Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An abdominal mass or abdominal fullness in a child usually becomes apparent when it enlarges enough to be visualized during bathing or palpable on physical examination. Masses may arise from intraperitoneal, retroperitoneal, or abdominal wall locations and emanate from both solid and hollow viscera ( Figs. 20.1 and 20.2 ). An abdominal mass may prove life threatening (e.g., malignant neoplasm, splenic sequestration crisis in sickle cell disease), arise from congenital malformation or disorganized development (e.g., mesenteric cyst, enteric duplication), or be benign or correctable nonoperatively (e.g., fecaloma, splenomegaly associated with infectious mononucleosis). Hepatomegaly and splenomegaly often indicate systemic illnesses such as infection, hemolysis, storage disease, or malignancy (see Chapter 17 ). A child with an abdominal mass requires a prompt and thorough work-up with testing guided by history, physical examination findings, age, and gender. Early surgical referral may assist in this work-up following a directed screening approach.
A child’s age, gender, and thorough clinical history help to identify the most likely disease category ( Tables 20.1 and 20.2 ). The duration and character of associated symptoms are important for narrowing the differential diagnosis (e.g., fatigue, fever, appetite changes, vomiting, stooling history, weight loss, night sweating, character and frequency of pain, hematuria, flushing, palpitations, lower extremity swelling, lymph nodal prominence, and jaundice). A history of abdominal trauma should be elicited, as solid organ injuries may result in hematoma, seroma, persistent pseudocyst, or arteriovenous malformation. Infectious disease may have sequelae of cyst, lymphadenopathy, or intraabdominal abscess. Some systemic diseases (e.g., glycogen storage, hereditary spherocytosis), genetic syndromes (e.g., Beckwith-Wiedemann, Peutz-Jeghers, von Hippel-Lindau, familial adenomatous polyposis, hereditary pheochromocytoma, PTEN hamartoma tumor), and anomalies (aniridia with Wilms tumor, isolated hemihypertrophy with neuroblastoma and Wilms tumor, Hirschsprung with neuroblastoma) are associated with intraabdominal tumors. A family history is pertinent as is a menstrual and sexual history, particularly in adolescent females. Prenatal imaging frequently identifies congenital malformations and neoplasms, requiring postnatal imaging and surgical assessment.
Clinical History
Physical Examination
Abdominal Palpation
Ultrasonography
Depending on the Clinical Suspicion, Evaluation Can Be Continued with One or More of the Following:
|
| Age | Benign | Malignant |
|---|---|---|
| Neonate (0–1 mo) | Congenital hydronephrosis Cystic or dysplastic kidney disease Intestinal duplication Mesenteric/omental cyst Neurogenic bladder Ovarian cyst Renal vein thrombosis Choledochal cyst Mesoblastic nephroma Meconium ileus Hematoma (adrenal, hepatic, splenic) |
Neuroblastoma |
| Infant (0–1 yr) | Intestinal duplication Mesenteric/omental cyst Ovarian cyst Hydronephrosis Mesoblastic nephroma Cystic-dysplastic kidney Liver hamartomas Hepatic cavernous hemangioma Liver hemangioendothelioma Teratoma Lymphatic malformation Intussusception Hepatosplenomegaly Choledochal cyst Megacolon Urachal cyst |
Neuroblastoma Hepatoblastoma Wilms tumor (rare) Teratoma |
| Child | Mesenteric/omental cyst Choledochal cyst Appendiceal abscess Lymphatic malformation |
Neuroblastoma (2–10 yr) Hepatoblastoma Wilms tumor Leukemia Lymphoma |
| Adolescent | Bezoar Hematocolpos Hydrometrocolpos Pregnancy Inflammatory bowel disease Retroperitoneal hematoma (hemophilia) Castleman disease Mesenteric fibromatosis (dermoid) |
Neuroblastoma (11–16 yr) Adrenal cortical carcinoma Hepatocellular carcinoma Ovarian or testicular neoplasm Lymphoma Rhabdomyosarcoma Inflammatory myofibroblastic tumor Desmoplastic small round cell tumor |
A complete physical exam should be performed in children with abdominal masses. Attention should be paid to the general condition (fever, pallor, petechiae, cachexia) of the child and to signs of metastatic disease. Enlarged lymph nodes and their locations should be noted, the skin inspected, and the lungs and heart auscultated. Extremities should be evaluated for evidence of swelling, venous phlegmasia, or evidence of embolic disease to bone, muscle, or skin. Genitourinary exam should make note of any inappropriate virilization, testicular changes, and hymenal patency in the case of a female with a low pelvic mass. In addition, a neurologic examination may reveal signs of central or peripheral nervous system involvement. The eyes should be carefully inspected for periorbital ecchymosis, proptosis, squint, opsoclonus-myoclonus syndrome, heterochromia of the iris, Horner syndrome, and scleral icterus. The patient’s blood pressure must be determined and may be elevated in patients with Wilms tumor, neuroblastoma, or pheochromocytoma.
To successfully perform abdominal palpation in a child, the clinician must approach the patient calmly and gently, as the most reliable exams are completed in cooperative and relaxed children. Enlarged organs may be missed in a struggling child who does not lie quietly. When cooperation proves difficult in an infant, the examining hand should be placed and remain static on the abdomen and the exam completed between cries or as the child calms in the parent’s arms. Creative play is sometimes necessary, with the use of pacifiers or bottles to distract the child from the exam. Re-examination after voiding or defecating elucidates contributions of constipation and urinary retention in the child’s presentation.
The abdominal quadrants should be examined systematically ( Table 20.3 ; see Figs. 20.1 and 20.2 ). With the patient in the supine position, the symmetry of the abdomen should be inspected, and any visible masses or the presence of ascites should be noted. A very enlarged spleen is frequently visible, with fullness of the left side of the abdomen. The presence of tense fluid-filled hernias or prominent periumbilical veins as sequelae of portal hypertension should be noted. The mass should be localized, and its size, shape, texture, mobility, tenderness, and relation to midline noted. The umbilical position is a useful marker of abdominal asymmetry.
| Organ | Congenital | Benign | Malignant | Acquired |
|---|---|---|---|---|
| Liver and biliary tract | Hemangioma | Hemangioendothelioma | Hepatoblastoma | Abscess |
| Choledochal cyst | Hamartoma | Lymphoma | Hematoma | |
| Leukemia | Parasitic disease | |||
| Hepatocellular carcinoma | Hydrops of the gallbladder | |||
| Spleen | Cyst | Sarcoma | Splenomegaly (e.g., mononucleosis) | |
| Kidney | Hydronephrosis Cystic disease Duplication |
Wilms tumor | Hematoma | |
| Adrenal gland | Neuroblastoma | Pheochromocytoma | Neuroblastoma | Hematoma |
| Pheochromocytoma | ||||
| Stomach | Duplication | Leiomyoma | Leiomyosarcoma | Bezoar |
| Teratoma | Inflammatory pseudotumor | Adenocarcinoma | ||
| Intestines | Duplication | Lymphangioma | Carcinoma | Appendiceal abscess |
| Megacolon | Hemangioma | Lymphoma | Intussusception Obstipation |
|
| Mesentery | Mesenteric/omental cyst | Inflammatory bowel disease Parasitic disease Tuberculosis |
||
| Pancreas | Cyst | Carcinoma | Pseudocyst | |
| Uterus | Hydrometrocolpos | Myoma | Rhabdomyosarcoma | Pregnancy |
| Ovaries | Cyst | Cyst | Yolk sac tumor | Tuboovarian abscess |
| Teratoma | Cystic teratoma | Embryonal carcinoma | ||
| Cystic adenoma | Dysgerminoma | |||
| Granulosa cell tumor | Choriocarcinoma | |||
| Bladder | Urachal cyst Posterior urethral valve |
Inflammatory pseudotumor | Rhabdomyosarcoma | Urinary retention |
| Retroperitoneum | Presacral teratoma | Ganglioneuroma | Neuroblastoma | Psoas abscess |
| Anterior myelomeningocele | Aortic aneurysm | |||
| Abdominal wall | Hernia | Hemangioma | Rhabdomyosarcoma | Hematoma |
| Omphalocele | Rectus sheath hematoma | |||
| Gastroschisis | Abscess |
Signs of peritoneal inflammation must be sought (see Chapter 13 ). Dull visceral pain conducted by slow C nerve fibers may be reported for inflammatory processes in the vascular distributions of the celiac, superior mesenteric, and inferior mesenteric arteries and referred to the epigastrium, umbilical region, or hypogastrium, respectively. When the inflamed process contacts the peritoneum, peritoneal fast A nerve fibers allow discrete localization of sharp pain to the abdominal wall. Ultrasound is often a very useful adjunct in the evaluation of an abdominal mass and is often available at the bedside.
Approximately half of abdominal masses in older children are caused by enlargement of the liver or spleen, or both. The liver is normally palpated in the right upper quadrant and epigastrium extending 1–2 cm below the costal margin. The inferior hepatic margin may be palpated in a thin child, is usually nontender, and moves with respiration. Detection of liver edge by auscultation using skin scratches has been proven unreliable and has been supplanted by the use of readily available ultrasound. Hepatomegaly is discussed in detail in Chapter 17 . The spleen is located in the left upper quadrant and is nonpalpable in most healthy children. To locate an enlarged spleen, the examiner must begin palpation in the patient’s left iliac fossa (to avoid missing a grossly enlarged spleen or liver with extension of the left hepatic lobe into the splenic area in the left upper quadrant); the examiner’s right hand should move toward the patient’s left upper quadrant to find the spleen’s lower pole or medial border. The examiner’s left hand is placed in the patient’s left flank, and gentle displacement of the thoracic cage toward the examiner’s right hand often displaces the spleen forward enough to make it appreciable. The spleen has a rounded tip and should move downward with inspiration and is more superficial than a renal mass. It is equally important for the examiner to palpate the spleen as it is for the spleen to “touch” the examiner during its descent with inspiration. Overaggressive palpation may push the spleen away, whereas gentle or light palpation permits the examiner to feel the spleen’s edge passively. Because the extent to which the spleen extends below the costal margin depends heavily on the patient’s position, the extent of the spleen below the costal margin should be measured with the patient in the supine position. Measurement from the left costal margin to the lower pole of the spleen defines the splenic axis. Ordinarily, the long axis of the spleen is along the length of the 10th rib. As it enlarges, it extends medially and downward. Masses in the left upper quadrant, especially left renal masses, may be difficult to distinguish from an enlarged spleen. In general, a splenic notch, if present, helps identify the mass as a spleen, but nodular masses, such as Wilms tumors of the kidney, neuroblastomas, and retroperitoneal teratomas, may masquerade as splenomegaly. Many enlarged spleens are not palpable on physical examination because of their relationship to other organs and the thoracic cage. Hyperinflation of the lungs (as occurs in asthma, bronchiolitis, and ipsilateral pneumothorax) may make a normal-sized liver or spleen palpable.
Flank masses are the next most frequent, particularly in newborn to toddler-aged children. Renal masses extend caudally, are fixed with respiration, and cause abdominal asymmetry. Lower abdominal masses are most commonly caused by constipation or urinary retention. These may be functional or secondary to neurogenic dysfunction. A perforated appendix with resulting abscess formation may create a tender right lower quadrant mass. Ovarian or uterine tumors often grow undetected in the pelvis until large enough to exit the pelvis as a large palpable abdominal mass.
Screening laboratory data, including CBC with differential and cell morphology, measurements of serum electrolytes, urinalysis, urine pregnancy test when appropriate, and inflammatory markers, are broadly applicable. Liver function tests, serum amylase, tumor marker levels ( Table 20.4 ), and renal function tests are often important initial objective data points.
| Tumor | Tumor Markers |
|---|---|
| Neuroblastoma | Urinary catecholamines LDH Ferritin Neuron-specific enolase |
| Wilms tumor | Erythropoietin |
| Hepatoblastoma, pancreatoblastoma, yolk sac tumors | α-Fetoprotein (AFP, AFP-L3), PIVKA |
| Germ cell tumors | β-hCG, α-fetoprotein, LDH |
| Pheochromocytoma, paraganglioma | Plasma metanephrine, normetanephrine |
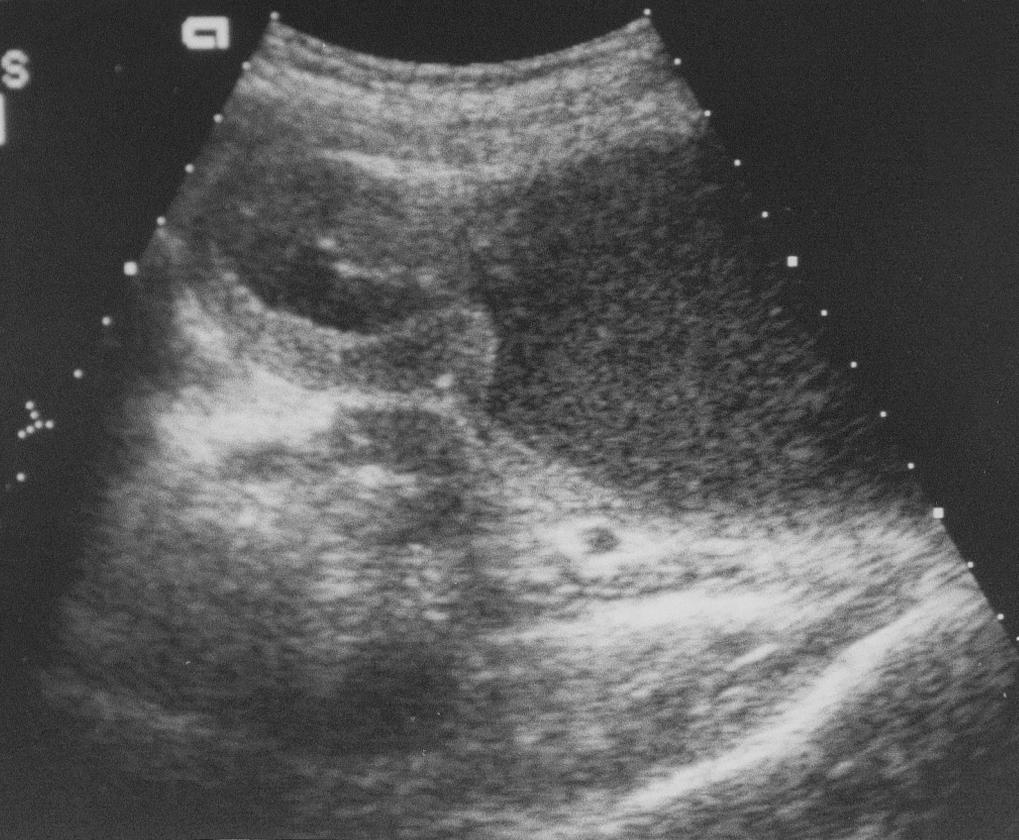
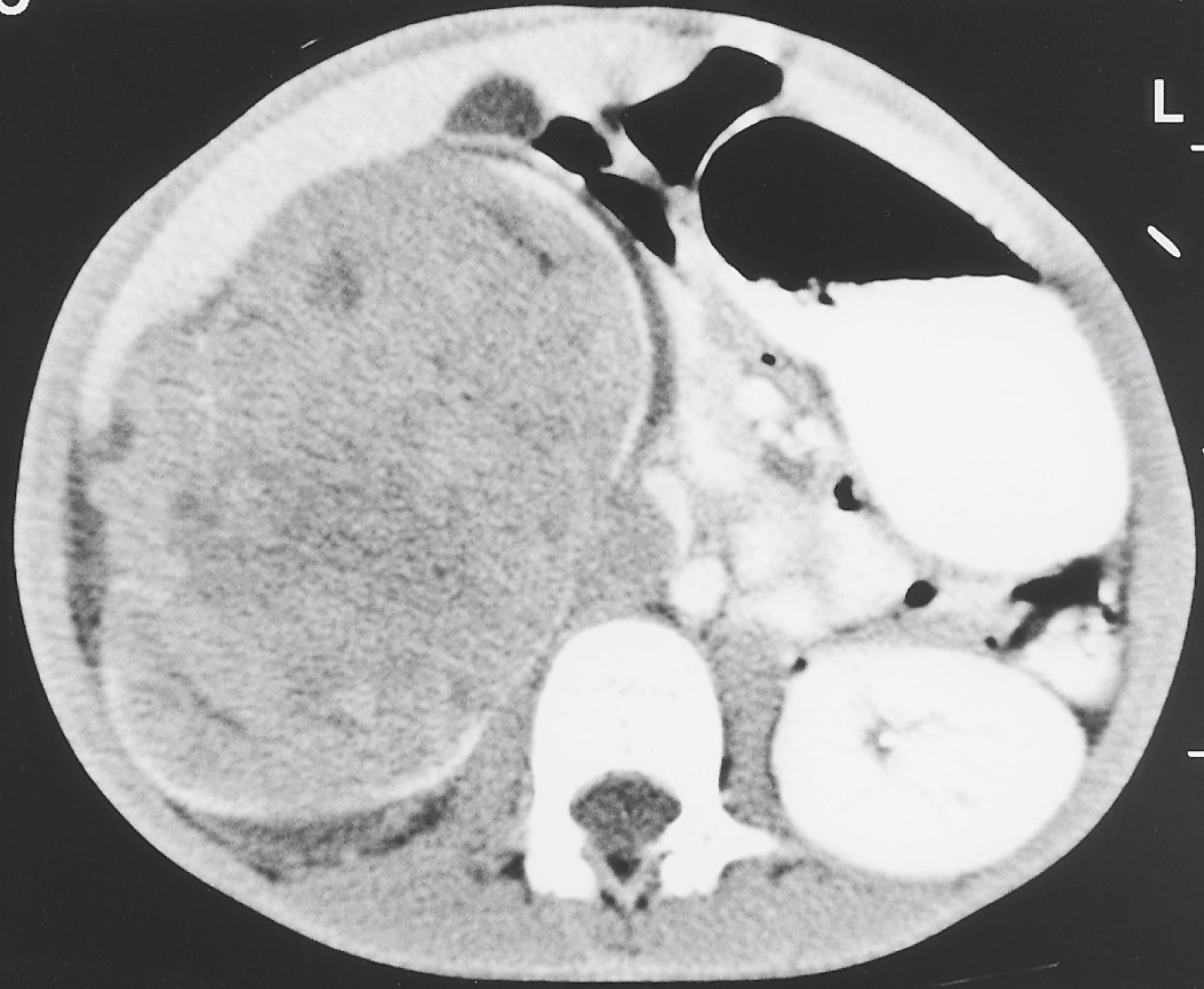
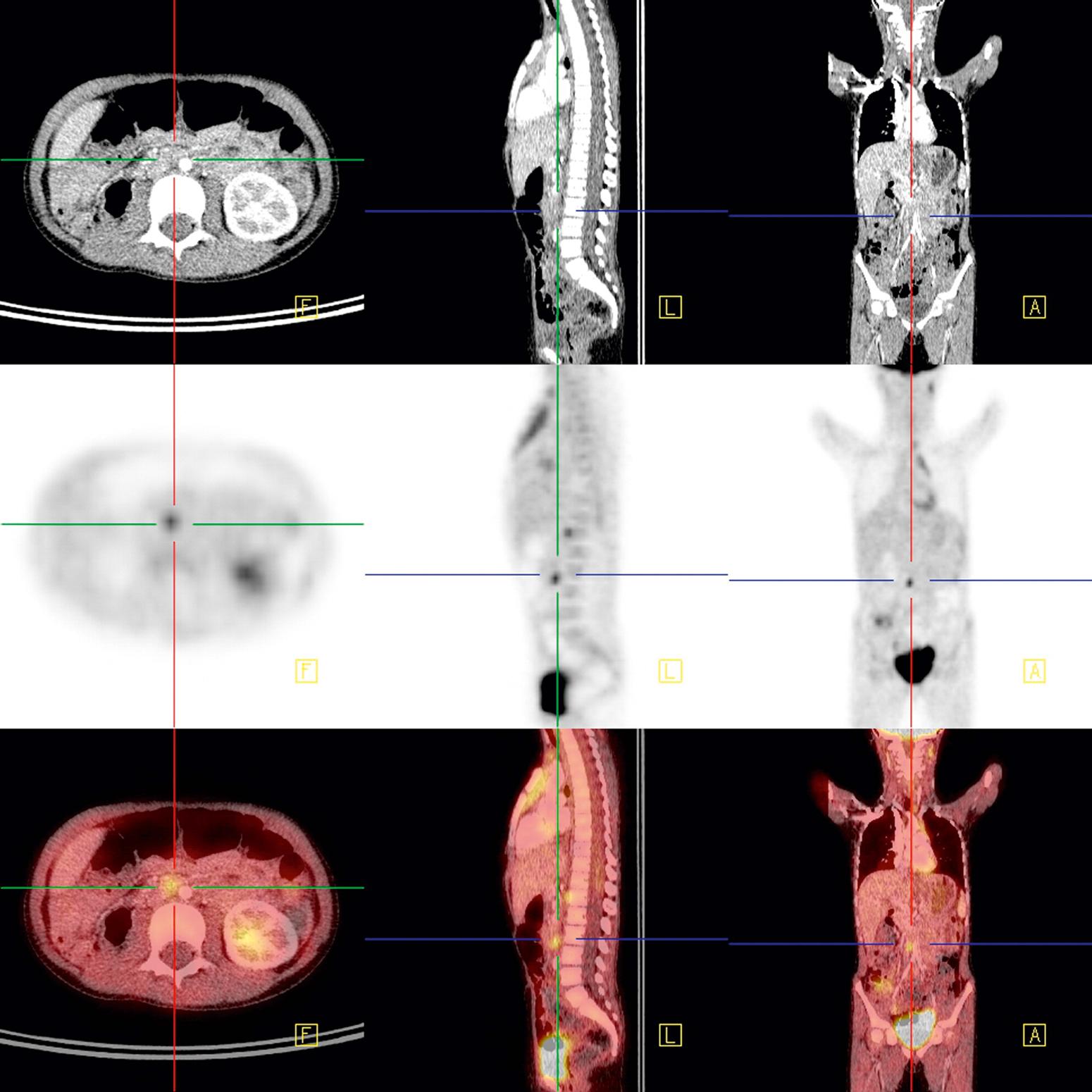
Plain abdominal radiographs may reveal tumor calcifications, organomegaly, excess fecal load, and mass effect upon intestines. Views in at least two different positions should be obtained to appreciate ascites or intestinal obstruction. As a screening modality, ultrasonography is a highly efficient, low-cost, and widely available test. It is noninvasive, is nonirradiating, and can give detailed information on the location, vascularity, and nature of the mass and adjacent structures ( Fig. 20.3 ). The most widely used defining imaging technique is CT ( Fig. 20.4 ), followed by MRI. These modalities often provide radiologic diagnosis, are invaluable for surgical planning, and are unhampered by bowel gas, a common limitation of ultrasound. MRI or CT when combined with functional PET scanning can define primary, metastatic, and recurrent disease ( Figs. 20.5 and 20.6 ).
Unlike many other abdominal masses, splenomegaly is usually secondary to a systemic process; it can be caused by diseases that result in hyperplasia of the lymphoid and reticuloendothelial systems (infections, inflammatory disorders), infiltrative disorders (Gaucher disease, leukemia, lymphoma, histiocytosis, hemophagocytic lymphohistiocytosis), hematologic disorders (thalassemia, hereditary spherocytosis), and conditions that cause distention of the sinusoids whenever there is increased pressure in the portal or splenic veins (portal hypertension) ( Table 20.5 ). In addition, palpable spleens in children and adolescents are not always indicative of disease. A palpable spleen (≤2 cm below the left costal margin) is a normal finding in a child younger than 3 years and may be a normal finding in an older child. Up to 15% of full-term neonates, 10% of children, and 3% of college freshmen have palpable spleens unassociated with an increase in lymphoreticular malignancy and with equivalent health. However, a spleen that is palpable >2 cm below the left costal margin should be evaluated further. Masses that may be mistaken for splenomegaly include the left lobe of the liver, a left upper quadrant tumor such as Wilms or neuroblastoma, a spleen that is displaced by obstructive lung disease such as asthma or bronchiolitis, or a gastric bezoar. Painful splenomegaly generally follows stretching of the splenic capsule with rapid enlargement of the spleen. Malfixed “wandering” spleens may undergo torsion, presenting the upper pole to the abdominal wall as a prominent painful abdominal mass. Splenic enlargement that is noted not in the context of an acute illness (i.e., it is noted incidentally, for instance, on a well-child examination or checkup) is more likely to be caused from a chronic process such as a storage disease than splenomegaly that is noted in the context of an acute illness. Acute onset of splenomegaly is most characteristic of an acute infection or a rapidly progressive malignancy (acute leukemia, lymphoma). Chronic splenomegaly (present for ≥1 month) is much more likely to represent a chronic process, such as storage diseases, congestive processes (portal hypertension, congestive heart failure), hemolysis, chronic infection, or inflammation. Massive splenomegaly (a spleen that crosses the midline or reaches the pelvis) indicates leukemia, lymphoma or other lymphoproliferative syndromes (post-transplant, familial hemophagocytic lymphohistiocytosis), hemoglobinopathy, Langerhans cell histiocytosis, visceral leishmaniasis, or a storage disease.
| Anatomic Lesions Cysts, pseudocysts Hamartomas Polysplenia syndrome Hemangiomas and lymphangiomas Hematoma or rupture (traumatic) Peliosis |
Parasitic Malaria Toxoplasmosis, especially congenital Toxocara canis, Toxocara cati (visceral larva migrans) Leishmaniasis (kala-azar) Schistosomiasis (hepatic-portal involvement) Trypanosomiasis Fascioliasis Babesiosis |
| Hyperplasia Caused By Hematologic Disorders Acute and Chronic Hemolysis ∗ Hemoglobinopathies (sickle cell disease in infancy with or without sequestration crisis and side variants, thalassemia major, unstable hemoglobins) Erythrocyte membrane disorders (hereditary spherocytosis, elliptocytosis, pyropoikilocytosis) Erythrocyte enzyme deficiencies (severe G6PD deficiency, pyruvate kinase deficiency) Immune hemolysis (autoimmune and isoimmune hemolysis) Paroxysmal nocturnal hemoglobinuria Chronic Iron Deficiency Extramedullar Hematopoiesis Myeloproliferative diseases: CML, juvenile CML, myelofibrosis with myeloid metaplasia, polycythemia vera Osteopetrosis Patients receiving granulocyte and granulocyte-macrophage colony-stimulating factors |
|
| Immunologic and Inflammatory Processes ∗ Systemic lupus erythematosus Juvenile idiopathic arthritis Mixed connective tissue disease Systemic vasculitis Serum sickness Drug hypersensitivity, especially to phenytoin Graft versus host disease Sjögren syndrome Cryoglobulinemia Amyloidosis Sarcoidosis Autoimmune lymphoproliferative syndrome Post-transplant lymphoproliferative disease Large granular lymphocytosis and neutropenia Histiocytosis syndromes Hemophagocytic syndromes (nonviral, familial) |
|
| Infections † Bacterial Acute sepsis: Salmonella typhi, Streptococcus pneumonia, Haemophilus influenzae type b, Staphylococcus aureus Chronic infections: infective endocarditis, chronic meningococcemia, brucellosis, tularemia, cat-scratch disease Local infections: splenic abscess ( S. aureus, streptococci, less often Salmonella spp., polymicrobial infection), pyogenic liver abscess (anaerobic bacteria, gram-negative enteric bacteria), cholangitis Viral ∗ Acute viral infections Congenital CMV, herpes simplex, rubella Hepatitides A, B, and C; CMV EBV Viral hemophagocytic syndromes: CMV, EBV, HHV-6 HIV Spirochetal Syphilis, especially congenital syphilisLeptospirosis Rickettsial Rocky Mountain spotted fever Q fever Typhus Fungal/Mycobacterial Miliary tuberculosis Disseminated histoplasmosis South American blastomycosis Systemic candidiasis (in immunosuppressed patients) |
Malignancies Primary: leukemia (acute, chronic), lymphoma, angiosarcoma, Hodgkin disease, mastocytosis Metastatic |
| Storage Diseases Lipidosis (Gaucher disease, Niemann-Pick disease, infantile GM1 gangliosidosis) Mucopolysaccharidoses (Hurler, Hunter-type) Mucolipidosis (l-cell disease, sialidosis, multiple sulfatase deficiency, fucosidosis) Defects in carbohydrate metabolism: galactosemia, fructose intolerance, glycogen storage disease IV Sea-blue histiocyte syndrome Tangier disease Wolman disease Hyperchylomicronemia type 1, IV |
|
| Congestive Disease ∗ Heart failure Intrahepatic cirrhosis or fibrosis Extrahepatic portal (thrombosis), splenic, and hepatic vein obstruction (thrombosis, Budd-Chiari syndrome) |
† Chronic or recurrent infection suggests underlying immunodeficiency.
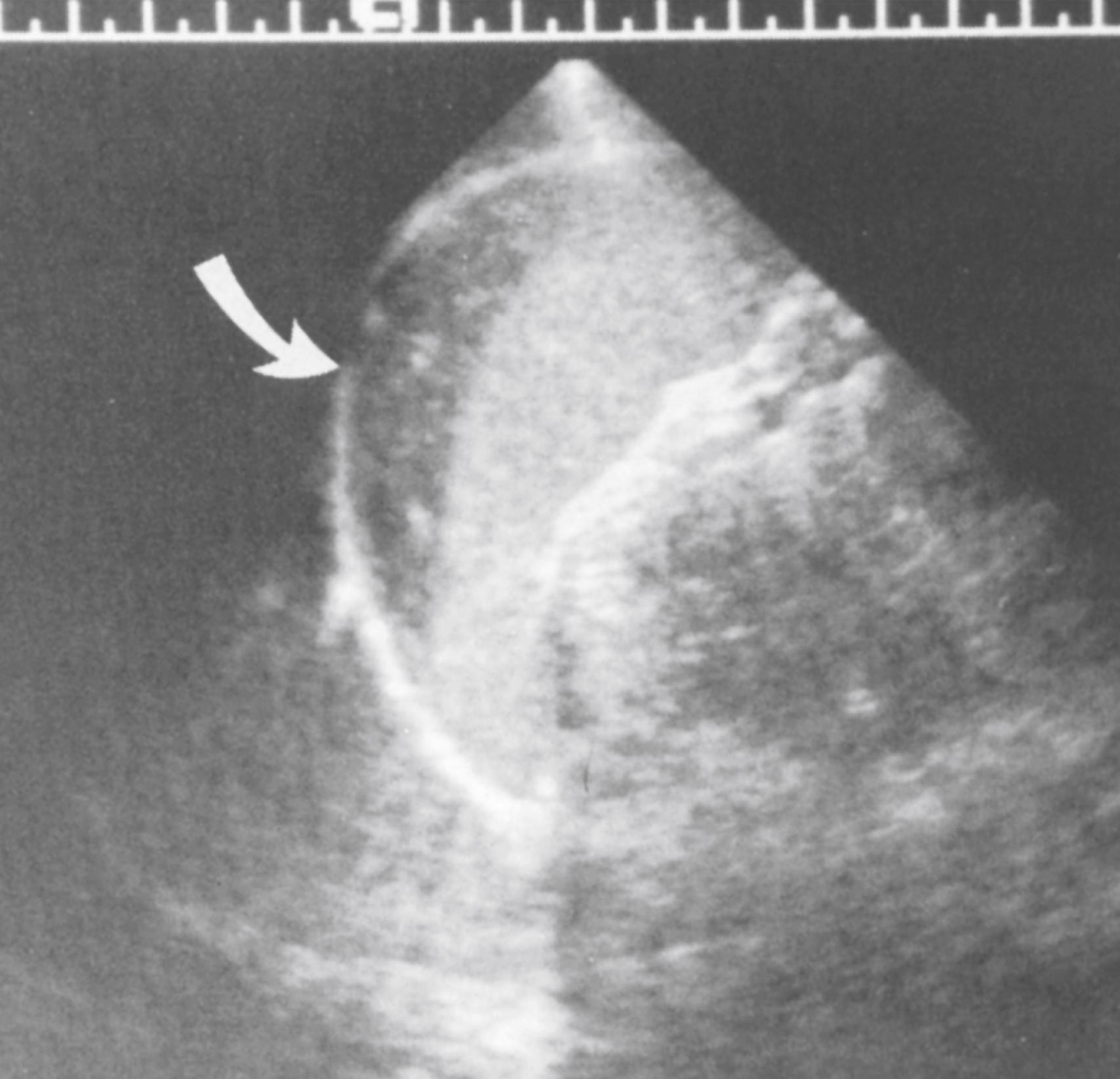
Birth and medical history, including transplacentally acquired infections, especially congenital syphilis, are often associated with splenic enlargement. History of a neonatal umbilical venous catheter, perhaps accompanied by occult portal venous injury or thrombosis, may progressively obstruct the extrahepatic portal vein, leading to congestive splenomegaly. Recurrent infections suggest immunodeficiency. Liver disease may lead to portal hypertension, which may in turn lead to splenomegaly. Congestive heart failure and associated congenital cardiac malformations have an increased risk of abdominal heterotaxia with splenic malformation and splenomegaly. Abdominal trauma may acutely produce a splenic hematoma ( Fig. 20.7 ) or may be followed by development of a chronic splenic pseudocyst.
A family history of anemia, transfusions, early biliary stones, cholecystectomy, and splenectomy may indicate hemolytic anemia. Identification of Mediterranean (thalassemia, glucose-6-phosphate dehydrogenase [G6PD] deficiency), African (sickle cell disease, G6PD deficiency), southern Asian (thalassemia, G6PD deficiency), or Ashkenazi Jewish (storage disease) ancestry in patients with splenomegaly is helpful in identifying an inherited process.
Social and behavioral issues of parents, children, and adolescents heavily affect certain risks and exposures. Sexual encounters and intravenous and illicit drug exposure may expose patients to hepatitis, cytomegalovirus, and HIV. Sexual abuse may place even young children at risk; it may be difficult to elicit an accurate history.
A review of systems in a patient with splenomegaly should elucidate related conditions. Systemic symptoms, such as fever or weight loss, are seen in many disorders that manifest splenomegaly, particularly infections, malignancies, and inflammatory or granulomatous processes, such as hemophagocytic lymphohistiocytosis (HLH) and sarcoidosis. When fever is acute in onset, infection is most likely. Chronic fever, often gradual in onset and not associated with chills, is more likely to be caused by inflammatory processes (systemic lupus erythematosus [SLE], juvenile idiopathic arthritis [JIA], sarcoidosis, Langerhans cell histiocytosis) or tumors (lymphomas, especially Hodgkin disease, or leukemia). Exposure to infectious agents or a travel history that might result in exposure to infectious agents unusual for the patient’s community (malaria, leishmaniasis, schistosomiasis, trypanosomiasis for U.S. citizens with a travel history to an endemic country) should be determined. Pallor suggests anemia (hemolysis, bone marrow infiltration, hypersplenism); purpura and petechiae suggest thrombocytopenia (bone marrow failure, autoimmune disorder, hypersplenism, bone marrow infiltration); and jaundice and conjunctival icterus suggest hemolytic anemia, liver dysfunction, or both. Rashes caused by a variety of acute and chronic infections and inflammatory diseases (SLE, JIA, infective endocarditis, HLH) and hemangiomata that are part of a systemic process involving the spleen may provide clues to splenic disease. Dyspnea, cough, orthopnea, and fatigue suggest respiratory disease (Langerhans cell histiocytosis or sarcoidosis), anemia, congestive heart failure, or malignancy (Hodgkin disease). Diarrhea caused by Salmonella infection or inflammatory bowel disease may be accompanied by splenic enlargement. Abdominal pain accompanied by splenomegaly may be attributable to acute splenic capsule distention or caused by coincident gallstones, hepatitis, or trauma. Joint pain resulting from SLE, JIA, and other autoimmune inflammatory diseases may be associated with splenomegaly. Bone pain is a feature of bone marrow infiltrative processes, particularly leukemia or neuroblastoma or storage disease (Gaucher disease). Poor vision in an infant with splenomegaly suggests osteopetrosis (with deafness) or uveitis-iritis (sarcoidosis, JIA). Loss of developmental milestones occurs with storage diseases. Splenomegaly may accompany neurologic paraneoplastic syndromes (such as myasthenia gravis) secondary to lymphoma or lymphoproliferative syndromes.
Nutritional status and growth parameters provide clues to disorders that affect the patient’s metabolic state and tissue oxygenation. Malnutrition (as evidenced by such problems as weight loss and failure to thrive) in a child with splenomegaly suggests malignancy, chronic hemolysis, immunodeficiency or chronic infection, a metabolic disorder, or liver disease. Pallor, petechiae, purpura, jaundice, hemangiomata, septic emboli to the skin, infiltrative lesions (leukemia cutis, solid tumors), seborrhea, or eczema (as occurs in Langerhans cell histiocytosis and immunodeficiency) should be noted. Cherry-red retinal spots or cloudy corneas suggest storage diseases. Conjunctival pallor, scleral icterus, fundal hemorrhages, evidence of sinus infection or otitis media, condition of gingivae, and evidence of salivary gland enlargement should be noted. The clinician should look for signs of heart failure or new or changing murmurs, which suggest valvular or other structural heart disease or endocarditis. Any respiratory distress, rales, rhonchi, or suggestion of pneumonia or asthma should be noted. Abdominal distention, prominent veins on the abdomen, hepatomegaly, fluid wave, tenderness, or rebound should be noted, as should specific characteristics and size of the spleen itself. A hard or nodular spleen suggests malignancy or chronic hemolysis. A tender spleen suggests either acute enlargement or infection, or both. A spleen that is more than 5 cm below the left costal margin is usually not transient and represents significant disease. Arthritis, splinter hemorrhages, and poor bone growth (as occurs in storage diseases and osteopetrosis) should be noted. Size, texture, mobility, tenderness, and distribution of lymph nodes should be noted. Enlarged (>1 cm), firm, fixed lymph nodes are suggestive of lymphoma or leukemia. Tender enlarged lymph nodes are suggestive of more common infections. Developmental delay suggests chronic infection, immunodeficiency, or storage diseases.
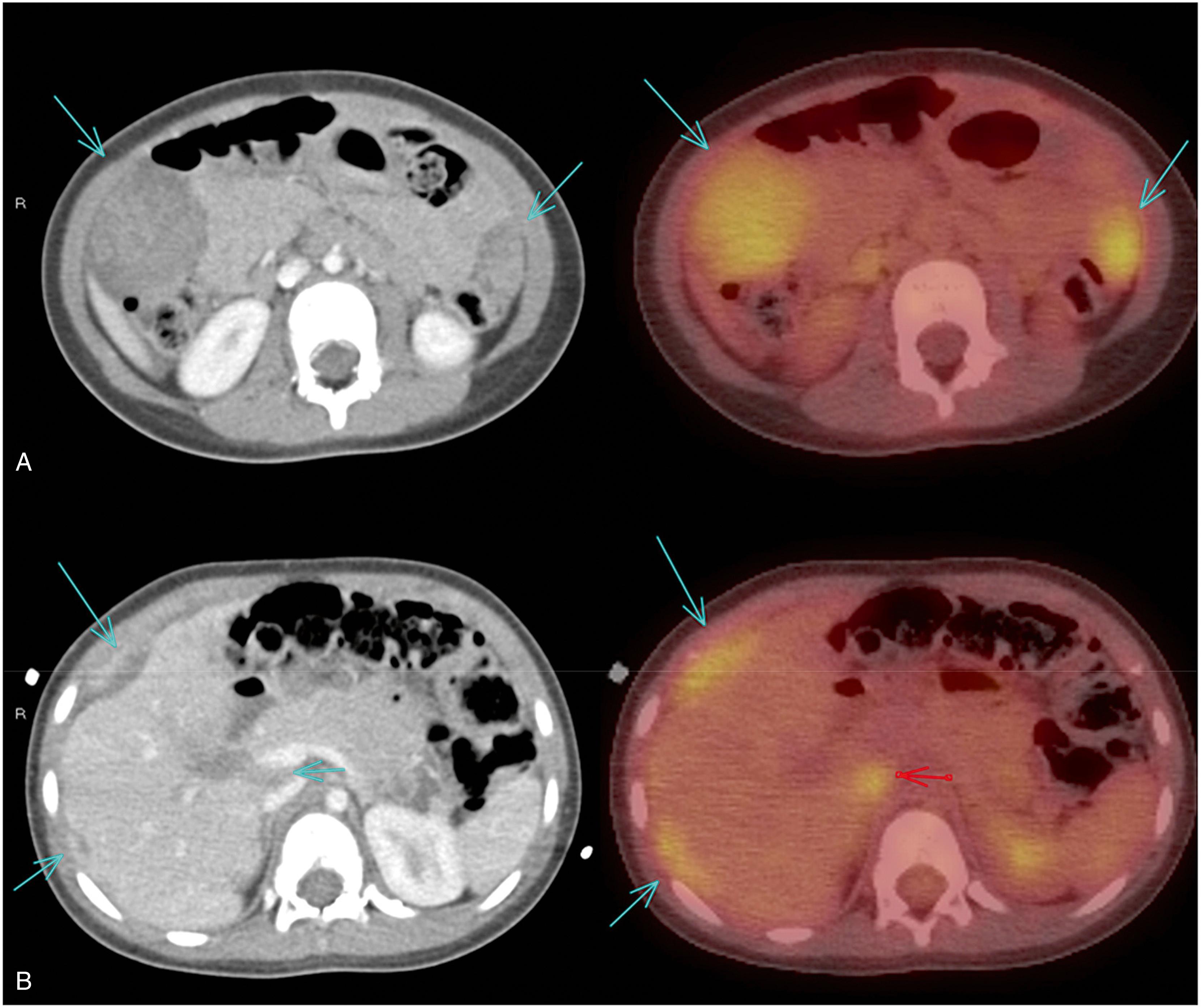
The most common cause of splenomegaly in childhood is viral infection, which should induce only moderate splenomegaly (<5 cm below the left costal margin) that is transient, lasting <4–6 weeks. Other common causes include autoimmune disorders and destruction of abnormal blood cells (such as brisk hemolysis). The approach to the child with splenomegaly is affected by several key factors, each of which indicates the probability of significant disease necessitating diagnosis and intervention ( Fig. 20.8 A and B ).
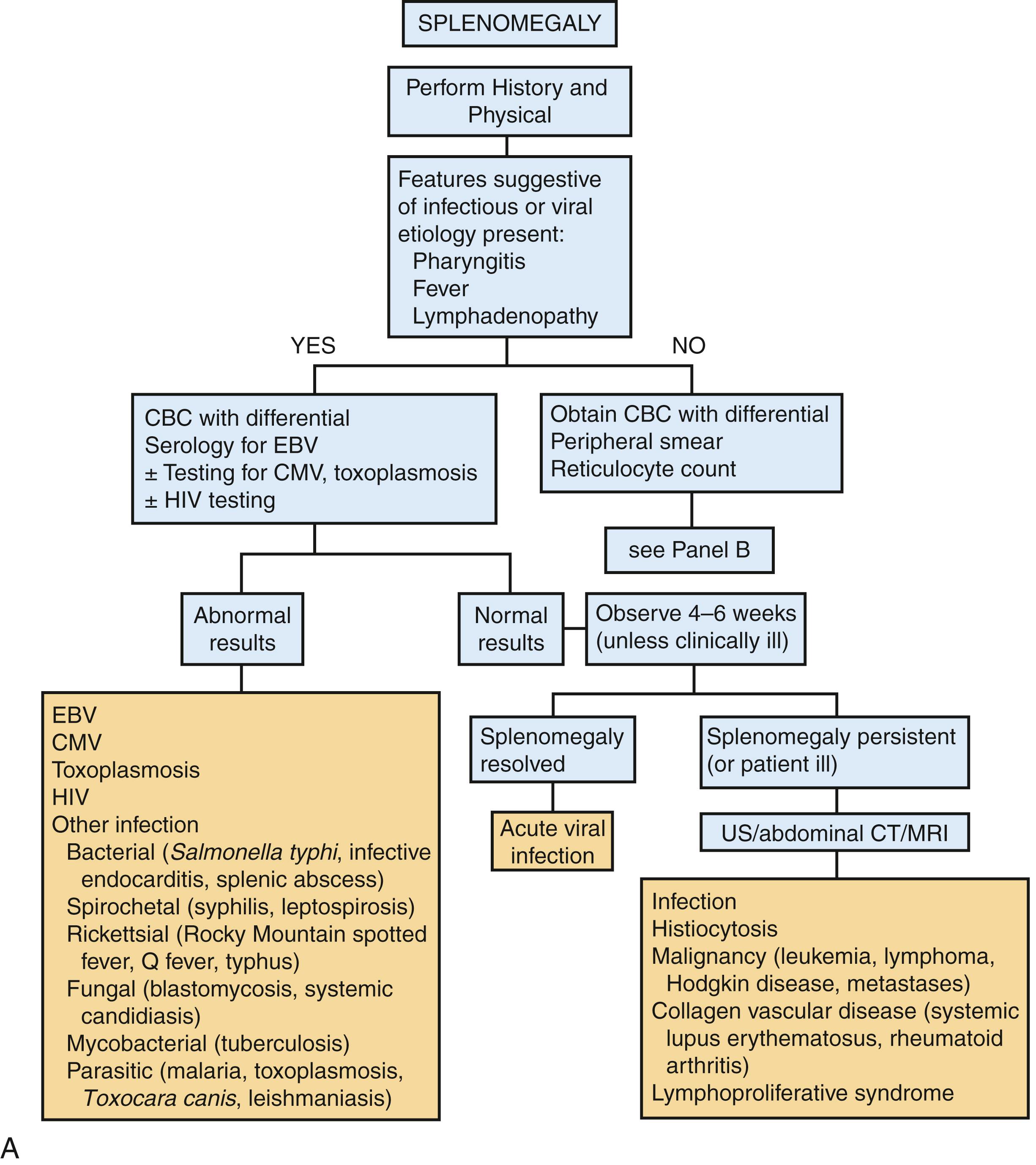
Table 20.6 summarizes key diagnostic laboratory investigations.
| Suspected Diagnosis | Tests to Be Performed |
|---|---|
| Hemolysis | CBC, reticulocyte count, blood smear, serum bilirubin measurement, Coombs test, osmotic fragility study, RBC enzyme assays, hemoglobin electrophoresis |
| Infection | CBC, differential, blood cultures, viral studies (EBV, CMV, HIV), toxoplasmosis, Bartonella titers, TB test, malaria blood smear, PCR testing and/or blood smear for babesiosis, CRP, procalcitonin |
| Liver disease | Liver function tests, albumin measurement, prothrombin time, α 1 -antitrypsin, serum copper, ceruloplasmin |
| Portal hypertension | Liver function tests; albumin measurement; prothrombin time; ultrasonography/CT of portal veins, liver, and spleen |
| Immunologic and inflammatory disease | ESR, CRP, C3, C4, antinuclear antibody, rheumatoid factor measurements; ferritin, urinalysis; BUN, serum creatinine, and immunoglobulin measurements |
| Infiltrative disease | CT, enzyme assay for Gaucher disease, tests as indicated for other storage diseases |
| Malignancy | CBC with differential, peripheral smear, CXR, uric acid, LDH, CT, bone marrow aspiration |
| Genetic syndrome | Molecular DNA testing or whole exome sequencing |
A CBC is the first test indicated in all patients with undiagnosed splenomegaly. This count provides extensive information about hematologic, infectious, and inflammatory processes; the result may also be abnormal in patients with hypersplenism caused by portal hypertension.
A white blood cell (WBC) count above or below the normal range for age, the neutrophil count, the lymphocyte count, and the presence of abnormal cells (atypical lymphocytes, blasts) should be noted. Viral infection is the most common cause of splenomegaly in children, and atypical lymphocytosis may be a clue. Viral infections may be associated with an increased (early) or decreased WBC count. Most significant bacterial infections produce neutrophilia and reactive changes in the neutrophils. Infections with intracellular bacteria or some viruses may produce neutropenia. Additionally, an elevated procalcitonin level may indicate bacterial infection. Leukemia can manifest with an increased or decreased total WBC count. The presence of blasts is confirmatory, but they are not always present.
Hemolytic anemia may be unsuspected without examination of the blood smear and the reticulocyte count (see Chapter 49, Chapter 50 ). Malarial parasites may be seen on the blood smear but may be missed unless a thick preparation is examined. Clues found on the blood smear include spherocytes (present in hereditary spherocytosis and hemolytic anemias); elliptocytes (present in hereditary elliptocytosis); polychromasia, poikilocytes, and fragmented cells (present in hemolytic anemias); sickled cells with target cells, spherocytes, and nucleated red blood cells (present in sickle cell anemia and variants); and microcytosis, hypochromia (present in thalassemias), and Howell-Jolly bodies (present in splenic dysfunction). Hypersplenism (pooling or sequestration of blood cells in an enlarged spleen) may cause anemia.
Thrombocytopenia (<150,000 platelets/mm 3 ) may be caused by decreased platelet production or increased platelet destruction. Production is diminished in conditions characterized by bone marrow infiltration (leukemia, neuroblastoma). Increased destruction accompanies immunologic processes, drug reactions, HLH, and viral infections. Thrombocytosis (>400,000 platelets/mm 3 ) often accompanies iron-deficiency or acute infection as an acute-phase reactant. Sequestration of platelets within an enlarged spleen may cause thrombocytopenia.
Pancytopenia implies bone marrow dysfunction, bone marrow infiltration, or portal hypertension with hypersplenic destruction of all the formed elements of the blood (see Chapter 50 ). A bone marrow aspiration and biopsy should be performed in any child with splenomegaly and pancytopenia. Tests of liver function, including prothrombin time and albumin, are indicated.
Viral antibody titers for Epstein-Barr virus (EBV) and cytomegalovirus should be obtained when a mononucleosis syndrome is present, especially when splenomegaly persists. Heterophile antibody tests are rapid and have a sensitivity of 85% for EBV in older children and adolescents but are not sensitive in children younger than 4 years. Specific serologic testing for EBV is useful in these younger children. The results of these tests rarely affect management but may permit a presumptive diagnosis of a self-limited process to be made, and they may preclude more invasive tests such as imaging and/or bone marrow examination. Toxoplasmosis should also be considered. Primary infection with HIV frequently causes splenomegaly. Acute infection with HIV may not be noted on screening labs, and therefore additional testing may be required.
Elevation of the ESR is nonspecific but suggests infection, especially bacterial, mycobacterial, or fungal infection, or an inflammatory process, such as JIA, HLH, or SLE. The ESR may be normal despite significant inflammation, for example, when there is severe anemia or hypofibrinogenemia. The CRP level may be elevated when the ESR is normal.
Liver function tests are indicated if splenomegaly is significant (>2 cm) or persists longer than 1 month. Portal hypertension is often asymptomatic until hepatic fibrosis is far advanced. Liver synthetic function (albumin, prothrombin time, fibrinogen), direct bilirubin levels, and transaminase levels should be assessed.
Immunologic evaluation is needed when autoimmune disorders (JIA, SLE) or immunodeficiency disorders (inherited or acquired) are suspected. This assessment includes measurements of antinuclear antibody titer, immunoglobulin levels, and immunoglobulin subclass levels; tests of neutrophil function; and measurements of T-cell subclasses. Repeated infections stimulate the immune system and may cause splenomegaly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here