Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Prosthetic valves have known thromboembolic complications, especially in the short term, with an incidence observed to be <1% to almost 8% in the first year after transcatheter aortic valve implantation (TAVI). However, the prevention of these complications with routine use of antithrombotic therapy is challenging in this patient population.
Risk factors that have been reported for stroke in the early period after TAVI include history of atrial fibrillation, female sex, renal dysfunction, and vascular complications.
Multiple risk factors for bleeding may also exist, including advanced age, frailty, and systemic medical comorbidities of noncardiac organ systems. These contribute to the risk of both long-term bleeding and immediate periprocedural and short-term bleeding.
Current guideline recommendations for antithrombotic therapy after TAVI ( Table 15.1 ) are predominantly based on nonrandomized data and expert consensus according to the protocols used in pivotal clinical trials of TAVI ( Table 15.2 ). , Randomized controlled data are now becoming available as well.
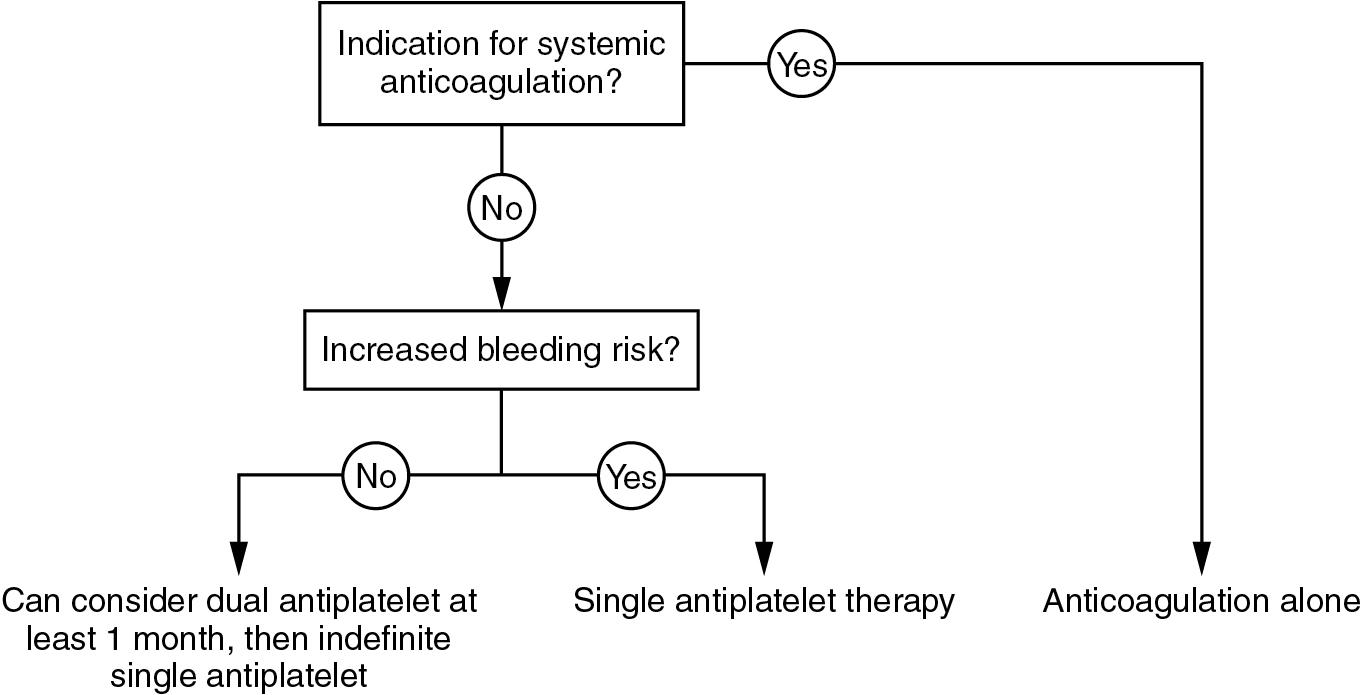
It is most common to use dual antiplatelet therapy for a limited number of months and indefinite aspirin in the absence of contraindications.
If systemic anticoagulation is indicated, additional single antiplatelet therapy can be considered for a limited period of time or not at all if the bleeding risks are likely to outweigh the benefit.
| ACC/AHA 2020 | Class | LOE | ESC/EACTS 2017 | Class | LOE | |
|---|---|---|---|---|---|---|
| Aspirin | Indefinitely | IIa | B-R | Indefinitely | IIa | C |
| Clopidogrel | Dual antiplatelet for 3–6 months may be reasonable if at low risk of bleeding | IIb | B-NR |
|
|
|
| Vitamin K antagonist | May be reasonable for at least 3 months if low risk of bleeding (INR 2.5) | IIb | B-NR | No recommendation | ||
| Direct oral anticoagulants | Low-dose rivaroxaban (10 mg daily) plus aspirin (75–100 mg) contraindicated if no indication for anticoagulation | III: Harm | B-R | No recommendation |
| PARTNER 1 | PARTNER 2 | PARTNER 3 | US CoreValve Pivotal | SURTAVI | Evolut Low Risk | |
|---|---|---|---|---|---|---|
| Aspirin | 6 months | Indefinitely | At least 1 month dual antiplatelet therapy | 3 months dual antiplatelet then single indefinitely | 3 months dual antiplatelet then single indefinitely | Not specified |
| Clopidogrel | 6 months | Minimum 1 month | ||||
| Vitamin K antagonist | If other indication present, then only single antiplatelet added | If other indication present, then only single antiplatelet added |
The objective of routine antiplatelet therapy after TAVI is to prevent the formation of thrombus on metallic stent structure before endothelialization, similar to what is done in the setting of stents used for percutaneous coronary intervention. Endothelialization of TAVI valves has been investigated in postmortem studies but the overall amount of data are limited. There is suggestion that although a significant portion of the outer stent structure of TAVI valves becomes endothelialized, areas that are not in direct contact with native tissue may not endothelialize at all in the short to medium term. Complete or near-complete endothelialization is expected in approximately 3 months after implantation.
Single versus dual antiplatelet therapy after TAVI has been evaluated in several observational and randomized controlled studies ( Table 15.3 ). It should be noted that the statistical power to detect differences in clinical outcomes was limited in some of these small studies.
Overall, these short-term studies suggest that single antiplatelet therapy does not appear to be inferior to dual antiplatelet therapy in preventing thromboembolic events or mortality.
The risk of bleeding complications was noted to be statistically significantly higher in one study (POPular TAVI EU), which was consistent with a trend noted in the earlier smaller ARTE study. This is not surprising given the body of evidence showing increased bleeding risk with more intensive antiplatelet and anticoagulation therapy observed in the setting of coronary artery disease, acute coronary syndrome, and percutaneous coronary intervention.
If there is an indication for systemic anticoagulation, usually atrial fibrillation, this should be continued after TAVI in the absence of any contraindication.
Antiplatelet therapy in addition to anticoagulation was evaluated in the randomized POPular TAVI EU trial, as well as small observational studies. In the POPular TAVI EU study, there was no reduction in a composite outcome including cardiovascular death, stroke, or myocardial infarction, but an increased risk of bleeding (predominantly at the TAVI access site) was seen.
| STUDY OR FIRST AUTHOR | ||||||
|---|---|---|---|---|---|---|
| POPular TAVI EU 1 | Ahmad et al. [CR] | Mangieri et al. [CR] | ARTE [CR] | SAT-TAVI [CR] | Ussia et al. [CR] | |
| Year | 2020 | 2018 | 2017 | 2017 | 2014 | 2011 |
| N | 690 | 11,781 | 439 | 222 | 120 | 79 |
| Design | Randomized open label | Meta-analysis | Observational | Randomized controlled | Randomized controlled | Randomized controlled |
| Intervention | Aspirin 80–100 mg daily | Dual antiplatelet | Dual antiplatelet | Dual antiplatelet | Dual antiplatelet | Dual antiplatelet |
| Comparison | Aspirin 80–100 mg daily plus clopidogrel 75 mg daily for 3 months, then aspirin alone | Single antiplatelet | Aspirin 75–160 mg daily | Aspirin 80–100 mg daily | Aspirin 75–160 mg daily | Aspirin 100 mg daily |
| Outcomes | All bleeding, nonprocedure-related bleeding, composite bleeding plus MACE, MACE | Stroke, death, bleeding | Morality, stroke | Composite major/life-threatening bleeding and MACE | Major stroke, cardiovascular death | Mortality, MACE, bleeding |
| Follow-up | 12 months | 3 months | 12 months | 3 months | 6 months | 6 months |
| Key finding | Less bleeding (all bleeding RR 0.57 [95% CI 0.42–0.77], p = 0.001) and composite bleeding plus MACE with aspirin alone; single antiplatelet noninferior for MACE | No difference | No difference | Trend toward worse outcome with dual antiplatelet (primary composite 15.3% vs. 7.2%, p = 0.065) | No difference | No difference |
| Other findings | No difference in death, MI, stroke/TIA individually | Less vascular complications with single antiplatelet | ||||
Brouwer J, Nijenhuis VJ, Delewi R, et al. Aspirin with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med . 2020;383(15):1447-1457.
Ahmad Y, Demir O, Rajkumar C, et al. Optimal antiplatelet strategy after transcatheter aortic valve implantation: a meta-analysis. Open Heart . 2018;5(1):e000748.
Mangieri A, Jabbour RJ, Montalto C, et al. Single-antiplatelet therapy in patients with contraindication to dual-antiplatelet therapy after transcatheter aortic valve implantation. Am J Cardiol . 2017;119(7):1088-1093.
Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) randomized clinical trial. JACC Cardiovasc Interv . 2017;10(13):1357-1365.
Stabile E, Pucciarelli A, Cota L, et al. SAT-TAVI (Single Antiplatelet Therapy for TAVI) study: a pilot randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. Int J Cardiol . 2014;174(3):624-627.
Ussia GP, Scarabelli M, Mulè M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol . 2011;108(12):1772-1776.
Observational data have noted that subclinical leaflet thrombosis is seen in some patients after TAVI, most notably through the finding of hypoattenuated leaflet thickening on computed tomography (CT). , This finding has also been associated with increased transvalvular gradients as assessed by Doppler echocardiography.
The prevalence of subclinical leaflet thrombosis is highly variable in the literature, ranging from <10% to as high as 40%, depending on the study design and medication use.
In multiple studies, these abnormalities seen on CT improve or completely resolve after initiation of systemic anticoagulation with an associated drop in transvalvular gradients.
There is also an observed association between the use of systemic anticoagulation and a lower incidence of leaflet thickening.
TAVI valves may be at higher risk of thrombus formation compared with surgical aortic valves for many reasons. Crimping causes some degree of trauma to the tissue leaflets, flow dynamics related to the native diseased aortic valve adjacent to the prosthesis may cause some degree of blood stasis, and prosthetic leaflet function may be affected by variability in the degree of prosthesis expansion because of the degree and pattern of native aortic valvular and annular calcification. The exact impact of each factor and strategies to reduce mechanical risk factors for prosthetic thrombus are not well studied. Most TAVI prostheses are manufactured using either bovine or porcine pericardial tissue that has been processed for sterility, immunogenicity, and durability. Although the final tissue characteristics may have implications on leaflet thrombogenicity, there are no studies investigating specific materials or processing in detail to fully understand the potential clinical impact.
Routine use of direct oral anticoagulants in the absence of other indications for anticoagulation is not well studied but has become an area of great clinical interest.
A single randomized trial (GALILEO) evaluated the use of the direct factor Xa inhibitor rivaroxaban after TAVI in patients without a specific indication for anticoagulation. In this study and the GALILEO-4D imaging substudy, whereas presence of subclinical leaflet thickening on CT was lower with rivaroxaban, the clinical outcomes of death combined with first thromboembolic event and overall bleeding were higher.
These findings highlight that the risks of anticoagulation in this patient population are very important to consider and are consistent with findings from other observational studies ( Table 15.4 ).
| STUDY | |||||
|---|---|---|---|---|---|
| POPular TAVI EU [CR] | GALILEO [CR] | FRANCE TAVI [CR] | RESOLVE/SAVORY 4 | ATLANTIS [CR] | |
| Year | 2020 | 2020 | 2019 | 2017 | 2022 |
| N | 326 | 1644 | 11,469 | 890 | Estimated 1510 |
| Design | Randomized open label | Randomized open label | Observational registry | Observational registry | Randomized open label |
| Key exclusion criteria | Intolerant to clopidogrel, drug-eluting stent within 3 months, bare-metal stent within 1 month | Indication for long-term anticoagulation or dual antiplatelet therapy | Lack of interpretable CT scans or CT imaging | Contraindication to oral anticoagulants | |
| Intervention | Oral anticoagulation alone | Rivaroxaban 10 mg daily (+ ASA 75–100 mg daily for 3 months) | Oral anticoagulation | Oral anticoagulation | Apixaban 5 mg twice daily |
| Comparison | Oral anticoagulation plus clopidogrel 75 mg daily for 3 months | ASA 75–100 mg daily (+ clopidogrel 75 mg daily for 3 months) | Antiplatelet therapy | Dual antiplatelet therapy | Antiplatelet therapy or vitamin K antagonist (stratified based on baseline indication for anticoagulation) |
| Outcome | All bleeding, nonprocedure-related bleeding, composite bleeding plus MACE, MACE | Death or first thromboembolic event | All-cause mortality, bioprosthetic valve dysfunction | Subclinical leaflet thrombosis, leaflet thrombosis resolution, stroke, TIA | Composite including death, MI, stroke, embolism, prosthesis thrombus, DVT, PE, major/disabling/life-threatening bleed |
| Follow-up | At least 1 year | Median 17 months | Mean 495 ± 3.5 days | 83 days (IQR 33–281 days) | Up to 13 months |
| Key finding | Less bleeding (all bleeding RR 0.63 [95% CI 0.43–0.9] p = 0.01) and composite bleeding plus MACE with anticoagulation alone; anticoagulation alone noninferior for MACE | Composite outcome worse with rivaroxaban (HR 1.35 [95% CI 1.01–1.81] p = 0.04) | Increased risk of mortality with oral anticoagulation (HR 1.25 [95% CI 1.08–1.44] p = 0.002) | Subclinical leaflet thrombosis less likely with anticoagulation (4% vs. 15%, p <0.0001) | No difference in primary outcome (HR 0.92 [95% CI 0.73–1.16]) and no interaction by stratum ( p = 0.57) |
| Other findings | Trend to higher bleed risk with rivaroxaban (HR 1.5, p = 0.08) | Reduced risk of bioprosthetic valve dysfunction with rivaroxaban (HR 0.51 [95% CI 0.33–0.79] p = 0.003) | Subclinical leaflet thrombosis was associated with increased rates of TIA (4.18 vs. 0.6 per 100 PY, p = 0.0005) but not with stroke ( p = 0.1) | In apixaban vs. antiplatelet stratum, obstructive valve thrombosis lower with apixaban (HR 0.19 [95% CI 0.08–0.46]) but signal for possible increased noncardiac death | |
Nijenhuis VJ, Brouwer J, Delewi R, et al. Anticoagulation with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med . 2020;382(18):1696-1707.
Dangas GD, Tijssen JGP, Wöhrle J, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med . 2020;382(2):120-129
Overtchouk P, Guedeney P, Rouanet S, et al. Long-Term Mortality and Early Valve Dysfunction According to Anticoagulation Use: The FRANCE TAVI Registry [published correction appears in J Am Coll Cardiol . 2019 Jun 4;73(21):2788]. J Am Coll Cardiol . 2019;73(1):13-21.
Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet . 2017;389(10087):2383-2392.
Collet JP, Van Belle E, Thiele H, et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: the ATLANTIS trial. Eur Heart J . 2022;ehac242. doi:10.1093/eurheartj/ehac242 . Online ahead of print.
Ongoing trials evaluating antithrombotic therapy after TAVI include ATLANTIS (apixaban vs. antiplatelet or vitamin K antagonist), AVATAR (vitamin K antagonist alone vs. vitamin K antagonist plus aspirin), AUREA (acenocumarol vs. dual antiplatelet therapy), ENVISAGE TAVI-AF (edoxaban vs. vitamin K antagonist), and ADAPT-TAVR (edoxaban vs. dual antiplatelet therapy).
Based on the current body of evidence and guideline recommendations (see Table 15.1 ), a proposed scheme for antithrombotic therapy considerations is summarized in Fig. 15.1 . Initiation of anticoagulation to replace antiplatelet therapy is also reasonable if subclinical leaflet thrombosis is detected through imaging studies.
Clinical follow-up after TAVI has been addressed in international valve disease guidelines ( Table 15.5 ).
Although a short-term baseline follow-up visit has not been specifically recommended, it is routine practice in many high-volume heart valve centers to have a first follow-up appointment with the patient at 4 to 8 weeks after the implantation ( Fig. 15.2 ).
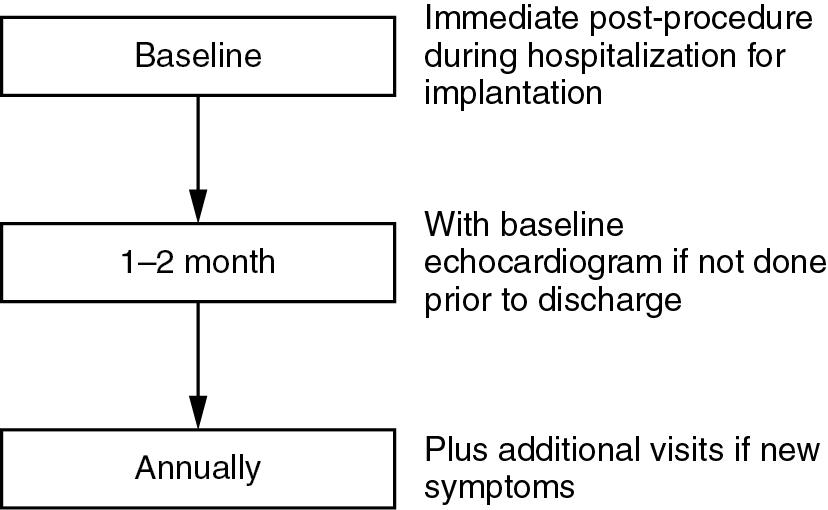
The first post-TAVI visit has the goals of ensuring good clinical recovery from the implantation procedure and the absence of symptoms to suggest a complication or early device dysfunction.
This early postprocedure visit also allows clinicians to review patient medications and ensure dual antiplatelet therapy is not prolonged unintentionally.
Annual routine follow-up appointments including a clinical history for cardiovascular symptoms and physical examination are then recommended to assess for potential prosthetic valve dysfunction.
If new symptoms develop between routine appointments, additional clinical follow-up with associated appropriate investigations is indicated. In particular, syncope and significant presyncope should prompt urgent clinical evaluation including potential inpatient hospital monitoring because of the known risk of conduction disease requiring a permanent pacemaker after TAVI.
| ACC/AHA 2020 | Class | LOE | ESC 2017 | Class | LOE | |
|---|---|---|---|---|---|---|
| Clinical follow-up | At least annually | None | Annually and if new symptoms | None | ||
| TTE in follow-up | Baseline after implant (ideally 6 weeks to 3 months after implant) | I | B-NR | Baseline within 30 days of implant | None | |
| 1 year after implant | None | |||||
| Symptoms or signs suggesting dysfunction | I | B-NR | Any new symptoms | None | ||
| Annually | IIa | C-LD | Annually after 1 year after implant | None | ||
| TEE in follow-up | Symptoms or signs suggesting dysfunction | I | B-NR | Consider if TTE poor quality or if dysfunction or endocarditis suspected | None | |
| Suspected valve thrombosis | IIa | C-LD | ||||
| Prosthetic valve with persistent fever without bacteremia or new murmur, TEE reasonable to aid in diagnosis of endocarditis | IIa | B-NR | ||||
| CT in follow-up | Symptoms or signs suggesting dysfunction | IIa | C-LD | If valve thrombus or pannus suspected | None | |
| Suspected valve thrombosis | IIa | C-LD | ||||
| Suspected paravalvular infection when anatomy cannot be delineated by echocardiography | IIa | B-NR | ||||
| Cardiac MRI in follow-up | No recommendation | No recommendation | ||||
| PET in follow-up | Adjunctive imaging for “possible endocarditis” by Modified Duke Criteria | IIa | B-NR | No recommendation |
Whether the follow-up appointments occur with the primary cardiac care provider, heart valve team performing the implantation (if separate from the primary cardiac care provider), or both is variable and depends on local practice culture, patient expectations, and logistic system-related factors. What is crucial is that follow-up should occur with at least one qualified cardiovascular health care professional if practical within the context of the individual health care system.
Imaging plays an important role in the follow-up of patients after TAVI because it allows for a noninvasive assessment of valvular function to complement the clinical history and physical examination findings. Most modalities are also readily available and pose little to no risk to the patient.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here