Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The patient was born at 40 weeks’ gestation and weighed 3150 g at birth. An emergency cesarean section was performed for suspected fetal compromise with bradycardia on the cardiotocogram because of a nuchal cord. Apgar scores were 1, 0, 0 at 1, 5, and 10 minutes, respectively. The infant was resuscitated for 15 minutes with intravenous adrenalin given once. The umbilical cord pH was 7.25, and base excess was –3.5. The first arterial lactate concentration was 26.4 mmol/L. The infant was cooled for 72 hours at 33.5°C. Seizures were treated with phenobarbital, midazolam, and lidocaine. Magnetic resonance imaging (MRI) on day 4 showed severe abnormalities in the basal ganglia and thalami. The infant died on day 5 after redirection of care.
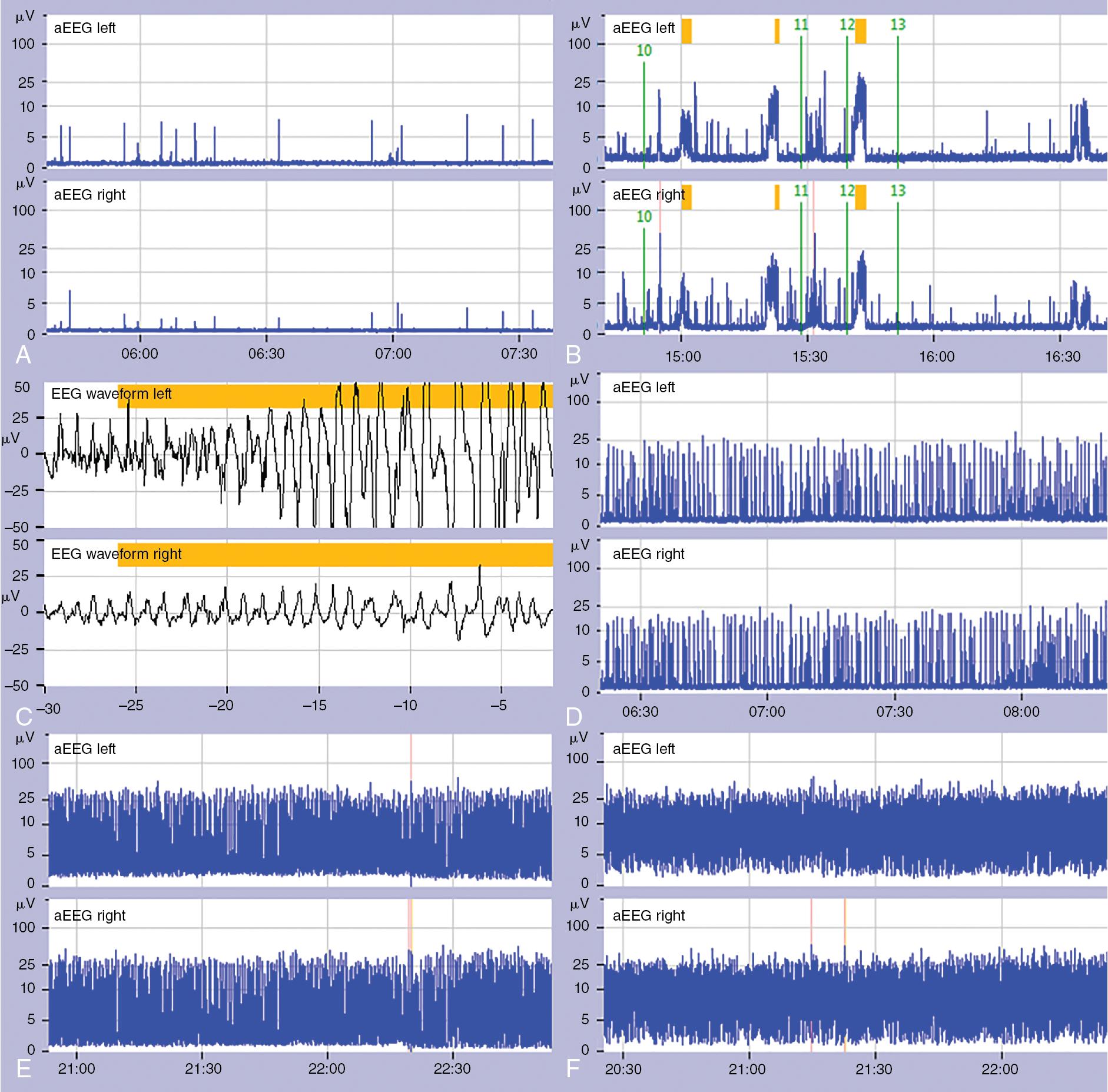
aEEG is easy to apply and interpret at the cotside.
The aEEG background pattern is a reliable marker for encephalopathy in full-term infants and for brain maturation in preterm infants and can guide prognostication.
Electrographic seizures detected with the aEEG compressed tracing should always be confirmed on the raw EEG, with two channels having greater reliability than single channel.
Factors influencing the aEEG such as interelectrode spacing, medications, and common artifacts should be considered when interpreting the aEEG.
Interest in the neonatal brain has increased considerably throughout the past decades. Imaging techniques such as ultrasound and magnetic resonance imaging (MRI) can evaluate the presence and extent of structural lesions of the brain. Near-infrared spectroscopy (NIRS) allows noninvasive monitoring of brain oxygenation and cerebral hemodynamics. Conventional multichannel electroencephalography (will be referred to as cEEG) and amplitude-integrated EEG (aEEG) provide information about brain function. EEG may detect epileptic discharges, reflect encephalopathy, from common etiologies such as hypoxic-ischemic encephalopathy (HIE, as illustrated in Fig. 14.1 ), and may give valuable information on brain maturation. Today, aEEG is used routinely in an increasing number of neonatal intensive care units (NICUs) due to its ease of use at the cotside. The extent of EEG monitoring in the NICU has been evaluated by analyzing 210 surveys (124 from Europe and 54 from the United States). Ninety percent of respondents had access to either cEEG or aEEG monitoring; 51% had both. The cEEG was mainly interpreted by neurophysiologists (72%), whereas aEEG was usually interpreted by the neonatologist (80%). However, as many as 31% of the respondents reported that they were not confident in their ability to interpret aEEG/cEEG.
Maynard originally constructed the cerebral function monitor (CFM) in the late 1960s for continuous brain monitoring. Prior developed the clinical application for monitoring adult patients during anesthesia and intensive care, after cardiac arrest, during status epilepticus, or after heart surgery.
The term aEEG is currently preferred to denote a method for encephalographic monitoring, whereas CFM refers to a specific type of equipment. The EEG signal for the single-channel aEEG is usually recorded from a pair of electrodes placed over the parietal lobes (corresponding to P3 and P4 according to the international EEG 10–20 classification, ground Fz, Fig. 14.2 A). Two-channel EEG places frontal-parietal or central-parietal leads (F3-P3 and F4-P4 or C3-P3 and C4-P4, ground Fz according to the international EEG 10–20 classification, Fig. 14.2 B and C) and is now predominantly used. This provides additional information about hemispheric asymmetry, which may be especially helpful in children with unilateral brain lesions. In the two-channel recording, the F3-P3 and F4-P4 position is preferred for assessment of the background pattern. This arrangement is in opposition to the short electrode distance of the C3-P3 and C4-P4 positions, which is better for seizure detection but may alter the background pattern.
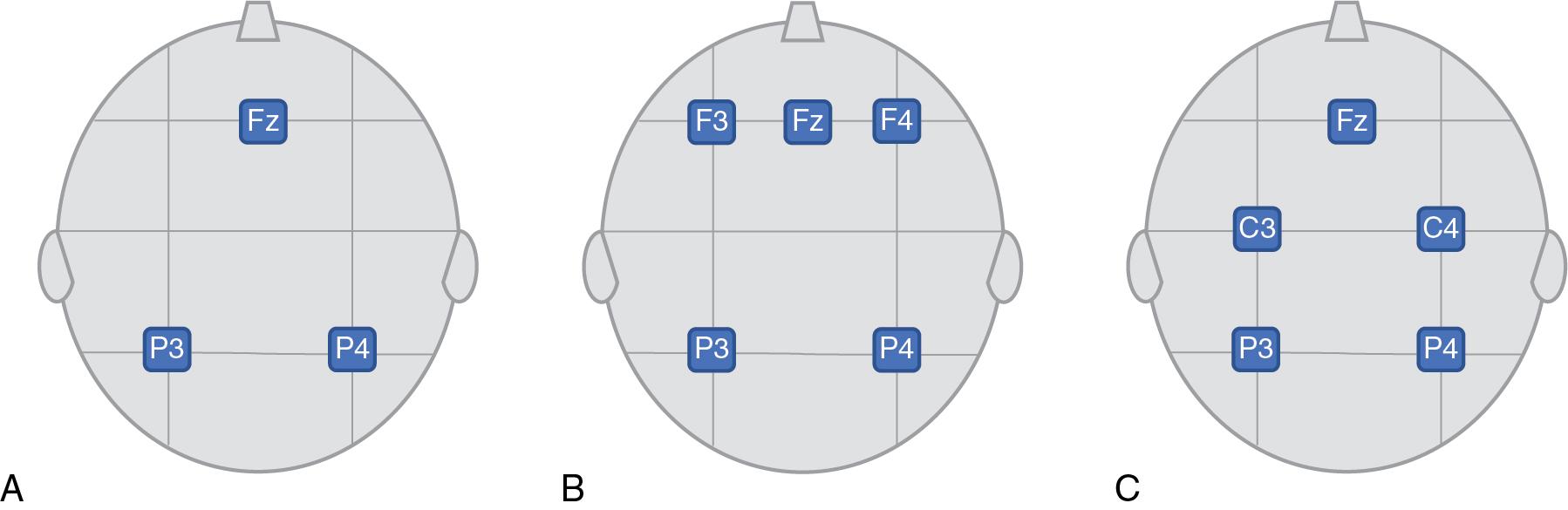
For aEEG processing, the raw EEG signal is amplified and passed through an asymmetric band-pass filter that prefers higher frequencies over lower ones and suppresses activity below two Hz and above 15 Hz to minimize artifacts from sweating, movement, muscle activity, and electrical interference. Additional processing includes rectification (negative waves become positive), smoothing, and considerable time compression. The signal is displayed on a semilogarithmic scale at slow speed (6 cm/hr) at the cotside. A second tracing continuously displays the original or raw EEG from either one or two channels. The electrode impedance is continuously recorded but not necessarily displayed; there is an alarm when the impedance is high, often as a result of a loose electrode. The bandwidth (BW) in the output reflects variations in minimum and maximum EEG amplitude, both of which depend on the maturity and severity of illness of the newborn. Because the semilogarithmic scale is used to plot the output, changes in background activity of very low amplitude (<5 μV) are enhanced.
The aEEG traces are assessed visually based on pattern recognition and are classified into the following five categories in full-term infants :
The continuous normal voltage (CNV) pattern is a continuous trace with a voltage between 10 and 25 (–50) μV ( Fig. 14.3 A).
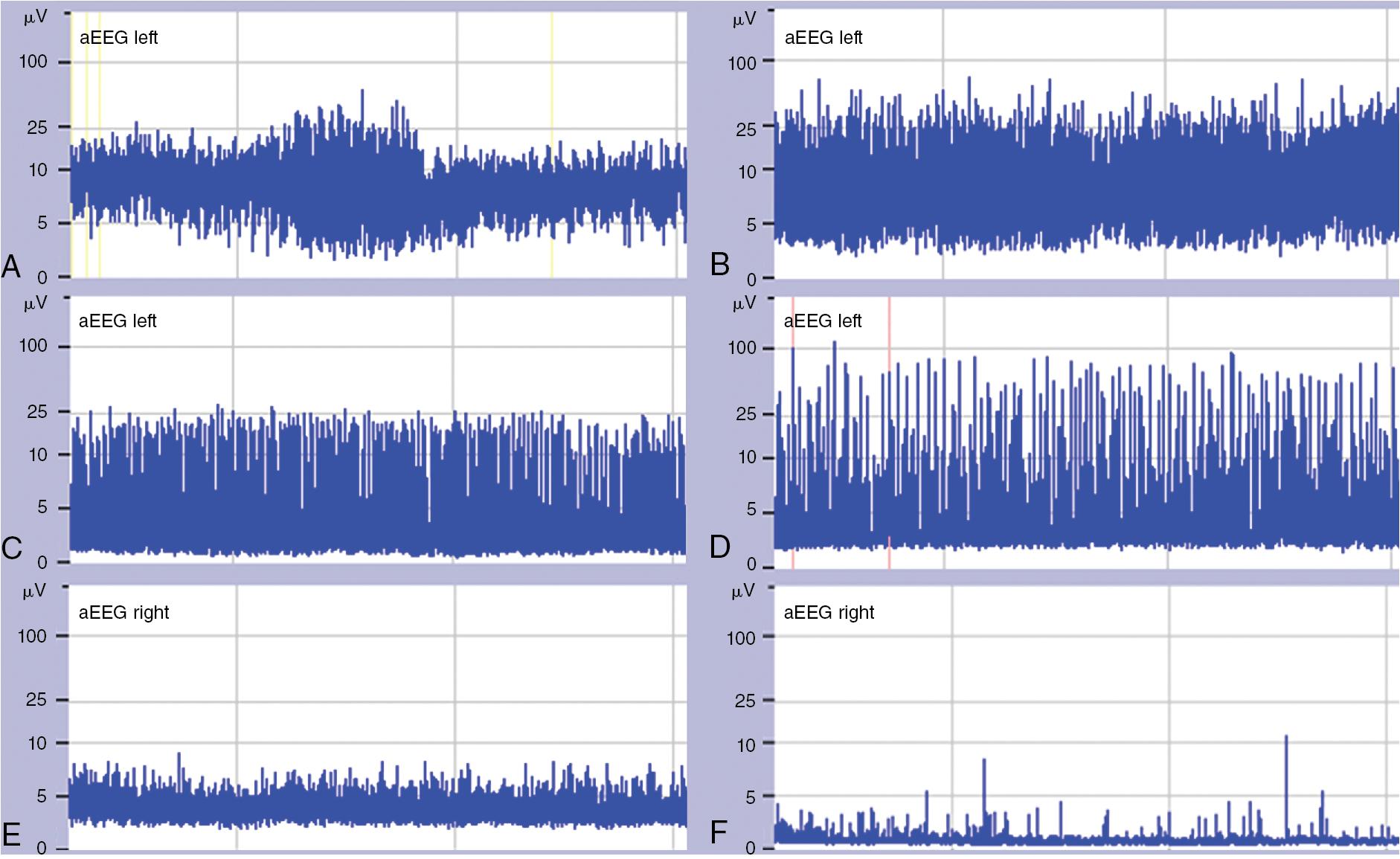
The discontinuous normal voltage (DNV) pattern in which the lower margin is predominantly below 5 μV (no burst suppression [BS]) ( Fig. 14.3 B).
The BS pattern with periods of low amplitude (inactivity) intermixed with bursts of higher amplitude (usually >25 μV). According to spacing between bursts, tracing can be classified either as sparse or dense BS pattern ( Fig. 14.3 C and D).
The continuous low voltage pattern (CLV) of very low voltage (around or below 5 μV) ( Fig. 14.3 E).
The flat tracing (FT) which has very low voltage, mainly inactive tracing with activity below 5 μV ( Fig. 14.3 F).
Another classification according to al Naqeeb uses absolute values for background patterns in term infants:
Normal: upper margin greater than 10 μV; lower margin above 5 μV.
Moderately abnormal: upper margin greater than 10 μV; lower margin less than 5 μV.
Severely abnormal: upper margin less than 10 μV; lower margin less than 5 μV.
We prefer the pattern recognition criteria because the background pattern may be influenced by a baseline drift ( Fig. 14.4 ). This drift is especially common in infants with very poor background activity where the lower margin is lifted upward by a high-frequency external signal, such as the ECG signal. When these two aEEG scoring systems were compared in the same dataset containing comparable normothermia and hypothermia-treated infants, it was noted that the pattern recognition method was superior for early outcome prediction in a subgroup of patients with HIE. Interobserver agreement was slightly higher using the voltage criteria compared with the pattern recognition method. However, both methods are equally good in determining the background pattern compared with the use of cEEG. The voltage classification system is easier to use for clinicians with little experience in reading aEEG, but one should always try to assess the underlying pattern. It has been shown that a BS pattern may be read as a normal voltage pattern when a drift of the baseline is bringing the lower margin above 5 μV. When this artifact is not recognized, the background pattern may be misclassified and consequently hypothermia may not be offered to eligible infants (see Fig. 14.4 ).
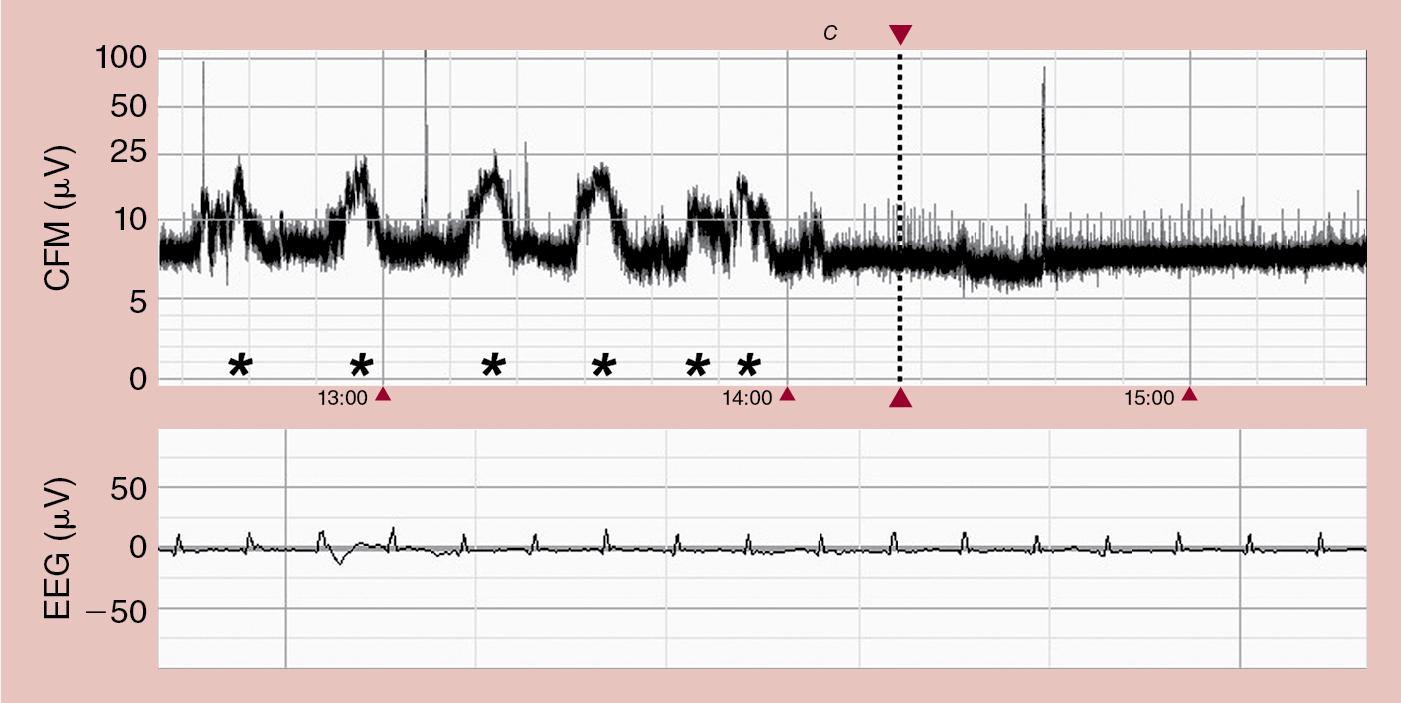
Several studies investigating simultaneous use of aEEG and cEEG have been performed to compare the two techniques for background pattern recognition. A good correlation between the aEEG background pattern and cEEG background activity was seen in full-term infants with moderate to severe neonatal encephalopathy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here