Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors acknowledge use of substantial material from this chapter in the previous edition.
Group A Streptococcus (GAS) is synonymous with Streptococcus pyogenes, the only species in this group of β-hemolytic streptococci. GAS is a leading pathogenic bacterium affecting children and adolescents, and it is associated with a wide spectrum of infections and diseases. Worldwide, >600 million cases of GAS pharyngitis (“strep throat”) and >700 million cases of GAS pyoderma are estimated to occur annually. Although uniformly and exquisitely susceptible to penicillin and many other antimicrobial agents, GAS infections continue to present formidable clinical and public health challenges. Although most GAS infections are relatively benign, invasive disease often is fulminant and life threatening. GAS also differs from other pyogenic bacteria in its potential to produce delayed, nonsuppurative sequelae, such as poststreptococcal acute glomerulonephritis (PSAGN) and acute rheumatic fever (ARF).
The importance of GAS infections in the US was reinforced in the late 20th century by resurgence of severe, invasive GAS infections (e.g., streptococcal toxic shock syndrome [STSS] and necrotizing fasciitis) with high morbidity and mortality. Globally, GAS is an important cause of morbidity and mortality, primarily in income-poor countries, causing >500,000 deaths per year due to rheumatic heart disease (RHD) and invasive infections.
GAS is a gram-positive coccus that grows in chains, producing small white to gray colonies with a clear zone of β-hemolysis on blood agar. GAS is distinguished from other groups of β-hemolytic streptococci by its group-specific cell wall polysaccharide (Lancefield A antigen). Serologic grouping by the Lancefield method is precise, but group A organisms can be identified more readily by any one of a number of latex agglutination, coagglutination, or enzyme immunoassay procedures. GAS has been universally highly susceptible to β lactum agents but very rare isolates with a penicillin-binding protein mutation in the pbp2x gene conferring slightly higher (but still susceptible) minimal inhibitory concentrations (MICs) have been reported recently.
GAS is subdivided into >240 immunologic serotypes by the cell surface M-protein antigen with fimbriae (hairlike structures) that project from the cell surface. Classically, typing of the surface M protein relied on available polyclonal antisera, but this technique has been largely supplanted by a molecular genotyping using polymerase chain reaction (PCR) and deoxyribonucleic acid (DNA) sequencing of the 5′ variable region of the emm gene encoding the M protein ( http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm ), with good correlation between known serotypes and emm types. ,
M serotyping or genotyping is valuable for epidemiologic studies; particular GAS diseases tend to be associated with certain M types. M types commonly associated with pharyngitis (e.g., 12, 1, 28, 4, 3, 2) rarely cause skin infections, and higher number M types commonly associated with skin infections (e.g., 49, 55, 57, 60) rarely cause pharyngitis. A few “pharyngeal” strains (e.g., M type 12) are associated with PSAGN, but far more of the “skin” strains are considered nephritogenic. Several pharyngeal serotypes (e.g., 1, 3, 5, 6, 14, 18, 19) are classically associated with ARF. However, the precise basis of rheumatogenicity remains unclear. Recent studies suggest that, in contrast to high income countries, most ARF-associated GAS in low to medium income countries are not the classic rheumatogenic types. Certain GAS M types are more strongly associated with invasive disease, including M1, M3, M6, M12, M18, and M28. A globally disseminated clone of the M1 serotype has been a leading cause of severe invasive GAS infections, such as necrotizing fasciitis, STTS, and scarlet fever, over several decades.
The GAS cell is a complex structure. In rapidly dividing organisms, the cell is covered with a hyaluronic acid capsule that gives the colonies a mucoid or water drop appearance on agar. Microscopic fimbriae protrude from the cell surface into the hyaluronic capsular layer, promoting the adherence of GAS to epithelial cells and extracellular matrix proteins. The fimbriae are composed of cell–wall-anchored M protein that adopts a coiled-coil structure and is closely associated with lipoteichoic acid polymers. GAS is now recognized to express surface pili, corresponding to the classic “T antigen” used in earlier serologic typing schemes. Roughly one half of GAS strains are able to opacify mammalian serum through the activity of surface-anchored serum opacity factor (SOF).
The group A carbohydrate, encoded by the gac operon, comprises 40%–50% of the GAS cell wall. The carbohydrate, a polymer of rhamnose units with side chains of N -acetyl-glucosamine, is responsible for its group (e.g., A) specificity. As with other gram-positive species, a peptidoglycan provides thickness and rigidity to the cell wall. M protein, pilus antigen, SOF, and other surface proteins are covalently attached to the GAS cell wall by sequences that interact with an anchoring enzyme called sortase.
GAS releases a large number of biologically active extracellular products locally, some of which are toxic to human and other mammalian cells. Streptolysin S (SLS) is a small, oxygen-stable toxin responsible for β-hemolysis on blood agar; and streptolysin O (SLO) is an oxygen-labile, cholesterol-dependent toxin related to staphylococcal α-hemolysin. Both SLS and SLO injure cell membranes and lysing red blood cells, while also damaging other eukaryotic cells and membranous subcellular organelles. SLO is antigenic, whereas SLS is not. Antibody to SLO (ASO) is clinically useful as an indicator of recent GAS infection. Streptococcal pyrogenic exotoxins (SPEs) are secreted factors that can act as superantigens and trigger T-lymphocyte proliferation and cytokine release. GAS expresses a broad-spectrum cysteine protease, SpeB, which has multiple host targets. Various other peptidases cleave host chemokines and complement factors. Several nucleases, including DNAse B, are produced, and like ASO, anti-DNAse B is useful clinically. GAS also produces bacteriocins, small proteins that kill a variety of other gram-positive bacteria and thus may promote infection or persistent colonization. Bacteriophages have played an important role in the evolutionary genetics of GAS, including the transfer of genes that encode antibiotic resistance, SPEs, and other virulence determinants.
GAS induces serious human disease by at least three mechanisms: suppuration, as in pharyngitis and pyoderma; toxin elaboration, as in STSS; and immune-mediated inflammation, as in ARF and PSAGN. No complete explanation is available for the predilection of certain body sites for GAS infection, nor for the ability of certain M-type strains to produce pharyngitis and of others to produce pyoderma.
The first step in the pathogenesis of GAS disease in humans is successful colonization of the upper respiratory mucosa or skin. A large number of adherence factors for epithelial cells and extracellular GAS have been described, including lipoteichoic acid, M protein, pili, and fibronectin- or laminin-binding proteins, including Sfb1, SOF, and Lbp. The formation of a GAS biofilm facilitates persistence in humans. Both M protein and fibronectin-binding proteins are important for subsequent endocytosis of GAS into respiratory epithelial cells, in addition to representing a proximal step in the pathogenesis of systemic infection. Intracellular invasion allows GAS access to a privileged intracellular niche, which has been postulated to contribute to repeated infections after antibiotic therapy. Repeated courses of antibiotics may not eradicate intracellular organisms, possibly leading to selection for more invasive pathogens.
After invasion, GAS pathogenesis is characterized by numerous mechanisms of immune evasion associated with virulence factors that vary across serotypes but are functionally redundant. Ability of GAS to resist innate immune clearance mechanisms that normally prevent microbial dissemination accounts for its signature propensity to cause serious infection in otherwise healthy children and adults. For example, when GAS gains access to deeper tissues through cellular invasion or a breach of epithelial integrity, it deploys specific peptidases that cleave and inactivate the neutrophil chemoattractants interleukin-8 (IL-8) and complement factor 5a. Likewise, SpeB can degrade the host’s complement components and cationic antimicrobial peptides. GAS also secretes three enzymes that degrade immunoglobulin, including Ide/Mac-1 produced by serotype 1, that can also reduce host defenses by inhibiting neutrophil activation, phagocytosis, and release of reactive oxygen species.
M protein is a multifaceted immune resistance factor that promotes GAS resistance to opsonophagocytosis through multiple mechanisms, including the binding of fibrinogen, complement inhibitory factor H, host antimicrobial peptides, and the Fc region of immunoglobulins. , M protein is essential to virulence in animal models, and immunization with M protein provides strong protection against infection with a type-specific strain. During invasive infection, significant quantities of M protein are released from the cell surface by proteolysis. Soluble M protein is proinflammatory by multiple mechanisms, including NLRP3 inflammasome activity, and can form a proinflammatory, clotlike complex with human fibrinogen, leading to uncontrolled neutrophil activation, vascular leakage, and toxic shock symptomatology. , M protein also collaborates with the GAS virulence factor streptokinase to bind host plasminogen to the GAS surface; this generates plasmin activity, effectively coating the bacterial surface with a powerful protease to facilitate tissue spread.
The pore-forming toxins SLS and SLO are toxic to multiple host cell types, including macrophages and neutrophils, and therefore promote GAS tissue damage and resistance to phagocytic clearance. SLO in particular can induce accelerated apoptosis of immune cells and inhibit neutrophil oxidative burst and neutrophil extracellular trap (NET) production. The GAS hyaluronic acid capsule is not immunogenic, mimicking a common human matrix component, and cloaks opsonic targets on the bacterial surface from phagocyte recognition. In the case of the highly invasive, globally disseminated M1T1 GAS clone, hyperinvasive forms bearing mutations in the CovR/S 2-component regulator arises in vivo under innate immune selection, leading to upregulation of capsule and other key virulence determinants. Specific virulence determinants present in the hyperinvasive M1T1 clone include the phage-encoded DNAse Sda1, which allows GAS to escape killing in DNA-based NETs, and the serum inhibitor of complement (SIC), which binds and inactivates terminal complement components and host defense peptides.
The SPEs are a family of >15 bacterial superantigens, including the bacteriophage-encoded SPE A and SPE C. These superantigens induce antigen-nonspecific T-lymphocyte activation, suppress antibody synthesis, potentiate endotoxic shock, induce fever, promote the release of proinflammatory cytokines, produce reticuloendothelial blockade, and may contribute to the multiorgan failure characteristic of STSS. In the US, STSS is commonly associated with infections caused by strains that produce SPE A. Susceptibility to STSS appears to be related to the absence of antibodies to both M protein and superantigens, in addition to the presence of specific human leukocyte antigen (HLA) haplotypes. The SPEs share homology with staphylococcal enterotoxins but not with staphylococcal toxic shock syndrome toxin-1. SPE A and SPE C are responsible for the rash of scarlet fever, stimulating the formation of specific antitoxin antibodies that provide immunity against future scarlatiniform rashes, but not against subsequent GAS infections.
Although many GAS constituents and extracellular products are antigenic, protective immunity is primarily type specific, mediated by opsonic anti-M-protein antibodies. These antibodies protect against infection with a homologous M type but confer little immunity against other M types. Therefore, multiple GAS infections attributable to different M types are common during childhood and adolescence. Anti-M antibodies persist for years, perhaps for life, protecting against invasive infection but not against pharyngeal carriage. Type-specific antibody may be transferred across the placenta from mother to fetus. Type-specific antibody against M protein usually is not detectable until as long as 6–8 weeks after infection ; therefore, its primary role may be in the prevention of reinfection by the same serologic type. Development of opsonic M-type-specific antibodies may be suppressed by early effective antimicrobial therapy.
Humoral antibodies to specific streptococcal extracellular products, such as antistreptolysin O (ASO) and anti-DNAse B, can be demonstrated readily by neutralization assays and are clinically important. These assays have been useful in defining recent GAS infection in clinical and epidemiologic studies and in documenting the occurrence of a recent preceding GAS infection in patients suspected of having a nonsuppurative complication, such as ARF. The ASO assay is the most commonly used streptococcal antibody test. Because SLO is also produced by group C and group G streptococci, the test is not 100% specific for GAS infections, and the response can be weak in patients with streptococcal impetigo. In contrast, the anti-DNAse B response is demonstrable after both skin and throat infections. Neutralizing antibody titers peak at about 3–6 weeks for SLO and at about 6–8 weeks for DNAse B. Antibody titers against other GAS extracellular antigens reported by clinical immunology laboratories may vary. The upper limits of normal are substantially higher for children than for adults; adult normal values should not be used to interpret in children results.
GAS has a narrow host range; it is identified almost exclusively in humans and only rarely in other species. GAS is highly communicable and can cause disease in individuals of all ages who lack type-specific immunity. Disease in neonates is uncommon in resource-rich countries. Significant differences exist between the epidemiology of common throat and skin infections due to GAS (see Chapter 27, Chapter 68 ).
Severe invasive GAS infections had become uncommon in the US and Western Europe during the second half of the 20th century. In children, the most frequent predisposing event was varicella prior to the varicella vaccine. However, since the late 1980s, a worldwide increase in severe invasive GAS infections has occurred, and the incidence in industrialized societies (2–4 per 100,000 population) is similar in the geographically distinct regions of Europe, North America, and Australia. This rate translates to about 10,000 cases of invasive GAS disease annually in the US. However, rates of invasive disease in indigenous populations in Africa, the Asian subcontinent, and the Pacific islands typically are much higher ; rates as high as 12–83 per 100,000 annually have been reported in indigenous populations in Australia. It was estimated in 2005 that at least 663,000 cases of invasive GAS disease occur globally each year, resulting in 163,000 deaths.
The stratified squamous epithelium of the oropharynx and skin is the principal barrier against invasive GAS disease. GAS can gain access to sterile sites by means of direct inoculation after an injury that breaches the mucous membranes or skin ; however, a substantial number of invasive streptococcal infections have no identifiable site of entry. Epidemiologic data suggest that the oropharynx and skin are the primary site of origin for systemic GAS infection; therefore, invasive disease is likely to occur as a result of transient bacteremia originating from the oropharynx or skin, possibly as a result of direct tissue penetration by GAS. Varicella is a particularly important risk factor for severe invasive GAS infections in previously healthy, unvaccinated children. Other risk factors more common in adults include diabetes mellitus, human immunodeficiency virus infection, obesity, intravenous drug use, alcoholism, and chronic pulmonary or cardiac disease.
GAS is a common cause of acute pharyngitis (see Chapter 27 ) ( Fig. 118.1 ) and pyoderma (impetigo) (see Chapter 68 ) in children and adolescents. GAS also produces a variety of other infections of the respiratory tract, including otitis media, retropharyngeal abscess, peritonsillar abscess, sinusitis, and mastoiditis; infections of the skin and soft tissues ( Figs. 118.2, 118.3, and 118.4 ), which include cellulitis, erysipelas, perianal cellulitis, vaginitis, and blistering distal dactylitis (see Chapter 68, Chapter 74, Chapter 75 ); invasive diseases, including STSS (see Chapter 13 ), necrotizing fasciitis, septicemia, meningitis, pneumonia, empyema, peritonitis, puerperal sepsis, neonatal sepsis, osteomyelitis, suppurative arthritis, myositis; and surgical wound infections. In addition, GAS is the cause of two potentially serious nonsuppurative complications, ARF and PSAGN, and another potential nonsuppurative complication, poststreptococcal reactive arthritis (PSRA). GAS is also alleged by some to be related to the hypothesized pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).


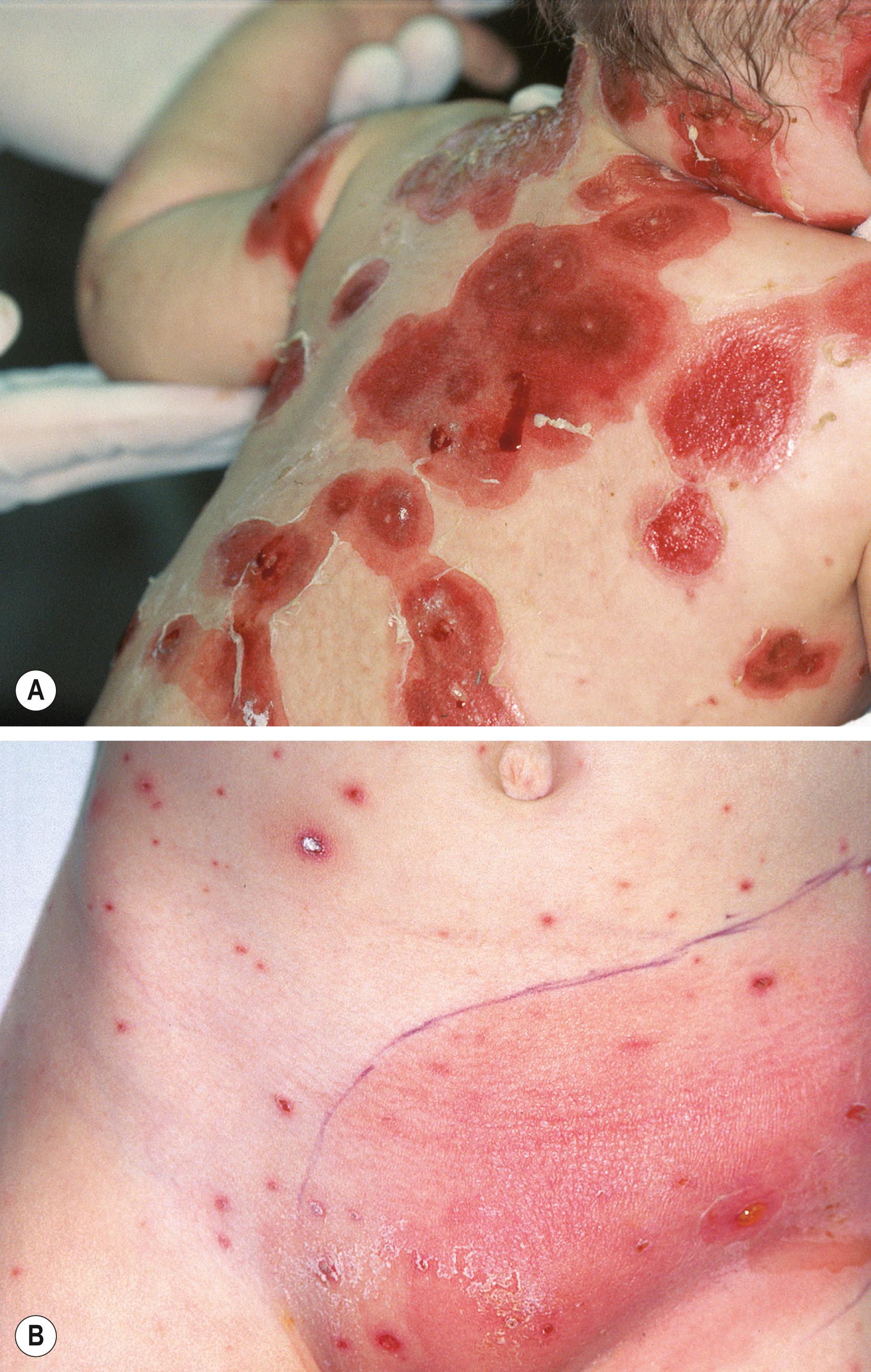
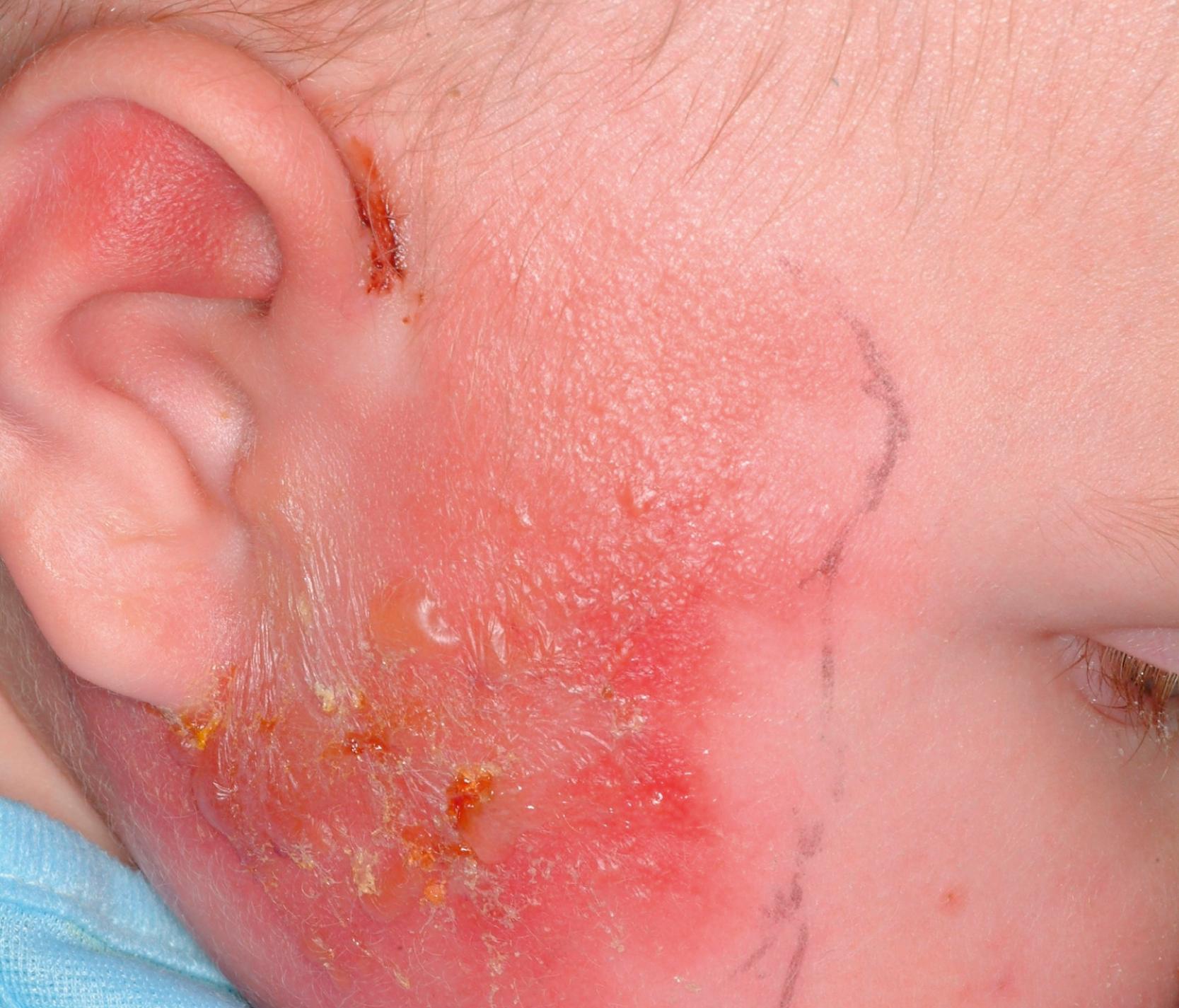
Severe invasive GAS infection is defined as the isolation of GAS from a normally sterile body site; it includes three overlapping clinical syndromes. Cellulitis and bacteremia are the most common GAS invasive diseases, each accounting for about 20%–40% of invasive GAS disease. , Clinically, cellulitis is characterized by the acute redness and inflammation of the skin, with associated fever, pain, and swelling. GAS bacteremia often triggers a rapid and robust proinflammatory cytokine response that results in a high fever, nausea, and vomiting and can progress to irreversible shock. Cellulitis and bacteremia also can be preludes to necrotizing fasciitis and STSS.
The GAS so called “flesh eating disease,” necrotizing fasciitis, is a devastating bacterial infection involving the skin, subcutaneous and deep soft tissues, and muscle. Necrotizing fasciitis involves rapid bacterial growth, spread along the fascial sheaths that separate adjacent muscle groups, breach of the fascial sheaths, and necrosis of adjacent tissues. The pathogenesis of necrotizing fasciitis is complex and not completely understood. However, the rapid tissue destruction and bacterial spread are thought to involve host and bacterial proteases (plasmin, SpeB), GAS pore-forming cytotoxins (e.g., SLO and SLS), and tissue-damaging enzymes released by host neutrophils in response to GAS cell wall components and superantigens. A major risk factor for the development of necrotizing fasciitis is blunt trauma, which may result in increased vimentin expression that attracts circulating GAS to the injured muscle.
Invasive GAS infections also can result in STSS, a “cytokine storm” produced in response to GAS superantigens that substantially increases the risk of death. , GAS superantigens simultaneously engage major histocompatibility complex (MHC) class II molecules and T-lymphocyte receptor (TCR) β-chain variable regions, resulting in antigen-independent activation of large numbers of T lymphocytes. The resulting cytokine response by T lymphocytes (lymphotoxin-α, interleukin-2 [IL-2], interferon γ) and by antigen-presenting cells (tumor necrosis factor [TNF], IL-1β, IL-6) causes widespread organ dysfunction, disseminated intravascular thrombosis, and tissue injury. The result is an acute, very rapidly progressive illness typified by high fever, rapid onset hypotension, and rapid multisystem failure. The magnitude of the STSS inflammatory response is closely linked to the severity of the disease. Specific criteria for the diagnosis of STSS have been established ( Box 118.1 ).
Hypotension (systolic pressure ≤90 mmHg for adults or <5th percentile by age for <16 years old) plus 2 or more of the following:
Renal impairment (creatinine ≥2× upper limit for age)
Coagulopathy (platelets <100,000/mm 3 or DIC)
Hepatic involvement (elevated ALT, AST, or bilirubin ≥2× upper limit for age)
Adult respiratory distress syndrome
Generalized erythematous macular rash
Soft tissue necrosis including necrotizing fasciitis or myositis or gangrene
Meets clinical criteria plus isolation of group A Streptococcus (GAS) from a normally sterile site
Meets clinical criteria plus isolation of GAS from a nonsterile site
The clinical course of severe invasive GAS infections often is precipitous, requiring rapid diagnosis and initiation of the appropriate therapy. Although the initial clinical findings of STSS are nonspecific, a high index of suspicion is required, particularly in patients who have an increased risk. Pain disproportionate to superficial signs of infection can be a clue to developing necrotizing fasciitis. Cultures of focal lesions are helpful in patients with skin or soft tissue involvement. Blood culture is often positive in patients with sepsis without an apparent focus of infection. A complete blood count can show a relatively normal or low leukocyte count, with a marked left shift to immature neutrophils. If necrotizing fasciitis is suspected, magnetic resonance imaging is helpful in confirming the diagnosis but should not delay therapy. Histologic examination of excised tissue shows extensive necrosis, inflammation, hemorrhage, and thrombosis of small vessels.
The initial management of a child or an adolescent with a severe invasive GAS infection includes hemodynamic stabilization and specific antimicrobial therapy to eradicate GAS. When necrotizing fasciitis is suspected, prompt surgical drainage, debridement, fasciotomy, or even amputation is often required. Patients with necrotizing fasciitis also require careful fluid and nutritional support and may require extensive skin grafting or other reconstructive surgery, as well as physical therapy. Multiple boluses of fluid may be required because of severe volume depletion and ongoing capillary leakage. When fluid resuscitation alone is insufficient to maintain adequate tissue perfusion, inotropic agents (e.g., dobutamine, dopamine, norepinephrine) often are required.
Parenteral antimicrobial therapy should include agents active against at least GAS and Staphylococcus aureus until the results of bacteriologic studies are available. Once GAS has been identified, intravenously administered penicillin G (200,000–400,000 U/kg/day in 4–6 divided doses) is the drug of choice. A mouse model of streptococcal myositis suggests that clindamycin may be more effective than penicillin in eradicating GAS in severe invasive infections. In addition, in susceptible organisms, clindamycin inhibits protein synthesis and the production of important virulence factors (e.g., M protein, SPEs) and overcomes the Eagle effect (decreased bacterial activity of β-lactams at high bacterial density). Therefore, many experts recommend intravenously administered clindamycin (40 mg/kg/day in 3 or 4 divided doses) in addition to penicillin empirically.
Several studies have described the use of immunoglobulin intravenous (IGIV) therapy in patients with severe invasive GAS infections. An observational comparative cohort study conducted in Canada reported significantly reduced mortality among IGIV-treated patients. However, this study had confounding factors that might have affected mortality data. In a recent comparative observational study in Europe of 67 patients with STSS, with 28-day survival as the measured endpoint, suggested a therapeutic benefit of both IGIV (odds ratio, 5.6; P = .030) and clindamycin (odds ratio, 8.6; P = .007). Most experts recommend use of IGIV for STSS.
The only specific indication for long-term use of an antibiotic to prevent GAS infection is for patients with a history of ARF or RHD. Measures to prevent the spread of GAS infections have variable effectiveness. The spread of throat or skin infection within a family often occurs before the index case is identified. In epidemic situations, especially when cases of ARF or PSAGN occur, screening and treatment of all individuals with positive culture results (mass prophylaxis) may be indicated.
Clusters of invasive GAS infections within a chronic care facility or among members of a single household have been reported. However, given the infrequency of these infections and the lack of a clearly effective chemoprophylactic regimen, the available data currently do not support a recommendation for routine testing for GAS colonization or routine chemoprophylaxis for otherwise healthy household contacts of a patient with invasive GAS disease. The Centers for Disease Control and Prevention (CDC) recommend that healthcare providers inform such household contacts about the clinical manifestations of pharyngeal and invasive GAS infections and emphasize the importance of seeking immediate medical attention if contacts develop such symptoms. A recent CDC study found that secondary household cases of invasive GAS are rare, occur within 30 days, and that those >65 years of age are at greatest risk.
Unfortunately, an effective vaccine to prevent GAS infection is not yet available. A major challenge is the great number and diversity of unique M types. Another challenge is to ensure that putative vaccine components do not cross-react with host tissues with potential for autoimmune phenomena. Recent investigations have discovered a hidden conserved motif within hypervariable N termini to which C4b protein binds and which could serve as a cross-reactive target. Molecular techniques have been used to genetically engineer a complex multivalent M protein–based vaccine (e.g., 30-valent) to optimize functional antibody responses. A major hurdle to vaccine development is the very great diversity of group A strains among various climates and global regions. Because GAS disease and particularly ARF/RHD are major causes of health inequality affecting indigenous children in Australia and New Zealand, the governments of the two countries formed the Coalition to Advance New Vaccines for group A Streptococcus (CANVAS) to assess several vaccine candidates and to fast-track development of a vaccine most likely to be safe, efficacious, and cost effective.
Suppurative complications from the spread of GAS to adjacent structures were common in the pre-antibiotic era. Cervical lymphadenitis, peritonsillar abscess, retropharyngeal abscess, otitis media, mastoiditis, pneumonia, and sinusitis related to GAS still occur in children. Nonsuppurative complications are discussed in the following sections.
ARF is an inflammatory disorder that commonly involves the joints and heart (and less commonly, the brain and skin). It develops as a complication of an untreated or incompletely treated GAS pharyngitis. The association of GAS pharyngitis with ARF first was suspected in the 19th century based on parallels in peak age (5–15 years) and seasonal (winter/spring) incidence, with observations that outbreaks of GAS pharyngitis in closed communities (e.g., boarding schools and military bases) often were followed by outbreaks of acute ARF. Subsequent serologic studies documenting anti-GAS antibodies in patients with ARF provided further evidence. Clinical trials showed that antimicrobial therapy that eliminates GAS from the pharynx also prevents initial attacks of ARF, and that long-term, continuous prophylaxis that prevents GAS pharyngitis also prevents recurrences of ARF.
The incidence of ARF has declined substantially over the past 8–9 decades in the US and other resource-rich countries, a decline that began even before the advent of penicillin. However, ARF remains a major public health burden in much of the income-poor world. Currently at least 33 million people worldwide have RHD, with 282,000 new cases and 319,000 deaths annually directly attributable to ARF or RHD.
The annual incidence of ARF in some countries exceeds 50 per 100,000 children. Some of the highest incidence rates reported are in school-aged Pacific Islanders in New Zealand (80–100 per 100,000) and in aboriginal children in central and northern Australia (150–380 per 100,000). In contrast, recent ARF incidence data for an industrialized country, like the nonindigenous population of New Zealand or the US, shows an annual rate of <2 per 100,000 children. The prevalence of RHD in children aged 5–14 years is highest in sub-Saharan Africa, the Pacific Islander and indigenous populations of New Zealand and Australia, and south central Asia with rates up to 20 per 1000. In contrast, the prevalence of RHD in resource-rich countries usually is ≤1 per 1000. A dramatic outbreak of acute ARF occurred in the area of Salt Lake City, Utah, in the mid-1980s, and elevated numbers of ARF cases persisted for many years. Smaller US outbreaks were reported in the same period in various communities and among recruits at military training centers, but these resurgences remained localized and did not lead to a nationwide increase in cases.
The observation that only a very small proportion of patients with GAS pharyngitis subsequently experience ARF suggests that specific characteristics of the organisms, the host, and the environment contribute to ARF.
Experts have long suspected that various strains of GAS differ in propensity to lead to ARF, and a limited number of classic rheumatogenic M serotypes are linked epidemiologically to outbreaks of ARF. Strains of certain rheumatogenic serotypes (e.g., types 1, 3, 5, 6, and 18), isolated infrequently during the 1970s and early 1980s, reappeared dramatically as causes of pharyngitis concurrently with localized US outbreaks of ARF in the mid-1980s. The marked decrease in ARF in the US in recent decades was strongly correlated with replacement of rheumatogenic types by nonrheumatogenic types in childhood acute streptococcal pharyngitis.
A genetic predisposition to ARF appears to be a factor. Studies in twins have shown a higher concordance rate of ARF in monozygotic than in dizygotic twin pairs. Recent genetic analyses have yielded an association between an immunoglobulin heavy chain allele and risk for RHD in Melanesians and Polynesians in Oceania, as well as HLA-DQA1-DQB1 in aboriginal Australians. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here