Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Immunization is the process of artificially inducing immunity or providing protection from disease. Active immunization is the process of stimulating the body to produce antibody and other immune responses (e.g., cell-mediated immunity) through administration of a vaccine or toxoid. Passive immunization is provision of temporary immunity via administration of preformed antibodies derived from humans or animals (see Chapter 5 ).
Vaccines are among the most effective means of preventing disease, disability, and death. Use of vaccines, initiated by Jenner in 1796, demonstrated that inoculation of material from cowpox lesions could prevent smallpox, and predated the germ theory of disease. The profound impact of vaccines on disease incidence is a result of both the availability of safe and effective vaccines as well as effective strategies and programs to deliver vaccines to target groups. Eradication of smallpox in 1977, one of the greatest public health achievements, required both disease surveillance and containment with a highly effective vaccine. Similarly, ongoing global efforts to eradicate poliomyelitis involve comprehensive disease control strategies, including national and multicountry mass vaccination campaigns, intensive surveillance for wild poliovirus (WPV), and house-to-house vaccination programs. These programs have succeeded in terminating transmission of WPV in the Americas, the Western Pacific, Europe, Southeast Asia, and Africa, and gains are being made in the remaining endemic countries of Pakistan and Afghanistan ( http://polioeradication.org/news-post/two-out-of-three-wild-poliovirus-strains-eradicated/ ). WPV types 2 and 3 have been certified as eradicated and WPV type 1 cases have been detected in only 2 countries (Pakistan and Afghanistan) since 2016. Efforts to reduce or even eliminate measles and neonatal tetanus, 2 of the major causes of global childhood mortality, also are progressing, although cases of measles continue to occur worldwide. , Gavi, a collaboration among public and private sectors to promote vaccination globally, provides support for introduction of vaccines in the world’s poorest countries (≤$1580 per capita income per year) that otherwise could not support their use. ( www.gavi.org ).
Benefits of successful vaccines include not only reductions in disease incidence, disability, pain, and suffering, but also savings in healthcare costs in both the economically developed and developing world. Studies in the US have reaffirmed benefits of childhood immunization. A cost-benefit analysis covering vaccine-preventable diseases of childhood, including diphtheria, tetanus, pertussis, poliomyelitis, hepatitis A, hepatitis B, Haemophilus influenzae type b (Hib) infection, measles, mumps, rubella, rotavirus infection, pneumococcal disease, and varicella, estimated that routine childhood immunization among members of the 2009 US birth cohort will prevent approximately 42,000 early deaths and 20 million cases of disease, with net savings of $13.5 billion in direct costs and $68.8 billion in total societal costs.
Biologic agents used to induce active immunization include vaccines and toxoids. Traditionally, a vaccine is defined as a suspension of live (usually attenuated) or inactivated microorganisms, or fractions thereof, which is administered to induce immunity and prevent infectious diseases or their sequelae; efforts to develop vaccines to increase the immune response to cancers or to treat diseases including diabetes mellitus necessitate reconsideration of this definition. Live, attenuated vaccines traditionally have been developed by means of serial passage (in culture or animals) of an initially pathogenic bacteria or virus strain with selection for strains that are less pathogenic for humans but that induce protective immunity (e.g., measles, mumps, and rubella [MMR] vaccine). Live, attenuated vaccines also can be developed with use of reassortants of attenuated animal or human virus strains with virus coat antigens from pathogenic strains (e.g., cold-adapted influenza, rotavirus vaccines). Inactivated vaccines can consist of the following: (1) whole organisms inactivated by heat, formalin, or other agents (e.g., polio, hepatitis A, rabies vaccines); (2) purified protein (e.g., acellular pertussis, influenza, and meningococcal serogroup B vaccines) or polysaccharide antigens (e.g., pneumococcal, meningococcal, and intramuscular typhoid vaccines) from whole organisms; (3) purified antigens produced by genetically altered organisms (e.g., hepatitis B and human papillomavirus [HPV] vaccines produced by yeast); (4) chemically modified antigens, such as polysaccharides conjugated to carrier proteins to increase immune response (e.g., conjugated Hib, pneumococcal, and meningococcal vaccines); or (5) messenger RNA encoding protein(s) of the organism, which then are transcribed in the cytoplasm of cells near the site of infection to express the antigen and then stimulate the immune response. Toxoids are bacterial toxins produced in bacterial culture that have been rendered nontoxic but retain the ability to stimulate formation of antitoxin.
Vaccine and toxoid preparations also contain other constituents intended to enhance immunogenicity and stability but that can be responsible for adverse reactions. Such constituents include the following: (1) suspending fluid, which can be saline or complex fluids containing constituents derived from the biologic system or medium in which the vaccine is produced (e.g., egg or serum proteins); (2) preservatives, stabilizers, or antimicrobial agents, which are used to inhibit bacterial growth in viral cultures or the final product or to stabilize antigens (e.g., mercurials, phenols, albumin, glycine, neomycin); and (3) adjuvants, which enhance response to inactivated antigens (e.g., aluminum phosphate, aluminum hydroxide, mixed aluminum salts). Concern about the theoretical possibility of adverse effects from cumulative exposure to mercury in the environment has led to removal of thimerosal as a preservative from most US vaccines recommended for children. The only vaccines administered to children that contain thimerosal as a preservative are some influenza vaccines (multidose vials). Physicians should be knowledgeable about the constituents of each vaccine, which are described in vaccine package inserts ( https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states ).
The two major approaches to active immunization are (1) live, attenuated vaccines and (2) inactivated or detoxified agents or their purified components, or the messenger RNA encoding these inactive agents. For some diseases, such as poliomyelitis and influenza, both approaches have been used.
Live, attenuated vaccines have the advantage of producing a complex immunologic response simulating natural infection. Because replication of the organism and processing of antigens mimic those of the natural pathogen, both humoral and cell-mediated responses can be generated to a variety of antigens. Generally, immunity induced by a single dose of a live, attenuated vaccine is long-lasting, possibly lifelong. However, the strength of response, particularly the humoral response, usually is less robust than the response following natural infection, and detectable antibodies can wane with time, resulting in some potential loss of protection. Induction of immunity by live vaccines can be inhibited by passive antibody, whether from transplacental acquisition from the mother or receipt of immunoglobulin-containing blood products. Thus, optimal response depends on ensuring that passive antibody levels have declined prior to vaccination (e.g., primary measles vaccination at 12 months of age instead of earlier, delay of measles vaccination after administration of blood products). In addition, because response may be only 90%–95% after a single dose, a 2-dose or multiple-dose regimen may be necessary to induce higher levels of protection in the community and prevent spread of disease if the population is exposed (community protection).
Inactivated or purified antigen vaccines induce response only to components present in the vaccine. Multiple doses, usually three or more, are generally necessary to induce satisfactory antibody levels that persist for long periods of time; booster doses at longer intervals (e.g., 10 or more years for tetanus and diphtheria toxoids) sometimes are required to ensure lasting protection. The nature of vaccine responses depends on antigen type. Protein and glycoprotein antigens usually induce both humoral immunity and memory (response through helper T lymphocytes) after multiple doses, as evidenced by more rapid, broad, and intense (anamnestic) response to successive doses. Polysaccharide antigens by themselves induce only humoral antibody without T-lymphocyte stimulation and fail to induce an anamnestic response with repeated antigenic challenge. This limitation can be overcome by conjugation of polysaccharides to protein carriers to induce both a stronger immune response in younger children and immunologic memory.
The general mechanism by which a long-term protective memory immune response is induced requires activation of T lymphocytes by antigen-presenting cells, which then provide help to B lymphocytes to develop into antibody-producing plasma cells (i.e., T-lymphocyte–dependent response). , Polysaccharide antigens bypass T-lymphocyte help (i.e., T-lymphocyte–independent response) and induce B-lymphocyte differentiation directly, but they do not induce immunologic memory. Initiation of a T-lymphocyte–dependent antibody response occurs by activation of naïve CD4 + helper T lymphocytes in response to antigen-presenting cells, generally dendritic cells. This interaction occurs through recognition of the major histocompatibility complex (MHC) on the antigen-presenting cells by the T-lymphocyte receptor and by secretion of cytokines that are necessary for maturation of naïve CD4 + helper T lymphocytes to differentiate toward a helper T-lymphocyte 1 (Th1) or Th2 response. Th1 responses are predominantly cell-mediated, whereas Th2 responses are predominantly humoral. In the presence of interleukin-12 (IL-12), Th1 response is differentiated and is associated with secretion of IL-2 and interferon γ (IFNγ). In the presence of IL-4, Th2 response is differentiated and is associated with secretion of IL-4 and IL-5. These two cytokines are essential for differentiation and maturation of B lymphocytes into antibody-secreting plasma cells. A small subset of CD4 + T lymphocytes does not differentiate into effector cells; instead, these T-lymphocytes mature to become long-lived memory cells, which form the nucleus of the secondary immune response on reexposure to antigen. The overall response is regulated by a further class of CD4 + T lymphocytes (Treg).
In a primary response, naïve B lymphocytes recognize a specific antigenic epitope on native antigen through the immunoglobulin receptor on the surface of the B lymphocyte, and they differentiate into antibody-secreting cells in response to help from CD4 + Th2 lymphocytes. A given B lymphocyte is activated by a T-lymphocyte responding to the same antigen. B-lymphocyte proliferation and maturation then occur in a clonal manner, and during this process, immunoglobulin (Ig) class switching (from IgM to IgG and IgA) and affinity maturation also occur. Antigen-specific plasma cells develop and secrete large amounts of clonal antibody.
The polyclonal response to a given immunogen reflects the sum of the multiple individual clonal B-lymphocyte responses that result in an antibody response. A few activated B lymphocytes mature into long-lived memory B lymphocytes, which form the basis of the rapid secondary response on the next encounter with antigen. Some plasma cells acquire the capacity to migrate toward long-term survival niches mostly located within the bone marrow, from where they may produce antibodies during extended periods. , The duration of antibody responses reflects the number or quality, or both, of long-lived plasma cells generated by immunization. The mechanism of maintenance of these lymphocytes is an area of active research because the ability to mount a strong secondary response after many years is a critical component of the adaptive immune response.
Initiation of the specific immune response to a pathogen depends on the innate immune response, which is rapid, does not exhibit memory, and historically has been considered nonspecific. However, discovery of germline-encoded pattern recognition receptors has led to an understanding that specific molecular structures are recognized by pathogen-associated molecular patterns. These specific structures include Toll-like receptors (TLRs), which are membrane associated, and nucleotide-binding oligomerization domain (NOD)-like receptors, which are found in the cytoplasm and include the NALP family of receptors (member of the nucleotide-binding domain and leucine-rich repeat containing gene family) and others, all of which contribute to immune activation by inducing proinflammatory cytokines, which in turn modulate the adaptive immune response. TLRs and NOD-like receptors are found on antigen-presenting lymphocytes and recognize a wide variety of molecules commonly found in pathogens. TLR4 binds lipopolysaccharide and TLR5 binds flagellin, both common bacterial components. The NALP3 inflammasome is activated by aluminum and has been shown to be the pathway through which aluminum adjuvants enhance immunogenicity.
Antibodies produced in response to immunization can mediate protection by a variety of extracellular mechanisms including (1) direct neutralization of bacterial toxin, (2) facilitation of intracellular digestion of bacteria by phagocytes (opsonization), (3) initiation of or combination with the complement pathway to cause lysis, or (4) sensitization of macrophages to promote phagocytosis. Antibodies cannot readily reach the intracellular space, which is the site of viral and some bacterial replication. However, antibodies are effective against many viral diseases by interacting with a virus before initial intracellular penetration occurs (neutralization) and by preventing locally replicating virus from disseminating from the site of entry to an important target organ, as in spread of poliovirus from the intestine to the central nervous system or spread of rabies from a puncture wound to peripheral neural tissue. Antigen-specific cytotoxic T-lymphocyte–mediated responses are important components of the response to viral infections.
The cell-mediated immune response also has an innate and memory effector arm. Natural killer (NK) cells recognize virally infected cells in a nonspecific manner, without generation of memory. Activated NK cells secrete a variety of soluble mediators, including IFNs that kill virus-infected cells and ILs that modulate differentiation and response of CD4 + helper T lymphocytes. Cytotoxic CD8 + T lymphocytes require the help of CD4 + T lymphocytes (generally Th1) to differentiate and mature. CD8 + cytotoxic T lymphocytes are an important part of the immune response to intracellular bacteria and viruses. As viruses replicate in a cell, viral proteins are processed and presented on the cell surface as an MHC class I/peptide complex. The complex is recognized by cytotoxic T lymphocytes. As with CD4 + T lymphocytes, a small subset develops into long-lived memory CD8 + T lymphocytes capable of rapid reactivation as part of a secondary immune response.
Rarely, vaccination can result in immune responses that alter the course of natural infection detrimentally. For example, killed measles vaccine, a formalin-inactivated vaccine administered to some children in the US from 1963 to 1967, sensitized some vaccine recipients so that when exposed to wild virus, they developed an atypical measles infection with enhanced severity of disease. The prevailing theory has been that failure of formalin-inactivated vaccine to produce response to the measles virus fusion protein led to altered immune response and atypical disease on subsequent challenge. More recent theories suggest that killed measles virus failed to induce high-avidity antibody, and immune-complex deposition was the major determinant of atypical measles.
Following primary immunization with inactivated antigens, antibody response develops in 2–6 weeks but can be incomplete even after two doses; after effective priming, booster responses occur within 4–14 days. The initial response usually is IgM antibodies, followed within weeks by IgG antibodies. For example, response to live vaccines requires one incubation period, followed by several weeks to months for development of a strong immune response. Response to measles vaccination usually is maximal by 6 weeks, but in younger children, antibody levels can continue to rise for several months. However, protection against measles begins much earlier, and some evidence suggests that disease can be prevented even when vaccine is administered within 72 hours of exposure.
Vaccine immunogenicity and response are determined by characteristics of the vaccine and the host. Vaccine dose, presence of an adjuvant, route and site of administration, timing of doses, and vaccine handling can affect response. Vaccine doses are adjusted before licensure to ensure a high level of response (generally >90% of subjects); adjuvants permit a better response with a lower dose of inactivated antigen. The route of vaccine administration (e.g., intradermal, subcutaneous, intramuscular, or mucosal) can determine the strength and nature of the immune response. Mucosal administration (intranasal or oral) stimulates higher levels of mucosal IgA antibodies that can inhibit disease transmission with greater effectiveness than parenteral administration, which induces limited or no mucosal response. Intradermal vaccination with lower antigen doses can induce antibody responses similar to responses induced by intramuscular or subcutaneous administration of larger doses, but intradermal vaccine is more difficult to deliver precisely and, in practice, achieves less predictable responses.
Intramuscular injections should be administered in the anterior thigh (infants and toddlers) or deltoid muscle (children and adults); injection into the buttocks can produce lower antibody response, which has been documented for hepatitis B and rabies vaccines in adults, probably as a result of delivery of vaccine into adipose tissue. , Vaccines with adjuvants should be administered into a muscle because subcutaneous or intradermal injection can induce local irritation, inflammation, granuloma formation, or skin discoloration.
Timing of doses of killed vaccines is important; a minimal interval of 1 month between primary doses is usual, as is delay of a fourth or reinforcing dose for 10 months or longer after the first dose to enhance response and duration of antibody persistence. The recommended routes and sites of administration and timing of doses are devised to ensure optimal effectiveness in disease prevention and should be used.
Intrinsic factors in the host that affect immune response include genetic factors, age, nutritional or disease status, primary or secondary immunodeficiency, sex, pregnancy, and smoking. , Although genetic factors such as MHC polymorphism are known to affect both cellular and humoral immune response at a molecular level for some vaccines, the precise mechanisms are unknown. Evaluating how host genetic markers alter vaccine response could identify differences that predict successful or adverse vaccine outcome. Studies of the expression of genetic information modified on a molecular level can reveal how environmental influences affect innate and adaptive immune responses. These considerations combined with system vaccinology approaches may lead to better understanding of immune responses. , , Age is an important factor in response to immunization. With killed vaccines, neonates generally do not develop as brisk a response as older infants or children (e.g., with hepatitis B), and with certain vaccines, too-early immunization can result in a suboptimal response or development of tolerance or interference (diphtheria and tetanus toxoids and acellular pertussis [DTaP]; inactivated poliovirus vaccine [IPV]; Hib conjugates). For live (and some killed) vaccines, inhibition of response by maternal antibodies determines the optimal timing for vaccination in early childhood (measles, hepatitis A). Generally, response to all vaccines is excellent in young children, adolescents, and young adults but diminishes with increasing age. In adults, smoking decreases response to hepatitis B vaccine. , Extreme debilitation, primary or secondary immunodeficiency disorders (including diseases or treatments that cause immunosuppression), and some chronic diseases (renal disease, diabetes mellitus) can diminish immune response. For people with such conditions, inactivated vaccines can be recommended, although higher or more frequent doses may be required; live vaccines are contraindicated for some immunocompromising conditions because of the risk of disseminated disease and possible death caused by the vaccine organism. ,
Ideally, reliable laboratory tests should be available to measure the presence and strength of each of the major effectors of protection against the disease for which the vaccination is administered. In routine practice, many different assays are available to assess the presence, absence, and level of antibodies. These include enzyme immunoassay (EIA), complement fixation, and immunofluorescent techniques. Assays for functional antibody, including neutralization or opsonophagocytosis, generally are performed in reference or research laboratories, as are assays for T-lymphocyte function. CD4 + Th1 and Th2 lymphocytes and CD8 + lymphocytes generally are characterized by their cytokine profiles by using assays such as enzyme-linked immunospot (ELISpot) or intracellular cytoplasmic staining.
For certain diseases, including hepatitis B, poliovirus, rubella, and measles, reliable assays exist, and antibody levels that correlate with protection are known. For other diseases, such as pertussis and HPV infection, no serologic correlates of protection have been defined. Development of improved laboratory methods to measure protection and to permit rapid diagnosis of acute disease continues to be a priority of vaccine-preventable disease control programs.
Before testing a vaccine in humans, a manufacturer files an investigational new drug (IND) application with the FDA, followed by three phases of clinical trials that are performed to study vaccine safety, immunogenicity, and efficacy. , On completion of the prelicensure clinical trials, the following steps generally occur for a vaccine to become available and used: (1) the manufacturer applies for a Biologics Licensure Application (BLA) with the FDA; (2) the FDA licenses the vaccine; (3) the Advisory Committee on Immunization Practices (ACIP), the Centers for Disease Control and Prevention (CDC), the American Academy of Pediatrics (AAP), and other professional medical societies make recommendations for use of the vaccine; and (4) financing is secured for the public and private sectors ( Fig. 6.1 ).
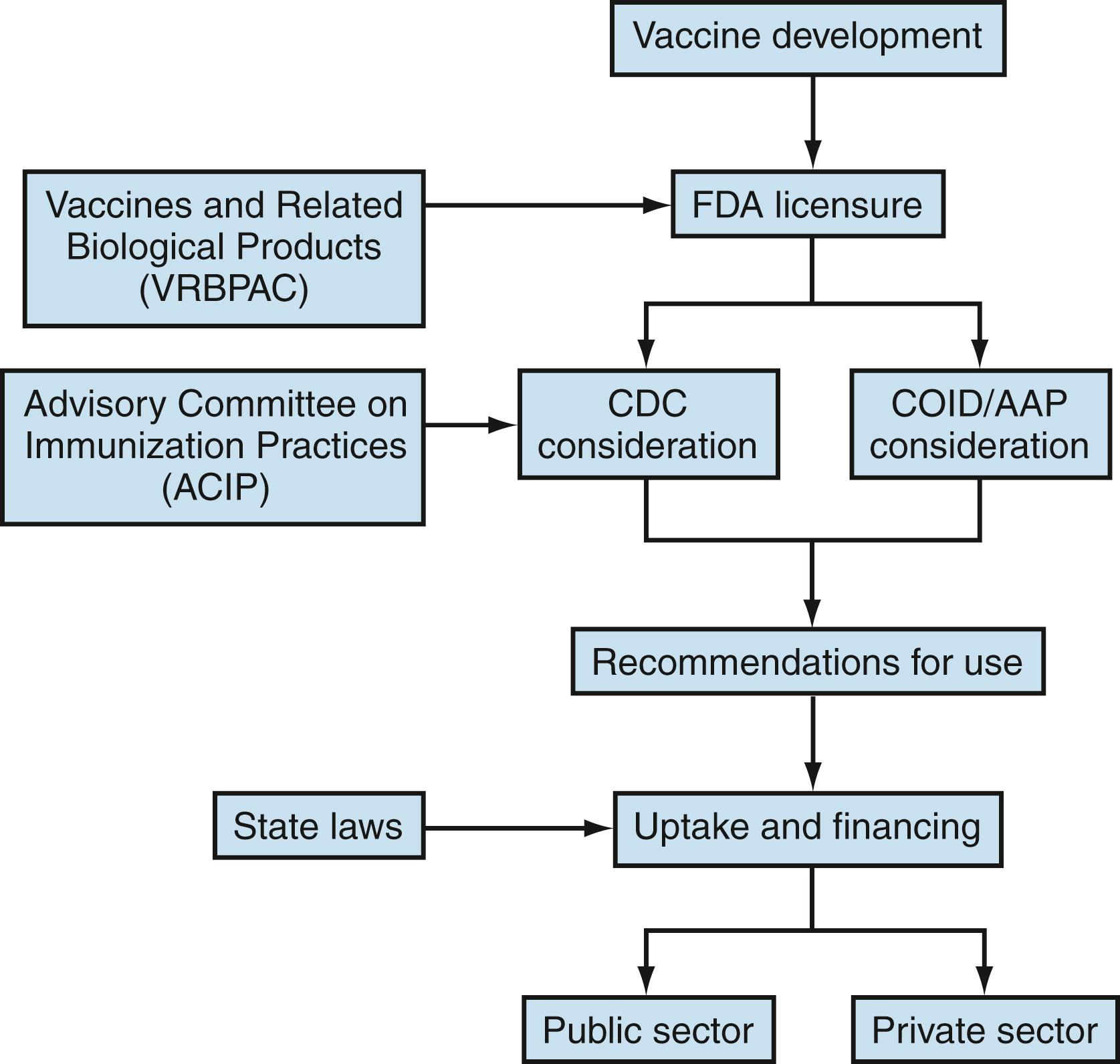
Following FDA licensure of a new vaccine, information about the vaccine is reviewed by the ACIP. The ACIP consists of 15 voting, nongovernment members appointed by the US Department of Health and Human Services for 4-year terms. In addition to the 15 voting members, ACIP includes 8 ex officio members who represent other federal agencies with responsibility for immunization programs in the US, and 30 non-voting representatives of liaison organizations that bring related immunization expertise. To formulate recommendations, the ACIP establishes subject-specific work groups to review and synthesize data months to years before presentation to the ACIP, where votes are taken on vaccine use. Data considered in making recommendations are subjected to the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) process ( https://www.cdc.gov/vaccines/acip/recs/grade/index.html ). ACIP recommendations are not official until approved by the CDC director and published in the Morbidity and Mortality Weekly Report (MMWR) ( https://www.cdc.gov/vaccines/acip/committee/charter.html ). Given that new vaccines and updated recommendations appear often, the reader is advised to follow the ACIP website for new recommendations at https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/index.html .
Following vaccine licensure, monitoring for rare adverse events continues for some vaccines through formal phase IV trials conducted by the manufacturer, which often are required and monitored by the FDA. In addition, post-marketing surveillance for adverse events is performed and permits detection of new or unanticipated adverse events. Physician and manufacturer reporting of certain adverse events observed after vaccine administration is required by the National Childhood Vaccine Injury Act for vaccines covered under the Vaccine Injury Compensation Program. The importance of post-marketing surveillance was demonstrated following licensure and wide use of tetravalent rhesus rotavirus vaccine for infants in the US in 1999. Surveillance of adverse events detected cases of intussusception within 1 week after receipt of the first or second doses of the rhesus rotavirus vaccine. Follow-up studies determined that risk of intussusception was approximately 1 case per 10,000 doses of vaccine administered. Subsequently, the vaccine was withdrawn from distribution, and the recommendation for universal use in infants in the US was withdrawn. Currently there is a universal recommendation for infants for two other rotavirus vaccines.
Childhood immunization programs have substantially reduced the occurrence of vaccine-preventable diseases in the US ( Table 6.1 ). Declines exceed 90% for all diseases for which universal vaccination has been well implemented, with the exception of hepatitis B, which remains an important clinical disease in adults not reached by universal vaccine programs, and pertussis. , Smallpox has been eradicated, poliomyelitis resulting from indigenous WPV has not occurred since 1979 in the US, and endemic rubella has been declared eliminated in the US. , Fewer than 10 cases each of diphtheria and tetanus (DT) in children are now reported each year and indigenous transmission of measles has been interrupted, notwithstanding the 2018–2019 measles outbreak in the New York area. Wide use of Hib conjugate vaccines has reduced Hib disease by >98% in the US and in some European countries. Use of pneumococcal conjugate vaccines (PCVs) has led to marked reductions in invasive pneumococcal disease (IPD) among vaccinated children as well as unvaccinated young infants, adolescents, and adults. Building on the success of the program to date, the US Public Health Service established 2010 goals to eliminate indigenous transmission of measles, rubella and congenital rubella syndrome (CRS), mumps, diphtheria, poliomyelitis, Hib disease in children <5 years of age, and tetanus in people <35 years of age, and to reduce hepatitis B in people 2–18 years old by 90%. These goals were achieved for diphtheria, measles, rubella, congenital rubella, poliomyelitis, and hepatitis B. The Healthy People 2020 objectives included specific goals for disease reduction and vaccination coverage. ( http://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives).49
| Disease | US, 20th-Century Annual Morbidity a | US, 2018 Morbidity b | Vaccine Coverage Levels, (Birth Years 2015–2016) % c | Healthy People 2020 Coverage Level Goals (%) | Reduction in Morbidity (%) |
|---|---|---|---|---|---|
| Diphtheria | 21,053 | 1 | 87.9 (≥4 doses) | 90 | 100 |
| Tetanus | 580 | 23 | 87.9 (≥4 doses) | 90 | 96.0 |
| Pertussis | 200,752 | 15,609 | 87.9 (≥4 doses) | 90 | 92.2 |
| Poliomyelitis (paralytic) | 16,316 | 0 | 94.3 d (≥3 doses) | 90 | 100 |
| Measles | 530,217 | 375 | 93.3 (≥1 dose) | 90 | 100 |
| Mumps | 162,344 | 2,515 | 93.3 (≥1 dose) | 90 | 98.5 |
| Congenital rubella | 152 | 0 | 93.3 (≥1 dose) | 90 | 100 |
| Acquired rubella | 47,745 | 4 | 93.3 (≥1 dose) | 90 | 100 |
| Haemophilus influenzae type b and unknown; <5 yr of age | 20,000 | 38 e | 94.1 (≥2 or 3 doses, depending on brand) | 90 | 99.8 |
| Varicella | 4,085,120 | 8,201 | 93.5 (≥1 dose) | 90 | 99.8 |
a Roush SW, Murphy TV; Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA . 2007;298(18):2155–2163
b Centers for Disease Control and Prevention. https://wonder.cdc.gov/nndss/static/2018/annual/2018-table1-H.pdf
c 2015–2016 Childhood Vaccination Coverage Combined Birth Year Dashboard. https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/interactive-reports/dashboards/2015-2016.html .
Proposed objectives for Healthy People 2030 include 4 doses of DTaP and 1 dose of MMR by the age of 2 years, maintaining two-dose coverage of MMR for children in kindergarten, and increasing the percentage of persons 6 months old and older vaccinated annually against influenza ( https://www.healthypeople.gov/sites/default/files/Report%207_Reviewing%20Assessing%20Set%20HP2030%20Objectives_formatted%20EO_508_05 ).
Vaccination coverage among preschool children has increased steadily after the measles resurgence that began in 1989 and stimulated unprecedented efforts to improve delivery of immunization. From 2016 through 2018, coverage with 3 doses of DTaP, poliovirus, and hepatitis B virus vaccines and 1 dose of MMR and varicella vaccines among children by 24 months of age each reached or exceeded 90%. School laws are often expanded to include newly recommended vaccines. In 2018, 88.9% of adolescents received 1 dose of Tdap, 85.1% received dose of MenACWY, and 53.1% of adolescent females and 44.3% of adolescent males were up-to-date with the HPV vaccine series. In 2019 90.2% of adolescents received 1 dose of Tdap, 88.9% received 1 dose of MenACWY, and 56.8% of adolescent females and 51.8% of adolescent males were up-to-date with the HPV vaccine series.
The CDC, AAP, American Academy of Family Physicians (AAFP), American College of Obstetricians and Gynecologists (ACOG), and American College of Nurse Midwives (ACNM) annually publish harmonized childhood and adolescent immunization schedules. The ACIP, AAFP, ACOG, ACNM, and American College of Physicians also publish an annual adult immunization schedule ( www.cdc.gov/vaccines ). The ACIP, with input from many liaison organizations, periodically reviews the schedules to ensure consistency with new vaccine developments and policies. The first harmonized childhood immunization schedule was published in 1995 and recommended 6 vaccines containing antigens against 9 infectious diseases. Since November 2010, vaccines have been recommended universally in the childhood and adolescent immunization schedule to protect against 16 infectious diseases ( Figs. 6.2 to 6.4 ). The harmonized schedule specifies the recommended and acceptable timing for each dose of universally recommended vaccine and for vaccines recommended for children and adolescents in selected high-risk populations.
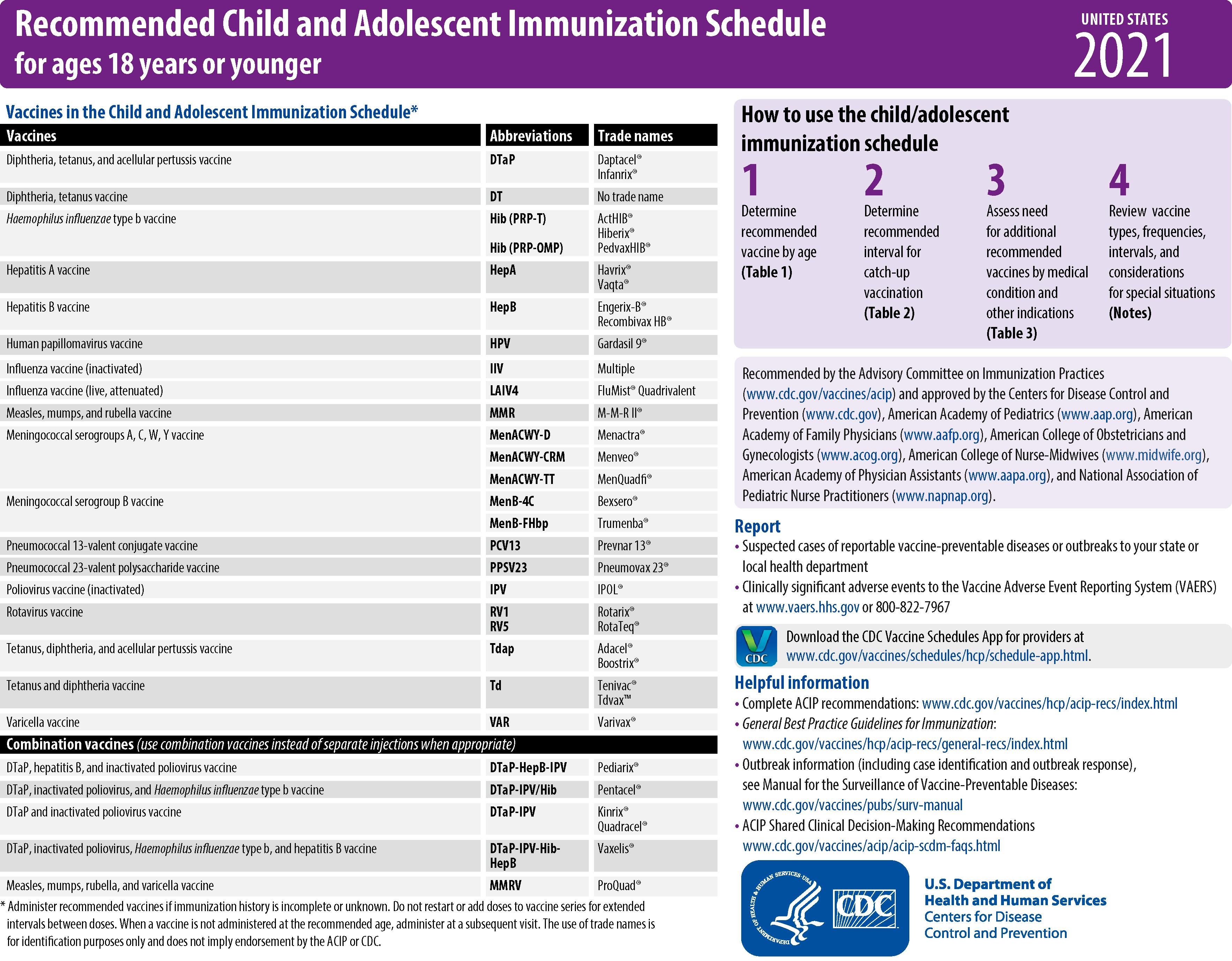
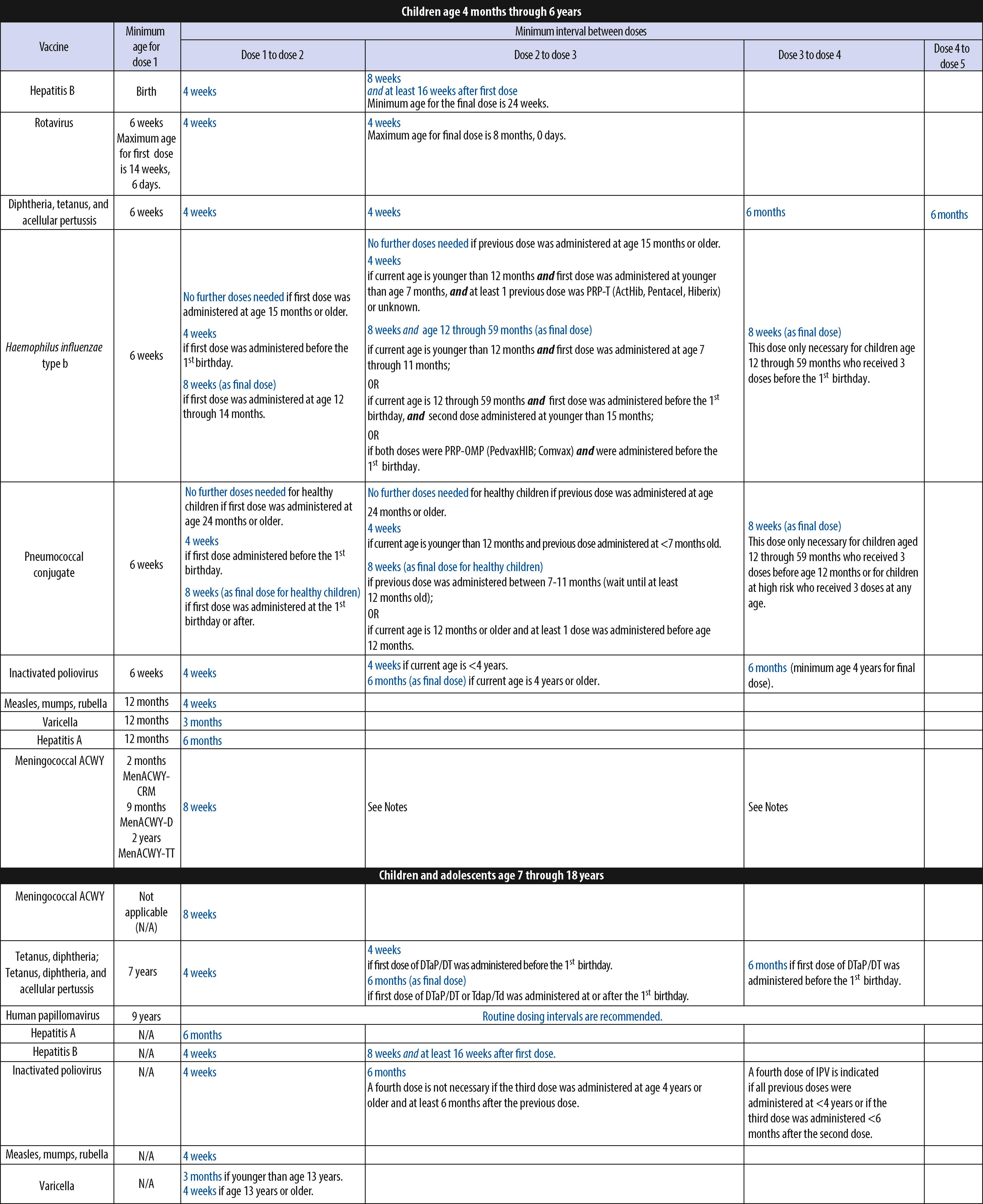
Since its inception, the US immunization program has focused on immunization of infants and young children. In 1996, following growing concern about morbidity associated with vaccine-preventable diseases in the difficult-to-reach adolescent population, the ACIP recommended expanding efforts to immunize adolescents by establishing a routine immunization visit at 11 through 12 years of age. In addition to providing Td and previously missed vaccinations, the report emphasized that this visit should be used to provide other important preventive health services. A decade ago the addition of several newer vaccines for adolescents (i.e., MenACWY, Tdap, and HPV) stimulated a reappraisal of approaches that continues to effectively and efficiently increase the proportion of adolescents who receive newly recommended vaccines and develop ways to integrate these approaches into other adolescent health, education, and development programs.
Minimal spacing of vaccine doses generally is 1 month for the initial doses of killed vaccines; longer intervals are needed for booster doses to provide optimal responses. For children with delayed initiation of immunization (after 6 months of age), an accelerated schedule is recommended ( Fig. 6.3 ). , To optimize adherence to the schedule in this circumstance, visits should be scheduled at 1-month intervals, and all recommended vaccines should be given at each visit. No need exists to restart any routine vaccine series in people with long delays between doses. The minimal spacing between nonsimultaneously administered live virus vaccines (e.g., MMR, varicella, and live, attenuated influenza) or repeated dose of the same live virus vaccine is 28 days.
All childhood vaccines can be administered simultaneously. This practice is based on extrapolation of data from multiple studies showing that most vaccines can be administered at the same time without compromising safety or immunogenicity. Thus DTaP, Hib, IPV, HBV, PCV, MMR, varicella, and rotavirus vaccines can be administered simultaneously, and for inactivated vaccines within any interval of one another when otherwise indicated. , Lower geometric mean concentrations (GMCs) of IgG antibodies to some pneumococcal serotypes were observed when 7-valent PCV (PCV7) was administered with Menactra (MenACWY-D), compared with corresponding IgG GMCs when PCV7 was administered alone. While there are no data on MenACWY-D and PCV13 co-administration, to avoid possible interference, children with functional or anatomic asplenia or HIV infection should not be immunized with MenACWY-D (Menactra) before 2 years of age to avoid interference with the immune response to the PCV series. , In patients with functional or anatomic asplenia or HIV infection, if PCV is delayed beyond 2 years of age and both PCV and Menactra are indicated, the PCV vaccination should be completed and then four weeks later Menactra should be administered. Data suggest possible immunologic interference in the meningococcal human complement serum bactericidal assay (hSBA) responses, resulting in decreased titers when MenACWY-D is administered 30 days after Daptacel (DTaP, Sanofi Pasteur). In contrast, coadministration of MenACWY-D and Daptacel was not associated with reduced hSBA responses to all four meningococcal serogroups. Available data do not allow evaluation of potential interference from other DTaP-containing vaccines on meningococcal seroresponse rates. If MenACWY-D is to be administered to a child at increased risk for meningococcal disease, the CDC recommends MenACWY-D be given either before or concomitantly with DTaP. Interference between live virus vaccines other than oral poliovirus vaccine (OPV) and rotavirus (e.g., MMR and varicella) theoretically can occur if they are given within a short interval; live virus vaccines should be given either simultaneously or at least 28 days apart. Vaccines should not be mixed in the same syringe unless specifically licensed for such use.
Immunoglobulins or blood products containing immunoglobulins inhibit response to certain live-virus vaccines (MMR and possibly varicella). The duration of inhibition of response is related to the dose of immunoglobulin delivered. Algorithms for calculating appropriate delays of MMR after receipt of such products are available. , , In general, MMR vaccines should be delayed 3 months or longer after administration of recommended doses of immunoglobulin (e.g., to prevent hepatitis A) or blood products and for longer periods after higher doses (e.g., 11 months after 2 g/kg of immune globulin intravenous administered for treatment of Kawasaki disease).
Available data support interchangeability of most vaccines produced by different manufacturers to prevent the same disease. The response to a 3-dose series using different Hib conjugate vaccines equals or exceeds response when the same vaccine is used for all doses. The ACIP and AAP recommend that, when feasible, the same vaccine should be used for the primary series but that 3 doses of any vaccine are sufficient. , Data are limited regarding safety, immunogenicity, and efficacy of using acellular pertussis (as DTaP) vaccines from different manufacturers for successive doses of the pertussis series. Data suggest that 2 of the current DTaP preparations can be used interchangeably for the first 3 doses of the DTaP series without affecting safety or immunogenicity. When the specific product is not known or not available, any DTaP vaccine should be used to continue or complete the series. Similarly, the series of hepatitis A vaccine does not have to include the same brand of vaccine for both doses. However, the two MenB vaccine products are not interchangeable. The same MenB vaccine product must be used for all doses on the MenB series.
In 1986, the National Childhood Vaccine Injury Act was enacted to create a compensation program for families affected by childhood vaccine-associated adverse events and help ensure safety of the vaccine supply ( Table 6.2 ).
| National Vaccine Injury Compensation Program |
|
| Vaccine Information Statement (VIS) |
|
| National Vaccine Program Office |
|
| Advisory Commission on Childhood Vaccines |
|
| National Vaccine Advisory Committee |
|
| Federal excise tax on childhood vaccines |
|
| Vaccine Adverse Events Reporting System |
|
| Committee from the Institute of Medicine (IOM) to review literature on vaccine reactions |
|
As many vaccine-preventable diseases approach or reach elimination in the US, continuing to balance the risks and benefits of each vaccine becomes increasingly important. For example, OPV was recommended formerly for routine use in the US but was associated with vaccine-associated paralytic poliomyelitis (VAPP) in 1 case per 2.5 million vaccine doses distributed. This rare adverse event was no longer considered acceptable following elimination of poliomyelitis in the US, and in 2000, the ACIP recommended using IPV for all doses of poliovirus vaccine. Public perceptions of vaccine safety are a challenge to the continued success of the vaccination program. New parents and younger physicians grew up without appreciating the morbidity and mortality of several vaccine-preventable diseases. Therefore, risk or perception of risk for adverse events becomes an important concern.
In the early 1990s, the Institute of Medicine (IOM) (renamed the National Academy of Medicine in 2015) reviewed available information regarding the possible causality of serious adverse events after administration of each of the then-licensed childhood vaccines. These reviews were updated in 2011. For many events, information was considered insufficient to determine causality. For some events, however, the investigating panels classified events more definitively, as follows: (1) evidence establishes definitively that vaccine plays a causal role; (2) evidence supports a causal role for the vaccine; and (3) evidence favors rejection of causation. These events are summarized in Table 6.3 . These investigations represented a comprehensive compilation of data on vaccine safety, although controversy persists regarding certain events. Reanalysis of available data on the occurrence of type 1 diabetes mellitus after MMR or DTaP vaccination suggests that evidence favors rejection of a causal relationship.
| Vaccine | Established Causation/Convincingly Supports | Favors Causation/Acceptance | Favors Rejection of Causation |
|---|---|---|---|
| DT/Td/TT | Anaphylaxis | GBS a Brachial neuritis |
Encephalopathy Type 1 diabetes Infantile spasms Death from SIDS |
| DTaP, Tdap (acellular pertussis) | — | — | Type 1 diabetes |
| DTP (whole cell pertussis) | Anaphylaxis; protracted, inconsolable crying | Acute encephalopathy Shock and unusual shock-like state (hypotonic-hyporesponsive episode) Chronic encephalopathy (after acute encephalopathy) |
Infantile spasms Hypoarrhythmia Reye syndrome SIDS |
| Influenza | Anaphylaxis AHRQ: GBS (after 2009 monovalent H1N1 vaccine only) |
Oculorespiratory syndrome Febrile seizures |
(Inactivated) Bell palsy Asthma exacerbation or reactive airway episodes in children and adults (Inactivated); cardiovascular and cerebrovascular events in older adults; serious adverse events in renal disease |
| IPV | — | — | — |
| Hepatitis B | Anaphylaxis | — | — |
| Hib (conjugate) | — | — | Early-onset Haemophilus influenzae b disease |
| HPV | Anaphylaxis | — | Type 1 diabetes, juvenile rheumatoid arthritis, GBS, appendicitis, seizures, stroke, syncope, venous thromboembolism |
| Measles | Death from measles vaccine strain in primarily immunocompromised persons Measles inclusion body encephalitis in persons with immunodeficiencies |
Anaphylaxis | — |
| Meningococcus | Anaphylaxis | — | — |
| MMR | Anaphylaxis Thrombocytopenia Febrile seizures |
— | Autistic spectrum disorders Type 1 diabetes |
| Mumps (see MMR) | — | — | — |
| OPV | Poliomyelitis Death from polio vaccine strain, mainly in immunocompromised people |
GBS a | — |
| Pneumococcal (conjugate) | — | Febrile seizures | — |
| Rotavirus | — | Intussusception | — |
| Rubella b (see MMR) | Anaphylaxis | Transient arthralgia in women Transient arthralgia in children |
— |
| Varicella | Disseminated Oka VZV with (only in persons with immunodeficiencies) and without other organ involvement (e.g., pneumonia, meningitis, hepatitis) Vaccine strain viral reactivation without and with subsequent infection (meningitis, encephalitis) Anaphylaxis |
— | — |
a The Advisory Committee on Immunization Practice of the United States Public Health Service disagreed with these IOM findings (reference 39).
b Data were reviewed by an earlier IOM committee. Initial report categories corresponding to those table headings were “Evidence indicates a causal relationship, Evidence is consistent with a causal relationship,” and “Evidence does not indicate a causal relationship.”
As a result of continued concerns about vaccine safety, in 2000 the CDC and the National Institutes of Health commissioned the IOM to convene an Immunization Safety Review Committee. From 2001 through 2011, this independent expert committee published nine reports related to various immunization safety concerns. The committee has made recommendations in the areas of public health response, policy review, research, and communications for each of the 9 subjects reviewed in the nine reports ( Box 6.1 ). The IOM concluded that the body of epidemiologic evidence favors rejection of a causal relationship between MMR vaccine and autism and concluded that no relationship exists between thimerosal-containing vaccines and autism. None of the 9 IOM reports recommended a policy review of the current vaccine recommendations or change in the immunization schedule. The most recent review by the IOM was charged to (1) review scientific findings and stakeholder concerns related to the safety of the recommended childhood immunization schedule and (2) identify potential research approaches, methodologies, and study designs that could inform this question, considering strengths, weaknesses, as well as the ethical and financial feasibility of each approach. The report concluded that the committee’s efforts to identify priorities for recommended research studies did not reveal evidence suggesting that the childhood immunization schedule is linked to autoimmune diseases, asthma, hypersensitivity, seizures or epilepsy, child developmental disorders, learning disorders or developmental disorders, or attention deficit or disruptive behavior disorders. While the committee found that there is no scientific evidence to justify the majority of safety concerns, perceptions dictate parental support and actions. Therefore, further study of the full immunization schedule as well as further study to understand stakeholder perceptions and how they are formed can help improve awareness and education efforts.
Measles-Mumps-Rubella Vaccine and Autism (April 2001)
Thimerosal-Containing Vaccines and Neurodevelopmental Disorders (October 2001)
Multiple Immunizations and Immune Dysfunction (February 2002)
Hepatitis B Vaccine and Demyelinating Neurological Disorders (May 2002)
SV40 Contamination of Polio Vaccine and Cancer (October 2002)
Vaccinations and Sudden Unexpected Death in Infancy (March 2003)
Influenza Vaccines and Neurological Complications (October 2003)
Vaccines and Autism (May 2004)
Adverse Effects of Vaccines: Evidence and Causality (August 2011)
Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies (January 2013)
In addition, the Agency for Healthcare Research and Quality commissioned an assessment of vaccine safety that evaluated evidence on the safety of vaccines recommended for routine use among adults, children, and pregnant women as of 2011. This report included an extensive literature search for clinical trials and observational studies with strong study designs and analysis method including: cohort studies comparing vaccinated and unvaccinated groups, case-control studies, self-controlled case series, and designs using multivariate risk factor analyses. The results support most findings of the 2011 IOM report for vaccines routinely recommended for children, with some additional findings:
“There is insufficient evidence to determine any potential association between trivalent inactivated (influenza) vaccine and asthma exacerbation, acute disseminated encephalomyelitis, and transverse myelitis.”
Febrile seizures have been reported after DTaP, MMR, measles, mumps, rubella, and varicella (MMRV), inactivated influenza vaccine (IIV), and PCVs. , An analysis suggests that for DTaP, influenza, and PCV vaccines, only PCV vaccine is likely to cause febrile seizures when given alone or when given concomitantly with influenza or DTaP vaccines. MMRV poses a higher risk of febrile seizures compared with concomitant MMR and varicella vaccines.
Large US post-licensure studies found a small increased risk of intussusception following vaccination with both Rotarix (GlaxoSmithKline, Brentford, London, UK) and RotaTeq (Merck, Kenilworth, NJ); patient-related risk factors were not reported. ,
Strong evidence for a lack of association of HPV vaccines with several serious medical conditions (juvenile rheumatoid arthritis, type 1 diabetes, Guillain-Barré syndrome [GBS]) has been found in large post-licensure studies.
For HPV, insufficient evidence has been found regarding other serious conditions such as multiple sclerosis, chronic inflammatory demyelinating polyneuropathy, amyotrophic lateral sclerosis, and pancreatitis.
Insufficient evidence has been found to determine the possible association, if any, between vaccines such as DTaP, meningococcal vaccine, and varicella vaccine and the onset of nervous system conditions such as acute disseminated encephalomyelitis, transverse myelitis, multiple sclerosis, and GBS. Because these medical conditions are extremely rare, it may not be possible to reach a level of evidence beyond insufficient.”
A robust infrastructure consisting of several systems has been established to monitor vaccine safety following licensure. The Vaccine Adverse Event Reporting System (VAERS), operated jointly by the CDC and the FDA, is a national passive surveillance system used to detect early warning signals and generate hypotheses about possible new vaccine adverse events or changes in frequency of recognized events. Intussusception associated with receipt of rhesus rotavirus vaccine and leading to its withdrawal from the market in 1999 was detected by the VAERS. , Physicians and vaccine manufacturers should report all suspected adverse reactions after vaccination to the VAERS and are required to report certain events. , Family members, patients, and others also can report adverse events to the VAERS. VAERS reports can be submitted electronically through a secure website at http://vaers.hhs.gov . A second CDC system is the Vaccine Safety Datalink (VSD), which consists of large, linked databases from integrated healthcare systems. , , Associations between medically attended health events and immunizations can be evaluated for causality through the VSD. This usually is done by comparing the incidence rate of a given adverse event following administration of a given vaccine in vaccinees versus non-vaccinees or the incidence rate in a given interval after vaccination, the risk interval, with incidence at other intervals. Higher incidence rates in vaccinees versus non-vaccinees or in the risk interval versus other intervals would be compatible with vaccine causing the adverse event. Conversely, if rates are similar, the vaccine is unlikely to be playing a causal role. A third system is the Clinical Immunization Safety Assessment (CISA) Centers network, which consists of selected clinical academic medical centers that work in partnership with the CDC to study the pathophysiology of vaccine reactions and develop clinical management protocols for affected patients. In addition, the FDA uses a sentinel surveillance program to study adverse events reported after vaccination, drug use, and use of medical devices. These systems are crucial to the vitality and strength of the US immunization program.
In addition to mandating the review of causality of adverse events and creating a unified reporting system for adverse events after vaccination, the National Childhood Vaccine Injury Act established a program to provide compensation to certain people who experience permanent injury after vaccination. A table of injuries eliciting automatic compensation was developed and has been revised on the basis of the findings of the IOM studies; in addition, any person who shows medical evidence of causality for non-table injuries also may be compensated. This program is funded by a special excise tax on each dose of vaccine ($0.75 per disease prevented) to which the program applies (i.e., diphtheria, tetanus, pertussis, Hib infection, poliomyelitis, measles, mumps, rubella, hepatitis A, hepatitis B, IPD, varicella, rotavirus infection, meningococcal disease, HPV infection, and influenza).
Studies show that infants with birth weights of <2000 g may have a diminished response after administration of HBV vaccine at birth. However, by 1 month chronologic age, all preterm infants, regardless of gestational age or weight at birth, are as likely to respond as older and larger infants. , Preterm infants born to hepatitis B surface antigen (HBsAg)–positive mothers and mothers with unknown HBsAg status should receive immunoprophylaxis with HBV vaccine and hepatitis B immunoglobulin (HBIG) within 12 hours of birth. If these infants weigh <2000 g at birth, the initial vaccine dose should not be counted toward completion of the hepatitis B vaccine series, and 3 additional doses of vaccine should be administered, beginning when the infant is 1 month of age. Preterm infants weighing <2000 g and born to HBsAg-negative mothers should receive the first dose of the HBV vaccine series at 1 month of postnatal age (if medically stable) or at hospital discharge if the infant is <1 month of age at discharge.
The risk of an adverse reaction from vaccination during pregnancy is largely theoretical. The benefit of vaccinating a pregnant woman outweighs the potential risk when the risk for disease exposure is high. Infection may harm the mother or infant, whereas the vaccine is unlikely to cause harm. Tdap and influenza vaccines are indicated for all pregnant women. One randomized controlled trial conducted in Bangladesh that provided IIV to pregnant women during the third trimester demonstrated that infants born to vaccinated women had a 63% reduction in laboratory-confirmed influenza illness during the first 6 months of life, compared with pregnant women receiving 23-valent pneumococcal polysaccharide vaccine. Maternal influenza vaccination during pregnancy was associated with significantly reduced risk for influenza virus infection (relative risk, 0.59; 95% confidence interval [CI], 0.37–0.93) and hospitalization for influenza-like illness (relative risk, 0.61; 95% CI, 0.45–0.84) among infants <6 months of age in a nonrandomized prospective cohort study. Women’s healthcare providers should provide a maternal Tdap vaccination to every pregnant woman during each pregnancy. Studies have demonstrated no statistically significant differences in rates of medically attended acute adverse events or adverse birth outcomes in pregnant women related to timing since previous administration of tetanus—toxoid containing vaccines. , To maximize the maternal antibody response and passive antibody transfer to the infant, optimal timing for Tdap administration is early during the period of gestational weeks 27–36, regardless of time since prior Td or Tdap vaccination. For women not previously vaccinated with Tdap, if Tdap is not administered during pregnancy, Tdap should be administered immediately postpartum. Hepatitis B, hepatitis A, meningococcal, and pneumococcal vaccines can be given to pregnant women at high risk for these diseases.
The greatest concerns have been raised about live vaccines. MMR vaccine is contraindicated in pregnant women on theoretical grounds; however, because no case of CRS has been reported after MMR vaccination among susceptible women exposed to rubella virus through MMR vaccine, inadvertent vaccination is not a reason to interrupt pregnancy. Varicella-containing vaccines also are contraindicated in pregnant women because of the theoretical risk that they can cause birth defects. The CDC and FDA will continue to monitor adverse events after vaccination with varicella-zoster virus–containing vaccines through VAERS. New cases of exposure immediately before or during pregnancy or other adverse events after vaccination with varicella-containing vaccines should be reported to Merck (telephone: 877-888-4231) and to VAERS ( https://vaers.hhs.gov/index ). Pregnancy in a household member is not a reason to postpone vaccination of other family members. In fact, vaccination of family members can be the best way to protect a pregnant mother from being exposed to natural infection. Breastfeeding does not adversely affect the responses to live or killed vaccines; breastfed infants should be vaccinated according to the recommended childhood and adolescent immunization schedule.
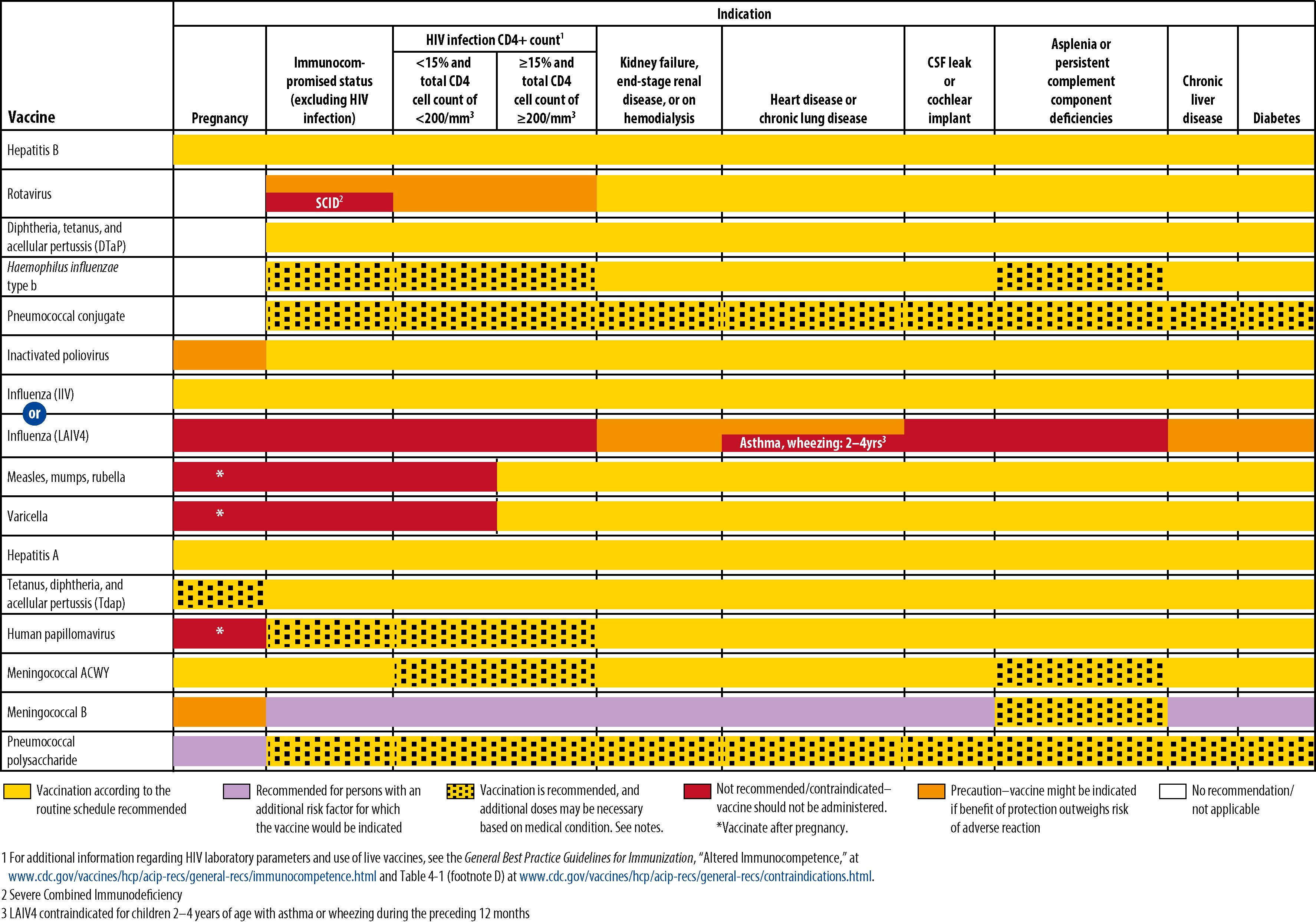
People with altered immunocompetence require special considerations for vaccination because they can be at increased risk for serious adverse consequences of disease or vaccination or poor response to vaccination. , , , , The safety and efficacy of vaccines in people with immunodeficiencies are determined by the nature and degree of immunosuppression. Immunodeficiency conditions can be grouped into primary and secondary (acquired) disorders. Primary disorders of the immune system generally are inherited as single-gene disorders, can involve any part of the immune system, and share the common feature of susceptibility to infection with various organisms, depending on the specific deficiency. Categories of immunocompromised people with acquired immunodeficiency disorders include people with the following conditions: human immunodeficiency virus (HIV) infection; hematopoietic or solid-organ transplants; malignant diseases; immunosuppression resulting from administration of chemotherapy, systemic corticosteroids, radiation, monoclonal antibodies, or other drugs with significant adverse effects; and other chronic conditions, including splenectomy. People in these categories can be vaccinated safely with killed vaccines, which usually are recommended in the same doses and on the same schedules as for immunocompetent people. Response to both killed and live vaccines can be suboptimal, and higher doses or additional doses may be needed to ensure protection. Live vaccines generally are not recommended for any of these groups because of known or theoretical risks of disseminated infection resulting from the vaccine. Exceptions are MMR and varicella vaccines, which are either recommended or can be considered for susceptible people with HIV infection with CD4 + T-lymphocyte counts ≥15% expected for age and no or mild symptoms of disease. , , , Table 6.4 shows recommendations for immunization of children and adolescents with primary and secondary immunodeficiencies.
| Type of Immunodeficiency | Specific Immunodeficiency | Contraindicated Vaccines a | Risk-Specific Recommended Vaccines a | Effectiveness and Comments |
|---|---|---|---|---|
| Primary | ||||
| B-lymphocyte (humoral) | Severe antibody deficiencies (e.g., X-linked agammaglobulinemia) | OPV b Smallpox LAIV BCG Ty21a (live typhoid) Yellow fever Measles Mumps Rubella Varicella |
Pneumococcal | The effectiveness of any vaccine is uncertain if it depends only on the humoral response (e.g., PPSV) IGIV interferes with the immune response to measles vaccine and possibly varicella vaccine |
| Less severe antibody deficiencies (e.g., selective IgA deficiency and IgG subclass deficiency) | OPV b BCG Yellow fever Other live vaccines appear to be safe |
Pneumococcal Hib (if unimmunized) c |
All vaccines likely effective; immune response may be attenuated | |
| T-lymphocyte (cell-mediated and humoral) | Complete defects (e.g., SCID disease, complete DiGeorge syndrome) | All live vaccines d , e , f | Pneumococcal | Vaccines may be ineffective |
| Partial defects (e.g., most patients with DiGeorge syndrome, Wiskott-Aldrich syndrome, ataxia-telangiectasia) | All live vaccines d , e , f , g | Pneumococcal Hib (if not administered in infancy) |
Effectiveness of any vaccine depends on degree of immunosuppression | |
| Complement | Persistent complement, properdin, or factor B deficiency | None | Pneumococcal Meningococcal conjugate ACWY, and B vaccines Hib (if unimmunized) c |
All routine vaccines likely effective |
| Phagocytic function | Chronic granulomatous disease, leukocyte adhesion defect, and myeloperoxidase deficiency | Live bacterial vaccines d | Pneumococcal h | All inactivated vaccines safe and likely effective Live viral vaccines likely safe and effective |
| Secondary | HIV/AIDS | OPV b Smallpox BCG LAIV Withhold MMR and varicella in severely immunocompromised persons Yellow fever vaccine may have a contraindication or a precaution depending on clinical parameters of immune function k |
Pneumococcal Hib (if unimmunized c ) Meningococcal conjugate ACWY |
MMR, varicella, rotavirus, and all inactivated vaccines, including IIV, may be effective i |
| Malignant neoplasm, transplantation, immunosuppressive or radiation therapy | Live viral and bacterial, depending on immune status d , e | Pneumococcal Hib |
Effectiveness of any vaccine depends on degree of immunosuppression | |
| Asplenia | LAIV | Pneumococcal Meningococcal conjugate ACWY and B vaccines Hib (if unimmunized c ) |
All routine vaccines likely effective | |
| Chronic renal disease | none | Pneumococcal Hepatitis B j |
All routine vaccines likely effective | |
| After allogeneic or autologous hematopoietic stem cell transplantation | LAIV Rotavirus |
IIV Pneumococcal Hib |
All other routinely recommended inactivated vaccines should be administered (see IDSA Clinical Practice Guideline for detailed scheduling ) | |
a Other vaccines that are universally or routinely recommended should be given if not contraindicated.
b OPV is no longer available in the US.
c Children who have not received a primary series and booster dose or at least 1 dose of Hib vaccine after 14 months of age are considered unimmunized.
d Live bacterial vaccines: BCG and oral Ty21a Salmonella Typhi vaccine.
e Live viral vaccines: MMR, MMRV, OPV, LAIV, yellow fever, zoster, rotavirus, varicella, and vaccinia (smallpox). Smallpox vaccine is not recommended for children or the general public.
f Regarding T-lymphocyte immunodeficiency as a contraindication for rotavirus vaccine, data exist only for SCID.
g Children with partial DiGeorge syndrome should undergo immune system assessment of lymphocyte subsets and mitogen responsiveness to determine whether they should be given live viral vaccines. Those with ≥500 CD3 T lymphocytes/mm 3 , ≥200 CD8 T lymphocytes/mm 3 , and normal mitogen response should receive MMR and varicella vaccines.
h Pneumococcal vaccine is not indicated for children with chronic granulomatous disease beyond age-based universal recommendations for PCV. Children with chronic granulomatous disease are not at increased risk for pneumococcal disease.
i HIV-infected children should receive IG after exposure to measles and can receive varicella and measles vaccine if CD4 + T-lymphocyte count is ≥15%.
j Indicated based on the risk from dialysis-based bloodborne transmission.
k Symptomatic HIV infection or CD4 + T-lymphocyte count of <200/mm 3 or <15% of total lymphocytes for children <6 years of age is a contraindication to yellow fever vaccine administration. Asymptomatic HIV infection with CD4 + T-lymphocyte count of 200–499/mm 3 for persons ≥6 years of age or 15%–24% of total lymphocytes for children <6 years old is a precaution for yellow fever vaccine administration. Details of yellow fever vaccine recommendations are available from the Centers for Disease Control and Prevention.
International travelers frequently have increased risk of exposure to vaccine-preventable diseases, even in economically developed countries. Parents and physicians of children and adolescents planning international travel should ensure that all routine childhood and adolescent immunizations are up to date and that adults are up to date on their immunizations. Infants ≥6 months of age should be administered MMR vaccine. The need for other destination-specific vaccines should be determined through consultation with specific guidelines for travelers. Additional information for international travelers can be found on the CDC website at wwwnc.cdc.gov/travel/page/yellowbook-home or the World Health Organization (WHO) website at www.who.int/ith/en .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here