Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Cardiac implantable electrical devices (CIEDs) refer to implanted devices that deliver therapeutic electrical stimuli and include permanent pacemakers and implantable cardioverter-defibrillators (ICDs).
Electrical therapy for cardiac arrhythmias includes low-voltage (typically 1 to 5 V) pacing stimuli (pulses) and high-voltage (typically 500 to 1400 V) stimuli (shocks). Pacemakers deliver pacing pulses to treat bradycardia. ICDs deliver shocks to defibrillate ventricular fibrillation (VF) or to cardiovert ventricular tachycardia (VT). ICDs also have antibradycardia pacing functions that can deliver pacing pulses to treat bradycardia, as well as antitachycardia pacing functions that can deliver sequences of rapid pacing pulses to treat ventricular or atrial tachyarrhythmias. Cardiac resynchronization therapy (CRT) pacemakers (CRT-P) or ICDs (CRT-D) also provide electrical therapy for heart failure in the form of pacing pulses that resynchronize the ventricular contraction sequence. This chapter covers antiarrhythmic electrical therapy delivered by CIEDs. See Chapters 50 and 58 for CRT in the treatment of heart failure and devices for hemodynamic monitoring, and see Chapter 61 for devices implanted for rhythm monitoring.
Chest and occasionally abdominal radiography can help to identify the type, manufacturer, and integrity of implanted devices. Transvenous system pulse generators are typically located subcutaneously in the upper chest, though in pediatric patients, some older larger devices, or devices with leads inserted via the femoral vein or epicardially, the pulse generator may be in the abdomen. Pacemaker pulse generators are smaller than ICD devices ( Figs. 69.1 and 69.2 and eFig. 69.1 ). Transvenous pacemaker leads are typically implanted with lead tips in the right ventricle and right atrium. Epicardial leads may be tunneled to the devices. ICDs are readily identified by the presence of defibrillation coils on the leads (see Fig. 69.2 ). For CRT, leads may be placed in a left ventricular branch of the coronary sinus ( Fig. 69.3 ) or on the LV epicardium. Some pacing leads are now being placed at the His bundle or deep septally from the right ventricle to capture the subendocardial left bundle branch conduction system ( Fig. 69.4 ). Subcutaneous ICDs are typically placed subcutaneously in the left chest along the axillary line with leads tunneled subcutaneously ( Fig. 69.5 ). Leadless devices can also be visualized radiographically ( Fig. 69.6B ).







Conventional pacemaker components include a pulse generator (PG) that contains the battery and circuitry and the lead system. The lead system consists of one to three leads that are connected to the pacemaker PG via the lead pin. The lead body includes conducting wires that are surrounded by insulating material and connected to sensing and stimulating electrodes. The tip of the leads connect to the heart via an active (screw) or passive (e.g., tine) fixation mechanisms ( Fig. 69.7 ). Conventional single-chamber systems have one lead that usually connect to the right ventricular (RV) endocardium or in some cases to the right atrium (RA). Dual-chamber systems usually have leads that connect to the right ventricle and RA (see Fig. 69.1 ). Cardiac resynchronization devices have a third lead that is placed to pace the left ventricle (LV) via a lead in a branch of the coronary sinus or implanted on the LV epicardial surface (see Fig. 69.3 ). (See Chapter 50 .) Newer pacemaker configurations include leads intended to pace the cardiac conduction system, using leads placed at the His bundle or into the interventricular septum to capture the left bundle branch (see Fig. 69.4 ). Leadless pacemakers are also commercially available and include self-contained devices implanted via a catheter to the right ventricle ( Fig. 69.6B ). These provide single chamber pacing; algorithms that are designed to detect atrial contraction may be able to provide atrial synchronous pacing as well. Investigational leadless devices include right atrial or left ventricular components.

Simply put, pacemakers are indicated for symptomatic bradycardia. In the early pacemaker era such patients mainly had complete AV block with resultant intermittent asystole known as Stokes-Adams attacks. In higher income countries, more pacemakers are implanted at present for symptomatic sinus node dysfunction. Patients at high risk of complete atrioventricular (AV) block due to advanced, infranodal conduction system disease may also receive a pacemaker prophylactically. There is a marked age-related pacemaker indication and implantation rate with an overall prevalence of pacemakers of 500/100,000 in the U.S. Medicare population (>65) but 40/100,000 for ages 18 to 64 and 2600/100,000 for age >75.
Transvenous pacing leads are insulated wires 6 to 8F (2.0 to 2.67 mm) in diameter that carry electrical signals between a pacemaker generator, usually in the prepectoral area (superficial to the pectoralis muscle) and the endocardial surface of the heart (see Fig. 69.7 ). Bipolar leads have a 1 to 2 mm metallic electrode tip and a slightly larger ring electrode situated about 10 to 15 mm proximally. The electrodes are made of polished titanium and platinum alloys. Unipolar leads, now used much less commonly, though occasionally used in coronary sinus or epicardial placement, have only the tip electrode and use the metallic pacemaker generator surface as the return electrode. The tip is secured to the myocardium with passive fixation (2 to 3 mm flexible polyurethane tines that embed in trabeculated myocardium) or active fixation (via a ∼1 mm screw that secures) to the endomyocardium ( Fig. 69.7A ). Epicardial leads are used primarily in patients with congenital heart disease, tricuspid valve replacement, endocarditis, or in the pediatric age range ( Fig. 69.7C ). They either screw into or are sewn onto the epicardial surface; the generator resides in the abdominal wall or pectoral region. Ventricular leads comprise RV and coronary sinus LV leads. Epicardial leads can be used on any chamber’s epicardial aspect. Leads are insulated with silicone and/or polyurethane and more recently polytetrafluoroethylene. Silicone is soft and flexible but needs to be thicker, has relatively high frictional surface resistance, and is prone to abrasions. Polyurethane is stiffer, more slippery, more durable, and can be made thinner but is prone to two forms of degradation, including environmental stress cracking (ESC) and metal ion oxidation (MIO). Nearly all transvenous leads have a hollow lumen for a thin malleable stylet or wire to shape or advance the lead for implantation. In cross section the lead design can be coaxial with the wire to the tip electrode in the center surrounded by insulation then the ring electrode surrounded by additional insulation or co-radial with the two different (insulated) coils wound together around the central lumen ( Fig. 69.7B ). Passive fixation leads are generally placed in the RV apex. Active fixation leads can be secured anywhere in the RV or in the RA. Passive fixation atrial leads have a J shape and are placed in the RA appendage, with the tines intended to secure to the trabeculated myocardium there. The connection between the lead and the generator is via a terminal pin that usually conforms to the 3.2 mm diameter IS-1 standard at present, but other size and configurations still exist. Attention to these details is critical when reoperating on patients with very old leads.
Pacemaker generators are hermetically sealed titanium casings of varying shapes with a volume of ∼10 to 15 cc housing the battery (typically lithium-iodine), sensing circuitry, activity sensors, hybrid circuit RF communication antenna, and logic boards (see eFig. 69.1 ). Glued to the generator is a clear epoxy header into which the leads are placed and secured using set screws.
Modern cardiac pacemakers perform a number of functions, but their most critical two actions remain pacing and sensing. Pacing means delivering a small (compared with a defibrillation shock) electrical stimulus of ∼1 to 5 V that captures a small myocardial region adjacent to the pacing electrode yielding a propagating wavefront in the chambers of interest (i.e., the atria or ventricles) ( eFig. 69.2 ).

A pacing stimulus that fails to capture is subthreshold ( eFig. 69.3 ). A stronger stimulus is required during the relative refractory period compared with electrically recovered tissue; cathodal versus anodal stimuli exhibit different characteristics, too. Events leading to capture are complex when the three-dimensional structure and fiber orientation is taken into account with some stimuli leading to hyperpolarization versus depolarization occurring in adjacent regions. Determinants of the threshold for a given pacing lead, above which “capture” occurs, include the stimulus strength (in voltage or current) and the pulse duration. An important relationship is Ohm’s law (V = IR, where V is voltage, I is current, and R is resistance).

The strength-duration curve describes the relationship between pulse strength (usually voltage) and duration and whether capture occurs or not ( Fig. 69.8 ). At an infinitely long pulse duration, practically estimated as about 1.5 to 2.0 msec, a minimal pulse strength (in V) eliciting capture is known as the rheobase. With shorter pulse durations, the threshold voltage (i.e., that required to capture) gradually and then abruptly increases at very short pulse durations (about 0.1 to 0.2 msec). The pulse duration at which the voltage threshold is twice the rheobase voltage is defined as the chronaxie. These concepts are important to understand threshold measurements, defining a safe margin for capture and optimizing battery longevity. Energy usage by pacing pulses are described by J = VIt = V 2 t(1/R) (where J is energy, V = voltage, I = current, t = pulse duration, and R = impedance). Monophasic pulses are used for pacing, whereas biphasic ones have advantages for defibrillation. Cathodal stimulation is used in pacing, so the distal electrode is the cathode. Pacing stimuli can be unipolar, wherein the return pole is the pacemaker generator housing or bipolar with an anodal ring electrode. Under certain circumstances, capture may occur at both sites (i.e., “anodal capture”). Of note, the capacitor-generated pulse declines over the course of the stimulus from a leading to a trailing edge voltage (see eFig. 69.2 ).

As illustrated in Figure 69.8 , increasing the pulse width beyond about 1 msec provides little safety margin. Similarly, increasing the voltage at very short pulse durations (0.1 to 0.15 msec) is ineffective due to the steep ascent of the curve. Examining the energy curve (see Fig. 69.8 ) illustrates that the optimal combination of safety margin, and efficiency usually is found near the chronaxie. Thresholds can be measured manually by reducing the voltage while maintaining the pulse width constant (or vice versa). A reliable electrocardiogram (ECG) is required to avoid prolonged loss of capture in device-dependent patients and correctly interpret the results for safety and battery longevity.
Thresholds are also measured automatically by most devices, in some or all chambers, usually by assessing for an evoked response indicating tissue capture, as distinguished from electrode polarization right after the pacing pulse ( eFig. 69.3 ). The device can be programmed to adjust the pacing output to achieve a desired safety margin automatically. In some devices, capture is confirmed on a beat-to-beat basis and using a pacing output only slightly (0.125 to 0.5 V) above threshold. In other devices and/or chambers, once the threshold is measured, pacing is set at a programmable amount, often two times the threshold, and the threshold is measured one or more times a day. In the latter scenario, beat-to-beat capture, using an evoked response, is not determined. For some devices, when AV conduction is present, atrial threshold is measured automatically by ascertaining a conducted ventricular event. Alternatively, the native atrial response to a premature atrial test pulse can determine whether capture occurs. LV threshold can be measured by evaluating whether an RV event is sensed after an LV pacing test pulse.
Pacing thresholds frequently vary over time early after implantation. For active fixation leads, transient myocardial injury elicited by securing them produces an elevated threshold for minutes to hours. Inflammation at the electrode tissue interface leads to a subacute threshold rise resolving in about 6 weeks. However, the magnitude of this rise has been minimized with corticosteroid-eluting leads. Nevertheless, even with such leads, a fibrous capsule develops around the implanted electrode. Smaller electrodes display a higher impedance thus favorably reducing current drain (Ohm’s law). However, they are more prone to threshold elevation and exit block due to the reactive fibrous cap. Electrode porosity, a fractal design in effect increasing the surface area, avoiding corrosion, and chemical composition are other important aspects of pacing electrode design.
Drugs, electrolyte perturbations, and metabolic changes also affect pacing thresholds ( eTable 69.1 ). Severe hyperkalemia results in an elevation of pacing thresholds; significant hyperglycemia, severe hypothyroidism, and also acidosis or alkalosis can elevate thresholds. Some studies, but not all, have found that sodium channel blocking drugs (flecainide, propafenone, and others, as well as amiodarone) may elevate the pacing threshold as well. Acute ischemia and chronic infarction may also result in loss of capture.
| Pacemakers |
| Effects on Pacing Threshold |
|
| Effect on Pacing Burden |
|
| ICDs |
| Frequency of VT or VF |
|
| Consideration for Detection of VT or VF |
|
| Therapy for VT or VF |
|
Apart from pacemakers functioning in the asynchronous modes (VOO, DOO; see Table 69.1 ), pacemakers need to accurately detect underlying atrial and/or ventricular native signals in order to know whether to and when to deliver a pacing stimulus. A stable position abutting viable myocardial tissue makes this possible, but numerous challenges exist. Sensing is dependent on the electrogram (EGM) characteristics, including amplitude and frequency content, but also filtering within the device.
The EGM derives from the temporal change in the local voltage between the two electrodes (for a bipolar lead or between the tip electrode and the generator for a unipolar sensing circuit) as the activation wavefront travels toward and then away from the electrode(s). , For a unipolar lead, as the wavefront moves toward the electrode, a positive EGM is inscribed and crosses baseline when it reaches the electrode; a negative signal is inscribed as the waveform moves away. With a bipolar lead the EGM is the difference between the two unipolar electrodes. Thus, the direction of the activation wavefront with respect to the bipolar pair influences the EGM size and shape, too. Bipolar leads in the ventricle sense the activation wavefront (commonly called the local R wave) but may also sense the repolarization signal (local T wave), and rarely the atrial activation event (e.g., if placed basally near the tricuspid annulus). Sensed signals vary in their timing, amplitude, and frequency content ( eFig. 69.4 ). Thus, bandpass filters are used to reduce the likelihood of sensing the wrong electrical events. Sensing of T waves can to some degree be avoided with a high pass filter above about 15 Hz (i.e., excludes lower frequencies). Local atrial and ventricular signals exhibit frequencies in the 5 to 50 Hz range, and T waves and far-field R waves exhibit frequencies in the 1 to 10 Hz range (see eFig. 69.4 ). The timing of events can also be used to exclude certain signals and thereby avoid inappropriate sensing, for example, the ventricular blanking and refractory periods and the postventricular atrial blanking and postventricular atrial refractory period ( eFig. 69.5 ). The signal from the lead undergoes amplification, filtering, and rectification and is then evaluated for whether it meets the sensing level programmed (see eFig. 69.5 ). Sensing in the atrial signals is more challenging due to the presence of large far-field ventricular signals. These far-field R waves exhibit a lower frequency range than the local atrial signal, as noted above. At implantation, P waves of about 1.5 mv or greater and R waves of 5 mv or greater are sought. Sensing thresholds for P waves thus are usually set at between 0.25 and 1.0 mv, and in the ventricle at about 2.0 mv. Setting the sensing threshold to a lower number increases the sensitivity reducing the chance of undersensing (but increasing the chance of oversensing). With unipolar leads, oversensing of pectoral myopotentials from the generator is a concern; in the ventricle this could lead to asystole in a pacemaker-dependent patient.


| Position | ||||
|---|---|---|---|---|
| I | II | III | IV | |
| Category | Chamber(s) paced | Chamber(s) sensed | Response to sensing | Rate modulation |
| O = None | O = None | O = None | O = None | |
| A = Atrium | A = Atrium | T = Triggered | R = Rate modulation | |
| V = Ventricle | V = Ventricle | I = Inhibited | ||
| D = Dual (A + V) | D = Dual (A + V) | D = Dual (T + I) | ||
| Manufacturers’ designation only | S = Single (A or V) | S = Single (A or V) | ||
Severe bradycardia and/or complete heart block with a junctional or ventricular escape adversely affects cardiac output. Instituting ventricular pacing at normal rates dramatically improves cardiac output by 25% to 30%. Restoring AV synchrony augments cardiac output still more by about 20%. Studies in the 1980s demonstrated that chronotropic response was dominant in improving exercise capacity over AV synchrony.
Nevertheless, there are additional advantages of maintaining the AV relationship. When patients were randomized to different rate responsive modes, most preferred a mode that maintained AV synchrony (i.e., DDDR rather than VVIR). Moreover, pacemaker syndrome occurs in 3% to 30% of patients who have ongoing sinus activity (i.e., not atrial fibrillation) when subjected to ventricular pacing. Its manifestations include fatigue, dyspnea, dizziness, neck pulsations, chest pain, and hypotension. It is most common when a fixed VA relationship is present, wherein the atrial contractions encounter closed AV valves. Dual-chamber as compared with single-chamber pacing leads to reduced occurrence of atrial fibrillation and of stroke and better quality of life in follow-up.
For dual-chamber pacing the AV interval is of importance. Pacemaker syndrome can occur with a severely prolonged PR interval analogously to the problem with VA conduction alluded to above. On the other hand, too short of an AV interval or a marked interatrial conduction delay adversely affects performance. A hemodynamically optimal AV interval is typically about 150 msec at rest and somewhat less with exertion.
The potential deleterious effects of RV pacing were initially obscured by the advantages of (1) any pacing over severe bradycardia, (2) AV sequential pacing over ventricular-only pacing, and (3) rate-responsive pacing over fixed-rate pacing. Although slightly reduced LV function occurs acutely, even in patients with normal ventricular function, RV pacing rarely exhibits clinically obvious adverse effects in the short term in such patients. Elegant studies of direct His bundle stimulation clearly identified that both the contribution of atrial systole (by varying PR interval) and ventricular activation sequence (by comparing atrial-His bundle with atrial-RV) influenced ventricular function.
Clinicians became much more aware of the adverse effects of (right) ventricular stimulation on ventricular function, especially over the long term in the early 2000s. The ameliorative role of biventricular pacing (CRT) in treating LBBB-related ventricular dysfunction cemented this observation since conduction with LBBB resembles RV apical pacing. Studies comparing ventricular pacing to native ventricular activation, especially in those with LV systolic dysfunction, demonstrated significantly increased heart failure events. The magnitude of the detriment with RV pacing worsened with greater QRS prolongation and worse baseline LV function.
The adverse effects of RV pacing were initially attributed to the RV apex in particular. However, targeting alternative sites such as the septum actually proved somewhat elusive and led to minimal advantages. Thus, algorithms emerged to limit RV pacing in dual-chamber pacemakers (as discussed later). These algorithms sometimes fostered very prolonged AV intervals, however, with potential hemodynamic consequences akin to pacemaker syndrome and an increased tendency toward atrial fibrillation and pause-dependent arrhythmias.
When ventricular pacing was unavoidable, as in AV block, achieving more physiologic ventricular activation has become important using either biventricular or His bundle or LBB-area pacing. , , ,
Permanent His bundle pacing was reported in a small cohort in 2000. However, owing to the perceived technical difficulty, rudimentary tools, and frequently elevated thresholds, uptake was modest. The field has exploded since 2014 when a cohort study reported high procedural success rates and better outcomes than with conventional DDD pacing. Dedicated sheaths and leads have eventually emerged and more improvements are expected. , An example is shown in eFigure 69.6 . His bundle capture can be selective (see eFig. 69.6 ) or nonselective wherein local ventricular tissue is captured in addition to the His bundle. The electrocardiography of His bundle pacing has been reviewed. His bundle pacing is being used in advanced AV block but also when atrial pacing is needed with marked first-degree AV block to avoid worsening AV dyssynchrony. In addition, it has been used to correct LBBB leading to corroboration of concepts that BBB could be very proximal or even within the His bundle. Elevated thresholds especially for correcting LBBB but even for His bundle capture in follow-up, and the potential for distal conduction system progression, have led to an iterative approach of targeting the LBBB, its fascicles, or the immediate region ( eFig. 69.7 ).


The current convention for naming pacemaker modes stems from 2001 consensus between the North American Society for Pacing and Electrophysiology (NASPE) and the British Pacing and Electrophysiology Groups (see Table 69.1 ). This convention specifies that five positions describe the functionality of pacemakers. The first position lists the chambers paced (A, V, or both, D). The second similarly describes the chambers sensed. The third letter specifies the device’s response(s) to sensed events. Position or letter IV specifies the presence or absence of rate response. Position V specifies the location or absence of multisite pacing (i.e., biatrial or biventricular pacing with at least two stimulation sites in each case).
Timing cycles, usually in msec, characterize the function of a pacemaker in different modes. Various periods, like a clock, run sequentially or simultaneously and specify what the pacemaker will do at the end of the period or during the period if another event occurs.
VOO is the simplest mode ( Fig. 69.9A ). From the first through third letter, respectively, the mode paces only in the ventricle, does not sense the atrium or ventricle (O), and behaves asynchronously (O). There is one timing clock, the ventricular escape interval. For example, for VOO 60 bpm, the ventricular escape interval is 1000 msec (60,000 msec/min/ 60/min = 1000 msec) (see eTable 69.2 ) Thus every 1000 msec the pacemaker delivers a ventricular pacing pulse (even if a native ventricular beat has occurred).

| Bradycardia | ||||||
| Interval (msec) | 1500 | 1200 | 1000 | 800 | 700 | 600 |
| Rate (bpm) | 40 | 50 | 60 | 75 | 86 | 100 |
| Tachycardia | ||||||
| Interval (msec) | 450 | 400 | 360 | 350 | 320 | 300 |
| Rate (bpm) | 133 | 150 | 167 | 171 | 188 | 200 |
VVI implies pacing in the ventricle (V) and sensing in the ventricle (V), and if a sensed event occurs, the next scheduled pacing pulse is inhibited (I) ( Fig. 69.9B ). The sensed event resets the ventricular escape interval. This mode adds a ventricular refractory period, during which sensed events are ignored. This prevents double counting ventricular events or local T waves and falsely inhibiting pacing.
In most pacemakers, applying a magnet converts the function from VVI to VOO. Depending upon the manufacturer, model, and programming magnet application may also change the pacing rate and/or initiate other temporary behavior (e.g., performing a pacing threshold test). The VOO mode is useful when oversensing is present as in a lead fracture or is anticipated due to electrocautery or other electromagnetic interference (EMI).
With respect to single chamber atrial pacing, AOO is analogous to VOO and AAI resembles VVI. In AAI, the device paces only in the atrium and senses in the atrium ( Fig. 69.9C ). In response to a sensed event, the next scheduled pacing pulse is inhibited, resetting the atrial escape interval. This mode, like VVI, has a refractory period, the atrial refractory period, during which sensed events are ignored. This prevents double counting atrial signals, or inappropriate sensing of far-field ventricular events that would falsely inhibit pacing. This mode is infrequently used due to the absence of ventricular support in the event of AV block.
Dual-chamber modes more than double the complexity. In DDD, the most commonly used one, pacing as well as sensing may occur in both the atrium and ventricle, as is evident from the first two letters ( Fig. 69.10 ). The third letter indicates that both inhibition and triggered events occur depending upon the circumstances. DDD preserves AV synchrony where possible. Unlike VVI or AAI there is an upper rate limit in addition to a lower rate limit. The occurrence of pacing versus native activity in the atrium and in the ventricle depends on the programmed rate and AV interval compared with the native sinus rate and AV conduction characteristics. Timing can be based on either the atrium or the ventricle. The AV interval may be different for sensed atrial versus paced atrial events and may shorten with faster rates.

An atrial paced atrial event initiates a post-atrial ventricular blanking period to minimize the chance of the ventricular channel sensing the atrial pacing spike, also called crosstalk, and inappropriately inhibiting resulting in ventricular asystole in the setting of complete AV block. Since crosstalk may even occur after the 40 to 60 msec post-atrial ventricular blanking period, DDD timing features a ventricular safety pacing interval (ending at about 100 to 110 msec after the atrial pacing spike). Should ventricular sensing occur between the end of the post-atrial ventricular blanking period and the end of the ventricular safety pacing interval (due to crosstalk, a premature ventricular complex [PVC], or very rapid native conduction), the device will V pace using an abbreviated AV interval (∼100 to 110 msec). If the sensing in this interval was due to crosstalk, asystole is prevented. If the sensing was due to a PVC, the abbreviated AV interval seeks to pace early enough after a PVC to avoid an R-on-T event ( eFig. 69.9 ). The post-atrial ventricular blanking tries to prevent crosstalk, while the safety pacing interval and function prevents asystole if crosstalk occurs. These blanking and safety pacing intervals don’t exist after atrial sensed events. The chance for crosstalk can be reduced by using bipolar rather than unipolar atrial pacing, reducing the atrial output to an appropriate safety margin, using bipolar ventricular (rather than unipolar) sensing, decreasing the ventricular sensitivity (increasing the numerical value), and increasing the post-atrial ventricular blanking period duration.

The ventricular sensed or paced event initiates an atrial refractory period, the postventricular atrial refractory period (PVARP). PVARP seeks to prevent the atrial channel from sensing either the far-field ventricular event or retrograde atrial events initiated by the ventricular event or atrial tachyarrhythmias. As in VVI, a ventricular refractory period is present as well to prevent double counting.
The DDI mode is used much less often ( eFig. 69.8 ). Unlike DDD, it does not exhibit atrial tracking. Thus, it can be useful in the setting of atrial oversensing to avoid inappropriate ventricular pacing. Another use of DDI is when there is intermittent atrial fibrillation but suboptimal atrial sensing prevents mode switching (in DDD); tracking of the AF is avoided in DDI. Operationally, in DDI, if the sinus rate is below the lower rate limit (LRL) the device will atrial pace at the LRL and then either native ventricular conduction will occur or the device will pace the ventricle at the conclusion of the AV delay. If the sinus rate is above the LRL the device will inhibit in the atrium; if AV conduction is absent it will pace the ventricle at the LRL (at a different rate than the atrium is firing, thus losing AV synchrony).
The VDD mode is adequate for patients with intact sinus node function but with AV block (see eFig. 69.8 ). However, should sinus bradycardia below the LRL develop, the device will pace the ventricle (only, as the initial letter implies) at the LRL losing AV synchrony.
Each of the aforementioned modes can function in a rate responsive fashion as well, provided the pacemaker generator has a sensor (or sensors) for rate adaptive pacing. As discussed above, the most important mechanism for increasing cardiac output with exercise is the capacity to double or triple the heart rate since stroke volume can only be increased slightly compared with rest. Most sensors attempt to detect motion by the patient, such as walking, via an accelerometer.
Limitations to the effectiveness of a motion-based sensor include the potential for a car passenger on a bumpy road or a patient with a tremor to experience a rate increase (worse with vibration sensors), or conversely, for a rock climber or cyclist (whose torso is moving little) to undergo negligible rate augmentation (e.g., with accelerometers). Consequently, numerous modalities have been attempted to detect the need for increased rate including respiratory volume change, catecholamine-induced increased myocardial contractility, the QT interval, pH, dP/dt, central venous temperature, oxygen saturation, peak endocardial acceleration as a measure of myocardial contractility, and RV lead impedance (correlated with contractility or inotropy). Of these, accelerometers are most widely used, with minute ventilation and RV lead impedance also in use.
For the single chamber modes (VOO, VVI, AAI, AOO), which do not have an upper rate limit and only an LRL, their rate-responsive forms (VOOR, VVIR, AAIR, AOOR) now add an upper rate limit. When the patient is at rest, the device paces at the LRL. Otherwise the pacing rate is determined by the sensor. The goal is the gradual increase in rate to match the activity and after return to rest a gradual (not immediate) decrease in rate to baseline. Sensor gain or function can be calibrated to the patient’s specific physiology and activities with thresholds and response curves and other adjustments. Devices with dual sensor can use one to cross check the other or to blend the input from the two. For dual-chamber modes, already featuring an upper (tracking) rate limit, a second upper rate limit is defined for the sensor as well as the one for the maximum rate allowed in tracking the atrial rhythm.
When first devised, the DDD mode was highly problematic for patients with atrial tachyarrhythmias because atrial fibrillation would cause the device to abruptly pace at or near the upper rate limit. This problem led to the development of an ability to detect the presence of atrial fibrillation (flutter or tachycardia) and when present “mode switch” to a non-tracking mode, DDI(R) ( Fig. 69.11 ). Practically DDI(R) functions as VVI(R) but allows ongoing surveillance of the status of the atrial arrhythmia.

To achieve mode switching, atrial events needed to be identified even within certain refractory zones such as the PVARP. A minimal atrial rate to define the arrhythmia is programmable. The device switches back to tracking (DDD or DDDR) when it confirms that the tachyarrhythmia has ended.
A related issue is nonphysiologic noise detection and response. External electromagnetic interference, such as electrocautery, could be sensed as intrinsic cardiac rhythm leading to inappropriate inhibition, and in the pacemaker-dependent patient, asystole. Noise detection and rejection algorithms can allow the device to pace asynchronously in this scenario; if noise is anticipated, the pacemaker can be programmed to an asynchronous mode (DOO, VOO, or AOO) or a magnet could be applied.
While dual-chamber pacing restores AV synchrony, it exhibits the disadvantage of a tendency to pace the right ventricle, even in the absence of complete AV block. As compared with native sinus rhythm, when the atrium is paced, intraatrial conduction is prolonged, and at faster rates AV nodal conduction may be prolonged. These two factors tend to result in ventricular pacing, unless the AV delay is set to a nonphysiologic long setting. Lengthening the AV delay also compromises the upper tracking rate. The heightened focus on the detrimental effect of RV pacing led to the development of modes or algorithms to reduce RV pacing for patients with generally intact AV conduction. Initially, these algorithms consisted of a programmable increase in AV interval. When conduction is lost, pacing occurs at a shorter, more physiologic AV interval, and periodic testing for AV conduction occurs by lengthening the AV delay. However, these proved only moderately effective at reducing RV pacing. Instead new modes that functioned essentially as single chamber atrial pacing (AAI/AAIR) but with backup ventricular pacing in the event of temporary AV block were devised. These can be described in the BPEG naming convention as ADI/ADIR ( Fig. 69.12 ). When persistent AV block develops, the device switches to DDD/DDDR. Some variation of their function among the different manufacturers exists.

Randomized trials have established that dual-chamber pacing is superior in reducing the occurrence of atrial fibrillation and possibly stroke, though a reduction in mortality has not been proven. Consequently a dual-chamber device is favored except when permanent atrial fibrillation is present. The recent availability of single chamber (ventricular) leadless pacemakers has led to a greater usage of these single chamber systems in some patients who would traditionally receive a dual-chamber device. Optimal leadless pacemaker candidates include those with increased infection risk, with limited vascular access, and who are expected to have a low (ventricular) pacing burden, as well as patients with minimal benefit from dual-chamber pacing due to advanced comorbidities or reduced activity. In addition, leadless devices with some atrial tracking capability have been developed, although atrial pacing is not available yet.
With growing complexity of pacemaker systems, many suspected pacing abnormalities are eventually deemed normal function after evaluation. Nevertheless, device dysfunction does occur and can result in serious consequence. Potential malfunction should be evaluated thoroughly via a multimodality approach harnessing all potential resources, such as telemetry, multichannel electrocardiography, the device programmer, and knowledge of the programmed parameters including active device algorithms. Moreover, it is important to take into account the patient’s location and potential external environment (EMI), radiography, stored device data, and provocative investigation such as pocket manipulation or arm movement. The most frequent issues are undersensing ( eFig. 69.9 ), failure to capture, failure to pace, and pacing at an unexpected rate ( Table 69.2 ).
| Failure to Capture |
| Pacing output below threshold Changes at electrode-myocardial interface Output programmed below threshold Lead dislodgement Lead insulation failure or conductor fracture Connection problem between header and lead Functional failure to capture (undersensing or asynchronous pacing) |
| Failure to Pace |
| Corrected by magnet or programming to asynchronous mode Oversensing of physiologic or nonphysiologic signals Not corrected by magnet or programming to asynchronous mode Failure in the pulse generator Lead conductor fracture Connection problem between header and lead |
| Pacing at a Rate Not Consistent with Programmed Rate |
| Shorter-than-expected escape interval: undersensing Longer-than-expected escape interval: oversensing Battery depletion |
| Unanticipated Rapid Pacing |
| Pacemaker-mediated tachycardia Inappropriate ventricular tracking of rapid sensed atrial rates, electromagnetic interference, or myopotentials Sensor-driven pacing unrelated to patient activity |

Especially in the acute period after implantation, pacing lead dislodgement or elevation of threshold (to a value greater than the programmed value) will cause failure to capture ( Fig. 69.13 ). Threshold elevation sometimes is mitigated by device-automated threshold determination and programmed output alteration. Lead fracture or other failure can cause loss of capture. Lead connection issues at the generator’s header are also important to consider. Pathologic capture failure must be distinguished from physiologic capture failure that occurs when a pacing impulse falls in the refractory period, sometimes due to undersensing.

The flipside of failure to capture is failure to pace. Either can result in ventricular asystole. Failure to pace usually stems from oversensing, either of physiologic signals (P, R, or T waves), of noncardiac or external noise (EMI), of lead fracture, or from a loose header connection ( Fig. 69.14 ). Advanced battery depletion or catastrophic device failure can cause loss of output, too. A special case of failure to pace, called crosstalk, is only possible in dual-chamber devices and is discussed above ( eFig. 69.10 ).

Several conditions are prominent causes of pacing at or near the upper rate limit ( eTable 69.3 ). One should recall that atrial fibrillation with a rapid, natively conducted ventricular response can result in rapid rates just as in patients without pacemakers. For rate responsive devices, unexpected activation of an accelerometer sensor can occur due to vibratory movement (e.g., transportation) or hyperventilation for minute ventilation devices. In dual-chamber devices, tracking nonphysiologic atrial arrhythmias, atrial lead noise (fracture or EMI), or pacemaker-mediated tachycardia are causes of unexpectedly rapid (ventricular) pacing. Tracking an atrial tachyarrhythmia may occur if mode switch is not programmed on, if the atrial arrhythmia is below the mode switch rate, or if the atrial arrhythmia is undersensed. A special example of the latter is when every other atrial flutter beat falls in the postventricular atrial blanking period. Rapid nonphysiologic signals due to atrial lead fracture can lead to tracking near the upper rate. Similarly, external electromagnetic noise detected by the atrial lead can produce rapid ventricular pacing.
| Ventricular Pacing at Upper Rate Limit in DDD Mode |
|
| Other Causes |
|
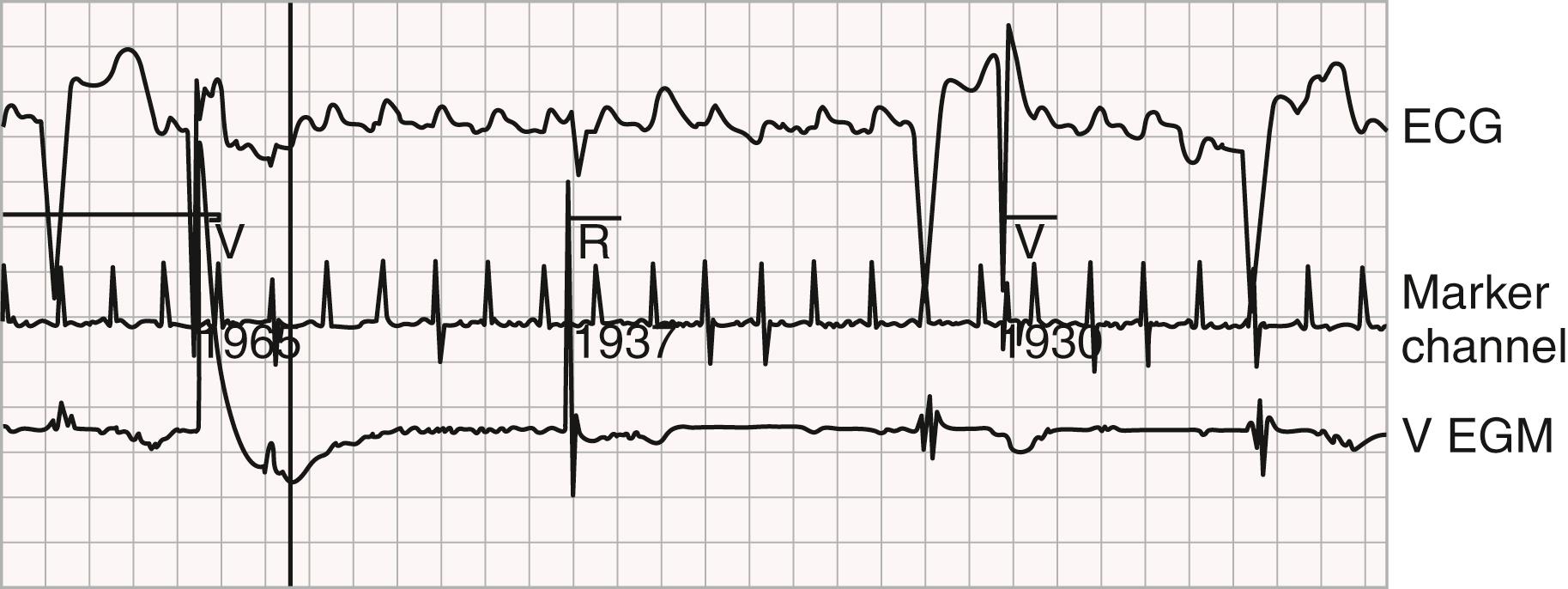
Pacemaker-mediated tachycardia (PMT), or endless loop tachycardia, originates with a ventricular impulse (especially a PVC) that conducts retrogradely to the atrium, where it is sensed and thus triggers a paced ventricular beat at the expiration of the AV delay ( eFig. 69.11 ). Subsequently, that ventricular-paced beat may again conduct retrogradely to the atrium and the “reentrant loop” continues. The ECG shows ventricular pacing at or near the upper rate limit, and retrograde P waves. PMT can be prevented by programming the PVARP to an interval longer than the observed VA conduction interval; in addition, PVARP extension can be programmed to occur for PVCs. Once begun, PMT termination algorithms function by omitting tracking for one atrial event. Applying a magnet will terminate it as well. PMT will not occur in the DDI mode since it is dependent upon atrial tracking, but it can occur in VDD, as well as DDD.

Non-reentrant ventricular-atrial synchrony, similar to PMT, also starts most commonly with a PVC that conducts retrogradely. However, due to timing and programming particulars, it falls in the PVARP. Atrial pacing then occurs at the LRL (or at the sensor indicated rate). However, in this scenario the atrial pacing pulse does not capture due to functional refractoriness from the preceding retrograde atrial activation. The sequence then repeats. It can be prevented by shortening the AV delay, reducing the PVARP and/or by reducing the lower rate, such as by inactivating rate-response pacing.
If the sinus rate exceeds the upper tracking rate in the setting of AV block, the pacing rate will fall. Dependent upon certain timing relationships (upper tracking rate [UTR], atrioventricular interval [AVI], PVARP), a phenomenon resembling AV Wenckebach or of 2:1 AV block can occur. Ideally, the UTR should be programmed high enough to avoid a drop in pacing rate with exercise. See Figure 69.15 for details.

Pacing-induced proarrhythmia includes several subtypes ( eFig. 69.12 ). The simplest form is an R-on-T ventricular pacing in the VOO mode triggering VT or VF. Any form of ventricular or atrial undersensing may allow pacing to trigger an arrhythmia. Even ventricular escape pacing at a low rate can rarely trigger ventricular tachyarrhythmias. A pause in pacing due to loss of capture during a threshold test or an RV pacing-minimization algorithm can initiate VT or VF by a short-long-short sequence. A pacing lead may mechanically trigger ectopic impulses, too. Competitive atrial pacing can trigger atrial tachyarrhythmias. Some manufacturers enable programming of a noncompetitive atrial pacing interval to minimize such events.

Numerous forms of pacemaker function can appear abnormal depending upon the type and amount of ECG data available, the presence or absence of marker channels, and the type of algorithms and programming in effect. ECG records may either suggest a pacing pulse is not present or fail to disclose one (bipolar pacing, dependent on lead vector, etc.). In-person threshold tests with loss of capture or change in rate may be noted on later review of telemetry as a possible abnormality. Similarly, automatic pacing thresholds or AV search algorithms may prompt concern when seen on a monitor strip.
Like implanted pacemakers, conventional ICD components include a PG that contains the battery and circuitry, and a lead system. The PG is larger than those for pacemakers, as the device must contain a larger battery and capacitors capable of generating higher voltage shocks. Transvenous ICDs incorporate fully functional antibradycardic pacing. The lead system consists of at least one lead that has one or two defibrillating coils along with pace/sense electrodes, typically placed at the RV apex ( Figs. 69.2 and 69.16 ). Dual-chamber ICDs include a pace/sense port that is usually connected to a lead placed in the RA, thus providing along with the RV lead, dual-chamber pacing capabilities. A third port for CRT can be connected to a pacing lead placed to pace the LV via a lead in a branch of the coronary sinus or implanted on the LV epicardial surface (see Fig. 69.3 ). Like pacing systems, newer pacemaker configurations may include a lead intended to pace the cardiac conduction system, using a lead placed at the His bundle or deep into the interventricular septum to capture the left bundle branch. Subcutaneous ICDs are also commercially available and consist of a PG typically placed subcutaneously in the left lateral chest connected to a subcutaneously tunneled sensing and defibrillation lead ( Fig. 69.5 ).

ICDs are indicated for prevention of sudden death from VT/VF, either as secondary prevention in patients who have been resuscitated from VT/VF or primary prevention in patients who have not had VT/VF but are at sufficiently high risk to warrant protection with an ICD.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here