into the cisternae chyli and thoracic duct. Lymph also leaves the liver in small lymphatics associated with the larger hepatic veins, which empty into larger lymphatics along the wall of the inferior vena cava. Lymphatics in the hepatic capsule drain to vessels either at the hilum or around the hepatic veins and inferior vena cava.
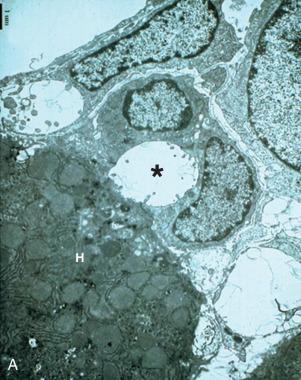
The biliary tree is the conduit by which fluid travels from the bile canaliculi between hepatocytes downstream to the lumen of the alimentary tract. The most proximal junction of the parenchymal canalicular network with the biliary tree is the canal of Hering , with half its circumference composed of one (or two) hepatocytes, and the other half composed of cholangiocytes—the polarized epithelial cells of the biliary tree ( Fig. 1-11, A ). These structures were originally thought to be visible only by electron microscopy. However, careful three-dimensional reconstruction histologic studies demonstrated that canals of Hering were visible by light microscopy and were shown to be present not just at the portal tract–parenchymal interface (as originally thought) but also extending into the hepatic parenchyma up to one third of the distance along the portal-to-central axis (see Fig. 1-11, B ). A further finding was that the canals of Hering are the anatomic compartment containing bipotential progenitor epithelial cells, which were capable of immense regenerative activity when the liver incurred severe damage—particularly if this damage was occurring at the portal-parenchymal interface. Hence, canals of Hering are not only the most peripheral compartment of the biliary tree but also play a key role in the hepatic regenerative response to injury.
The biliary channel that traverses the short space between the portal-parenchymal interface and the bile duct is the bile ductule ( Fig. 1-12 ). The bile ductule connects with the terminal bile duct at a roughly perpendicular angle—on three-dimensional step sections the angle of intersection is slightly off-perpendicular. The bile ducts converge down the length of the biliary tree, ultimately exiting the liver hilum as the right and left bile ducts to form the common hepatic bile duct just outside the liver corpus. Owing to normal variation in human bile duct anatomy, in 30% to 40% of individuals the confluence of the common hepatic bile duct is just internal to the liver.
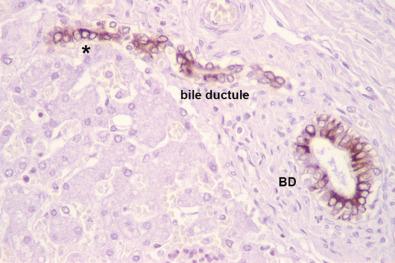
During hepatic morphogenesis, bile ductules and ultimately bile ducts arise from bipotential primordial cells at the interface of the embryonic portal tract mesenchyme and the parenchyma—termed the ductal plate . As noted earlier, the penetration of hepatic artery branches into the embryonic portal tract mesenchyme appears to act as an organizing influence for ontogeny of the terminal bile ducts, and for creation of the arterial vascular supply for bile ducts. This in-growth of hepatic arterial elements and maturation of bile ducts begins at the hilum of the liver and extends outwards not only during the fetal period of liver development but also well into the growing years of childhood as the liver continues to enlarge to reach its adult size by early adolescence. This ontogeny is evident in the mature adult liver by the uniform 1 : 1 pairing of hepatic arteries and terminal bile ducts in cross-sections of terminal portal tracts (see Fig. 1-7 ).
As regards the branching of the intrahepatic biliary tree, retrograde injection studies can demonstrate approximately 10 orders of dichotomous branches. However, in three-dimensional studies of the human adult liver, one terminal bile duct is observed for every 2 mm 3 to 3 mm 3 of the adult liver, therefore representing the volume of a liver microarchitectural unit. Based on the average size of the adult liver, between 17 and 20 orders of branching of the intrahepatic biliary tree would be expected. Given the limitations of retrograde filling techniques, and uncertainty in whether the terminal biliary tree branches symmetrically (dichotomously) or asymmetrically, the exact geometry of the intrahepatic biliary tree remains conjectural. Regardless, the adult liver must be supplied by 400,000 to 500,000 terminal bile ducts, corresponding to the estimated 440,000 microarchitectural units (defined as lobules or otherwise, see later) estimated to exist in the adult liver.
Cholangiocytes constitute 3% to 5% of the endogenous liver cell population, beginning with the canals of Hering, bile ductules, and then lining the intrahepatic and extrahepatic bile duct system. Cholangiocytes are not inert; they modify the composition of bile during its transit through bile ducts by the secretion and absorption of water, electrolytes, and other organic solutes. Indeed, in humans up to 40% of basal bile flow is produced by the ductal epithelium. This secretion is driven by sodium and bicarbonate transport out of the cholangiocyte into the duct lumen, followed by regulated passage of water through the intercellular tight junctions. Cholangiocyte secretion is under hormonal control by secretin and somatostatin. The duct epithelium also secretes IgA and IgM (but not IgG). Cholangiocytes reabsorb solutes from bile: glucose, glutamate, urate, and especially bile acids. Bile acids resorbed by the biliary epithelium are recirculated to hepatocytes via the peribiliary capillary plexus, creating a cholehepatic shunt pathway and promoting bile acid–dependent bile flow.
Cholangiocytes display phenotypic heterogeneity along the length of the biliary tree, including their becoming larger in basal-to-apical diameter with increasing diameter of the biliary lumen, concurrent with their acquiring increasing cytoplasmic complexity as evidenced by intracellular organelles such as the Golgi complex, intracytoplasmic vesicles, and mitochondria. Cholangiocytes express receptors for epidermal growth factor, secretin, and somatostatin and may secrete proinflammatory cytokines. As would be expected for polarized epithelial cells residing on a basement membrane, cholangiocytes express cell-matrix adhesion molecules such as integrins in abundance.
Extrahepatic bile ducts and the large bile ducts of the perihilar intrahepatic biliary tree have companion peribiliary glands, composed of branched tubule-alveolar seromucinous glands, ( Fig. 1-13 ). Along with mucin, these glands secrete substances such as lactoferrin and lysozyme. They are reported to be stem cell niches of the biliary tree capable of differentiating into hepatobiliary and pancreatic cells. Thus, in addition to providing their seromucinous secretions to the flow of bile, cells of the peribiliary glands may play a key role in normal tissue turnover and injury repair.
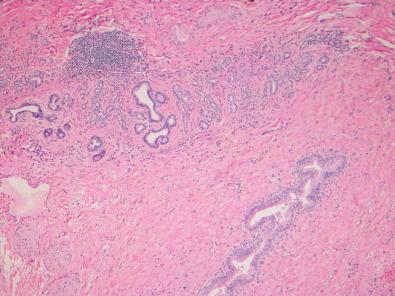
The liver has only a small amount of connective tissue in relation to its size: 5% to 10% of total protein and less than 3% of the relative area on a routine histologic section. Extracellular matrix underlies the capsule of the liver (Glisson's capsule), ensheathes the intrahepatic venous system, and under normal conditions is found within the space of Disse only as delicate bundles of type IV collagen—the so-called reticulin network of the parenchyma. However, most of the extracellular matrix visible on histologic sections of the normal liver is within portal tracts, surrounding the normal vascular and biliary structures. This mesenchymal investment is minimal in the most terminal portal tract branches (see Fig. 1-7 ). The mesenchymal content is more prominent in the larger sublobular portal tracts.
Portal tracts contain fibroblasts, which encircle bile ducts and bile ductules and may also be found elsewhere in portal tracts. In the first instance, the basement upon which cholangiocytes reside is surrounded directly by peribiliary fibroblasts. In the settings of bile duct inflammation and/or obstruction, these fibroblasts are rapidly activated with acquisition of smooth muscle actin, generating a myofibroblast phenotype. In other settings, such as hepatitis, other populations of fibrogenic cells in portal tracts play a role. These include myofibroblasts loosely placed around the portal vein and hepatic artery, and fibrocytes in the loose connective tissue of the portal tract, especially at the interface between the portal tract and parenchyma. Thus, in discussions of hepatic fibrogenesis, it is important to keep these particular cell types in mind.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here