Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
One of the fundamental responsibilities of the anesthesiologist is to mitigate the adverse effects of anesthesia on the respiratory system by maintaining airway patency and ensuring adequate ventilation and oxygenation. The term airway management refers to this practice and is a cornerstone of anesthesia.
Successful airway management requires a range of knowledge and skill sets—specifically, the ability to predict difficulty with airway management and to formulate an airway management strategy, as well as the skills to execute that strategy using the wide array of airway devices available.
The American Society of Anesthesiologists’ Practice Guidelines for Management of the Difficult Airway and the accompanying Difficult Airway Algorithm provide guidelines for the evaluation of the airway and preparation for difficult airway management and can guide clinical decision making when an anesthesiologist is faced with a known or potentially difficult airway. Cognitive aids, such as the Vortex approach, are useful to help implement airway algorithms in an emergency situation.
A detailed understanding of airway anatomy is essential for the anesthesia provider.
A complete evaluation of the airway and knowledge of difficult airway predictors can alert the anesthesiologist to the potential for difficulty with airway management and allow for appropriate planning.
Apneic oxygenation can be used to prolong the duration of apnea without desaturation and is increasingly being adopted during the management of both difficult and routine airways.
Application of local anesthesia to the airway or induction of general anesthesia is usually required to facilitate airway management, to provide comfort for the patient, and to blunt airway reflexes and the hemodynamic response to airway instrumentation.
Over the past 30 years, the laryngeal mask airway (LMA) has emerged as one of the most important developments in airway devices.
Tracheal intubation establishes a definitive airway, provides maximal protection against aspiration of gastric contents, and allows for positive-pressure ventilation with higher airway pressures than via a face mask or supraglottic airway.
Flexible scope intubation of the trachea in an awake, spontaneously ventilating, and cooperative patient is the gold standard for the management of the difficult airway.
Invasive airways are indicated as a rescue technique when attempts at establishing a noninvasive airway fail. The anesthesia practitioner should become proficient with techniques for transtracheal jet ventilation and cricothyrotomy.
Extubation is a critical component of airway management with the potential for significant complications. The plan for extubation of the trachea must be preemptively formulated and includes a strategy for reintubation should the patient be unable to maintain an adequate airway after extubation.
General anesthesia is associated with various effects on the respiratory system, including the loss of airway patency, loss of protective airway reflexes, and hypoventilation or apnea. Therefore one of the fundamental responsibilities of the anesthesiologist is to establish airway patency and to ensure adequate ventilation and oxygenation. The term airway management refers to the practice of establishing and securing a patent airway and is a cornerstone of anesthetic practice. Traditionally, ventilation via a mask and tracheal intubation have been the foundation of airway management; however, in the past 30 years, the laryngeal mask airway (LMA) has emerged as one of the most important developments in airway devices.
Because failure to secure a patent airway can result in hypoxic brain injury or death in only a few minutes, difficulty with airway management has potentially grave implications. Analysis of the American Society of Anesthesiologists (ASA) Closed Claims Project database has demonstrated that the development of an airway emergency increases the odds of death or brain damage by 15-fold. Although the proportion of claims attributable to airway-related complications has decreased over the past 3 decades, airway complications are still the second-most common cause of claims. In 2011, the Royal College of Anaesthetists and the Difficult Airway Society (DAS) of the United Kingdom reported the results of the 4th National Audit Project (NAP4), a 1-year audit aimed at determining the incidence of major complications of airway management in anesthesia. NAP4 identified 133 major airway-related events in the perioperative period resulting in 16 deaths—a mortality incidence of 1 per 180,000 anesthetics—a number that could be as high as 1 per 50,000 anesthetics when underreporting is considered. The most common airway problems in the NAP4 study were failure, delay, or difficulty in securing the airway; aspiration of gastric contents; and extubation-related complications. Poor assessment of the airway, poor planning, and a lack of personal and/or institutional preparedness for managing difficulty with airway management were the most common contributing factors.
Studies such as these highlight the importance of successful airway management, which requires a range of knowledge and skill sets—specifically, the ability to predict difficulty with airway management, to formulate an airway management strategy, and to have the skills necessary to execute that strategy using the wide array of available airway devices. Development of these skills should be an ongoing endeavor for all anesthesiologists. As with any manual skill, continued practice improves performance and may reduce the likelihood of complications. New airway devices are continually being introduced into the clinical arena, each with unique properties that may be advantageous in certain situations. Becoming familiar with new devices under controlled conditions is important for the anesthesia practitioner—the difficult airway is not an appropriate setting during which to experiment with a new technique.
In 1993, the ASA published the first Practice Guidelines for Management of the Difficult Airway , which was written with the intent to “facilitate the management of the difficult airway and to reduce the likelihood of adverse outcomes.” The most recent update to this report, published in 2013, defines the difficult airway as “the clinical situation in which a conventionally trained anesthesiologist experiences difficulty with ventilation of the upper airway via a mask, difficulty with tracheal intubation, or both” and provides guidelines for the evaluation of the airway and preparation for difficult airway management, including a Difficult Airway Algorithm (DAA) intended to guide clinical decision making when an anesthesiologist is faced with a known or potential difficult airway ( Fig. 44.1 ). The ASA DAA begins with a consideration of the relative clinical merits and feasibility of four basic management choices: (1) awake intubation versus intubation after induction of general anesthesia, (2) noninvasive techniques versus invasive techniques (i.e., surgical or percutaneous airway) for the initial approach to intubation, (3) video-assisted laryngoscopy (VAL) as an initial approach to intubation, and (4) preservation versus ablation of spontaneous ventilation.
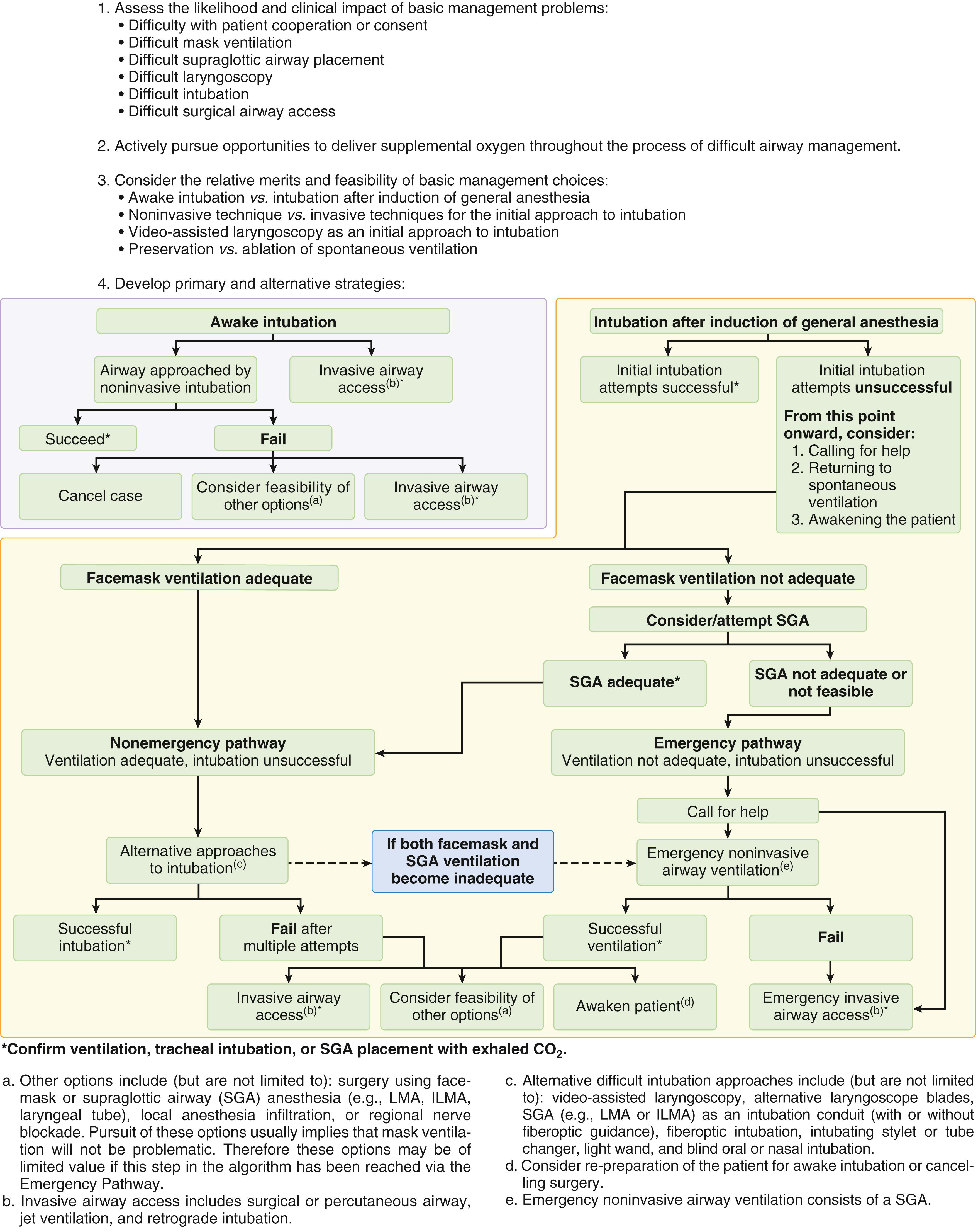
The ASA DAA does not follow a linear decision-making tree, as the advanced cardiac life support (ACLS) algorithms do. It can be better understood and remembered by considering it as three separate scenarios: (1) predicted difficult airway (awake intubation), (2) difficult intubation with adequate oxygenation/ventilation (the “ non-emergency ” pathway), and (3) difficult intubation without adequate oxygenation/ventilation (the “cannot intubate, cannot oxygenate” [CICO] scenario or the “ emergency ” pathway).
In addition to the ASA, several different national anesthesia societies have published their own guidelines for management of the difficult airway, including the Difficult Airway Society (DAS) from the United Kingdom, the Canadian Airway Focus Group (CAFG), the French Society of Anesthesia and Intensive Care (SFAR), the German Society of Anesthesiology and Intensive Care Medicine (DGAI), the Italian Society for Anesthesia and Intensive Care (SIAARTI), and the Japanese Society of Anesthesiologists. All of these include recommendations for the prediction of the difficult airway and suggest awake intubation as a management strategy (with the exception of the DAS guidelines) and all incorporate algorithms for both unanticipated difficult intubation with adequate oxygenation and the CICO scenario. Common elements include a focus on awakening the patient in the setting of a difficult intubation with adequate ventilation, the use of the LMA as a rescue for difficult mask ventilation, and emergency front of neck access (FONA) in the CICO scenario. The primary differences in these algorithms are in specific details, such as the number of intubation attempts suggested, the specific alternate devices recommended for difficult intubation, and the organization of the algorithm.
There has been growing attention to the influence of “human factors” on difficult airway management—namely, human behaviors, abilities, shortcomings, and biases as well as individual and team performance. Studies such as NAP4 have shown that these human factors contribute to an adverse airway outcome in over 40% of cases. The use of airway checklists, preprocedural team briefings, and cognitive aids are all strategies for addressing human factor challenges.
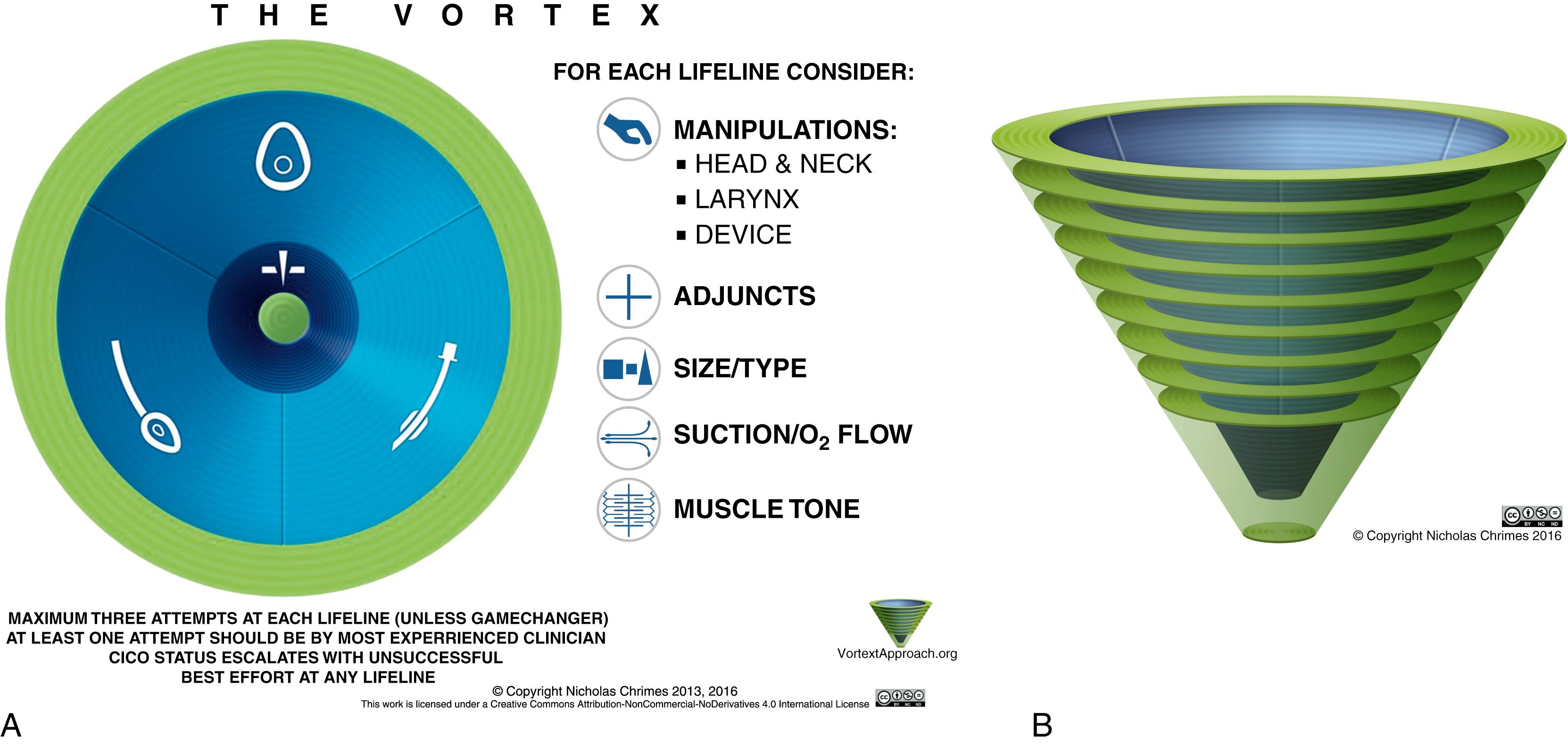
The Vortex approach, conceived by Dr. Nicholas Chrimes, a specialist anaesthetist in Melbourne, Australia, is one such cognitive aid designed to facilitate management of the unanticipated difficult airway. Rather than relying on complex algorithms that are based on decision trees, the Vortex model utilizes a visual aid in the shape of a funnel or vortex ( Fig. 44.2 ) to guide the airway practitioner through the three basic nonsurgical airway techniques (face-mask ventilation, supraglottic airway [SGA], and tracheal intubation). If after an “optimal attempt” at each of these nonsurgical modalities alveolar oxygen delivery has not been achieved, then one “travels down the vortex,” and an emergency surgical airway is indicated. Because this strategic approach is more conceptual, it is simple enough to be utilized and recalled during a stressful airway emergency.
A detailed understanding of airway anatomy is essential for the anesthesiologist. Various aspects of airway management depend on a working knowledge of the anatomy involved, including airway assessment, preparation of the airway for awake intubation, and the proper use of airway devices. Knowledge of normal anatomy and anatomic variations that may render airway management more difficult helps with the formulation of an airway management plan. Because some critical anatomic structures may be obscured during airway management, the anesthesiologist must be familiar with the interrelationship between different airway structures.
The airway can be divided into the upper airway, which includes the nasal cavity, the oral cavity, the pharynx, and the larynx; and the lower airway, which consists of the tracheobronchial tree.
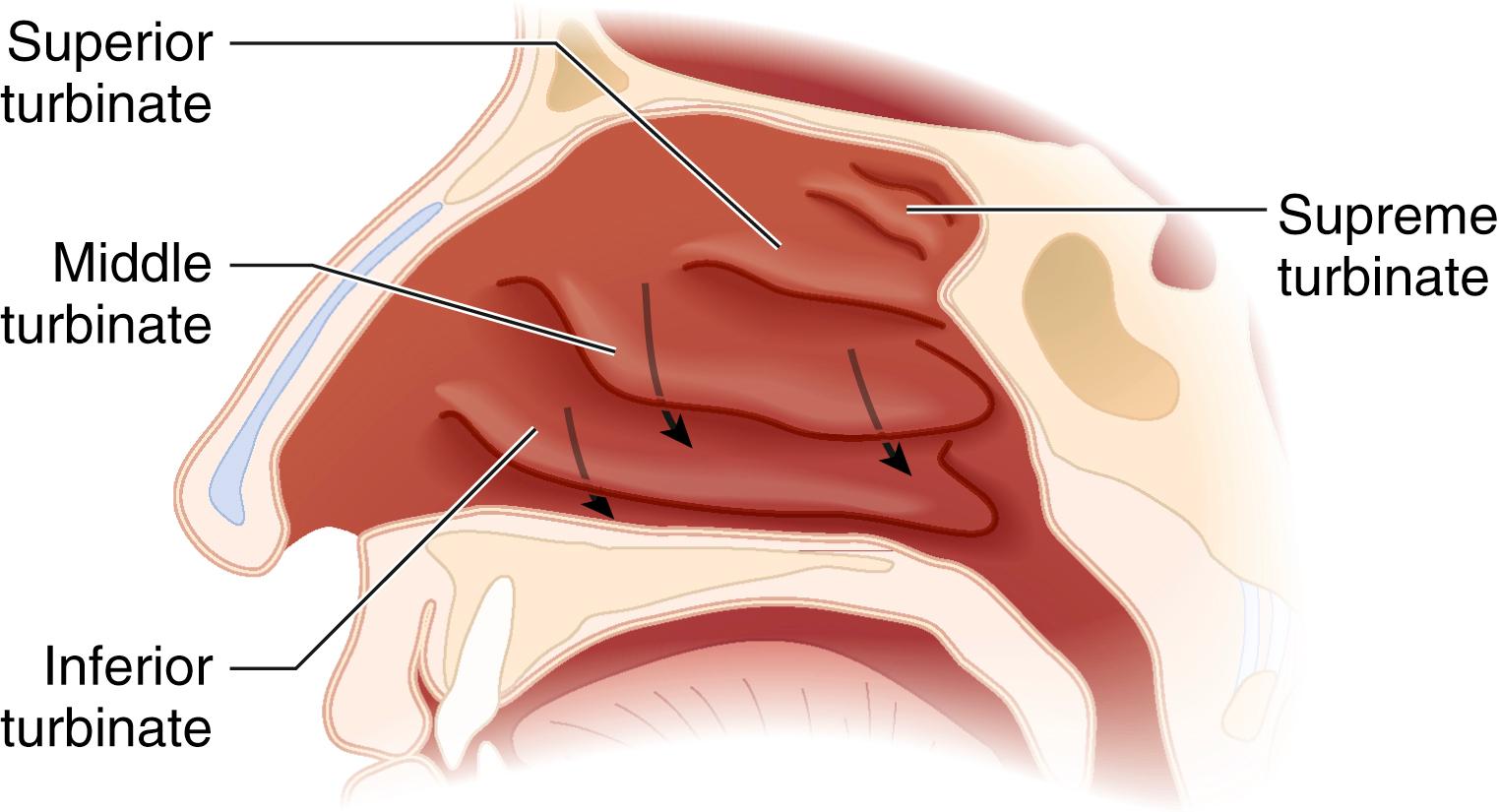
The airway begins functionally at the naris, the external opening of the nasal passages. The nasal cavity is divided into the right and left nasal passages (or fossae) by the nasal septum, which forms the medial wall of each passage. The septum is formed by the septal cartilage anteriorly and by two bones posteriorly—\the ethmoid (superiorly) and the vomer (inferiorly). Nasal septal deviation is common in the adult population ; therefore the more patent side should be determined before passing instrumentation through the nasal passages. The lateral wall of the nasal passages is characterized by the presence of three turbinates (or conchae) that divide the nasal passage into three scroll-shaped meatuses ( Fig. 44.3 ). The inferior meatus, between the inferior turbinate and the floor of the nasal cavity, is the preferred pathway for passage of nasal airway devices ; improper placement of objects in the nose can result in avulsion of a turbinate. The roof of the nasal cavity is formed by the cribriform plate, part of the ethmoid bone. This fragile structure, if fractured, can result in communication between the nasal and intracranial cavities and a resultant leakage of cerebrospinal fluid. Because the mucosal lining of the nasal cavity is highly vascular, vasoconstrictor should be applied, usually topically, before instrumentation of the nose to minimize epistaxis. The posterior openings of the nasal passages are the choanae, which lead into the nasopharynx.
Because of the relatively small size of the nasal passages and the significant risk of trauma, the mouth is often used as a conduit for airway devices. Many airway procedures require adequate mouth opening, which is accomplished by rotation within the temporomandibular joint (TMJ) and subsequent opening by sliding (also known as protrusion or subluxation ) of the condyles of the mandible within the TMJ.
The oral cavity leads to the oropharynx and is inferiorly bounded by the tongue and superiorly by the hard and soft palates. The hard palate, formed by parts of the maxilla and the palatine bone, makes up the anterior two thirds of the roof of the mouth; the soft palate (velum palatinum), a fibromuscular fold of tissue attached to the hard palate, forms the posterior one third of the roof of the mouth.
The tongue is anchored to various structures by its extrinsic musculature; of these, the most clinically relevant to the anesthesiologist is the genioglossus, which connects the tongue to the mandible. The jaw-thrust maneuver uses the sliding component of the TMJ to move the mandible and the attached tongue anteriorly, thereby relieving airway obstruction caused by the posterior displacement of the tongue into the oropharynx.
Beneath the tongue, the mylohyoid muscles separate the floor of the mouth into the sublingual space superiorly and the submental space inferiorly. Cellulitis (Ludwig’s angina) or hematoma formation in these spaces can cause elevation and posterior displacement of the tongue and resultant airway obstruction.
The pharynx is a muscular tube that extends from the base of the skull down to the level of the cricoid cartilage and connects the nasal and oral cavities with the larynx and esophagus. The posterior wall of the pharynx is made up of the buccopharyngeal fascia, which separates the pharynx from the retropharyngeal space. Improper placement of a gastric or tracheal tube can result in laceration of this fascia and the formation of a retropharyngeal dissection. The pharyngeal musculature in the awake patient helps maintain airway patency; loss of pharyngeal muscle tone is one of the primary causes of upper airway obstruction during anesthesia. A chin lift with mouth closure increases longitudinal tension in the pharyngeal muscles, counteracting the tendency of the pharyngeal airway to collapse.
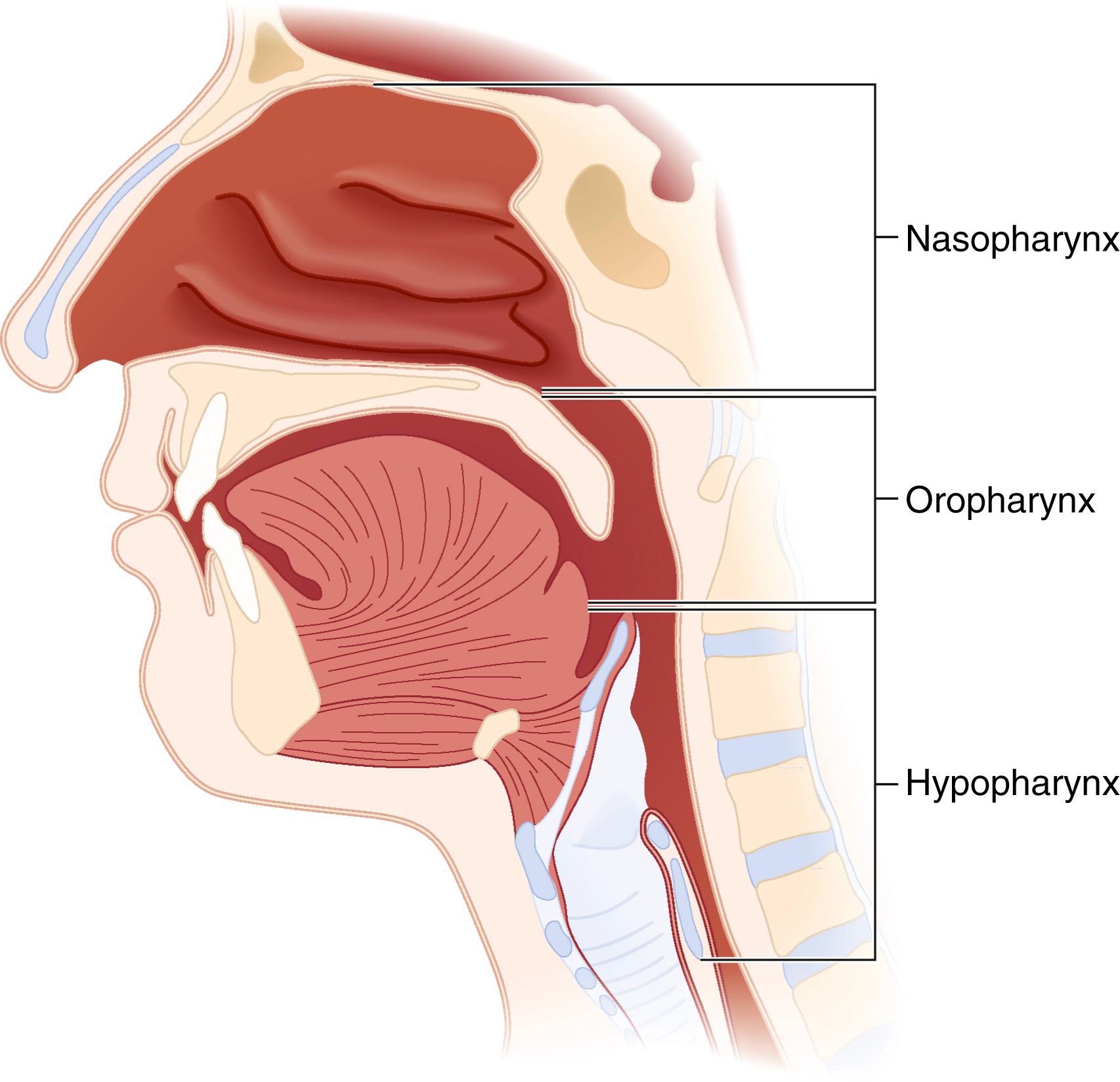
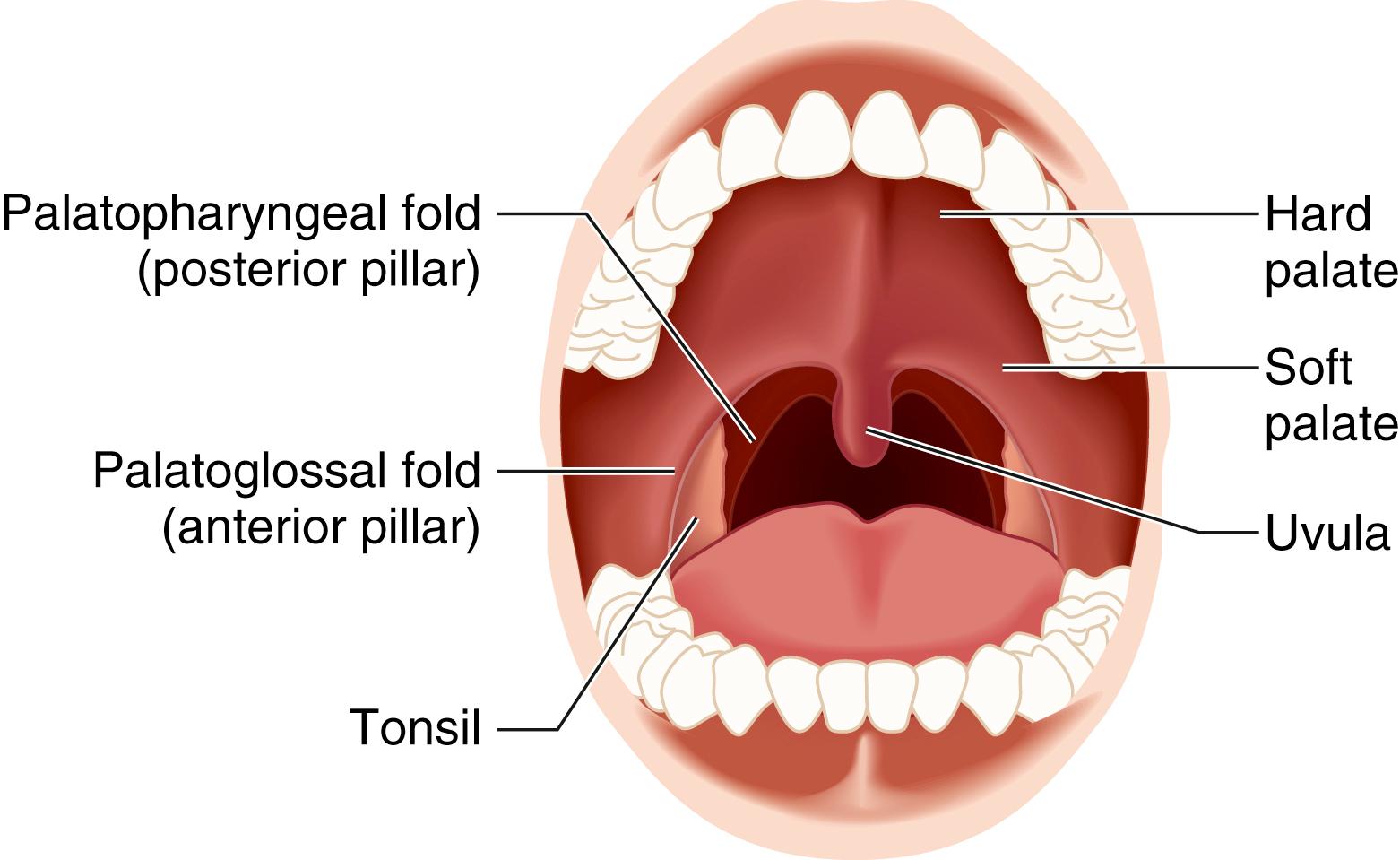
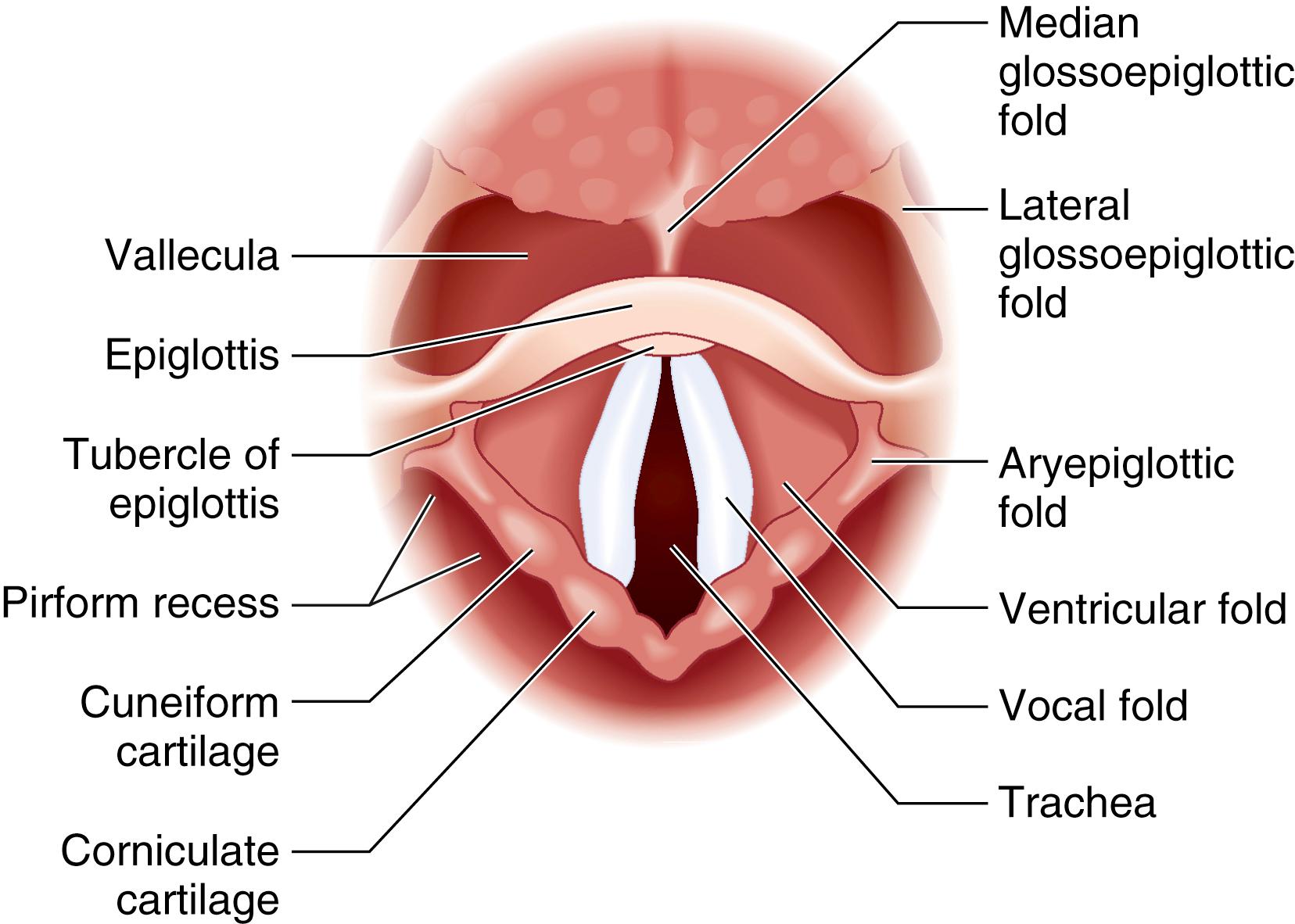
The pharynx can be divided into the nasopharynx, the oropharynx, and the hypopharynx ( Fig. 44.4 ). Along the superior and posterior walls of the nasopharynx are the adenoid tonsils, which can cause chronic nasal obstruction and, when enlarged, can cause difficulty passaging airway devices. The nasopharynx ends at the soft palate; this region is termed the velopharynx and is a common site of airway obstruction in both awake and anesthetized patients. The oropharynx begins at the soft palate and extends inferiorly to the level of the epiglottis. The lateral walls contain the palatoglossal folds and the palatopharyngeal folds, also termed the anterior and posterior faucial (tonsillar) pillars , respectively; these folds contain the palatine tonsils, which can hypertrophy and cause airway obstruction ( Fig. 44.5 ). The base of the tongue lies in the anterior aspect of the oropharynx, connected to the epiglottis by the glossoepiglottic folds, which bound paired spaces known as the valleculae (although these are frequently referred to as a single space called the vallecula ). The hypopharynx begins at the level of the epiglottis and terminates at the level of the cricoid cartilage, where it is continuous with the esophagus. The larynx protrudes into the hypopharynx, creating two piriform recesses on either side ( Fig. 44.6 ).
The larynx is a complex structure of cartilage, muscles, and ligaments that serves as the inlet to the trachea and performs various functions, including phonation and airway protection. The cartilaginous framework of the larynx is made up of nine separate cartilages: the thyroid and cricoid cartilages; the paired arytenoid, corniculate, and cuneiform cartilages; and the epiglottis. They are joined by ligaments, membranes, and synovial joints, and are suspended by the hyoid bone via the thyrohyoid ligaments and membrane ( Fig. 44.7 ).
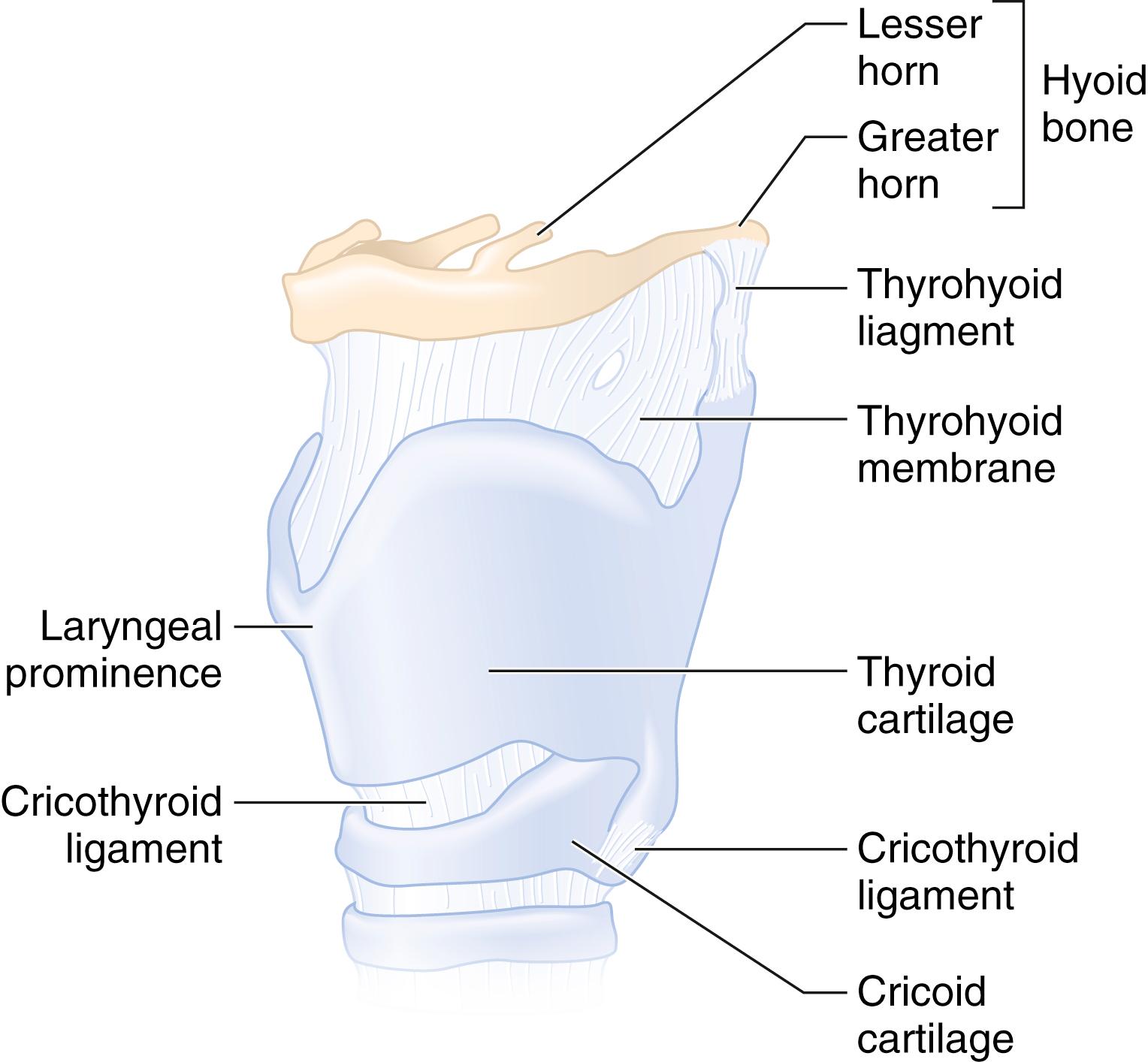
The thyroid cartilage is the largest of these cartilages and supports most of the soft tissues of the larynx. The superior thyroid notch and the associated laryngeal prominence ( Adam’s apple ) are appreciable from the anterior neck and serve as important landmarks for percutaneous airway techniques and laryngeal nerve blocks. The cricoid cartilage, at the level of the sixth cervical vertebra, is the inferior limit of the larynx and is anteriorly connected to the thyroid cartilage by the cricothyroid membrane (CTM). It is the only complete cartilaginous ring in the airway. The arytenoid cartilages articulate with the posterior cricoid and are the posterior attachments for the vocal cords.
When viewed from the pharynx, as during direct laryngoscopy (DL), the larynx begins at the epiglottis, which is a cartilaginous flap that serves as the anterior border of the laryngeal inlet. It functions to divert food away from the larynx during the act of swallowing, although its role in this regard is not essential to prevent tracheal aspiration. The anterior surface of the epiglottis is attached to the upper border of the hyoid bone by the hyoepiglottic ligament. The laryngeal inlet is bound laterally by the aryepiglottic folds, and posteriorly by the corniculate cartilages and the interarytenoid notch (see Fig. 44.6 ).
The space inferior to the laryngeal inlet down to the inferior border of the cricoid cartilage is the laryngeal cavity. The ventricular folds (also referred to as the vestibular folds or false vocal cords ) are the most superior structure within the laryngeal cavity. Beneath these are the true vocal cords, which attach to the arytenoids posteriorly and the thyroid cartilage anteriorly, where they join together to form the anterior commissure. The space between the vocal cords is termed the glottis ; the portion of the laryngeal cavity above the glottis is known as the vestibule , and the portion inferior to the vocal cords is known as the subglottis .
The trachea begins at the level of the cricoid cartilage and extends to the carina at the level of the fifth thoracic vertebra; this length is 10 to 15 cm in the adult. It consists of 16 to 20 C -shaped cartilaginous rings that open posteriorly and are joined by fibroelastic tissue; the trachealis muscle forms the posterior wall of the trachea. At the carina, the trachea bifurcates into the right and left mainstem bronchi. In the adult, the right mainstem bronchus branches off at a more vertical angle than the left mainstem bronchus, resulting in a greater likelihood of foreign bodies and endotracheal tubes (ETTs) entering the right bronchial lumen.
Although the anesthesia provider should always be prepared for potential difficulty with airway management, the ability to predict the difficult airway in advance is obviously desirable. Certain physical findings or details from the patient’s history can be prognostic of difficulty with mask ventilation, supraglottic airway placement, laryngoscopy, tracheal intubation, or the performance of a surgical airway. No single test has been devised to predict a difficult airway accurately 100% of the time; however, a complete evaluation of the airway and knowledge of the difficult airway predictors can alert the anesthesiologist to the potential for difficulty and allow for appropriate planning.
Airway assessment should begin with a directed patient history whenever possible. One of the most predictive factors for difficult intubation is a history of previous difficulty with intubation. On the other hand, a history of a previously easy airway does not rule out the possibility of difficulty with ventilation or intubation. In either case, the patient interview should specifically address changes in weight, symptomatology, and pathologic conditions since the last induction of an anesthetic (if there was one), and attempts should be made at obtaining prior anesthetic records—they may yield useful information concerning airway management. The presence of pathologic states that increase the risk of a difficult airway should be elicited by performing a medical history. A focused review of systems can alert the anesthesiologist to other potential factors that may predict difficult airway management; for example, a history of snoring has been shown to be predictive of difficult mask ventilation.
A physical examination of the airway should be preoperatively performed, when possible, to detect any physical characteristics that may suggest a difficult airway. The specific characteristics that should be evaluated in this examination are listed in Box 44.1 .
Visual inspection of the face and neck
Assessment of mouth opening
Evaluation of oropharyngeal anatomy and dentition
Assessment of neck range of motion (ability of the patient to assume the sniffing position)
Assessment of the submandibular space
Assessment of the patient’s ability to slide the mandible anteriorly (test of mandibular prognathism)
The visual inspection of the face and neck should focus on any physical characteristics that may indicate the potential for difficulty with airway management. These include obvious facial deformities, neoplasms involving the face or neck, facial burns, a large goiter, a short or thick neck, or a receding mandible. The presence of a beard has been shown to be associated with difficult ventilation attributable to the difficulty in obtaining a mask seal. Cervical collars or cervical traction devices can interfere with both mask ventilation and DL. A neck circumference greater than 43 cm (17 inches) is associated with difficulty with tracheal intubation ; Brodsky demonstrated that a large neck circumference is, in fact, more predictive of difficulty with tracheal intubation than a high body mass index (BMI).
Assessment of mouth opening and inspection of the oropharyngeal anatomy is achieved by instructing the patient to open his or her mouth as wide as possible. An interincisor distance of less than 3 cm (or 2 fingerbreadths), as measured from the upper to the lower incisors with maximal mouth opening, can suggest the possibility of difficult intubation ; some studies have used 4 or 4.5 cm as the cutoff. A thorough inspection of the oropharynx can help identify pathologic characteristics that may result in difficulty with intubation, such as neoplasm, a high arched palate, or macroglossia. In 1983, Mallampati and associates described a clinical sign to predict difficult tracheal intubation based on the size of the base of the tongue. A Mallampati classification of I to III is assigned, based on the visibility of the faucial pillars, uvula, and soft palate when the patient is seated upright with the head neutral, the mouth open, the tongue protruded, and no phonation. Higher scores on the Mallampati classification indicate poor visibility of the oropharyngeal structures attributable to a large tongue relative to the size of the oropharyngeal space, and, subsequently, a more difficult laryngoscopy. The modified Mallampati classification described by Samsoon and Young, which adds a fourth classification, is the most commonly used airway assessment test in current anesthesia practice and is defined as follows ( Fig. 44.8 ):
Class I: Faucial pillars, uvula, and soft palate are visualized.
Class II: Base of the uvula and soft palate are visualized.
Class III: Soft palate only is visualized.
Class IV: Hard palate only is visualized.
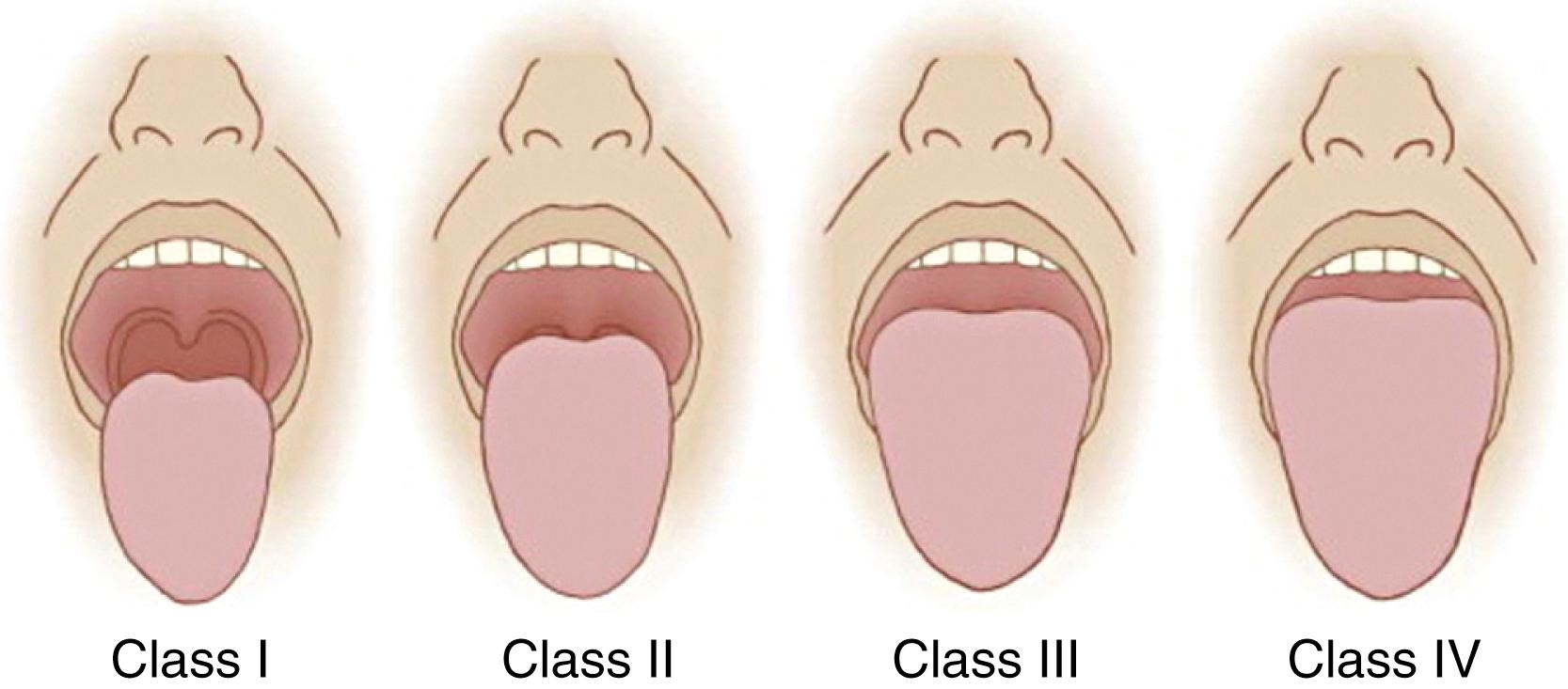
As a stand-alone test, the modified Mallampati classification is insufficient for accurate prediction of difficult intubation; however, it may have clinical utility in combination with other difficult airway predictors. Some studies support obtaining a Mallampati score with the head in full extension to improve the predictive value of the test. A Mallampati zero classification has been proposed when the epiglottis can be visualized during examination of the oropharynx; this finding is usually associated with easy laryngoscopy, although difficulty with airway management attributable to a large, floppy epiglottis in patients with a Mallampati zero classification can occur.
An examination of dentition should be performed when the oropharyngeal anatomy is being evaluated. Relatively long upper incisors can impair DL. Poor dentition and loose teeth increase the risk of dental trauma and present a risk of tooth dislodgment with subsequent aspiration; very loose teeth should be removed before laryngoscopy. Cosmetic dental work, such as veneers, caps, crowns, and bridges, are particularly susceptible to damage during airway management. Edentulousness is predictive of easy tracheal intubation but potentially difficult mask ventilation.
The ideal positioning for DL is achieved by cervical flexion and atlantooccipital extension and is most commonly referred to as the sniffing position (see Direct Laryngoscopy: Preparation and Positioning). Assessment of a patient’s ability to assume this position should be included in the airway examination; an inability to extend the neck at the atlantooccipital joint is associated with difficult intubation. Head and neck mobility can also be quantitatively assessed by measuring the sternomental distance between the sternal notch and the point of the chin with the head in full extension and the mouth closed. Distances less than 12.5 cm are associated with difficult intubation. An assessment of overall neck range of motion can be performed by measuring the angle created by the forehead when the neck is fully flexed and then fully extended; a measurement of less than 80 degrees is predictive of difficult intubation.
During DL, the tongue is displaced into the submandibular space; glottic visualization may be inadequate if this space is diminished because of a small mandible. This scenario is frequently referred to as an anterior larynx . A thyromental distance of less than 6.5 cm (3 fingerbreadths), as measured from the thyroid notch to the lower border of the mentum, is indicative of reduced mandibular space and may predict difficulty with intubation. Compliance of this space should also be assessed; a lack of compliance or the presence of a mass is a nonreassuring finding.
Tests of the ability for mandibular protrusion (prognathism) have predictive value and should be included in the airway assessment. The inability to extend the lower incisors beyond the upper incisors may be indicative of difficult laryngoscopy. A similar evaluation, the upper lip bite test (ULBT) described by Khan and colleagues, has been shown to predict difficult laryngoscopy with higher specificity and less interobserver variability than the Mallampati classification; an inability of the lower incisors to bite the upper lip is associated with more difficult laryngoscopy.
Although individual airway tests are limited by low sensitivity and positive predictive value, some multivariable assessments have been shown to have higher predictive power. The Mallampati score has been shown to have improved predictive value when combined with thyromental, sternomental, and/or interincisor distances. Models that use several risk factors, such as the Wilson risk sum score (weight, head and neck movement, jaw movement, receding mandible, and buck teeth) and the El-Ganzouri risk index (mouth opening, thyromental distance, Mallampati class, neck movement, prognathism, weight, and history of difficult intubation) have been developed in an attempt to improve the predictive value of airway assessment. On the other hand, a recent large database study of an airway risk index that utilizes seven independent risk factors found that it does not improve prediction of difficult intubation. Langeron and associates developed a computer-assisted model that uses complex interactions among several risk factors (BMI, mouth opening, thyromental distance, Mallampati class, and receding mandible) to predict difficult intubation more accurately than other models based on simpler statistical analyses.
Owing to the poor sensitivity and specificity of traditional metrics for airway assessment, a number of new modalities are being studied. The use of point-of-care ultrasonography for the prediction of difficult laryngoscopy and intubation has shown some promise in small studies, but its overall value has yet to be established. Computed tomographic images of the head and neck can be used to create three-dimensional virtual endoscopic images that can be used for planning difficult airway management, particularly for patients with complex airway pathology. Early studies of facial image analysis have also shown promise for the use of this technology in predicting difficult intubation.
With the induction of anesthesia, hypoxemia can quickly develop as a result of hypoventilation or apnea in combination with decreases in functional residual capacity (FRC) attributable to the supine position, muscle paralysis, and the direct effects of the anesthetic agents themselves. Preoxygenation, the process of replacing nitrogen in the lungs with oxygen, provides an increased length of time before hemoglobin desaturation occurs in an apneic patient. This lengthened apnea time provides an improved margin of safety while the anesthesiologist secures the airway and resumes ventilation. Adequate preoxygenation is essential when mask ventilation after the induction of anesthesia is contraindicated or anticipated to be difficult, when intubation is anticipated to be difficult, and in patients with a smaller FRC (i.e., patients who are obese or pregnant). Because difficulty with airway management can unexpectedly occur, routine preoxygenation before induction of general anesthesia is recommended.
Preoxygenation is typically performed via a face mask attached to either the anesthesia machine or a Mapleson circuit. To ensure adequate preoxygenation, 100% oxygen must be provided at a flow rate high enough to prevent rebreathing (10 to 12 L/min), and no leaks around the face mask must be present. An end-tidal concentration of oxygen greater than 90% is considered to maximize apnea time. With maximal preoxygenation, the time to oxyhemoglobin desaturation below 80% can vary from 9 minutes in a healthy, nonobese adult to 3 minutes or less in children or obese adults.
Two primary methods are used to accomplish preoxygenation. The first method uses tidal volume ventilation through the face mask for 3 minutes, which allows the exchange of 95% of the gas in the lungs. The second method uses vital capacity breaths to achieve adequate preoxygenation more rapidly. Four breaths over 30 seconds is not as effective as the tidal volume method but may be acceptable in certain clinical situations; eight breaths over 60 seconds has been shown to be more effective.
Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) at 60 L/min for 3 minutes has been demonstrated to be as effective as tidal volume preoxygenation by face mask (see Apneic Oxygenation). Head-up positioning has been shown to improve the quality of preoxygenation in both obese and nonobese patients. The use of noninvasive positive-pressure ventilation (PPV) for preoxygenation also prolongs apnea time.
Apneic oxygenation is a physiologic phenomenon by which oxygen from the oropharynx or nasopharynx diffuses down into the alveoli as a result of the net negative alveolar gas exchange rate resulting from oxygen removal and carbon dioxide excretion during apnea. Assuming the airway is patent and oxygen is insufflated through the nose and/or mouth, oxygenation occurs, prolonging apnea time beyond that of standard face-mask preoxygenation.
Oxygen can be insufflated at up to 15 L/min with nasal cannulae (nasal oxygen during efforts securing a tube [NO DESAT]) or with a catheter placed through the nose or mouth with the tip in the pharynx (pharyngeal oxygen insufflation). Studies have demonstrated that these techniques are effective in delaying oxyhemoglobin desaturation in morbidly obese patients and during emergency tracheal intubation.
THRIVE involves the administration of warmed, humidified oxygen, allowing higher oxygen flow rates than the previously described techniques—up to 70 L/min. These higher flows extend the apnea time even further and improve the clearance of carbon dioxide, preventing the potential development of severe respiratory acidosis. In 25 patients with a difficult airway at risk for rapid desaturation, THRIVE was used to achieve a median apnea time of 14 minutes, with a range of 5 to 65 minutes, and an average rate of carbon dioxide rise of only 1.1 mm Hg per minute.
In 1946, Mendelson was the first to describe aspiration pneumonitis attributable to the pulmonary aspiration of acidic gastric secretions in pregnant women undergoing anesthesia. This potentially fatal complication, occasionally referred to as Mendelson syndrome, has since been the intense focus of preventive efforts among the anesthesia community. Prevention of aspiration of gastric contents is primarily accomplished by adherence to established preoperative fasting guidelines, premedication with drugs that may decrease the risk of aspiration pneumonitis, and specialized induction techniques, which are discussed later in this chapter.
Traditionally, patients who were scheduled for elective procedures requiring sedation, regional anesthesia, or general anesthesia were instructed to remain NPO (Latin for nulla per os or nothing by mouth ) after midnight to ensure an empty stomach to decrease the risk of regurgitation. Based on evidence that allowing ingestion of clear liquids 2 to 4 hours before surgery resulted in lower gastric volumes and higher gastric pH, the ASA published Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration in 1999 that liberalized the traditional NPO policy and allowed clear liquids up to 2 hours before beginning elective procedures requiring anesthesia. The guidelines, most recently updated in 2017, recommend 4 hours of fasting from breast milk and 6 hours of fasting from solid foods, infant formula, and nonhuman milk. Fried or fatty foods may require longer fasting times (e.g., 8 hours or more). Although the ASA guidelines do not specifically address chewing gum, hard candies, or smoking, guidelines published by the European Society of Anaesthesiology on the topic do not recommend delaying the start of anesthesia if a patient has consumed any of these immediately before the induction of anesthesia.
The routine use of drugs as prophylaxis against aspiration pneumonitis is not recommended by the ASA guidelines but may be beneficial in patients with specific risk factors for aspiration, such as a full stomach, symptomatic gastroesophageal reflux disease (GERD), hiatal hernia, presence of a nasogastric tube, morbid obesity, diabetic gastroparesis, or pregnancy. The goal of aspiration prophylaxis is twofold: to decrease gastric volume and to increase gastric fluid pH. Commonly used agents include nonparticulate antacids (e.g., Bicitra), promotility drugs (e.g., metoclopramide), and H 2 -receptor antagonists. These drugs may be used alone or in combination.
One of the most important teleologic functions of the larynx is that of airway protection, which is primarily provided by the glottic closure reflex. This reflex is triggered by sensory receptors in the glottic and subglottic mucosa and results in strong adduction of the vocal cords. An exaggerated, maladaptive manifestation of this reflex, referred to as laryngospasm , is a potential complication of airway management. Laryngospasm is usually provoked by glossopharyngeal or vagal stimulation attributable to airway instrumentation or vocal cord irritation (e.g., from blood or vomitus) in the setting of a light plane of anesthesia (stage II of the Guedel classification), but it can also be precipitated by other noxious stimuli and can persist well after the removal of the stimulus. Treatment of laryngospasm includes removal of airway irritants, deepening of the anesthetic, and the administration of a rapid-onset neuromuscular blocking drug (NMBD), such as succinylcholine. Continuous positive airway pressure with 100% oxygen is commonly cited as a therapeutic maneuver, although the pressure may push the aryepiglottic folds closer together and may actually promote laryngospasm by acting as a mechanical stimulus. Bilateral pressure at the laryngospasm notch between the condyle of the mandible and the mastoid process can be effective at treating laryngospasm by causing an intense, painful stimulus, which may function to terminate laryngospasm by arousing a semiconscious patient or by activating autonomic pathways.
The tracheobronchial tree also possesses reflexes to protect the lungs from noxious substances. Irritation of the lower airway by a foreign substance activates a vagal reflex–mediated constriction of bronchial smooth muscle, resulting in bronchospasm. Untreated bronchospasm can result in an inability to ventilate because of an extremely elevated airway resistance. Treatment includes a deepening of anesthetic with propofol or a volatile agent and the administration of inhaled β 2 -agonist or anticholinergic medications. Administration of intravenous (IV) lidocaine has been studied, but the evidence does not support its use for treatment of bronchospasm.
Tracheal intubation, as well as laryngoscopy and other airway instrumentation, provides an intense noxious stimulus via vagal and glossopharyngeal afferents that results in a reflex autonomic activation, which is usually manifested as hypertension and tachycardia in adults and adolescents; in infants and small children, autonomic activation may result in bradycardia. Hypertension and tachycardia are usually of short duration; however, they may have consequences in patients with significant cardiac disease. Central nervous system activation as a result of airway management results in increases in electroencephalographic (EEG) activity, cerebral metabolic rate, and cerebral blood flow, which may result in an increase in intracranial pressure in patients with decreased intracranial compliance.
To facilitate airway management, some form of anesthesia is usually required to provide comfort for the patient, to blunt airway reflexes, and to blunt the hemodynamic response to airway instrumentation. Most commonly, airway management is performed after induction of general anesthesia. Alternatively, an awake technique, which entails establishing an airway (including tracheal intubation) by using local anesthesia of the airway and/or sedation, can be used to meet these goals when clinically indicated. In emergency scenarios where the patient is obtunded or comatose, such as in the event of acute respiratory or cardiac arrest, anesthetic drugs may not be required.
Airway management is usually performed after the induction of general anesthesia if the anesthesiologist determines that it is safe to do so. Several pharmacologic techniques are used for the induction of anesthesia, each with its own implications for airway management. The decision of which induction technique to use should be made with careful consideration of the specific clinical circumstances at hand.
The most common technique for induction of general anesthesia is the standard IV induction, which entails the administration of a rapid-acting IV anesthetic, followed by an NMBD. Muscle relaxation achieved by the administration of NMBDs improves intubating conditions by facilitating laryngoscopy, preventing both reflex laryngeal closure and coughing after intubation.
Propofol is the most frequently used IV anesthetic drug; other options include etomidate, ketamine, thiopental, and midazolam. The choice of drug depends on a variety of factors including the patient’s hemodynamic status, comorbidities, and allergies, as well as drug pharmacokinetics, side effects, physician preference, and availability. Whether the choice of an anesthetic drug has any effect on the quality of intubating conditions when NMBDs are also administered is not well established. Studies comparing propofol, etomidate, and thiopental in combination with NMBDs showed no difference in intubating conditions between the different anesthetics. On the other hand, one study, during which patients received cisatracurium, showed that larger doses of propofol were associated with improved intubating conditions, as compared with smaller doses.
For many years, succinylcholine was the most frequently used NMBD for routine IV induction ; however, nondepolarizing NMBDs have gained greater popularity attributable to the risk of adverse effects from succinylcholine administration, including bradycardia, myalgia, hyperkalemia, increased intracranial pressure, and increased intragastric pressure. Succinylcholine, the only depolarizing NMBD in clinical use, has the benefit of a rapid onset combined with a short duration of action, and it is currently used most often when those properties are desired. Most notably, succinylcholine is still commonly used in the setting of a suspected difficult airway; its short duration of action theoretically allows for the resumption of spontaneous ventilation before severe hypoxia develops in a preoxygenated patient, although evidence suggests that this may not predictably occur .
Nondepolarizing NMBDs are the more frequently used relaxants for routine IV induction of anesthesia. The most commonly used nondepolarizing NMBDs in current practice—rocuronium, vecuronium, and cisatracurium—are notable for having a favorable safety profile with relatively few side effects. The primary limitation of these drugs is a significantly longer duration of action; once administered, a functional airway must be established within minutes to avoid life-threatening hypoxia. Sugammadex is a selective relaxant-binding agent for rocuronium that has the ability to reverse profound neuromuscular blockade rapidly in a time comparable with spontaneous recovery from succinylcholine (also see Chapter 28 ).
Traditional teaching in the United States has advocated withholding NMBDs until the ability to mask ventilate has been established. If ventilation via a mask cannot be achieved, a preoxygenated patient can then theoretically resume spontaneous ventilation or be awakened before the onset of hypoxia. This practice has been increasingly questioned in the literature in part because of a number of studies demonstrating that ventilation via a mask is not rendered more difficult by muscle relaxation ; rather, mask ventilation is, in fact, facilitated by muscle relaxation. One issue with the traditional paradigm is that the theoretical advantage of the practice—the ability to awaken the patient if mask ventilation fails—is rarely used. The desire to preserve that ability may, in fact, result in giving an inadequate dose of anesthetic during induction, resulting in a difficult mask ventilation situation when one would not have otherwise occurred. Delaying the administration of NMBDs can result in the onset of hypoxia before spontaneous recovery (with succinylcholine) or reversal (with sugammadex) is possible.
The authors do not recommend withholding NMBDs in patients who are predicted to be easy to mask ventilate and/or intubate. For patients in whom difficulty with both mask ventilation and intubation are predicted, awake intubation or inhalation induction of anesthesia should be considered, and the administration of NMBDs is best withheld until the ability to ventilate is proven.
Rapid-sequence induction and intubation (often simply referred to as rapid sequence induction [RSI] in the anesthesia literature) is a specialized method of IV induction commonly used when an increased risk of gastric regurgitation and pulmonary aspiration of gastric contents exists. After adequate preoxygenation and while cricoid pressure is applied, an induction dose of IV anesthetic is rapidly followed by 1 to 1.5 mg/kg of IV succinylcholine, and the trachea is intubated without attempts at PPV. The goal is to achieve optimal intubating conditions rapidly to minimize the length of time between the loss of consciousness (LOC) and securing of the airway with a cuffed endotracheal tube (ETT). Cricoid pressure, eponymously referred to as the Sellick maneuver after the physician who first described it, involves the application of pressure at the cricoid ring to occlude the upper esophagus, thereby preventing the regurgitation of gastric contents into the pharynx. The recommended force to be applied is 10 Newtons (N) while the patient is awake, increased to 30 N after LOC. These values are based on esophageal manometry on patients undergoing induction of anesthesia and cadaver studies of safe amounts of pressure. RSI is widely practiced and approaches a standard of care in patients with a full stomach (i.e., when NPO guidelines have not been observed) and in the setting of bowel obstruction. RSI has historically been recommended for patients who are pregnant, starting in the second trimester, but this dogma has been called into question. Other clinical situations for which RSI may be considered due to a higher than normal risk for aspiration of gastric contents, include poorly controlled GERD, presence of a nasogastric tube, morbid obesity, and diabetic gastroparesis. RSI is also a useful induction technique when mask ventilation is predicted to be difficult, but intubation is not, such as with an edentulous, bearded patient with an otherwise reassuring airway examination.
Some common variations to RSI have developed from the technique first described in 1970. When succinylcholine is contraindicated or its side effects are undesired, RSI can be accomplished using nondepolarizing NMBDs (rocuronium 1.0 to 1.2 mg/kg or vecuronium 0.3 mg/kg); these doses provide adequate intubating conditions in less than 90 seconds. The primary disadvantage with the nondepolarizing NMBDs used to be the prolonged duration of neuromuscular blockade; however, since the introduction of sugammadex these agents are increasingly employed in place of succinylcholine for RSI (also see Chapter 27, Chapter 28 ). Although traditional RSI calls for induction with a fixed dose of thiopental, the use of other anesthetics such as propofol, etomidate, or ketamine is common. Some advocate for the titration of the chosen anesthetic agent to LOC rather than the delivery of a fixed, predetermined dose.
The application of cricoid pressure is the most controversial aspect of RSI. Opponents point to studies demonstrating that cricoid pressure results in a decrease in lower esophageal sphincter tone, potentially increasing the risk for regurgitation, and to magnetic resonance imaging (MRI) studies showing that cricoid pressure does not, in fact, result in compression of the esophagus, but rather a lateral displacement. Cricoid pressure also worsens laryngeal visualization during DL, potentially lengthening the time to intubation and increasing the risk of pulmonary aspiration, and can result in occlusion of the subglottic airway, resulting in difficulty with tracheal intubation or mask ventilation. On the other hand, advocates argue that properly applied cricoid pressure is effective in reducing the risk of aspiration and that reports of problems are due to incorrect application. The authors of an MRI study of cricoid pressure argue that the position of the esophagus is irrelevant because the effectiveness of cricoid pressure is due to occlusion of the hypopharynx. In general, because of the relatively low risk of application of cricoid pressure, its use is encouraged for RSI unless glottic visualization proves difficult, in which case it can be easily released.
The term modified RSI is frequently used, but no standardized definition exists. A survey of anesthesia residents and attending anesthesiologists in the United States showed that the term was most commonly used to refer to the use of mask ventilation in conjunction with cricoid pressure. Indications for this technique include patients at risk for rapid development of hypoxemia (e.g., patients who are obese, pregnant, or critically ill; pediatric patients) in emergent situations during which preoxygenation cannot be satisfactorily completed, or when a longer time to acceptable intubating conditions is required because of the use of standard doses of nondepolarizing NMBDs. Although the effect of PPV with cricoid pressure applied in terms of gastric insufflation of air is not definitively known, gentle PPV (inspiratory pressure <20 cm water [H 2 O]) in conjunction with cricoid pressure may be acceptable in these clinical scenarios.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here