Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ovary is a solid organ composed of (1) surface epithelium , an extension of the pelvic peritoneum; (2) the cortex , composed of cellular stroma, follicular units (comprised of a central oocyte surrounded by granulosa and theca cells), corpora lutea, and corpora albicantia; and (3) the medulla , composed of large vessels that merge in to the ovarian hilum , which is the point of attachment of the ovarian vessels (artery and vein) and the broad ligament. These compartments harbor elements that can be confused with benign and malignant epithelial lesions, including the following:
Endosalpingiosis: benign cortical glandular elements with tubal differentiation, including ciliated, nonciliated, and peg cells. When cystic, denominated Müllerian inclusion cysts (described later).
Peritoneal inclusions: benign cortical elements lined by flat to low cuboidal bland epithelium. When cystic, denominated peritoneal inclusion cysts (described later).
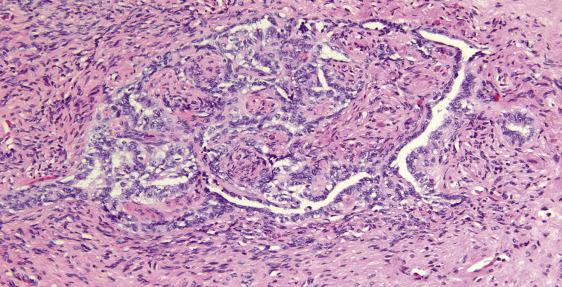
Rete ovarii: embryologic equivalent of the rete testis, seen in the hilar portion of the ovary as an area of compressed and irregularly shaped glandular structures with narrow lumens and low cuboidal bland epithelium ( Fig. 13.1 ).
Nonneoplastic lesions of the ovary may be entirely asymptomatic incidental findings identified on gross or microscopic examination of the ovary, or they may be associated with a pelvic mass, pain, or manifestations of abnormal hormonal regulation. Many occur during the reproductive years and may be associated with infertility. They are an important category in neonates, children, and reproductive-aged women, and should always be considered in the differential diagnosis of cystic masses at this site.
Solitary ovarian cysts of follicular origin occur at three different age peaks: neonates (rare after 6 months of age), around menarche (often associated with endocrine manifestations) and in perimenopause but may occur anytime during reproductive age as a transient physiologic finding. In neonates, the cysts are thought to develop secondary to in utero stimulation by maternal hormones. Multiple follicular cysts associated with sclerosis of the superficial cortical stroma (sclerocystic ovaries) are usually indicative of chronic anovulation. Multiple follicular cysts secondary to excess of gonadotropins (hyperreactio luteinalis or ovarian hyperstimulation syndrome) are discussed later in this chapter, under the heading “Pregnancy-Associated Changes.” In contrast to follicular cysts, luteal cysts are much less frequent and typically occur in reproductive-age women as the normal corpus luteum undergoes hemorrhage and involution.
Solitary follicular cysts are usually an incidental finding; when persistent, they may cause symptoms secondary to a pelvic mass and are typically excised to exclude malignancy. Acute abdominal pain secondary to torsion, with or without hemoperitoneum, may occur, particularly in neonates, although most follicular cysts that occur during the neonatal period regress within the first 6 months. Occasionally, follicular cysts may be associated with manifestations of excess estrogen production (isosexual pseudoprecocity in children or irregular menses related to disordered proliferative endometrium and/or endometrial hyperplasia in women of reproductive age). Postpubertal women with cystic fibrosis may have a predisposition to developing solitary follicular cysts.
Multiple follicular cysts are part of the spectrum of abnormalities seen in polycystic ovary syndrome (PCOS), a common disorder characterized by hyperandrogenism of ovarian origin resulting in virilization, infertility, obesity, and insulin resistance (Stein–Leventhal syndrome). Only up to 10% of women with polycystic ovaries have clinical manifestations. A family history is common, but the genetic factors in this disease are poorly understood. The most common etiology is insulin resistance of peripheral tissue and/or an abnormality of the hypothalamic–pituitary–ovarian axis. The findings of multiple ovarian cysts associated with cortical sclerosis, although part of the spectrum of PCOS, is not specific and can be seen in other causes of chronic anovulation; therefore, clinical and biochemical assessment is required for this diagnosis. Solitary or multiple follicular cysts may also occur in association with McCune–Albright syndrome (polyostotic fibrous dysplasia, cutaneous melanin pigmentation, and endocrine organ hyperactivity) and in prepubertal patients with hypothyroidism.
Cystic corpora lutea are a frequent finding in residual ovarian tissue in the setting of ovarian remnant syndrome. They may manifest as menstrual irregularities and rarely hematoperitoneum.
Transvaginal ultrasound shows simple thin-walled cysts; however, if there is intracystic hemorrhage, the appearance is that of a complex adnexal mass and may suggest a neoplasm. Small cysts can be followed with ultrasound; their disappearance over a period of weeks allows a presumptive benign diagnosis. The ultrasound appearance of multiple cysts in the ovarian cortex, associated with PCOS, is characteristic but, as noted previously, not specific.
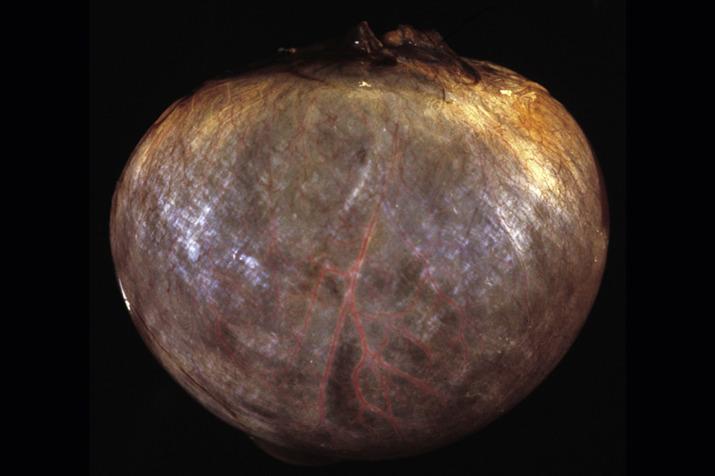
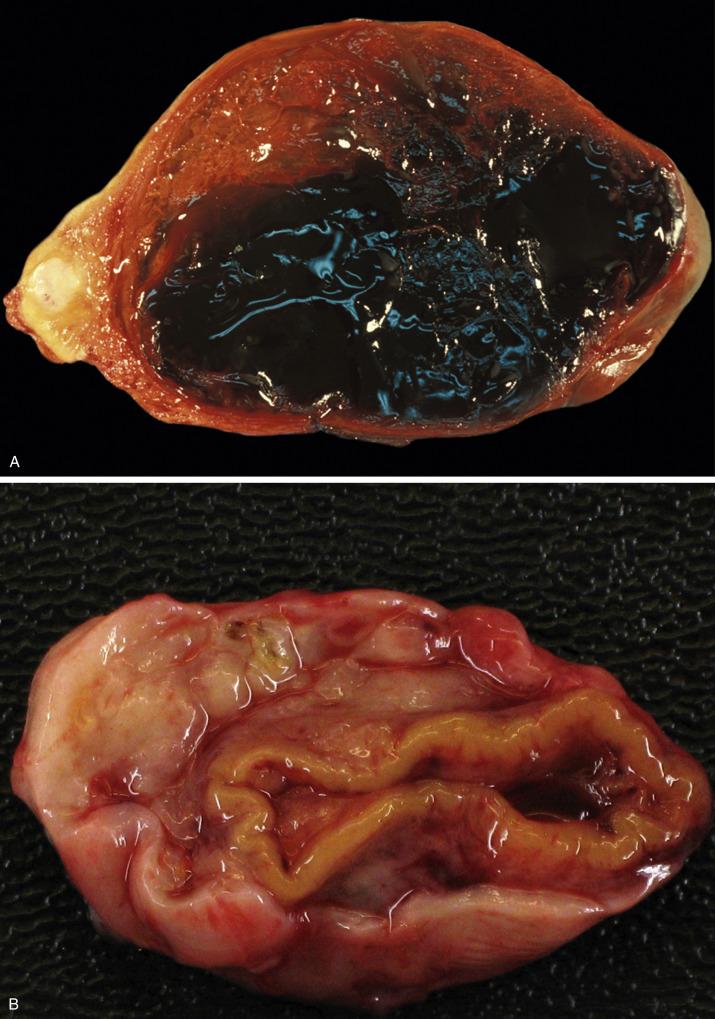
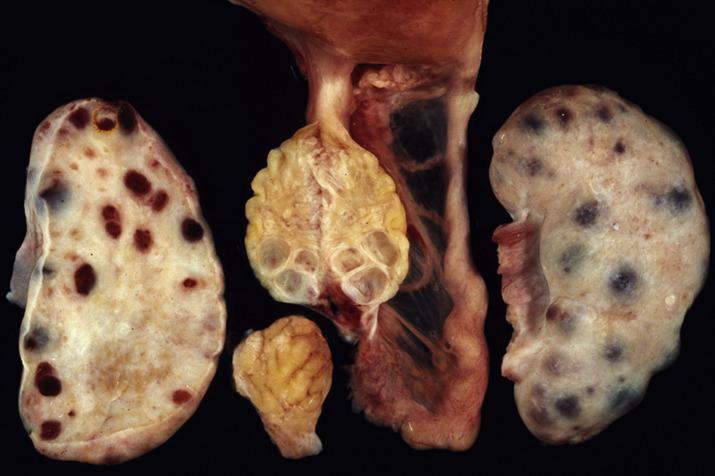
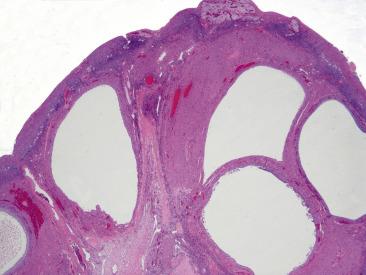
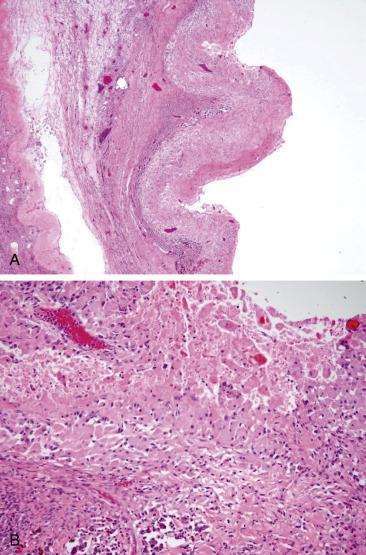
Solitary follicular cysts ( Fig. 13.2 ) have smooth surfaces, are thin walled, and are, by definition, >3 cm in diameter (but usually <10 cm). Cysts in neonates can be larger (average 8 cm). Cysts <3 cm are referred to as “cystic follicles” and are considered to be within the range of normal follicular development. Cyst contents are typically serous or serosanguinous. A corpus luteum cyst, also, by definition, >3 cm in diameter, more commonly contains intracystic hemorrhage and exhibits an undulating yellow rim of variable thickness ( Fig. 13.3A,B ). As follicular and luteal cysts are often received fragmented at the time of gross examination, exact measurement is not possible and designation as a cyst should rely on clinical/radiologic impression. Ovaries in PCOS typically show bilateral enlargement with numerous cortical cysts, most measuring <3 cm, and a band of white firm tissue that replaces the superficial cortex overlying the cysts ( Fig. 13.4 ).
Follicular cysts, whether solitary or multiple, are lined by an inner layer of granulosa cells and surrounded by an outer layer of theca interna cells ( Fig. 13.5 ). Both are composed of round cells in a stratified or layered configuration. Granulosa cells have round nuclei with very scant clear cytoplasm, whereas theca cells have more abundant eosinophilic cytoplasm and less hyperchromatic nuclei. Both granulosa and theca interna layers can display variable degrees of luteinization. Sometimes, the distinction between the two cell layers may be difficult and a reticulin may be of help as theca cells are typically surrounded by individual reticulum fibers whereas granulosa cells lack reticulin. If torsion occurs, the cyst, as well as the surrounding ovary, may infarct and the cyst contents have a grumous appearance ( Fig. 13.6A ). If infarction occurs, careful scrutiny of the cyst should be performed in order to exclude malignancy, and extensive sampling is indicated. Ovarian parenchyma near the cyst may show ghost cells including primary follicles ( Fig. 13.6B ).
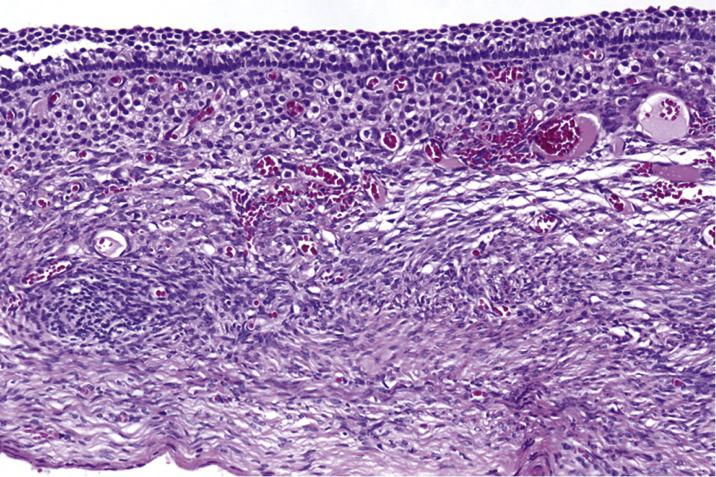
Corpora lutea cysts have undulating contours and show prominent luteinization of granulosa and, to a lesser extent, theca interna cells ( Fig. 13.7 ). In ovaries removed from patients with PCOS, there is prominent sclerosis of the superficial cortex, visible as a pale rim of heavily collagenized stroma underlying the surface epithelium, associated with multiple relatively uniformly sized and evenly spaced follicular cysts ( Fig. 13.8 ). Usually, the theca interna is thicker than in a normal follicle and the theca cells often appear luteinized. The granulosa cell layer may be of variable thickness, but it is typically not luteinized. Evidence of prior ovulation (e.g., corpora albicantia or lutea) is sparse. It is also common to find increased numbers of hilar cells.
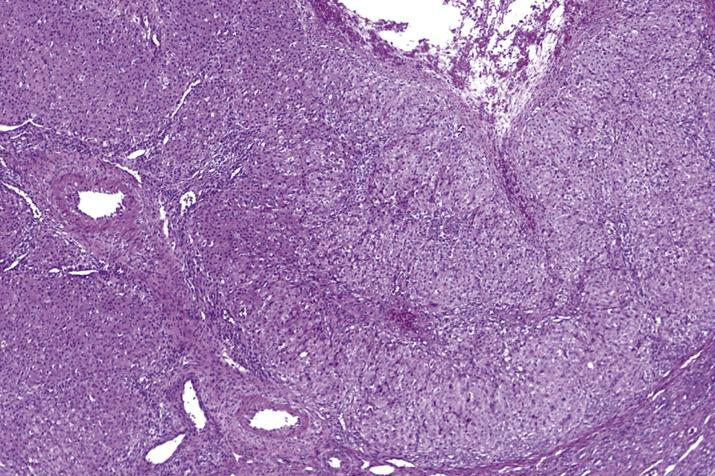
The granulosa and theca cells are positive for inhibin, calretinin, and SF-1.
Normal and cystic follicles do not harbor mutations in FOXL2 .
The chief differential diagnosis is with epithelial cysts , particularly when the lining is partially denuded. The most helpful features in establishing the diagnosis of a follicular cyst are the findings of surrounding theca cells, the stratification of round nuclei devoid of significant cytoplasm, and the lack of cilia. Cystic struma ovarii may also mimic a follicular cyst but will typically show small follicles, which may contain eosinophilic colloid within the wall. Immunohistochemistry can be used in difficult cases as epithelial cells are positive for epithelial membrane antigen and negative for inhibin; cystic struma is positive for TTF1 and thyroglobulin. Solitary follicular cysts must also be distinguished from unilocular cystic granulosa cell tumors , which are typically larger and have a thicker granulosa cell lining (average 10 cells thick), which sometimes displays papillary or microfollicular patterns (the former with fibrous cores, the latter with Call-Exner bodies). An important pitfall to keep in mind is that cystic granulosa cell tumors can have luteinized theca cells at the periphery, although most often the wall of the cyst is composed of fibrous tissue. Sampling of the adjacent parenchyma is important, as it may reveal more typical areas of granulosa cell tumor. In difficult cases, the presence of a FOXL2 mutation will be confirmatory of adult-type granulosa cell tumor.
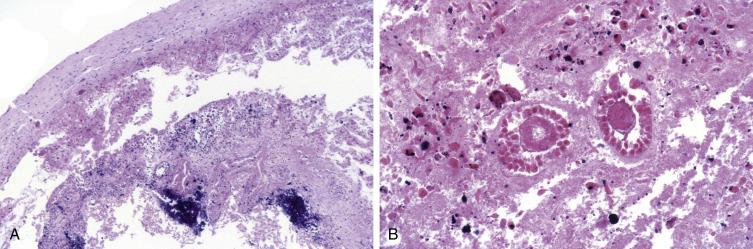
The main differential diagnostic consideration for a corpus luteum cyst is with a steroid cell tumor as the periphery of the former can have a nested architecture and cells with abundant pale eosinophilic cytoplasm. However, the low-power undulating contours, as well as the central cystic component often filled with recent or clotted blood, will help in establishing the diagnosis of a corpus luteum cyst. In addition, patients with steroid cell tumors often present with hormonal manifestations (typically androgenic). The lining of endometriotic cysts is often denuded and may be replaced by abundant foamy cells with ample eosinophilic cytoplasm, thus mimicking the appearance of a corpus luteum cyst ( Fig. 13.9A,B ). The presence of recent or old hemorrhage should prompt careful scrutiny for endometrial-type epithelium and stroma.
Although the histologic findings of PCOS are classic, diagnosis should not be based on histopathologic findings alone as similar findings can be seen in other diseases that may be associated with chronic anovulation including autoimmune oophoritis, multiple follicular cysts secondary to hypothyroidism, and adrenal disorders including congenital adrenal hyperplasia; therefore, clinical-pathologic correlation is essential.
Solitary follicular and corpora lutea cysts will usually resolve spontaneously. Management of PCOS depends on the severity of the clinical manifestations and whether pregnancy is desired. Depending on the symptoms and the fertility goals, patients undergo hormonal therapy (oral contraceptives or progestins), ovulation induction, weight loss, and medical treatment of insulin resistance and hirsutism.
Physiologic (functional) follicle cyst or corpus luteum cyst measuring >3 cm (smaller lesions arbitrarily designated as “cystic follicles”)
Common, especially during neonatal (<6 months), peripubertal, and reproductive years
Often self-limited, but acute abdomen may occur secondary to rupture or torsion with or without hematoperitoneum
If persistent, may require surgical removal to exclude a neoplasm
Three age peaks: neonates (<6 months), peripubertal, reproductive age
Asymptomatic, pelvic mass, and/or pain
Menstrual irregularities, occasionally amenorrhea
Precocious pseudopuberty in prepubertal patients
Acute abdominal pain ± hemoperitoneum, if rupture or torsion
Benign
Most solitary cysts resolve spontaneously
Cystectomy if persistence or rupture
Solitary, fluid-filled cyst measuring >3 cm in diameter
Follicular cyst lined by granulosa cells, with surrounding layer of theca cells; both layers may show luteinization
Corpus luteum cyst composed of thick undulating layer of granulosa and theca cells with prominent luteinization
Granulosa and theca cells are positive for inhibin, calretinin, and SF-1.
Absence of FOXL2 mutations
Cystic granulosa cell tumor
Surface epithelial cystadenoma
Unilocular cystic struma ovarii
Steroid cell tumor
Endometriotic cyst
Clinicopathologic syndrome characterized by anovulation, menstrual dysfunction, hyperandrogenism, and enlarged polycystic ovaries (Stein–Leventhal syndrome). Varied etiology, but more commonly insulin resistance of peripheral tissue and/or abnormality of the hypothalamic–pituitary–ovarian axis
Affects up to 20% of reproductive-age women
Increased incidence among first-degree relatives suggesting autosomal-dominant mode of inheritance
Infertility
Metabolic syndrome, hypertension, dyslipidemia, glucose intolerance, and/or diabetes, secondary to insulin resistance (up to 50%–70%)
Endometrial proliferation secondary to hyperestrogenism (disordered proliferative endometrium, endometrial hyperplasia and/or endometrioid adenocarcinoma)
Perimenarchal onset
Anovulation, infertility, menstrual disturbances
Obesity
Hirsutism, acne, hair loss
Ovulation induction, weight loss, hormonal therapy
Treatment of underlying insulin resistance or hirsutism if indicated
Enlarged ovaries with multiple, often uniformly sized follicular cysts
Thickened and white appearing superficial cortex
Collagenized superficial cortex
Multiple uniformly sized and spaced follicular cysts present close to ovarian surface
Follicular cysts with prominent thick and luteinized theca interna layer
Prominent luteinization of ovarian stroma
Hilus cell hyperplasia
Multiple follicular cysts secondary to hypothyroidism
Adrenal diseases such as congenital adrenal hyperplasia
Autoimmune oophoritis
Stromal hyperplasia is an exaggerated nonneoplastic proliferation of ovarian stromal cells. Stromal hyperthecosis is defined as stromal cell luteinization in the ovarian parenchyma away from the follicular units; it commonly happens in the setting of stromal hyperplasia.
Stromal hyperplasia and hyperthecosis are most common in patients in their sixth to seventh decade; in this age group, stromal hyperplasia is seen in up to one-third of women. Many patients are asymptomatic. Symptoms, which are more common in hyperthecosis, include:
Androgen manifestations, characteristically virilization and acne.
Estrogenic manifestations, including abnormal uterine bleeding due to disordered proliferative endometrium, endometrial hyperplasia, or even well-differentiated endometrioid adenocarcinoma.
Other symptoms such as obesity, hypertension, and hyperinsulinemia. A small percentage of patients have HAIR-AN syndrome, which consists of hyperandrogenism (HA), insulin resistance (IR), and acanthosis nigricans (AN).
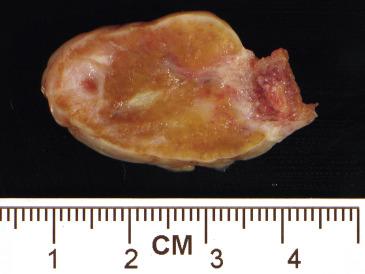
Stromal hyperplasia and stromal hyperthecosis typically involve both ovaries, which may be normal sized or enlarged (up to 7.0 cm). They display a diffuse or nodular, firm cut surface that is white to yellow (stromal hyperplasia) or yellow to brown (stromal hyperthecosis) ( Fig. 13.10 ).
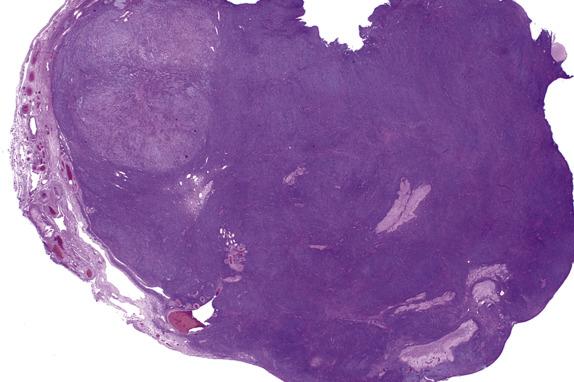
Stromal hyperplasia is characterized by a nodular and/or diffuse, cortical and medullary (latter more frequent) proliferation of mitotically inactive stromal cells with ovoid nuclei and indistinct nucleoli ( Fig. 13.11 ). In stromal hyperthecosis, nodular aggregates (up to 0.5 cm; Fig. 13.12A ), nests, or isolated luteinized cells are present in a background of stromal hyperplasia ( Fig. 13.12B ). The luteinized cells have abundant eosinophilic or vacuolated cytoplasm and central, round nuclei with small nucleoli. Hilar cell hyperplasia may be seen with stromal hyperplasia or stromal hyperthecosis. In addition, in patients with HAIR-AN syndrome, the ovaries show multiple follicle cysts associated with cortical sclerosis, akin to that seen in PCOS.
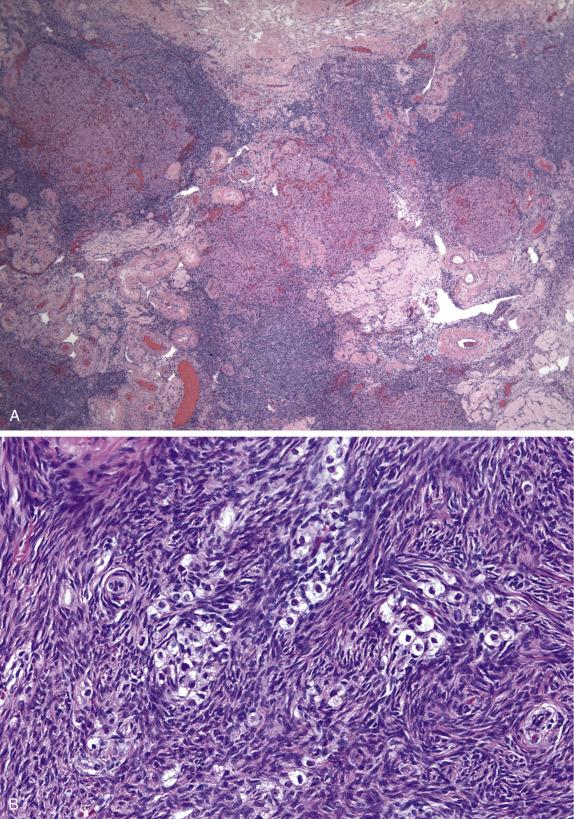
The spindle cells of stromal hyperplasia and the luteinized stromal cells of hyperthecosis are positive for calretinin, WT1, and SF-1. Luteinized stromal cells can also be positive for Melan-A and inhibin ( Fig. 13.12C ).
Stromal hyperthecosis should be distinguished from other processes characterized by lipid-laden cells such as thecoma and steroid cell tumors, including stromal luteoma. In the past, stromal luteoma was defined as a tumor composed of lutein cells <2 cm in size that typically arose in a background of stromal hyperthecosis. Currently, stromal luteoma does not represent a distinct entity since it was incorporated into the steroid cell tumor category. In contrast to stromal hyperthecosis, thecoma and steroid cell tumor form a distinct mass or nodule on macroscopic examination and are typically unilateral.
Patients with hyperandrogenic manifestations may respond to gonadotropin-releasing hormone (GnRH) agonists or oral contraceptives. Bilateral oophorectomy results in complete resolution of the androgenic symptoms, but the insulin resistance in HAIR-AN syndrome usually persists.
Stromal hyperplasia: nonneoplastic exaggerated proliferation of ovarian stromal cells
Stromal hyperthecosis: luteinized stromal cells dispersed throughout parenchyma away from follicles and theca layers, typically in setting of stromal hyperplasia
Common
Secondary to severity and complications of hormonal manifestations
More common in sixth to seventh decades
Often asymptomatic
Estrogenic manifestations (disordered proliferative endometrium, endometrial hyperplasia/carcinoma)
Androgenic manifestations (virilization, acne)
Obesity, hypertension, glucose intolerance
Rarely HAIR-AN syndrome (hyperandrogenism, insulin resistance, and acanthosis nigricans)
GnRH agonists or oral contraceptives if hyperandrogenic manifestations
Bilateral oophorectomy leads to complete resolution of androgenic symptoms (except insulin resistance in HAIR-AN syndrome).
Normal or enlarged ovaries (up to 7 cm) with diffuse or nodular firm, white or yellow (hyperplasia) or yellow to brown (hyperthecosis) cut surface
Stromal hyperplasia:
Involvement of medulla > cortex
Diffuse or nodular architecture
Oval to spindled cells with scant cytoplasm
May be associated with hilar cell hyperplasia
Stromal hyperthecosis:
Single cells or aggregates of large rounded cells with abundant eosinophilic or vacuolated cytoplasm and central, round nuclei with small nucleoli
Typical background of stromal hyperplasia
In the setting of HAIR-AN syndrome, numerous follicular cysts and cortical sclerosis (similar to PCOS)
May be associated with hilar cell hyperplasia
Stromal and luteinized cells are positive for calretinin, WT1, and SF-1.
Hyperthecosis cells can express Melan-A and inhibin.
Stromal hyperplasia: ovarian fibroma
Stromal hyperthecosis: thecoma, steroid cell tumors
This is a benign proliferation of hilar cells (also termed Leydig cells) that most commonly occur within the ovarian hilus or much less frequently within the ovarian stroma or in the wall of the fallopian tube.
Hilar cell hyperplasia is often an incidental finding in ovaries removed for an unrelated gynecologic disorder. It typically occurs in postmenopausal women in association with stromal hyperplasia or stromal hyperthecosis as well as in pregnant women. Mild hyperplasia is very common and usually subclinical. More significant forms of hilar cell hyperplasia are associated with virilization and most frequently occur in younger women, occasionally during pregnancy.
Ovaries with hilar cell hyperplasia, in the absence of associated stromal hyperplasia or stromal hyperthecosis, are grossly unremarkable.
Hilar cell hyperplasia is located in the interface between ovarian parenchyma and the supporting vascular and connective tissues (the ovarian “hilum”). It is typically seen as nodular aggregates, nests, clusters or, less commonly, diffuse sheets of polygonal cells ( Fig. 13.13A ). The cells have abundant eosinophilic cytoplasm and rounded, hyperchromatic nuclei, usually monomorphic but occasionally displaying nuclear pleomorphism. Intracytoplasmic elongated, eosinophilic Reinke crystals, when present, are pathognomonic ( Fig. 13.13B ). Mitotic figures may be present. Oftentimes, the cells are in close association with peripheral nerve bundles.
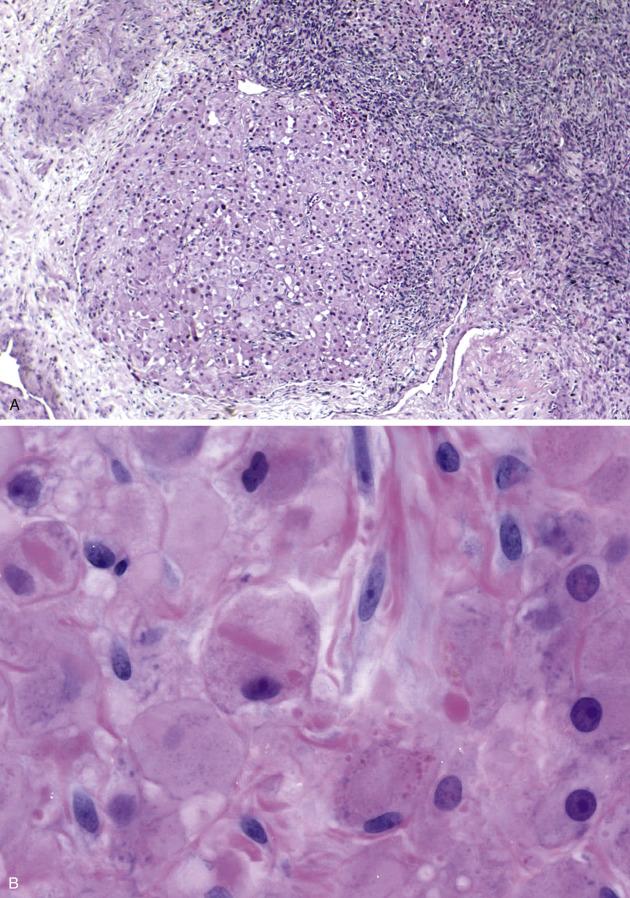
The hilar cells are positive for inhibin, calretinin, SF-1, and Melan-A.
The high-power features of hilar cell hyperplasia closely overlap with those seen in a hilar (Leydig) cell tumor ; however, the latter forms a mass, it may have fibrinoid necrosis of blood vessels, often shows aggregation of nuclei alternating with nuclear free areas, and is usually associated with endocrine manifestations. Hilar cell hyperplasia involving the ovarian cortex can be indistinguishable from stromal hyperthecosis , unless Reinke crystals are identified.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here