Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Yellow fever virus (YFV) is the prototype member of the Flaviviridae (Latin flavus , “yellow,” after the jaundice seen with the disease) family of viruses. The virus causes a viral hemorrhagic fever, a systemic illness characterized by high viremia, hepatic, renal, and myocardial injury; hemorrhage; and high lethality. A highly effective vaccine (17D), developed in 1936, is widely used to protect travelers to and residents of endemic regions of tropical South America and sub-Saharan Africa. Although the vaccine provides long-lasting immunity, rare but serious side effects have emphasized the importance of determining the need for vaccination on a case-by-case basis and of continued exploration for next generation vaccines.
The early history of yellow fever (YF) is uncertain owing to the inexactness of clinical and epidemiologic descriptions. Carter found the earliest record in a Mayan manuscript describing an epidemic with hematemesis ( xekik , “black vomit”) in Yucatan in 1648. , The nosologic term “yellow fever” was first used in 1750 during an outbreak in Barbados. YF became a major problem in the 18th century in colonial settlements in the Americas and West Africa.
Until the 20th century, YF was widely believed to be an airborne miasma arising from filth, sewage, and rotting organic matter. Several physicians, most notably Carlos Finlay in Cuba, suggested that YF was transmitted by mosquitoes. Proof was not obtained until 1900, when Walter Reed and colleagues conducted experiments on human volunteers in Cuba and demonstrated that the agent was a filterable virus transmitted by Aedes aegypti mosquitoes. This led to successes in preventing disease through mosquito abatement during the first 20 years of the 20th century.
In 1925, the Rockefeller Foundation’s West African YF Commission laboratory in Yaba (Lagos), Nigeria, set out to determine the etiology of YF, using imported monkeys for isolation of the causative agent. On June 30, 1927, blood of a 28-year-old man called Asibi, a resident of the village of Kpeve, Ghana, was inoculated into a rhesus monkey. The animal was moribund 4 days later and had hepatic lesions consistent with YF. Blood from this monkey was inoculated intraperitoneally into a second animal, which developed YF. Stokes and associates established the Asibi strain by continuous direct passage in monkeys and indirect passage through Ae. aegypti mosquitoes. Contemporary efforts at the Institut Pasteur in Dakar led to isolation of the “French” strain from a Syrian (François Mayali) with mild YF, which was later termed the French viscerotropic virus (FVV). Isolation of the Asibi and FVV strains in 1927 enabled the development of vaccines with research initiated immediately in the United Kingdom, the United States, West Africa, and Brazil.
Vaccine development was spurred, in part, by a growing number of laboratory infections. In the 5 years following isolation of YFV in 1927, 32 cases (five fatal) had occurred among laboratory workers. In 1928, Edward Hindle of the Wellcome Research Laboratories in London described the first attempt to produce an inactivated vaccine. This and many other contemporary efforts on inactivated YF vaccines were, however, unsuccessful. In 1931, Sawyer and associates at the Rockefeller Institute in NY first vaccinated humans with a live-attenuated virus, the mouse brain neuroadapted French strain (also known as the French neurotropic vaccine [FNV]), mixed with immune serum.
In 1932, Sellards and Laigret tested FNV without immune serum in humans, and in 1934, Mathis, Laigret, and Durieux described the first field trial of FNV. Believing the mouse brain tissue virus to have safety concerns for use in humans, Theiler and Smith at the Rockefeller Foundation developed a live vaccine (17D) attenuated by serial passage of the Asibi strain in tissue cultures prepared from embryonated chicken eggs. In 1936, 17D vaccine was tested in a small number of human volunteers in NY, and it entered field trials in Brazil the following year. By 1939, more than one million Brazilians had received the 17D vaccine and more than 100,000 persons in French West Africa had received FNV. During this era, important discoveries surrounding the epidemiology of YF were made, first in South America and then in Africa, showing that YFV was transmitted between monkeys and forest mosquitoes. These findings suggested that eradication of YFV was unlikely and established a mandate for vaccinating human populations wherever there was a risk of exposure to the zoonotic cycle.
During the 1940s, control of YF at a population level was achieved in francophone Africa by a program of compulsory vaccination with FNV administered by scarification. Immunization of laboratory workers, travelers, and military and expatriate residents in endemic areas removed the threat of acquiring the disease, and the disease faded from public view, having been transformed from a major human plague to a medical curiosity by the end of World War II.
Despite the availability of a highly effective vaccine, full control of the disease has continually failed owing to incomplete implementation of routine vaccination, the movement and migration of unvaccinated people into endemic areas, and the maintenance of YFV in an enzootic cycle.
In 2001, a previously unrecognized, serious adverse event (SAE) following 17D vaccine was first described. The viscerotropic reaction resembled wild-type YF and has similar lethality, but it is, fortunately, rare and all data to date suggest it is due to host genetic factors rather than reversion of the vaccine virus. The awareness of this SAE has led to the need for a more careful risk-to-benefit analysis for vaccine use in individual persons and populations, and continued exploration into the vaccine attenuation and molecular determinants of both virulence as well as host factors involved in immune responses.
This chapter provides background information on the basic virology, virus transmission, clinical disease presentation, pathogenesis, immune response, and epidemiology of YF before presenting detailed information on the development, immunogenicity, and reactogenicity of existing YFV vaccines. Finally, ongoing work to develop second-generation vaccines, including inactivated vaccines, and alternative delivery routes for dose sparing are discussed.
YFV is the prototype member of the Flaviviridae (Latin flavus , “yellow”) family, which includes approximately 60 positive-sense, enveloped, single-stranded RNA viruses, most of which are transmitted by mosquitoes or ticks. For reviews of flavivirus genome and protein structure, virus entry, and replication and assembly see Chambers and Monath, Bollati and colleagues, Kaufmann and Rossmann, and Perera and Kuhn.
Members of the Flavivirus genus were originally distinguished serologically by the neutralization test and were originally classified into eight antigenic complexes. , In the current classification that synthesizes epidemiologic, antigenic, and genetic phylogeny, YFV is considered the type species in a complex of Aedes -borne flaviviruses that includes Wesselsbron, Sepik, Edge Hill, Bouboui, Uganda S, Banzi, Jugra, Saboya, and Potiskum viruses.
YF viruses belong to a single serotype but seven distinct genotypes have been found by sequencing wild-type YFV strains of different geographic origin. , There are five African genotypes that differ in nucleotide sequence by 0–26% and in amino acid sequence by 0–9%. , , These are West African type I (represented mainly by strains from eastern areas of the region, e.g., Nigeria) and genotype II (represented mainly by strains from the western West Africa, e.g., Senegal, Guinea Bissau); the East and Central Africa genotype (Central African Republic, Ethiopia, Sudan, Zaire, Uganda); the East Africa genotype (Uganda, Kenya); and the Angola genotype (1971 and 2015–2016 outbreaks). The Central African, East African, and Angolan genotypes are notably different from the West African genotypes (7% amino acid differences), consistent with their ecological separation, namely different mosquito vectors.
In contrast to the situation in Africa, only two genotypes (I and II) are found in South America. Genotype I is widely dispersed in South America, including Brazil, Bolivia, Colombia, Ecuador, Panama, Venezuela, and Trinidad, whereas the (apparently older) genotype II circulates in the western part of the continent (Peru, Bolivia, portions of western Brazil) divergent by 0–4.6% at the amino acid sequence level. ,
The genome of the prototype YFV vaccine strain 17D-204 contains 10,862 nucleotides, composed of a 5′-terminal type I cap structure, a short 5′ noncoding region, a single open reading frame of 10,233 nucleotides, and a 3′ noncoding region. , The open reading frame encodes three structural proteins at the 5′ end (capsid [C], premembrane [prM], and E proteins), followed downstream by seven NS proteins. The proteins are encoded in the order C-prM/M-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5 ( Fig. 64.1 ).
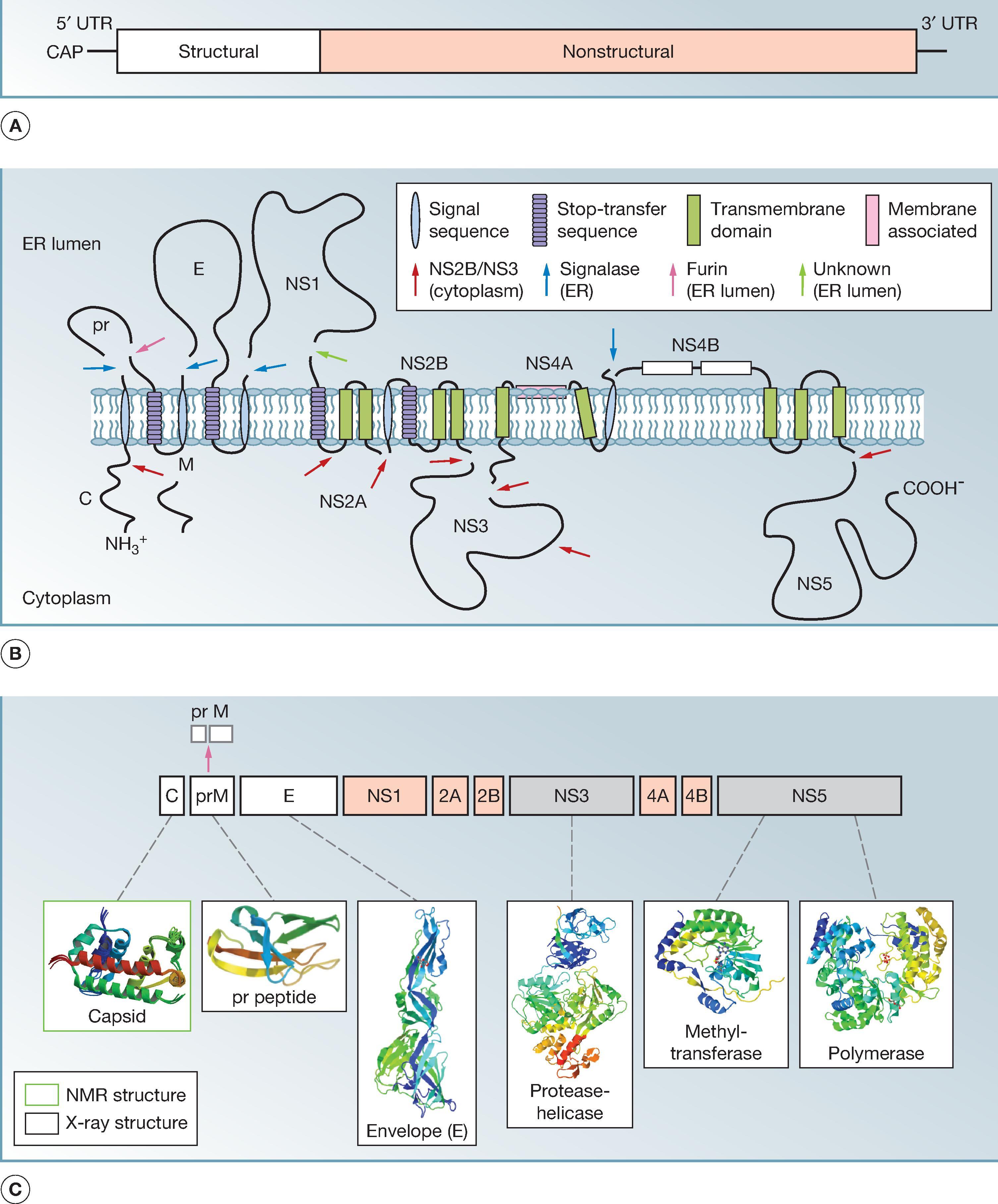
YFV has been found to be both viscerotropic, referring to the ability to cause viremia and to infect and damage liver, spleen, heart, and kidneys, and neurotropic, referring to the ability to infect the brain parenchyma and cause encephalitis. To determine the potential molecular determinants of virulence, the entire genomes of both the 17D and the FNV vaccines and their wild-type progenitors, Asibi and FVV, have been sequenced and compared. This includes examples of the two substrains of 17D (17D-204 and 17DD) used by many of the producers. , Because a large number of mutations occurred during the more than 230 passages that separate vaccines from their parental strains and the lack of availability of the initial derivatives, it is impossible to define those responsible for attenuation, nor is it clear which determinants encode viscerotropism and neurotropism.
It is clear that attenuation of the live vaccines is multigenic, determined by contributions of both nonstructural and structural genes of the virus. It is also important to note that nearly all studies on the molecular determinants of virulence have employed wild-type mouse models, which reveal only one of the two biological properties of the virus (neurotropism). Mice deficient in the interferon-alpha/beta (IFN-α/β) receptor (A129) or the STAT1 signaling molecule (STAT129) were found to be highly susceptible to infection and disease, succumbing within 6–7 days to wild-type YFV strains. Importantly, these transgenic mice developed viscerotropic disease reminiscent of human YF, instead of the encephalitic signs typically observed in wild-type mice. Wild-type Asibi virus caused a lethal infection in these transgenic models, but infection with 17D-204 vaccine virus was subclinical, suggesting that this model might have applications for investigating the molecular basis of attenuation and virulence of YFV. Hamsters were shown to be susceptible to a lethal disease with hepatic dysfunction and necrosis resembling wild-type YF after infection with virus strains adapted by serial passage in hamster liver. , This model also permits dissection of the molecular determinants associated with viscerotropism (at least for the hamster).
The first comparison of nucleotide and amino acid differences between 17D-204 (ATCC) and Asibi virus was made by Hahn and associates. Of a total of 10,862 nucleotides, 67 changes (0.62%) were identified, resulting in mutations in 31 (0.91%) of 3411 amino acids. As additional sequences of 17D-204 and 17DD substrain vaccines became available, the number of amino acid differences between parental Asibi virus and attenuated 17D viruses, and shared by vaccines from different sources, was reduced from 31 to 20, and the number of nucleotide differences in the 3′ noncoding region was reduced from six to four ( Table 64.1 ). , , Although the possibilities have been reduced to a limited number of mutations, it has not been possible to determine precisely which are responsible for the attenuation of virulence of the vaccine strain.
| Nucleotide | Gene | Amino Acid | Asibi | 17D-204 and 17DD Vaccines |
|---|---|---|---|---|
| 854 | M | 36 | Leu | Phe |
| 1127 | E | 52 | Gly | Arg |
| 1482 | 170 | Ala | Val | |
| 1491 | 173 | Thr | Ile | |
| 1572 | 200 | Lys | Thr | |
| 1870 | 299 | Met | Ile | |
| 1887 | 305 | Ser | Phe | |
| 2112 | 380 | Thr | Arg | |
| 2193 | 407 | Ala | Val | |
| 3371 | NS1 | 307 | Ile | Val |
| 3860 | NS2A | 118 | Met | Val |
| 4007 | 167 | Thr | Ala | |
| 4022 | 172 | Thr | Ala | |
| 4056 | 183 | Ser | Phe | |
| 4505 | NS2B | 109 | Ile | Leu |
| 6023 | NS3 | 485 | Asp | Asn |
| 6876 | NS4A | 146 | Val | Ala |
| 7171 | NS4B | 95 | Ile | Met |
| 10,142 | NS5 | 836 | Glu | Lys |
| 10,338 | 900 | Pro | Leu | |
| 10,367 | (3′ NCR) | U | C | |
| 10,418 | U | C | ||
| 10,800 | G | A | ||
| 10,847 | A | C |
The role of the E protein in neurovirulence is illustrated by a study in which heterologous YF chimeras were constructed having a YF 17D backbone and prM and E genes from flaviviruses with different neurovirulence profiles. Mutations in three principal areas of E protein alter virulence properties of YF and other flaviviruses. These include the tip of the fusion peptide in domain II, the molecular hinge between domains I and II, and the upper lateral surface of domain III, containing the putative receptor-ligand. , Among the eight amino acids in the E protein that distinguish Asibi and 17D viruses (see Table 64.1 ), four are nonconservative changes: E52 Gly→Arg, E200 Lys→Thr, E305 Ser→Phe, and E380 Thr→Arg. , ,
Amino acid residues E52, E173, and E200 are in the molecular hinge region and are also located in domain II at the top of the virion, where they are exposed to interactions with cell receptors and binding moieties. Mutations in the hinge region could alter the acid-dependent conformational change in E protein required for virus entry. Neuroadaptation of 17D virus resulted in an increase in neurovirulence of the virus for mice and was associated with a reversion (Ile→Thr) at residue E173. , Moreover, residue E173 is implicated in an epitope binding to wild-type specific monoclonal antibody (MAb 117).
A chimeric virus composed of Japanese encephalitis (JE) prM-E genes in the YF 17D backbone having a single amino acid change to the wild-type JE amino acid at E279 in the molecular hinge caused an increase in neurovirulence for mice but reduced viscerotropism for monkeys. This result suggested that neurovirulence and viscerotropism of YFV strains might not be linked at the molecular level. This may explain why FNV, which was developed by adapting the virus during over 128 passages in mouse brains, was highly neurovirulent for mice but had lost the ability to cause hepatitis in monkeys and humans.
Vaccine-specific mutations at positions E305 and E380 are located in domain III, and the mutation at position E299 is located at the interface of domains I and III. E305 and E380 are located at the top surface of the virion in domain III. Because domain III contains ligands for cell-receptor interactions, these mutations could alter tropism of the vaccine virus. YF 17D strains that were neuroadapted and neurovirulent had reversions at E305, E325, and E380 (as well as the reversions to wild-type residues at E52 and E173 noted earlier). , , , Moreover, the possible role of the E305 mutation was illustrated by sequence analysis of 17D virus recovered from the brain of a child with a fatal YF vaccine–associated neurotropic adverse event. , The 17D virus recovered had increased neurovirulence for mice and monkeys. The isolate differed from 17D vaccine at E303 Glu→Lys, located in domain III very near the 17D-204–substrain-specific E305 amino acid. However, two other mutations (at E155 and NS4B76) were also present in the brain isolate and could have played a role in reversion to virulence.
The mutation at amino acid E380 of 17D occurs in the moiety that is a putative integrin cell-receptor ligand; the sequence change in this motif is from Thr-Gly-Asp in Asibi to Arg-Gly-Asp (RGD) in 17D vaccine. Although mutations in the RGD sequence predicted to alter integrin binding did not interfere with 17D virus replication, , studies with another flavivirus (Murray Valley encephalitis) also showed that RGD mutations attenuated neurovirulence for mice. , Multiplication of the Murray Valley encephalitis mutant viruses were also inhibited by heparin, suggesting that cell receptors other than integrin bind the viral ligands.
Two groups were able to further elucidate a role for mutations in domain III in the attenuation of 17D. The first investigated three amino acid substitutions (305 Phe→Val, 326 Lys→Glu, and 380 Arg→Thr) as critical determinants of mouse neuroinvasiveness in a severe combined immunodeficiency model. Virus containing vE326-Glu caused a rapid lethal encephalitis, but mutants with either E380-Thr or E305-Val did not independently affect neuroinvasiveness. Testing a panel of viruses with various amino acid substitutions at E326 revealed that attenuation of neuroinvasiveness required a positively charged residue (Lys or Arg) at this position. Molecular-modeling studies of protein domain III indicate that E326 and E380 contribute to charge clusters on the lateral surface of domain III that constitute putative heparin-binding sites. The second group investigated E305, E325, and E380 and showed that these residues inhibit spread of 17D in extraneural tissues and attenuate virulence in types I/II IFN-deficient mice. One of these residues (E380-Arg) was a dominant GAG-binding determinant, which mediated more-rapid in vivo clearance of 17D from the bloodstream in comparison to 17D-derived variants with wild-type–like E protein. However, these studies did not include wild-type strain Asibi for comparison.
The stem-anchor region of the E protein is involved in the reconfiguration of the E protein from dimeric to trimeric structure during low-pH–induced fusion and virus entry and also plays a role in localizing and retaining the envelope proteins in the endoplasmic reticulum during morphogenesis. The Val→Ala mutation at amino acid E407 of Asibi virus occurs in the N-terminal stem-anchor region of the E protein. Mutations in this region can affect the structural integrity and three-dimensional structure of the prM-E heterodimer and are known to attenuate flaviviruses, including dengue, tickborne encephalitis group virus, JE, and YF–JE chimeras. In addition to the E protein, the M protein also contains a single amino acid substitution at residue 36 (L36F) and is found in a proapoptotic sequence described for dengue virus.
The 17D vaccine is not fixed with respect to neurovirulence, so that sequential mouse-brain passage of the vaccine results in increasing mouse virulence. The neuroadapted 17D virus reverted to the wild-type (Asibi) sequence at amino acid residues E52 and E173 and also accumulated mutations at the putative virulence determinant E305 (Ser→Val) and in nonstructural genes (NS1, NS2A, NS4A, NS4B, and NS5). , A chimeric virus with the original 17D backbone and the neuroadapted E sequence did not have increased neurovirulence compared to original 17D. In contrast, when all mutations in E and NS genes of the neuroadapted strain were introduced into the 17D infectious clone, neurovirulence was increased, illustrating that multiple genes were involved in virulence and implicating mutations in the NS proteins or the 3′ noncoding region of the virus. Studies with other flaviviruses also have shown that mutations in the NS coding region might reduce neurovirulence, probably by reducing the rate of replication. ,
Eleven amino acid changes in the NS proteins of Asibi viruses occurred during the derivation of 17D vaccine (see Table 64.1 ): one in the NS1 protein; four in NS2A; one each in NS2B, NS3, NS4A, and NS4B; and two in NS5. Although the role of NS2A mutations is not known, this protein plays roles in viral RNA replication, and virion assembly and release. The change in NS3 occurs at residue 485, in the region coding for the RNA helicase and triphosphatase enzymes for unwinding RNA during replication. The two mutations in the NS5 RNA dependent RNA polymerase potentially influence replication efficiency and might contribute to attenuation of 17D.
The 3′ noncoding region plays a critical role in replication, and changes in this sequence can contribute to attenuation. The 3′ noncoding proximal region is variable in length among YF strains and contains one to three repeat sequence elements. The 3′ terminal region contains a 90- to 120-nucleotide conserved region involved in folding of the stem-loop structure and serving as a promoter for minus strand synthesis during replication. Mutations in the stem-loop region can interfere with virus replication. , In the case of dengue-4 virus, the proximal region of the 3′ noncoding region does not appear to be critical to replication, but mutations or deletions in this region can nevertheless attenuate virulence. It is clear that mutations in the 3′ noncoding region of 17D present in both the variable and the conserved proximal region might have contributed to attenuation of the vaccine virus.
Much less is known about the determinants of YFV viscerotropism, principally because of the difficulty of assessing this property in nonhuman primates. As noted earlier, the neurotropism and viscerotropism properties of YFV might reside in distinct regions, and thus one cannot conclude that attenuation of one feature correlates with attenuation of the other.
In addition to attenuation of viscerotropism and neurotropism, a critical property of 17D vaccine is its inability to disseminate from the midgut to other tissues in the mosquito, and thus it is not transmissible from the mosquito to vertebrate hosts. Higgs and coworkers have used infectious clone technology to generate chimeras of Asibi and 17D viruses containing swapped residues in NS2A (four amino acids), NS4B (one amino acid), and the 3′ untranslated region (four nucleotides) to identify viral genes involved in dissemination in Ae. aegypti . , Dissemination was found to be related to whether the chimera had an Asibi or 17D E protein domain III, but E domain III appeared not to be the only genetic factor controlling dissemination.
While the precise molecular determinants of attenuation and virulence have not been elucidated, recent data have shed light on the molecular basis of attenuation of 17D vaccine. It is known that RNA viruses, including YFV, exist as a quasispecies, or a group of nucleotide sequences related by a similar mutation or mutations that compete within a viral population, because of the high error rate of the RNA-dependent RNA polymerase. Until recently, nucleotide sequences were obtained by “first-generation sequencing,” such as Sanger sequencing (chain-termination method), where the nucleotide sequence of the dominant member of a population was obtained and the quasispecies were seen as a “consensus” sequence. However, “next-generation sequencing” (NGS) has enabled amplification of a single molecule to obtain nucleotide sequence of the quasispecies population. Comparison of a 17D-204 commercial lot with wild-type Asibi strain was undertaken by NGS and revealed that the wild-type virus population structure differs from that of the vaccine. Specifically, Asibi virus had the typical quasispecies structure of a RNA virus while in contrast the vaccine virus was relatively homogeneous suggesting that the lack of diversity in the 17D vaccine virus population may contribute to the attenuated phenotype.
Monoclonal antibodies (MAbs) recognize structurally distinct regions in the E protein of YFV, including vaccine strain–specific epitopes and YFV–specific epitopes, as well as determinants cross reactive with specific heterologous flaviviruses and with broad flavivirus-group epitopes. Antibodies against vaccine strain–specific, virus-specific, and flavivirus group–reactive epitopes neutralize virus, and many passively protect mice against intracerebral challenge. Interestingly, MAbs generated following immunization with 17D virus neutralized wild-type (Asibi) virus but not 17D, and flavivirus group–reactive monoclonals generated after immunization with 17D, Asibi, or heterologous flaviviruses neutralized wild-type virus. , , This multiplicity of neutralizing determinants helps explain the broad protective immunity afforded by 17D vaccine against wild virus strains and the partial cross-protection by heterologous flaviviruses against YF.
Additional studies have defined epitopes that are substrain specific, differentiating 17D-204 vaccine from other YF viruses and 17D-204 from 17DD vaccines and even distinguishing between vaccines of the same substrain from different manufacturers. , Plaque-size variants purified from 17D vaccine can also be distinguished in neutralization and hemagglutination inhibition tests. , , Some monoclonals are specific for 17D and do not recognize wild-type virus. , The antigenic heterogeneity of 17D vaccine is due to the uncloned nature and different passage histories during manufacture and laboratory manipulation. At present, there is no recognized practical consequence, with respect to protective immunity, of the absence of some wild-type antigenic determinants in 17D vaccines.
A neutralization determinant in the E protein of both wild-type and 17D was identified at residues E71/72 in domain II of the E protein. , Ryman and colleagues found an additional neutralizing epitope at either E155 or 158 in domain I. These data were confirmed using human MAb fragments that neutralized 17D and wild-type YFVs belonging to West African and to Central and East African genotypes. This study suggested that amino acids E71 and E155 formed part of a single conformational epitope in the E protein dimer. Recent studies have used X-ray crystallography to demonstrate the interaction between a human Mab prepared against YFV with neutralizing antibody to domain II of the E protein.
In a later analysis, Ryman and colleagues selected escape mutants from three 17D substrains with a MAb, namely MAb 864, specific for 17D-204 virus that had a number of functional activities including neutralization, hemagglutination, and hemolytic and passive protection. The escape mutants were characterized with respect to mouse neurovirulence and prM-E nucleotide sequence. One series of escape mutants had reduced neurovirulence for mice compared to the parental virus and had a Ser→Leu mutation at E325. E325 is the site of a Pro→Ser mutation that occurred in the derivation of 17D-204 from Asibi but is not present in the 17DD substrain and thus is not considered to be relevant to the attenuated vaccine phenotype. ,
In contrast, another neutralization escape mutant was neurovirulent for mice and contained an amino acid mutation at E305 (Phe→Ser), which is a conserved substitution across all 17D strains (see Table 64.1 ). This mutation represents a reversion to the wild-type residue at E305. The E305 and E325 residues are spatially adjacent within domain III of the E glycoprotein and thus represent a conformational epitope recognized by the MAb 864 used to generate the different escape mutants. , The location of the epitope is consonant with the effector roles of antibody in blocking cell attachment and intracellular uncoating events. Clearly, this epitope also is critical to pathogenesis and neurovirulence.
Although an exhaustive search for neutralization and protective epitopes has not been made, YFVs (like other flaviviruses) contain only a few such determinants in the E protein, which are structurally diverse. These epitopes must be conserved across wild-type strains, consistent with the broad protective activity of YF vaccine against wild virus strains.
Antigenic determinants involved in cell-mediated immunity have been localized in YF 17D virus, dengue virus, and Murray Valley encephalitis viruses. YF cytotoxic T-lymphocyte determinants are found in the E proteins and in multiple nonstructural proteins: NS1, NS2B, NS3, NS4B, and NS5. These T-cell epitopes are highly conserved and probably contribute to the cross-protective activity of 17D vaccine against all geographic variants of wild-type YFV. In the case of YF, cytotoxic T-cell epitopes were found on E, NS1, NS2B, and NS3, and these epitope sequences were conserved across multiple YF strains. T-cell responses tend to be cross-reactive. , Cytotoxic T-effector cells raised to Murray Valley encephalitis virus demonstrated significant cross-reactivity with target cells pulsed with YF–derived NS3 peptides, despite the relative low sequence homology of the determinants.
The primary mode of transmission of YFV to humans is through the bite of an infected mosquito. Various mosquitoes are involved in the maintenance and transmission of YFV and the virus is maintained in an enzootic cycle by these mosquitoes and wild nonhuman primates. Besides mosquito-borne transmission, a few other modes of transmissions have been documented (see “Other Modes of Human Infection” Section).
The enzootic transmission cycle involves monkeys and diurnally active tree-hole–breeding mosquitoes ( Haemagogus spp. in South America and Aedes africanus in Africa) ( Fig. 64.2 ). Humans are sporadically exposed to infected mosquitoes when they encroach on this cycle during occupational or recreational activities (“jungle YF”). This transmission cycle accounts for jungle YF cases in South America and in the rainforest zone of Africa. In the moist savanna regions of Africa, tree-hole-breeding Aedes mosquitoes reach very high densities and are implicated in endemic and epidemic transmission, transferring virus from monkeys to humans and between humans. Ae. aegypti , a domestic mosquito that breeds in human-made containers and achieves highest densities in urban environments, can transmit YFV between humans (“urban YF”).
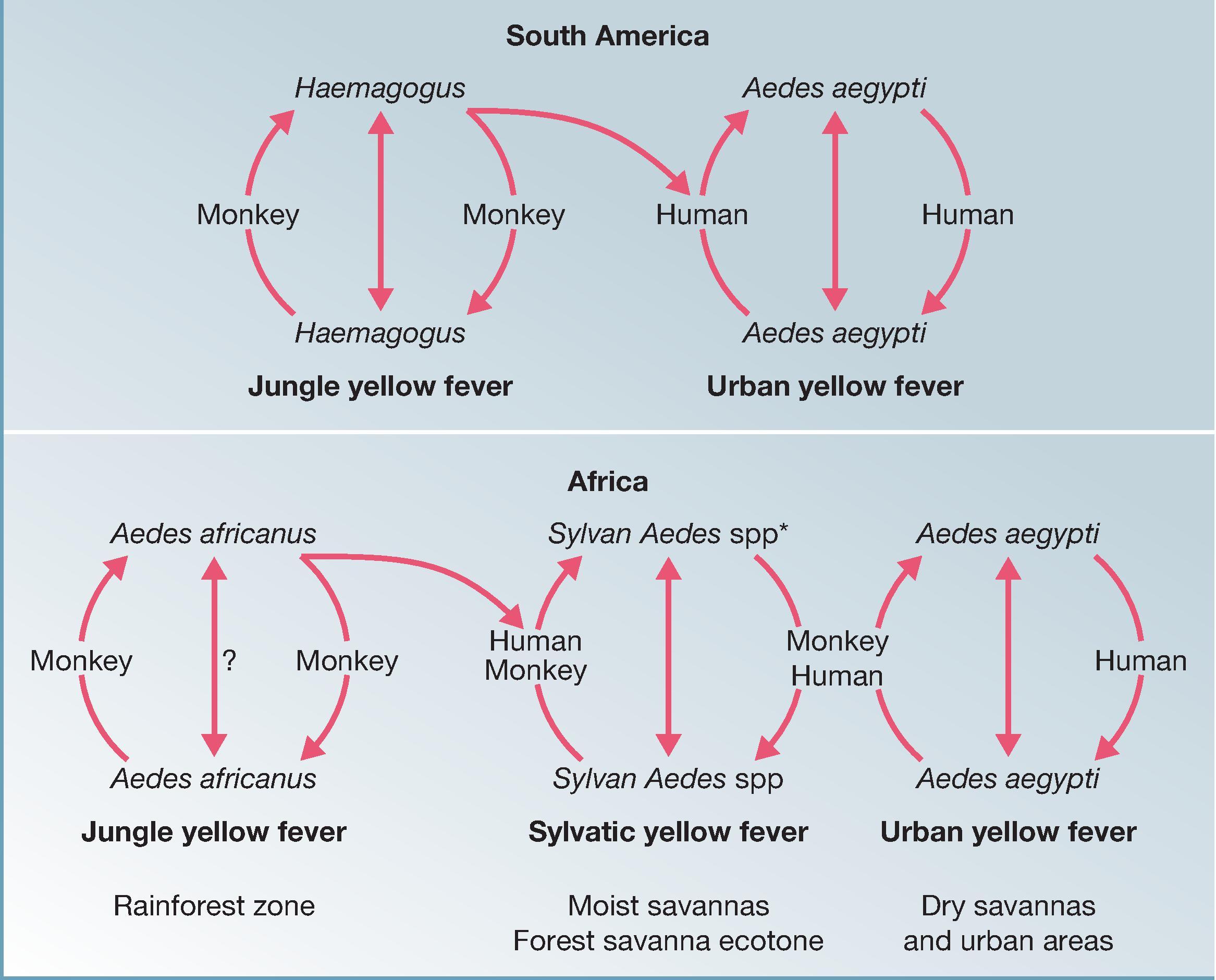
Many species of nonhuman primates are susceptible to YFV infection. The majority of African species have viremic infections sufficient to infect mosquitoes without developing clinical illness, whereas some neotropical species (e.g., howler monkeys) develop fatal infections. Depletion of vertebrate hosts through natural immunization and death during epizootic waves is a factor in the cyclic appearance of YF activity. In many areas, deforestation and hunting pressure have markedly reduced monkey populations, and human beings become the host in the YFV transmission cycle. , Although this point is still debated, there is little evidence that nonprimate vertebrates are involved in enzootic transmission. , ,
The pattern of YFV activity in South America is characterized by intermittent emergence around the edges of the Amazon region. These outbreaks are preceded by evidence for increased virus transmission between monkeys and Haemagogus spp. within the Amazon basin, which moves in a circular fashion within the forest or along gallery forests of river courses. A detailed analysis of the patterns of virus activity preceding and during epidemics in Brazil is provided by Mondet, Vasconcelos and colleagues, and de Oliveira Figueiredo.
The ecology of YFV differs in areas bordering the rainforest in Africa. A mosaic of savanna and forest in galleries along rivers characterizes this transitional vegetation zone. In this region and surrounding moist (Guinea) savanna, YFV transmission is effected by a wide variety of tree-hole–breeding mosquito vectors. , In West Africa, the principal vectors responsible for YFV transmission in the savanna zone include Aedes furcifer, Aedes vittatus, and Aedes luteocephalus, as well as Ae. Africanus. In East Africa, Ae. africanus and human-biting populations of the Aedes simpsoni species complex ( Ae. simpsoni, Aedes lilii, Aedes bromeliae ) play a similar role. During the wet and early dry seasons, vector density reaches high levels. Vectors are active in plantation areas and in proximity to human dwellings and can enter houses. Both humans and nonhuman primates may be involved as hosts in the transmission cycle, and the rate of virus transmission far exceeds that found in the rainforest zone. The savanna-forest ecotone and surrounding Guinea savanna has been described as the “zone of emergence” of YF and represents the region principally affected by epidemics in Africa. ,
An epidemiologically distinct transmission cycle involves Ae. aegypti , which breeds in containers used to store water or in artificial containers that collect rainwater around human habitations and predominantly feeds on humans. Ae. aegypti transmits YFV between humans, the sole viremic hosts in the cycle. The vector occurs in dry areas and heavily settled areas but is also widely dispersed in settlements in rural areas. Urban outbreaks have followed introduction by viremic persons from areas of jungle YFV activity. , In the Americas, urban YF outbreaks were common prior to the successful eradication of the vector, , and outbreaks currently show signs of reoccurring owing to the repopulation of most urban areas with Ae. aegypti.
Africa suffers many Ae. aegypti –borne epidemics, because the vector is prevalent in urban and rural areas. , In dry areas (Sudan and Sahel savanna zones), where domiciliary Ae. aegypti might represent the only species capable of sustaining YFV transmission, outbreaks occur after introduction of the virus by viremic persons. In Africa, some feral populations of Ae. aegypti feed preferentially on mammals other than humans. Aedes spp. other than Ae. aegypti are responsible for interhuman YFV transmission, as noted earlier.
The means of survival of YFV across the long dry season, when sylvatic mosquito vectors are virtually absent, remains incompletely understood. Aedes and Haemagogus eggs survive desiccation in tree holes and hatch with the return of rain. Experimental and field studies indicate that transovarial transmission is a means of virus survival across the dry season. However, the filial infection rate of YFV by vertical transmission in mosquitoes is too low for this to maintain the virus indefinitely, and annual amplification in nonhuman primates is thus imperative. Low-level horizontal transmission by drought-resistant vectors and alternative horizontal and vertical transmission cycles involving ticks have been suggested as ancillary mechanisms for survival across the dry season. Persistent infection of experimentally infected nonhuman primates has been documented, , but such infections are probably not accompanied by viremias sufficient to infect vectors.
Infection of mosquitoes is initiated by ingestion of a blood meal containing a threshold concentration of virus (∼3.5 log 10 /mL), resulting in infection of the midgut epithelium. The virus is released from the midgut into the hemolymph and spreads to other tissues, notably the reproductive tract and salivary glands. The time elapsed between ingestion of virus and secretion in saliva (the extrinsic incubation period) is temperature-dependent and has a median duration of 10 days at 25°C. After the extrinsic incubation period, the vector is capable of transmitting virus when she refeeds on a susceptible host. Infection of reproductive tissues of the mosquito provides a mechanism for vertical transmission of YFV from the female mosquito to her progeny and from congenitally infected males to females during copulation. , ,
The use of a live vaccine theoretically engenders a risk of secondary spread by mosquitoes, and passage of vaccine virus could result in a reversion to a more-virulent phenotype. This is unlikely for two reasons. First, viremia following 17D vaccination is very low and below the threshold of oral infection of the vector, , with a number of caveats including viremia may be higher in patients with viscerotropic adverse events, and viremia has not been measured in infants or in immunosuppressed persons. Second, 17D virus is poorly infectious for mosquitoes. Whitman infected Ae. aegypti larvae with 17D virus after immersion in virus, but infected adult progeny were incapable of transmitting the virus. Bhatt and colleagues reported that 17D vaccine virus inoculated by the intrathoracic route (thus bypassing the midgut barrier) replicated to a low level in Ae. aegypti and Aedes albopictus , but orally exposed mosquitoes contained no detectable virus after a 22-day extrinsic incubation period. Jennings and colleagues showed that 45% of adult female Ae. aegypti that fed on a high concentration of 17D vaccine in an artificial blood meal developed midgut infections, but no virus was detected in head tissue. In another study, only 1 of 32 Ae. aegypti orally exposed to 17D virus developed infection in head tissue, and none of the mosquitoes transmitted the virus. A more recent study confirmed 17D virus has lost its ability to be transmitted by Ae. aegypti , owing to inability of the virus to cross the midgut barrier.
Approximately 10 3 virions are inoculated during mosquito feeding. Salivary virus is deposited mainly in the extravascular tissues of the skin of the host during probing, because saliva that is injected intravascularly is apparently reingested by the mosquito during blood feeding. Virus replication is initiated in the epidermis and dermis at the site of inoculation, probably in Langerhans cells, and spreads through lymphatic channels to regional lymph nodes and then to other lymphoid organs and viscera via the bloodstream. In the immunized host, the small mosquito inoculum would encounter a vast excess of antibodies in extracellular transudate and lymph. This suggests that a low level of immunity is sufficient to protect the host against disease. It is not known whether immunity is sufficient to sterilize the mosquito inoculum.
Laboratory infections with YFV were common in the prevaccine era and remain of concern today, particularly where unvaccinated clinical laboratory personnel encounter blood from patients during the early stage of illness. On numerous occasions, transmission has occurred between separately caged monkeys housed in a single room, possibly by aerosol spread. Findlay and MacCallum infected monkeys by a mucosal (intragastric) route and Bauer and Hudson transmitted YFV to monkeys by rubbing virus on unbraided skin, a technique that might have exposed epidermal Langerhans cells to infection. Some infections in laboratory workers may be explained by contact with viremic blood, but it remains unclear whether infection occurred via intact skin, via abrasions, or by contact with mucosal membranes.
There has been at least two cases of perinatal transmission of wild-type YFV from women who developed their initial symptoms of YF 3 days prior to giving birth. , Both infants developed symptoms 3–6 days after birth and had viral RNA detected in their blood samples. The infants unfortunately died of fulminant YF 12 and 16 days after birth. In addition to perinatal transmission, the recent transmission of 17D vaccine to several infants via breastfeeding and the identification of wild-type YFV in breast milk suggests that the wild-type virus might be spread the same way via either breast milk, in ingested blood, or possibly by inhalation. The 6-month-old, breast-fed infant of the woman where wild-type virus was detected in her breast milk at 5 days after illness onset had high fever, vomiting and prostration, with elevated white blood cells and platelets, and leukocytes on her urinalysis. Sample obtained on the day of illness onset for the infant was RT-PCR negative for YF; no serology was performed.
YFV was extensively investigated in the U.S. Biological Weapons Program. Whereas the virus could infect by the aerosol route, it was considered too thermolabile for successful weaponization. Instead, extensive studies were undertaken on the use of Ae. aegypti as an entomologic weapon capable of delivering the virus. ,
The clinical spectrum of YF is very broad, including subclinical infection, abortive infection with non-specific flu-like illness, and potentially lethal hemorrhagic disease. This variability makes the clinical diagnosis of sporadic cases difficult and is responsible for the underestimation of morbidity and inflation of case-fatality rates when only cases of full-blown YF are enumerated. As with many other infections, this variability in response is due to intrinsic and acquired host resistance factors and probably to differences in the pathogenicity of virus strains.
After an incubation period of 2–9 days (median, 4.3 days), clinical disease begins abruptly with rigors and headache. The illness is often characterized by three stages. , The first stage, referred to as the period of infection, lasts 3–4 days and is marked by the presence of viremia. , Levels of the viremia usually peak around days 2–3 of illness with titers of up to approximately 6.6 log 10 PFU in cell culture. This stage of illness is characterized by fever, malaise, prostration, headache, photophobia, lumbosacral pain, pain in the lower extremities (particularly the knee joints), generalized myalgia, anorexia, nausea, vomiting, restlessness, irritability, and dizziness. On physical examination the patient appears toxic, with hyperemia of the skin; congestion of the conjunctivae, gums, and face; epigastric tenderness; and tenderness and possible enlargement of the liver. The tongue is characteristically small and pointed and bright red at the tip and sides, with a white coating in the center. Initially, the pulse rate is high, but by the second day there is bradycardia relative to fever (Faget sign). The average fever is 38.9–39.4°C and lasts 3.3 days, but temperature can rise as high as 40.6°C. Young children can experience febrile convulsions. Laboratory abnormalities include leukopenia (1.5–2.5 × 10 9 cells/L) with a relative neutropenia. The leukopenia occurs precipitously, in concert with onset of illness. Between 48 and 72 hours after onset, serum transaminase levels are often elevated and usually precede the appearance of jaundice.
The period of infection may be followed by a distinct period of remission (also referred to as a period of calm), with abatement of fever and symptoms lasting up to 48 hours. The remission is often subtle or very brief. In cases of abortive infection, the patient simply recovers at this stage. Such cases typically remain anicteric, and the non-specificity of the syndrome makes it impossible to diagnose YF clinically except during an epidemic. It is estimated that 55% of YFV infections are asymptomatic and 33% result in mild disease.
The third stage of the disease, the period of intoxication, occurs on the third to sixth days after onset and is characterized by jaundice and the development of often life-threatening signs and symptoms. Approximately 12% of persons infected with YFV will enter the period of intoxication. , , During this period, fever can reoccur, and there is relative bradycardia, nausea, vomiting, epigastric pain, jaundice, oliguria, and a hemorrhagic diathesis. Virus disappears from blood and antibodies appear. The subsequent course reflects dysfunction of multiple organ systems, including the liver, kidneys, and cardiovascular system. High levels of circulating proinflammatory cytokines are present, and the overall picture has the clinical characteristics of the systemic inflammatory response syndrome and multiple organ failure.
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) peak early in the second week of illness and fall rapidly over a few days in patients who recover. AST levels typically exceed ALT, probably owing to direct viral injury to myocardium and skeletal muscle. This distinguishes YF from viral hepatitis, in which ALT levels typically exceed AST levels. Alkaline phosphatase levels are normal or only slightly elevated. Direct bilirubin levels are typically between 5 and 10 mg/dL.
Kidney dysfunction is marked by an increase in albuminuria, reduction in urine output, and rising azotemia. Albumin levels in the urine typically range between 3 and 5 g/L but can reach 20 g/L. Serum creatinine levels are three to eight times normal. In some patients who survive the hepatitis phase, kidney failure predominates. Death is preceded by virtually complete anuria. A hemorrhagic diathesis is manifested as coffee-grounds hematemesis, melena, hematuria, metrorrhagia, petechiae, ecchymoses, epistaxis, oozing of blood from the gums, and excessive bleeding at needle-puncture sites. Laboratory correlates include thrombocytopenia, prolonged clotting and prothrombin times, and reductions in clotting factors synthesized by the liver (factors II, V, VII, IX, and X). Some patients have clotting abnormalities that suggest disseminated intravascular coagulation (DIC), including diminished fibrinogen and factor VIII and the presence of fibrin split products.
The electrocardiogram can show sinus bradycardia without conduction defects, ST-T abnormalities, particularly elevated T waves, and extrasystole, , presumably the result of virus replication and direct viral injury to the myocardium. Bradycardia can contribute to the physiologic decompensation associated with hypotension, reduced perfusion, and metabolic acidosis in severe cases. Acute cardiac enlargement can occur during the course of YF infection.
Central nervous system (CNS) signs include delirium, agitation, convulsions, stupor, and coma. In patients dying of YF, CNS signs appear to result from cerebral edema or metabolic factors, based on the virtual absence of inflammatory changes in brain tissue. True wild-type YFV encephalitis is exceedingly rare, with few extant clinical case reports of paralysis, optic neuritis, and cranial nerve palsy suggesting neurologic infection, but without substantiating virologic evidence to differentiate encephalitis from encephalopathy. ,
The critical phase of the illness occurs between the fifth and 10th days, at which point the patient either dies or rapidly recovers. , , A model incorporating data from several outbreaks estimated the case-fatality rate for severe YF cases of 47%. Potential risk factors for death include male gender, older age (>40 years), jaundice, leukocytosis, and higher levels of liver transaminases, bilirubin, blood urea nitrogen, lipase, and lower Factor V levels. , , ,
Although convalescence may be associated with weakness and fatigability lasting several weeks, healing of the liver and kidney is typically complete, without postnecrotic fibrosis. In some cases, jaundice and elevations in serum aminotransferases persist for months after onset. , , It is uncertain whether patients with such atypical signs have had other underlying hematologic or hepatic diseases. In one study, the outcome of YF in hepatitis B surface antigen–positive and –negative patients was similar.
Complications of YF include superimposed bacterial pneumonia, parotitis, and sepsis associated with recovery from renal tubular necrosis. , Late deaths during convalescence have been ascribed to myocarditis, arrhythmia, or heart failure, but documentation of these events is incomplete.
The age, sex, and occupational distribution of YF in South America and Africa differ. In South America, most disease is caused by the jungle transmission cycle where humans are infected by tree-top dwelling mosquitoes that previously fed on viremic monkeys. Therefore, occupational activities, such as forest clearing, logging, and road construction, increase the risk of the disease. Most of these activities are performed by young male adults. , The prevalence of immunity in male residents exceeds that in female residents by 2.5–7.5-fold. The age and sex distribution of jungle YF cases differs from that observed in South America during Ae. aegypti –borne epidemics in the early 20th century. In the urban transmission cycle, Ae. aegypti are breeding in and around houses and sustaining interhuman transmission of virus; thus, a high prevalence of infection is often seen in children and women who are more likely to be in homes when the mosquitoes are active.
In Africa, the prevalence of natural immunity accumulates rapidly with age, so that children are at highest risk ( Table 64.2 ). , , , , A high attack rate in children (>70%) typically reflects an area where older persons are protected by previous YF vaccination campaigns (e.g., Senegal, 1965; Burkina Faso and Ghana, 1983; Mali, 1987; Guinea 2020). In Africa, a slight excess of cases among male residents has been observed during epidemics ( Table 64.2 ). This pattern was seen regardless of the role of domiciliary Ae. aegypti or sylvatic vectors, and it is thus difficult to explain by differences in human behavior or exposure to mosquito bites. The higher percentage of male patients has been observed, not only among notified or hospitalized patients, but also in population-based surveys. Serologic data have shown neither a consistently higher incidence of infection nor susceptibility to illness among male residents, , but limited sample size and sampling biases might preclude detection of small differences.
| Epidemic | Age Distribution | Male/Female Ratio | Presumed Vectors ( Aedes spp.) | Reference | |||
|---|---|---|---|---|---|---|---|
| Year | Country | Cases in Children (Age Range [y]) | Total Cases | Children as Percentage of Total | |||
| 1926–1928 | Ghana, Nigeria | 32 (0–19) | 122 | 26 | 2.3 : 1 | ?Ae. aegypti | Beeuwkes, 1936 |
| 1940 | Sudan (Nuba Mountains) | 110 (0–19) | 306 | 36 | 1.7 : 1 | Ae. aegypti, Ae. vittatus, other sylvatic vectors | Kirk, 1941 |
| 1965 | Senegal (Diourbel) | 86 (0–19) | 89 | 97 | — | Ae. aegypti | Brès et al., 1967 |
| 1969 | Nigeria (Jos Plateau area) | 38 (0–19) | 209 | 18 | 2.5 : 1 | Ae. luteocephalus | Carey et al., 1972 |
| 1969–1970 | Ghana | 99 (0–15) | 164 | 60 | — | Ae. aegypti | Addy et al., 1986 |
| 1970 | Nigeria (Okwoga District) | 35 (0–19) | 76 | 46 | ∼ 1 : 1 | Ae. africanus | Monath et al., 1973 |
| 1977–1980 | Ghana (Volta and Eastern Regions) | 87 (0–15) | 294 | 30 | — | Ae. aegypti | Addy et al., 1986 |
| 1982 | Côte d’Ivoire (Mbahiakro Subprefecture) | 43 (0–15) | 90 | 48 | — | Ae. aegypti | Lhuillier et al., 1985 |
| 1983 | Burkina Faso (Manga and Fada N’Gourma Regions) | 40 (0–15) | 45 | 89 | ∼ 1 : 1 | Ae. furcifer | Baudon et al., 1986 Roux et al., 1984 |
| 1983 | Ghana (Northern Region) | 61 (0–14) | 76 | 82 | — | Ae. aegypti | World Health Organization, 1985 |
| 1986 | Nigeria (Oju LGA) | 20 (0–19) | 39 | 51 | 2 : 1 | Ae. africanus | De Cock et al., 1988 |
| 1987 | Nigeria (Oyo State) | 72 (0–19) | 102 | 71 | 1.4 : 1 | Ae. aegypti | Nasidi et al., 1989 |
| 1987 | Mali (Kati Cercle) | 100 (0–15) | 143 | 70 | 2.1 : 1 | Ae. furcifer, Ae. aegypti | Kurz, 1988 |
| 1990 | Cameroon (Extreme North Province) | 91 (0–9) | 182 | 73 | — | Ae. aegypti | Vicens et al., 1993 |
| 1991 | Nigeria | 1209 (0–15) | 2229 | 54 | 1.1 : 1 | Ae. aegypti | WHO, 1993 |
| 1992–1993 | Kenya (Baringo and Elgeyo Marakwet Districts) | 18 (0–19) | 54 | 33 | 1.8 : 1 | Ae. africanus | Sanders et al., 1998 WHO, 1993 |
| 1993–1994 | Ghana (Upper West Region) | 47 (0–15) | 103 | 46 | 2 : 1 | Not known | WHO, 1994 |
| 1994 | Nigeria (Imo State) | 37 (0–15) | 116 | 32 | 1.3 : 1 | ?Ae. africanus | WHO, 1995 |
| 1995 | Senegal (Koungeheul District) | 58 (0–19) | 110 | 53 | 1.4 : 1 | Ae. aegypti; Ae. furcifer; Ae. metallicua, and Ae. luteocephalus | Thonnon et al., 1998 |
a Table includes data from outbreaks that have clearly documented the age and sex of yellow fever disease cases. For more recent outbreaks (e.g., Uganda, Sudan, Ethiopia, Angola, and Democratic Republic of Congo), these data are not available. WHO, World Health Organization.
The older literature makes repeated reference to racial differences in the lethality of YF, rates being lower in blacks than whites during outbreaks in West Africa, tropical America, and the United States. , , It is uncertain whether the apparent increased resistance of blacks reflects acquired immunity or is a result of genetic factors. The question of racial differences in susceptibility to YF will be resolved only by well-controlled epidemiologic and serologic studies in the setting of an outbreak affecting both races.
It is expected that YF would be more severe in individuals who are immunosuppressed either due to underlying disease (e.g., HIV) or medications. However, there are very limited data on individuals who are immunosuppressed and develop wild-type YF disease. Following the large outbreaks in Brazil, a few case reports of YF were reported in renal transplant patients. , Of the two renal transplant patients with YF, the one who was still receiving immunosuppressive medication 24 years post-transplant died. The other patient who was 5 years post-transplantation survived; information on the treatment regimen for second renal transplant patient was not provided.
No data exist on the potential virulence of the seven different genotypes of wild-type YFV. , , , In animals, these wild-type YFV strains differ with respect to neurovirulence for mice or viscerotropism for monkeys Deubel and colleagues found that South American strains were neuroinvasive for 8-day-old mice, whereas African viruses were not. On the other hand, South American viruses were found to be less often lethal for Rhesus monkeys. Miller and colleagues showed that the mosquito responsible for epidemic transmission in Nigeria had a low vector capacity, and they proposed that the vector served as a genetic bottleneck for selection of a virulent virus strain able to elicit high levels of viremia in humans.
Wild-type YFV strains are predominantly viscerotropic in primates, including humans. With susceptible nonhuman primates, even if the virus is inoculated intracerebrally, death is from hepatitis rather than encephalitis. However, when a small amount of antibody is given to a monkey shortly before or at the same time as the virus is injected intracerebrally, the animal dies of encephalitis, presumably because the antibody is able to protect peripheral organs but not the brain. This experiment reveals the essential duality of wild-type YFV (viscerotropism and neurotropism).
Antibody and presumably cellular responses occur coincident with the clinical crisis (period of intoxication), and both free virions and hemagglutinating, complement-fixing, or immune precipitating antigen, likely NS1, may be found in blood together with antibody, suggesting that immune clearance of infected cells, associated with release of cytokines, might play a role in the pathogenesis of capillary leak and shock. , Patients with fatal YF have a pattern of elevated pro- and anti-inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor [TNF]-α, monocyte chemoattractant protein [MCP]-1, IL-1–receptor antagonist, IL-10), resembling bacterial sepsis, whereas patients with nonfatal YF and without hemorrhagic diathesis only had anti-inflammatory cytokine elevations. Similar findings have been reported in cases of viscerotropic disease associated with 17D vaccine. Overall, these findings suggest that a cytokine storm, and in particular, the vasoplegic response to the exuberant immune response, can contribute to lethality.
The cell types infected in vivo in viscerotropic infections are only partially defined. A study that compared histopathologic features and immunohistochemical staining on tissues from persons who died following either wild-type YFV infection or viscerotropic disease secondary to 17DD vaccination found similar histopathology and immunohistochemical staining of hepatocytes and Kupffer cells for both conditions. However, with viscerotropic disease cases, there was also staining of mesenchymal calls in the liver as well as other tissues, suggesting that mesenchymal cell staining is unique and pathognomonic for viscerotropic disease caused by 17DD virus.
In general, YFV is not neurotropic in humans. However, similar to what is seen with young mice, human infants are at increased risk of developing neurotropic disease following vaccination with 17D. Cases of encephalitis in young infants who acquired YF 17D vaccine virus infection orally via breast milk from recently vaccinated lactating mothers emphasizes the susceptibility of very young infants to neurologic infection. , The pathway whereby virus invades the brain after parenteral inoculation is not established but is likely to be via the bloodstream, whereas in the case of exposure of infants through breastfeeding, the route is less clear. It could be via the olfactory apparatus or a mucosal route to the bloodstream. The basis for age-related susceptibility is uncertain. The blood–brain barrier is incomplete and matures during the first year of life. Zisman and colleagues suggested that development of resistance with age was related to maturation of macrophages involved in YFV clearance.
The pathophysiology of YF in rhesus monkeys, humans, and mice engrafted with human hepatocytes is characterized by liver dysfunction, kidney failure, coagulopathy, and shock , , , ; monkeys develop a more-fulminating illness than humans, lasting only 3–4 days. Higher viral doses shorten the incubation period but do not alter the duration or outcome of illness, implying that innate immune responses (e.g., IFN, cytokines, and natural killer [NK] cells) are downregulated and insufficient to clear even a minimal infection. The virus is rapidly internalized and cells are activated and serve to present antigens even though replication may be restricted. Human vascular endothelial cells are susceptible to infection in vitro, with release of RANTES and IL-6, suggesting that endothelial infection and the resulting inflammatory response could mediate vascular leak in vivo.
Infection with YFV induces pathologic changes in several organs, including liver, kidney, heart, and lymphoid tissues. The outcomes are a result of direct viral cytopathic effect, the necrosis and apoptosis of hepatocyte cells in the midzone, accompanied by minimal inflammatory response as well as low-flow hypoxia and cytokine overproduction. YFV infections typically affect the midzone of the liver lobule, with sparing of cells bordering the central vein and portal tracts. , Midzonal necrosis has been described in low-flow hypoxia, due to adenosine triphosphate depletion and oxidative stress of marginally oxygenated cells at the border between anoxic and normoxic cells, and a similar mechanism might contribute to injury in YFV infection. However, YFV antigen and RNA have been observed principally in hepatocytes in the midzone, , suggesting a predilection of these cells for virus replication.
Injury to hepatocytes is characterized by eosinophilic degeneration with condensed nuclear chromatin (Councilman bodies), indicating apoptosis, rather than by ballooning and rarefaction necrosis seen in virus hepatitis and Labrea hepatitis. , The apoptotic mode of cell death might explain the minimal inflammation, principally characterized as CD4 + T cells, in hepatic tissue affected by YF. The midzonal cellular response associated with apoptosis includes macrophages, NK cells, and CD4 + , CD8 + , CD68 + Kupffer, and CD20 + cells. , , The portal tracts also show evidence of sequential involvement of innate and adaptive immune activation mechanisms related to YFV infection. Because little inflammation occurs, the reticulin framework is preserved and complete healing results without residual fibrosis.
Kidney pathology is also characterized by eosinophilic degeneration and fatty change of renal tubular epithelium without inflammation. These changes might represent late-stage injury following shock as was documented in monkeys. However, YFV antigen was found in renal tubular cells of fatal human cases and in monkeys, suggesting that direct viral injury is responsible and accounts for albuminuria in advance of kidney failure (Monath TP, unpublished observation, 1980). ,
Direct virus injury to myocardial fibers, which show cloudy swelling and fatty changes and viral antigen, can contribute to shock. , , DIC and decreased synthesis of vitamin K–dependent coagulation factors by the liver contribute to the bleeding diathesis.
Most of our knowledge about the innate and adaptive immune response to YFV infection is derived from studies of patients receiving the live 17D vaccine (see “Immune Responses to Vaccination” Section). However, what is known about the kinetics of the antibody response following wild-type infection is briefly reviewed here.
The humoral response to wild-type YFV is characterized by the appearance of immunoglobulin (Ig) M antibodies, typically detected either with an enzyme-linked immunosorbent assay (ELISA) or indirect immunofluorescence assay (IFA), during the first week of illness. , , IgM levels peak during the second week, and decline rapidly over 30–60 days. IgM antibodies have been found to persist for prolonged periods after 17D vaccination, and they might also persist following wild-type YF. The magnitude of the IgM response in cases of primary YF infection is significantly greater than in patients with prior flavivirus exposure, in whom the ratio of IgM to IgG is low. Neutralizing antibodies persist for many years, if not lifelong, after natural YFV infection, and they provide complete protection against disease on re-exposure to the virus. Neutralizing antibodies have been documented as long as 78 years after illness. , No documented case of a second clinical YF infection has been reported.
Antibody responses following primary YF are specific for YFV antigen. With affinity maturation, specificity declines, and cross reactions with related flaviviruses appear during the second week after onset. Patients with prior heterologous flavivirus immunity develop broadly cross-reactive antibody responses.
Previous infection with some flaviviruses might ameliorate the clinical severity of YF though the evidence is conflicting. Observational studies going back to 1815 suggested that flavivirus immunity (e.g., to dengue) was the basis for resistance to YF in long-term residents of endemic areas, and it was later proposed as a barrier to introduction of YF into Asia. Early experiments indicated that passive transfer of dengue antibodies did not protect monkeys against challenge with YFV. In contrast, monkeys actively immunized with dengue virus were relatively resistant to challenge with YFV, suggesting that cellular immunity played a role in cross protection. Monkeys actively immunized with two African flaviviruses (Zika and Wesselsbron), but not with West Nile or Banzi viruses, resisted challenge with YFV.
Clinical experience as well as a recently retrospective analysis of thousands of dengue cases in Brazil indicate that there is no evidence that YF vaccination increases the risk of severe dengue disease. This is a question of immense importance, because dengue viruses are encroaching on YF endemic areas in South America, where routine 17D vaccination is performed. In a small study of Zika virus-infected travelers who were either flavivirus-naïve or had received YF vaccine and/or tick-borne encephalitis (TBE) vaccine suggested that the risk of antibody-dependent enhancement was mitigated by IgM antibodies, which were found to be elevated in individuals who were previously vaccinated compared to naïve individuals. Preexisting immunity to JE does not increase viremia following 17D vaccination.
The preliminary diagnosis of YF is based on the patient’s clinical features, YF vaccination status, and history of residence or recent travel to a YF–endemic zone. Mild YF cannot be distinguished clinically from a range of other infections and is unlikely to be recognized as YF. Cases of YF with jaundice must be differentiated from viral hepatitis, malaria, leptospirosis, louseborne relapsing fever ( Borrelia recurrentis ), Congo-Crimean hemorrhagic fever, Rift Valley fever, typhoid, Q fever, and typhus, as well as surgical, drug-induced, and toxic causes of jaundice. It also needs to be differentiated from other viral hemorrhagic fevers, which usually manifest without jaundice, include dengue hemorrhagic fever, Lassa fever, Marburg and Ebola virus diseases, and Bolivian, Argentinean, and Venezuelan hemorrhagic fevers.
Specific laboratory diagnosis is made by detection of virus, viral RNA, or viral antigen in blood or by serology. Virus is readily isolated from blood during the first 4 days after onset, but isolations as late as 12 days or longer are recorded. The virus can also be recovered from postmortem liver tissue.
Virus isolation was traditionally accomplished by intracerebral inoculation of suckling mice or intrathoracic inoculation of Toxorhynchites mosquitoes. Currently, cell culture is most often used to isolate the virus and is more sensitive than previous techniques. Aedes pseudoscutellaris (AP61) cells are more sensitive than other in vitro methods for primary isolation of YFV and show cytopathic effects within 5–7 days after inoculation and viral antigen detectable by immunofluorescence in advance of cytopathic effects (e.g., day 3 after inoculation). Ae. albopictus (C6/36) cells, Toxorhynchites amboinensis cells, and mammalian cells (e.g., Vero, SW13) may be used, particularly if combined with reverse-transcription polymerase chain reaction (RT-PCR) or detection of viral antigen.
RT-PCR is now more commonly used than virus isolation to diagnose YF disease in the acute period owing to its rapid turnaround time and the increased sensitivity it provides over viral isolation. RT-PCR has been used to detect wild-type YFV genome and 17D viral genome in serum and human tissues (liver, spleen) and a variety of tissues from nonhuman primates infected with 17D virus. RT-PCR and sequencing is the preferred method for distinguishing wild-type YF from a case of vaccine-associated viscerotropic disease; however, RT-PCR assays have been developed to distinguish between WT and vaccine infections without sequencing. , Although RT-PCR has been used to characterize the viremia seen following vaccination, only a limited number of YF cases have been evaluated with RT-PCR. A quality assessment of laboratories performing RT-PCR testing for YFV RNA did suggest problems with sensitivity for wild-type infection with 38% (12/32) of laboratories not detecting wild-type YFV strains. There was also problem with specificity as 78% (25/32) of laboratories had false-positive RT-PCR result for YF. These data suggest noticeable variability between laboratories performing RT-PCR testing and emphasizes the need for confirmatory testing at reference laboratories.
Rapid, early diagnosis is also possible by measurement of YFV antigen in serum by immunoassay. , However, the sensitivity of this assay is significantly lower than with RT-PCR.
Ideally, diagnostic samples should be kept cold or kept frozen on dry ice or liquid nitrogen to maintain stability of infectious virus and RNA. However, a suitable alternative may be to dry the sample on filter paper discs, a procedure commonly used for antibody samples. When maintained at room temperature, YFV infectivity and RNA was still detectable by culture and RT-PCR after 90 days. Samples for RT-PCR and ELISA do not need to be handled in a way that preserves infectivity; specimens may also be intentionally inactivated as a safety precaution where the diagnosis of dangerous pathogens (e.g., other viral hemorrhagic fevers) is considered.
Identification of YFV has been accomplished by a highly sensitive microarray amplification and hybridization method as well as NGS. , Using microarrays with multiple probes or NGS could be useful for differential diagnosis, particularly when the etiology is unclear or there might be several pathogens cocirculating in an area at one time but would require specialized equipment.
Examination of liver reveals the typical pathoanatomic features of YF, including midzone necrosis. , Liver biopsy should never be performed during the illness , because fatal hemorrhage can ensue. Histopathologic diagnosis may be difficult in patients who die after the second week of illness. Electron microscopy can reveal typical flavivirus particles in intracellular vacuoles. Definitive postmortem diagnosis may be made by immunocytochemical staining for YFV antigen in liver, heart, or kidney, , , even in specimens stored for years at ambient temperature. The distribution of virus in the liver is midzonal, suggesting that hepatocytes bordering the central veins and portal tracts undergo less-active virus replication. Viral genome may also be detected in formalin-fixed embedded tissues by hybridization. RT-PCR can be used on RNA extracted from fixed tissues to obtain YFV sequences from fatal cases as well as historical materials for epidemiologic and evolutionary studies; however, the quality of the formalin-fixation is critical.
Although older methods for serologic diagnosis (hemagglutination-inhibition [HI] and CF) are useful, they have been replaced by the IgM-capture ELISA and newer IFAs. , The presence of IgM antibodies in a single sample provides a presumptive diagnosis, and confirmation is made using a plaque-reduction neutralization test (PRNT) to document a rise in virus-specific titers between paired acute and convalescent samples or a fall in titer between early and late convalescent samples. In general, the sensitivity of YF IgM assays that are currently used is high for wild-type infection (though the sensitivity is lower for IgM formed following vaccination). The specificity of the IgM ELISA is also high in primary infections and in many cases of secondary infection. However, cross reactivity complicates the diagnosis of YF by all serologic methods, particularly in Africa, where multiple flaviviruses cocirculate. In a study conducted in Colombia, roughly 40% of persons infected with wild-type YFV and those who received 17D vaccine were found to be dengue IgM–positive. Furthermore, the phenomenon of “original antigenic sin” complicates flavivirus serologic investigations. Patients who have had prior heterologous infection and who develop YF can have higher responses to the original virus, and those who have had prior YFV infection or vaccination and who are subsequently infected with another flavivirus can have higher responses to YF. ,
Detection of NS1 antigen in serum during the acute phase of illness is an important method that has been successfully applied to the diagnosis of primary infections with other flaviviruses. A YFV-specific quantitative NS1 capture-ELISA was recently developed and shown to detect NS1 in both cell culture and serum with 80% sensitivity and 100% specificity.
An important diagnostic issue, in situations where 17D vaccine is used in areas endemic for YF, is the differentiation of YF vaccine–associated viscerotropic disease (YEL-AVD) from wild-type YF, because these conditions cannot be distinguished clinically. A case in point is the identification of a case of YEL-AVD during a retrospective study of genomic sequences of (supposedly) wild-type YF strains. The differential diagnosis can be made only by genomic sequencing of virus isolate or RT-PCR amplicon, or by MAb analysis of an isolate.
Although multiple drugs have been evaluated or used empirically to treat YF, to date, none have demonstrated specific benefit. , Management is supportive and based on symptoms and the organ systems involved.
In 1984, an expert panel recommended measures to improve the care and management of patients with YF. The recommendations included maintenance of nutrition and prevention of hypoglycemia; nasogastric suction to prevent gastric distention and aspiration; treatment of hypotension by fluid replacement and, if necessary, vasoactive drugs; administration of oxygen; correction of metabolic acidosis; treatment of bleeding with fresh-frozen plasma; dialysis if indicated by renal failure; and treatment of secondary infections with antibiotics. Use of heparin to reverse DIC is reserved for patients with documented consumption of clotting factors and activation of fibrinolytic mechanisms.
The final stage of YF disease has features resembling a cytokine storm or septic shock. Stress-dose corticosteroids (200–300 mg/day) have been shown to have therapeutic benefit in patients with septic shock, at least for those with adrenal insufficiency and appear to have also benefited patients with YEL-AVD. Corticosteroids are indicated for treatment of selected patients with acute disseminated encephalomyelitis (ADEM) associated with YF vaccine. There are no experimental or clinical data on other treatment approaches, such as MAbs against cytokines or hemofiltration, for late-stage YF disease. Finally, orthotropic liver transplantation theoretically might be considered as a clinical intervention in fulminant hepatitis caused by YFV as apoptotic cell death is central to the pathogenesis of YF. However, recent attempts to improve the survivial of patients with severe YF in Brazil with liver transplantation had mixed success, with some patient’s newly transplanted livers becoming infected (Erin Staples, personnel communication).
Treatment by administration of immune serum or by cross-circulation from an immune donor animal after clinical onset of YF has minimal therapeutic effect. One patient with YEL-AVD was treated with intravenous immunoglobulin (IVIg) during the acute illness and failed to survive suggesting no benefit of the treatment. Furthermore, treatment with a mouse monoclonal neutralizing antibody that was given as a last resort to a YF patient in late-stage hepatorenal failure did not provide any beneficial effect. However, a human/mouse chimeric IgG monoclonal (but not IgM) was shown to protect IFN-receptor knockout mice when given before or up to 48 hours after lethal challenge with 17D virus. A fully humanized IgG1 anti-YFV MAb was recently developed and its reactogenicity and ability to limit viral replications of 17D was assessed in healthy adults. Overall, the MAb was well tolerated and no virus was detectable after 17D vaccination in persons receiving IgG1 MAbs when compared to a placebo control. Despite these recent and promising findings, it is unclear that antibody would be useful except in the setting of postexposure prophylaxis when given before onset of clinical disease or as treatment given during the earliest stages of illness or potentially to an immunocompromised individual. ,
There are several reports in the literature on treatment of flavivirus infections of humans (dengue, JE, and St. Louis encephalitis viruses) with IFN-α claiming improvement or reduced mortality, although the studies were small and not controlled. IFNs have been investigated for the prevention and treatment of YF in various animals. Overall, the studies showed that IFNs needed to be administered very shortly before infection or during the incubation period to reduce viremia, morbidity, and mortality suggesting that it might not be a viable treatment option for wild-type YF. ,
Ribavirin is active against YFV in vitro but has had mixed success in animal models. There are no clinical data to support safety of high doses of intravenous ribavirin to treat YFV infection. Synergistic effects of ribavirin and related compounds, such as tiazofurin and selenazole, have been demonstrated in vitro but have not been investigated in vivo.
Molecular targets for rational design and development of antiviral drugs against flaviviruses include both virus-specified functions (e.g., NS5 polymerase and methyltransferase) and host cell enzymes. , This field is expanding owing to the interest in developing drugs against dengue and hepatitis C virus; a comprehensive review is not within the scope of this chapter. ,
A detailed comprehension of the geography, seasonality, and incidence of YFV is critical to the proper use of YF vaccine for both endemic and traveler populations. For more detail on risk factors for disease, see “Risk Factors for Developing Disease and Factors Impacting Disease Severity” under “Clinical Description” Section.
The current YF risk maps incorporate all available data to establish risk categories of endemic, transitional, low, and no risk ( Fig. 64.3 ). However, it is difficult to determine current areas of risk in real time and in some areas based on scanty serologic data.
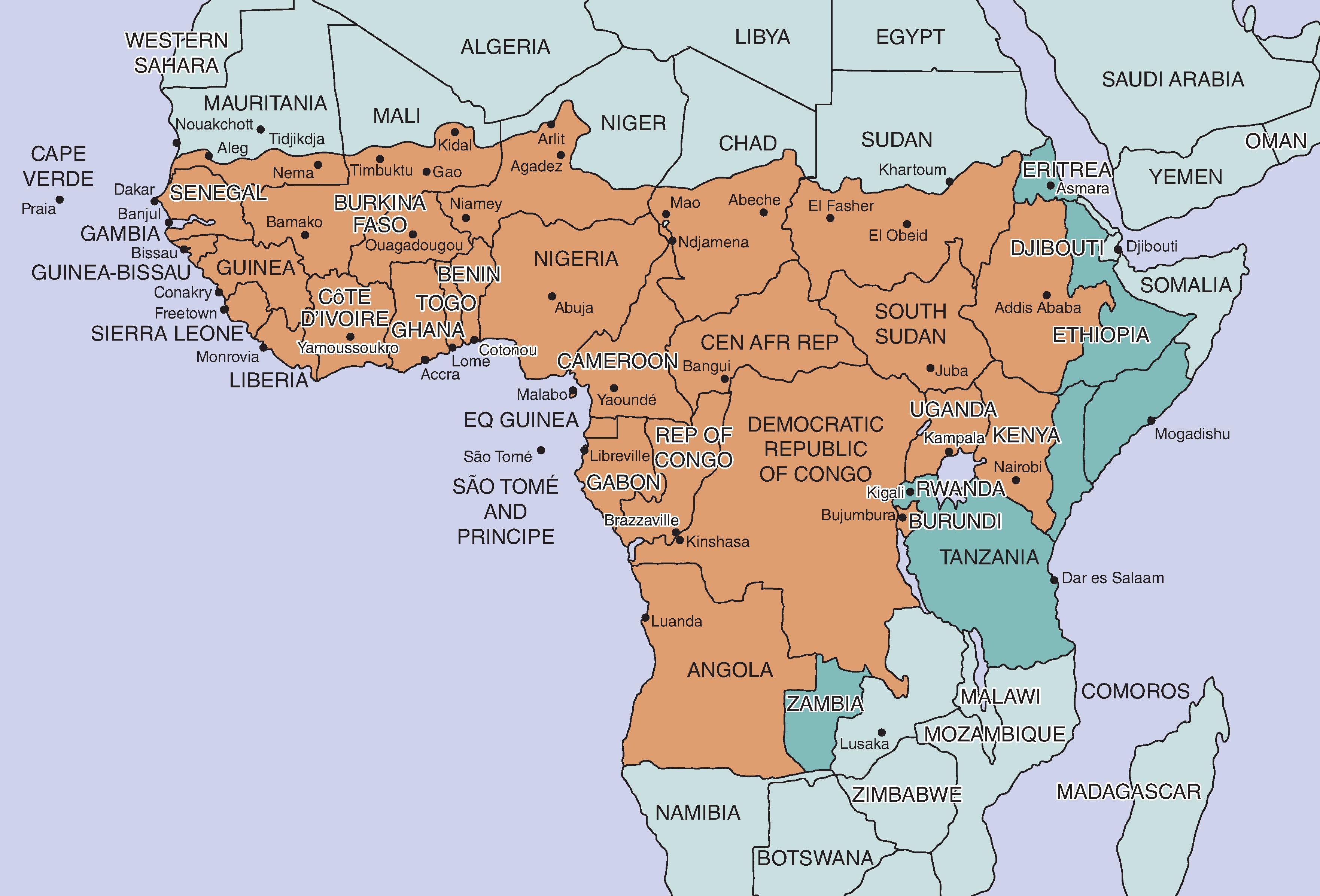
In South America, the incidence of YF is highest during months of high rainfall, humidity, and temperature (January to May; peak incidence, February and March), corresponding to the activity of Haemagogus mosquitoes, which breed in tree holes and are thus dependent on rainwater. Human exposure during agricultural activities is also increased at this time of the year and is correlated with YF disease-occurrence. In the savanna zone of West Africa, cases appear during the mid-rainy season (August) and peak during the early dry season (October), corresponding to the period of maximum longevity of sylvatic mosquito vectors. The domiciliary vector Ae. aegypti breeds in receptacles used by humans for water storage and is thus less dependent on rainfall. Where this mosquito is involved in virus transmission, YF can occur in the dry season in rural areas and in heavily settled urban areas. Thus, season is only a partially reliable guide to determining the risk of exposure and to making decisions on the need for immunization of travelers.
Fluctuations in rainfall profoundly affect the abundance of mosquito vectors and the potential for YF epidemics. , , ,
Temperature influences the transmission rates of several arboviruses. The extrinsic incubation period of YFV in the mosquito vector is very sensitive to temperature, and an increase of a few degrees can shorten the extrinsic incubation period by days, resulting in a significantly increased rate of transmission. , Even brief exposure to high temperatures (e.g., in a sunlit forest clearing) can have this effect. Warm temperature also increases biting and reproductive rates of Ae. aegypti. Thus, long-term environmental change (global warming) is likely to increase transmission rates of YFV with recent modeling work suggesting that climate change will result in an increase in annual deaths due to YF by 2050. ,
In the early 1990s, WHO estimated that there were roughly 200,000 cases and 30,000 deaths occurring annually from YF. Following the more recent efforts to improve vaccination coverage in high risk countries in Africa, the global estimates have been revised with an estimated 109,000 severe infections and 51,000 deaths occurring in Africa and South America due to YF during 2018. , Overall, the 2018 estimates of disease were less than those from the 1990s, but the estimates of deaths were increased likely secondary to more precise estimates of disease occurrence and severity. ,
The disease burden estimates are substantially higher than the actual number of confirmed YF disease cases and deaths reported from endemic regions on an annual basis. From 2001 through 2020, there has been a median of 241 confirmed YF disease cases (range, 21–2794) and a median of 64 deaths (range, 8–511) reported annually ( Table 64.3 ). This notable difference between the estimated and reported disease burden is thought to be partly because of occurrence of the disease in remote areas, late recognition of outbreaks, and lack of diagnostic facilities. Where specific investigations have been undertaken, the ratio of actual cases to official notifications varies between 1 and 311 to 1 ( Table 64.4 ). , , , , , , , ,
| Country | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
| Africa | |||||||||||||||||||||
| Angola | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 884 | 0 | 0 | 0 | 0 | 884 |
| Burkina Faso | 22 | 0 | 29 | 10 | 609 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 678 |
| Cameroon | 0 | 0 | 3 | 6 | 196 | 1 | 3 | 2 | 1 | 7 | 23 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 252 |
| Central African Rep | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 12 |
| Chad | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 139 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 140 |
| Congo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 |
| Côte d’Ivoire | 280 | 156 | 158 | 92 | 126 | 16 | 0 | 2 | 3 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 847 |
| Dem Rep Congo | 0 | 0 | 29 | 0 | 140 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 7 | 7 | 0 | 79 | 0 | 0 | 5 | 0 | 269 |
| Ethiopia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 141 | 0 | 0 | 0 | 0 | 5 | 1 | 2 | 149 |
| Gabon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| Gambia | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Ghana | 1 | 7 | 61 | 1 | 492 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 566 |
| Guinea | 172 | 20 | 60 | 6 | 263 | 1 | 0 | 25 | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 573 |
| Liberia | 59 | 0 | 25 | 5 | 41 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 136 |
| Mali | 0 | 0 | 0 | 2 | 97 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 108 |
| Nigeria | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 106 | 191 | 145 | 495 |
| Senegal | 1 | 134 | 1 | 2 | 105 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 255 |
| Sierra Leone | 0 | 0 | 90 | 0 | 3 | 0 | 0 | 9 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 104 |
| South Sudan | 0 | 0 | 178 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 2 | 186 |
| Sudan | 0 | 0 | 0 | 0 | 605 | 0 | 0 | 0 | 0 | 0 | 0 | 847 | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1500 |
| Togo | 8 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16 |
| Uganda | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 13 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 10 | 10 | 55 |
| Subtotal | 550 | 338 | 639 | 124 | 2677 | 28 | 11 | 47 | 20 | 25 | 58 | 987 | 206 | 7 | 0 | 970 | 33 | 118 | 216 | 178 | 7232 |
| South America | |||||||||||||||||||||
| Argentina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 |
| Bolivia | 4 | 15 | 6 | 13 | 16 | 16 | 6 | 2 | 0 | 3 | 2 | 3 | 1 | 1 | 0 | 0 | 8 | 1 | 2 | 0 | 99 |
| Brazil | 41 | 17 | 61 | 6 | 3 | 2 | 13 | 47 | 46 | 3 | 0 | 0 | 3 | 1 | 16 | 53 | 800 | 1389 | 431 | 19 | 2951 |
| Colombia | 8 | 20 | 108 | 34 | 20 | 5 | 6 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 6 | 0 | 0 | 0 | 222 |
| Ecuador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 4 |
| French Guiana | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 4 |
| Paraguay | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 |
| Peru | 29 | 52 | 25 | 68 | 66 | 63 | 23 | 15 | 8 | 18 | 13 | 15 | 22 | 12 | 15 | 62 | 9 | 10 | 4 | 7 | 536 |
| Suriname | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Venezuela | 0 | 3 | 34 | 5 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 55 |
| Subtotal | 82 | 107 | 234 | 126 | 117 | 86 | 49 | 111 | 59 | 24 | 15 | 19 | 26 | 14 | 31 | 122 | 828 | 1401 | 438 | 28 | 3917 |
| TOTAL | 632 | 445 | 873 | 250 | 2794 | 114 | 60 | 158 | 79 | 49 | 73 | 1006 | 232 | 21 | 31 | 1092 | 861 | 1519 | 654 | 206 | 11,149 |
a Only countries reporting disease cases from 2001 to 2020 are listed in the table; consequently, several countries that previously reported yellow fever disease cases (e.g., Benin, Guinea Bissau) and which might be considered to be endemic for YFV activity are not listed. Data from WHO weekly epidemiologic record https://www.who.int/wer/en/ ; WHO disease outbreak news https://www.who.int/csr/don/en/ ; PAHO Yellow Fever: Number of confirmed cases and deaths https://ais.paho.org/phip/viz/ed_yellowfever.asp . If number were inconsistent between WHO and PAHO; PAHO numbers were used for South American data.
| Epidemic Year | Country | Official Notification | Estimated by Direct Investigations | Ratio of Actual to Reported Cases | Reference |
|---|---|---|---|---|---|
| 1960–1962 | Ethiopia | 3010 | 100,000 | 33:1 | Serié et al., 1968 |
| 1965 | Senegal | 243 | 2000–20,000 | 8–82:1 | Brès et al., 1967 |
| 1969 | West Africa | 322 | >100,000 | 311:1 | Carey et al., 1972 |
| 1970 | Nigeria | 4 | 786 | 197:1 | Monath et al., 1973 |
| 1977–1978 | Ghana | 713 | 2400 | 3:1 | Addy et al., 1986 |
| 1978–1979 | Gambia | 30 | 8400 | 280:1 | Monath et al., 1980 |
| 1987 | Mali | 305 | 1500 | 5:1 | World Health Organization, 1989 |
| 1986 | Nigeria | 1289 | 9100 | 7:1 | De Cock et al., 1988 |
| 1987 | Nigeria | 2676 | 120,000 | 45:1 | Nasidi et al., 1989 |
| 1990 | Cameroon | 173 | 5000–20,000 | 29–116:1 | World Health Organization, 1992 |
| 1992–1993 | Kenya | 54 | 55 | 1:1 | Sanders et al., 1998 |
| 1994 | Nigeria (Orsu LGA) | 120 | 775 in one community representing 22% of the total population | >6:1 | World Health Organization, 1995 |
a List includes outbreaks where an epidemiologic investigation was performed to estimate the actual number compared to the number of cases that were officially notified.
In Africa, there are 33 countries that are considered to have endemic transmission of YF, including 27 with a high risk for YF outbreaks. , However, risk of YF disease varies among countries, , and often within a country, as many countries are not holoendemic. The highest risk for disease occurs in West Africa where disease cases are typically reported on an annual basis, especially prior to improved vaccination coverage in the region (see Table 64.3 ). Central and East African countries have a lower disease risk, often with no disease cases reported for several decades. , For instance in Ethiopia, the first recognized outbreak of disease occurred in the early 1960s and no disease activity was reported again until 2013. Another example is Angola, which experienced one of the largest disease outbreaks in recent history in 2015–2016. Prior to this they last reported cases in 1988. The differential risk of YF, falling as one moves west to east and south in Africa, is thought to be potentially related to differences in the strains circulating in the region, the frequency humans come into contact with sylvatic cycles, population immunity, and seasonal variations. ,
From 2001 to 2020, there were 7232 cases and 716 deaths (case-fatality ratio of 10%) reported from Africa countries ( Fig. 64.4 and see Table 64.3 ). The case-fatality rate in Africa is likely underestimated by official reports, because data are unavailable on the number of deaths. The annual incidence varies by country and region, reflecting periodic expansions of YFV activity, but accuracy of reporting limits interpretation.
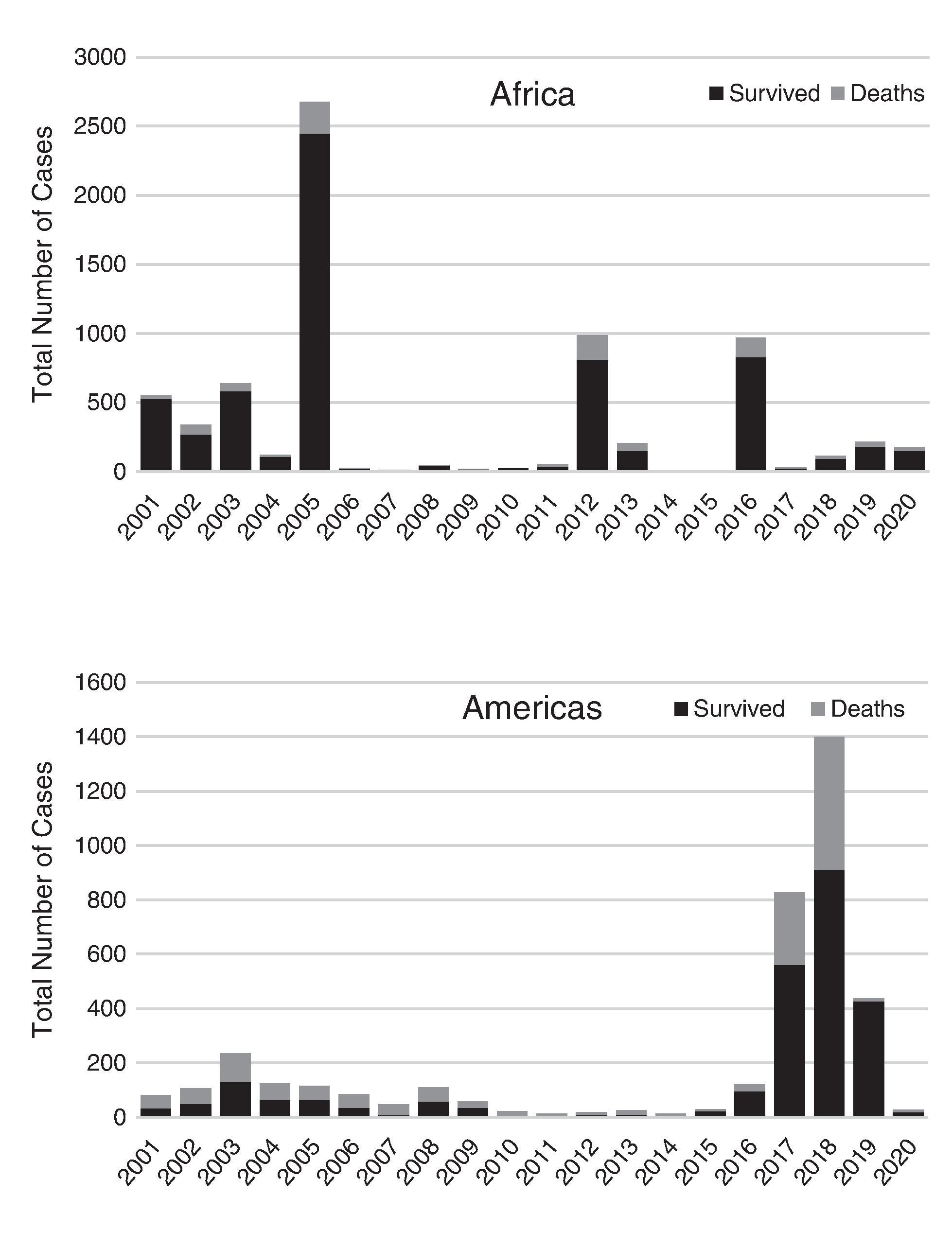
During outbreaks of disease in Africa, it has been estimated through serosurveys that roughly 20–40% of a population will become infected with YF. , , , , , , , , In comparison, the burden of endemic YFV infection has been less-well characterized. In Nigeria (1970–1971), a laboratory diagnosis of YF was made in two of 205 patients (1%) hospitalized with jaundice in areas without epidemic activity. Using data from serologic surveys in Nigeria and an estimated 7 : 1 infection-to-illness ratio, the annual incidence of overt infection with jaundice was estimated to be between 1.1 and 2.4 per 1000 population, and YF deaths were estimated to be between 0.2 and 0.5 per 1000 population. This estimate of endemic disease is likely lower as one moves eastward in Africa. Based on a serologic survey conducted in Central African Republic during 2009, the rate of disease with jaundice was 0.9 per 1000 population and death was 0.2 per 1000 population. It is likely that endemic YF activity varies considerably from year to year and that it causes thousands of unrecorded deaths annually in West Africa. This provides a strong rationale for preventive immunization.
There are 13 countries in South and Central America that are considered endemic for YF disease and at high risk for YF outbreaks. Although only sporadic cases and small outbreaks are reported in these regions, nearly all major urban areas in the American tropics have been reinfested with A. aegypti and many persons living in these regions have not been vaccinated. Improvements in roads, increased settlement within the Amazon region, and more ready movements between these areas have led to more unvaccinated people moving in and out of endemic zones.
Between 2001 and 2020, 3917 cases and 1362 deaths (case fatality ratio of 35%) were reported from South American countries (see Table 64.3 and Fig. 64.4 ). The annual incidence varies by country owing to fluctuating wandering epizootic activity that depends on herd immunity levels in monkey populations. The lower case numbers and incidence of YF in South America than in Africa is likely a consequence of several factors. The monkey and mosquito hosts are different on the two continents plus less transmission by enzootic vectors (principally from monkey to human), low densities of vectors, susceptible monkeys and human hosts, and relatively high vaccination coverage. The higher case-fatality rate in South America probably reflects hospitalization of severe cases and surveillance based on death reports and the postmortem examination of livers rather than the South American virus strains being more virulent than those in Africa.
Increased virus transmission, particularly in the endemic areas of South America, occurs with a periodicity of 7–10 years, in part because immunologically susceptible monkey populations are replenished at a slow rate after epizootics. Larger expansions of the disease into areas on the edge of the endemic region, such as Argentina, Paraguay, Trinidad, and coastal Brazil, occur less frequently, often at intervals of more than 30–40 years, and was last seen from late 2016 to early 2017.
In South America, disease cases are reported routinely from Bolivia, Brazil, Colombia, and Peru and sporadically in Ecuador, French Guyana, Guyana, Suriname, and Venezuela ( Table 64.3 ). Argentina and Paraguay last reported cases in 2008, which was the first disease activity reported since the 1960s and 1970s, respectively. In comparison, Panama and Trinidad have not reported cases for several decades.
For additional reviews on YF epidemiology see References ,
From 1970 through 2015, 11 cases of YF were reported in travelers from the United States and Europe who traveled to West Africa (6 cases) or South America (5 cases) ( Table 64.5 ). , , , Eight of 11 travelers (73%) died. Only one traveler had a documented history of YF vaccination; that patient survived. Starting in 2016, the number of travel-associated YF cases increased substantially, primarily because of outbreaks in Angola and Brazil. From 2016 through mid-2021, more than 37 travel-associated cases were reported in unvaccinated travelers who were residents of nonendemic areas or countries, including at least 15 European travelers and 1 American traveler to Peru.
| Month and Year | Age (y) | Sex | Vaccinated | Residence | Exposure | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Oct 1979 | 42 | M | No | France | Senegal | Died | Bendersky et al., 1980 |
| Oct 1979 | 25 | M | No | France | Senegal | Died | Bendersky et al., 1980 |
| Aug 1985 | 27 | F | No | Netherlands | Guinea-Bissau, Gambia, Senegal | Survived | World Health Organization, 1986 |
| Oct 1988 | 37 | F | Yes | Spain | Niger, Mali, Burkina Faso, Mauritania | Survived | Nolla-Salas et al., 1989 |
| Apr 1996 | 53 | M | No | Switzerland | Brazil (Amazonas) | Died | Barros and Boecken, 1996 |
| Aug 1996 | 42 | M | No | United States | Brazil (Amazonas) | Died | McFarland et al., 1997 |
| Aug 1999 | 40 | M | No | Germany | Côte d’Ivoire | Died | Bae et al., 2005 ; World Health Organization, 2000 |
| Sep 1999 | 48 | M | No | United States | Venezuela | Died | World Health Organization, 2000 |
| Nov 2001 | 47 | F | No | Belgium | Gambia | Died | Bae et al., 2005 ; Parent et al., 2005 ; Berneau, 2006 |
| Mar 2002 | 47 | M | No | United States | Brazil (Amazonas) | Died | CDC, 2002 |
The risk of a traveler acquiring YF is determined by vaccination status, geographic location, season, duration of travel, activities that lead to exposure to mosquito bite, and the intensity of YFV transmission occurring at the time of travel. Exposure in an area undergoing an outbreak, even for a short period, may be associated with a high risk of infection. Although nearly all recent episodes of YF among unvaccinated travelers have occurred in conjunction with increases in YF activity, information about increased risk was often not readily available at the time. Increased YFV activity in a region often spans 2 or more years and affects contiguous areas in an expanding fashion, but such extensions in time and place may be invisible to passive surveillance systems. Moreover, in areas where vaccination is widely practiced, YFV can circulate silently between monkeys and mosquitoes, with few human cases in the indigenous population. Season is also an important risk factor for YF with most cases in travelers occurring during the period of virus amplification (late rainy season to early dry season).
During interepidemic periods in Africa, the incidence of overt disease is below the threshold of detection by existing surveillance. Such interepidemic conditions can last years or even decades in specific countries or regions. Epidemiologic silence can provide a sense of false security and lead to travel without the benefit of vaccination. Surveys in endemic areas of rural West Africa during silent periods indicate that the annual incidence of YF illness approximates 1.1–2.4 per 1000 population and YF death 0.2–0.5 per 1000 population, below detection by existing surveillance. In unvaccinated travelers to these areas, the risk of YF illness may be estimated at one per 1000 population per month and death at one per 5000 (1:2000 risk of illness and 1:10,000 risk of death from YF for a typical 2-week journey), but risk varies considerably with season and location (e.g., West Africa having a higher risk than East or Central Africa). These estimates, which are based on risk to local residents, might overestimate the risk to travelers who take precautions against mosquito bites and have less outdoor exposure than the indigenous population.
In South America, the incidence of disease is lower than in Africa. The risk of illness and death for the traveler is probably 10 times lower than in rural West Africa—approximately 1 per 20,000 population for illness and approximately 1 per 100,000 population for death per 2-week journey—but the risk varies greatly with specific location and season. As in Africa, zoonotic virus transmission is often epidemiologically silent. The low reported incidence of YF has diminished concern among travelers. In Brazil, for example, where the majority of the population lives in coastal regions outside the endemic zone, unimmunized recreational or vocational travelers to the interior are the usual victims of YF. , Many of the recent travel-associated cases were in travelers to South America.
Current YFV activity is found on the WHO’s Disease Outbreak News and CDC’s Travel Health Notices website. Annual or biannual summaries of morbidity and epidemiologic trends are also published in the Weekly Epidemiological Record. Although these materials have significant limitations owing to underreporting, they provide a picture of current hot spots of YFV activity. The user should keep in mind that endemic (and even epidemic) YF occurs in areas that are silent with respect to official reports.
Many nonendemic countries with Ae. aegypti require a valid YF International Certificate of Vaccination or Prophylaxis (ICVP) for entry from a YF–endemic region. A current listing of receptive countries that require a valid certificate is provided in the most recent WHO International Travel and Health (green book) and the CDC’s Health Information for International Travel (yellow book). ,
Areas at highest risk for the introduction and secondary spread are areas with Ae. aegypti and tend to be in the Americas including coastal regions and interior towns infested by this vector in Argentina, Brazil, Peru, Bolivia, Ecuador, Venezuela, Colombia, the Guyanas, Central America, the West Indies, Mexico, and the southern United States. All these areas have been affected by YF in the past and have had recent circulation of dengue and Zika viruses, related flaviviruses with a similar urban transmission cycle involving Ae. aegypti . Within individual countries currently affected by YF, the disease may be acquired by unvaccinated residents who travel from an uninfected area to a region of endemic activity. This is a recurring theme in South America, where unvaccinated migrant workers move from a coastal area or the Andean highlands into the Amazon region. In Brazil during the first half of 2000, 11 (14%) of 77 reported YF cases were in tourists from coastal areas to the endemic part of the country. , In Africa, similar episodes undoubtedly are common, but tend only to be recognized when larger clusters of disease cases are detected by the surveillance system or viral activity is recognized first in sylvatic areas before being introduced into an urban area with Ae. aegypti. ,
The risk that urban YF will reemerge continues to exist owing to the expansion of urban centers, increased air travel, and the continued spread of Ae. aegypti. , , , These factors have also likely led to the rising global incidence of other Ae. aegypti –transmitted viruses, such as dengue, chikungunya, and Zika. , , Ae. aegypti –infested regions of southern Europe, the Middle East, Asia, Australia, and Oceania are also at potential risk for introduction of YFV. The virus has never been recorded in India or other parts of Asia. The reasons are unknown but might include both demographic and biological factors. The most likely mode of introduction of YF from endemic areas to Asia is by air travel of viremic humans, and all receptive areas can be reached by air from an endemic region within less than the incubation period of YF. However, YF occurs in remote areas and affects persons engaged in subsistence farming, who are infrequent international travelers. Even if the disease occurs in more urban areas, like it did in Luanda, Angola, in 2016, there will need to be some degree of unchecked expansion of the virus (e.g., no vaccination coverage in the population) to increase the risk of introducing the virus to a nonendemic area in Asia, and the virus will need to be introduced during a time when Ae. aegypti are active. , During the 2016 Angola outbreak, 11 Chinese workers flew from Angola to China and were diagnosed with YF in China, yet there were no secondary cases reported. Biological factors that could limit the risk of introduction include cross-protection from dengue or JE viruses, as the majority of persons residing in Asia are immune to dengue or frequently are immunized against JE. A third hypothesis is that Ae. aegypti strains in Asia could have low vector competence for YFV. , It is possible that all of these mechanisms combine to reduce the likelihood of introduction and spread of YFV in Asia.
Spread of YFV outside its traditional boundaries and reemergence of the urban disease in Africa or the Americas would greatly increase the demand for YF vaccine, as was seen in 2016 when demand outstripped supply during the response in Angola. During periods of increased epizootic transmission, a high risk of introduction of YFV into large unvaccinated coastal populations juxtaposed to endemic areas has been postulated. Ecological changes resulting from deforestation are also changing the landscape of sylvatic YF. Destruction of habitats of nonhuman primates and certain vectors might reduce transmission in some areas, but in general such effects are counterbalanced by increased human encroachment into enzootic areas and opportunities for interhuman transmission of the virus.
Antiserum to YFV produced in horses, monkeys, or chimpanzees protects rhesus monkeys against lethal YF when given 1–3 days after challenge. , , Before vaccines were developed, passive immunization was widely used for pre-exposure prophylaxis. The concept was established in the 19th century, and convalescent serum was first used in the early 20th century. Experimental validation occurred after isolation of YFV. In 1928, Stokes and colleagues reported that pretreatment with a small volume of convalescent serum protected monkeys against lethal challenge. Because of an increasing number of laboratory infections, standard practice was to administer convalescent serum to at-risk laboratory workers and to inject large amounts after accidental exposures. However, such antisera are no longer available.
The development of humanized mouse MAbs that target epitopes present in both wild-type and vaccine YFVs holds some promise as a potential passive immunization strategy. These antibodies provide several potential advantages such as being easily produced, being more capable of quantitating the amount delivered, and being relatively safe compared with IVIg, which is a blood product. Studies of a humanized MAb (2C9-cIgG) that targets the YFV-specific E protein domain II epitope in various animal models conferred increased survival in the animals when challenged. , However, more work is needed to determine if these antibodies will provide adequate protection if given to humans who cannot safely receive vaccine.
Transplacental transfer of YFV–specific neutralizing antibodies has been documented in monkeys and humans, and antibody has also been found in breast milk of immune mothers. Because YF 17D vaccine is not administered to infants younger than 6 months of age for safety reasons, maternal immunity typically does not pose an obstacle to effective immunization but should, in endemic areas, offer infants some initial protection against wild-type disease.
Methodological problems in antigen production, virus inactivation and potency assays, ignorance about immunologic principles, and the lack of adjuvants were obstacles to the early development of inactivated vaccines. However, with the recognition of SAEs following the 17D vaccine, there is renewed interest in inactivated whole virus and recombinant subunit vaccines. Four companies (Xcellerex, Bio-Manguinhos, Najit Pharma, and the Chumakov Insitute) have reported development of whole-virus inactivated vaccines prepared from the 17D or 17DD strains propagated in Vero cells, purified, and inactivated by different methods. Analysis of the inactivated vaccine developed by Xcellerex identified mutations in E protein and NS4B protein that supported viral adaptation to Vero cells. At least three vaccines inactivated with β-propiolactone and adjuvanted with aluminum hydroxide have been developed. , , All of these vaccines induced neutralizing antibodies in rodents and protected them against lethal challenge. In addition, the vaccine developed by Xcellerex was also found to protect hamsters against lethal challenge and induce neutralizing antibodies in monkeys and humans. , , In a Phase I clinical trial, two doses of 4.8 µg (a dose similar to that in a commercial vaccine against JE) spaced 21 days apart induced neutralizing antibodies within 10 days of the second vaccination in all subjects. Neutralizing antibody titers were similar to those induced by commercial inactivated vaccines against JE and TBE.
In the 1930s, several groups worked on developing an attenuated live viral vaccine from wild-type FVV strain. , , , , Its development is reviewed in more detail in reference. In 1946, the UNRRA Standing Technical Committee on Health approved the FNV, and the WHO granted similar approval in 1948.
FNV was prepared at the Pasteur Institute in Dakar by the intracerebral inoculation of 2.5–3-month-old mice with approximately 20,000 LD 50 (median lethal dose) of virus. A seed lot system was not used, although passage level was restricted. Mice showing illness were killed and the brains were aseptically removed and lyophilized. After sterility testing, brains were pooled, ground to powdered form, and again tested for sterility. The vaccine powder was filled into ampoules containing one-tenth of a mouse brain (0.4 g), equivalent to 100 doses, and tested for sterility and potency. After reconstitution in 2 mL of diluent, the recommended minimum potency was 5000 mouse LD 50 /dose. The vaccine was quite stable and was stored at 4°C but shipped at ambient temperature. After reconstitution in a solution of gum arabic, a drop of the solution was placed on the skin, and scarification was performed with a scarification devise or similar instrument typically used to perform smallpox vaccinations.
By 1953, 56 million doses had been delivered in francophone Africa (twice the population of the region). As a result the incidence of YF declined in francophone countries ( Fig. 64.5 ), but not in neighboring Nigeria and Ghana, where immunization was not practiced. Seroconversion rates were shown to exceed 95%, and population surveys in French West Africa showed that the prevalence of immunity rose from approximately 20% before vaccination to 86% in 1952–1953.
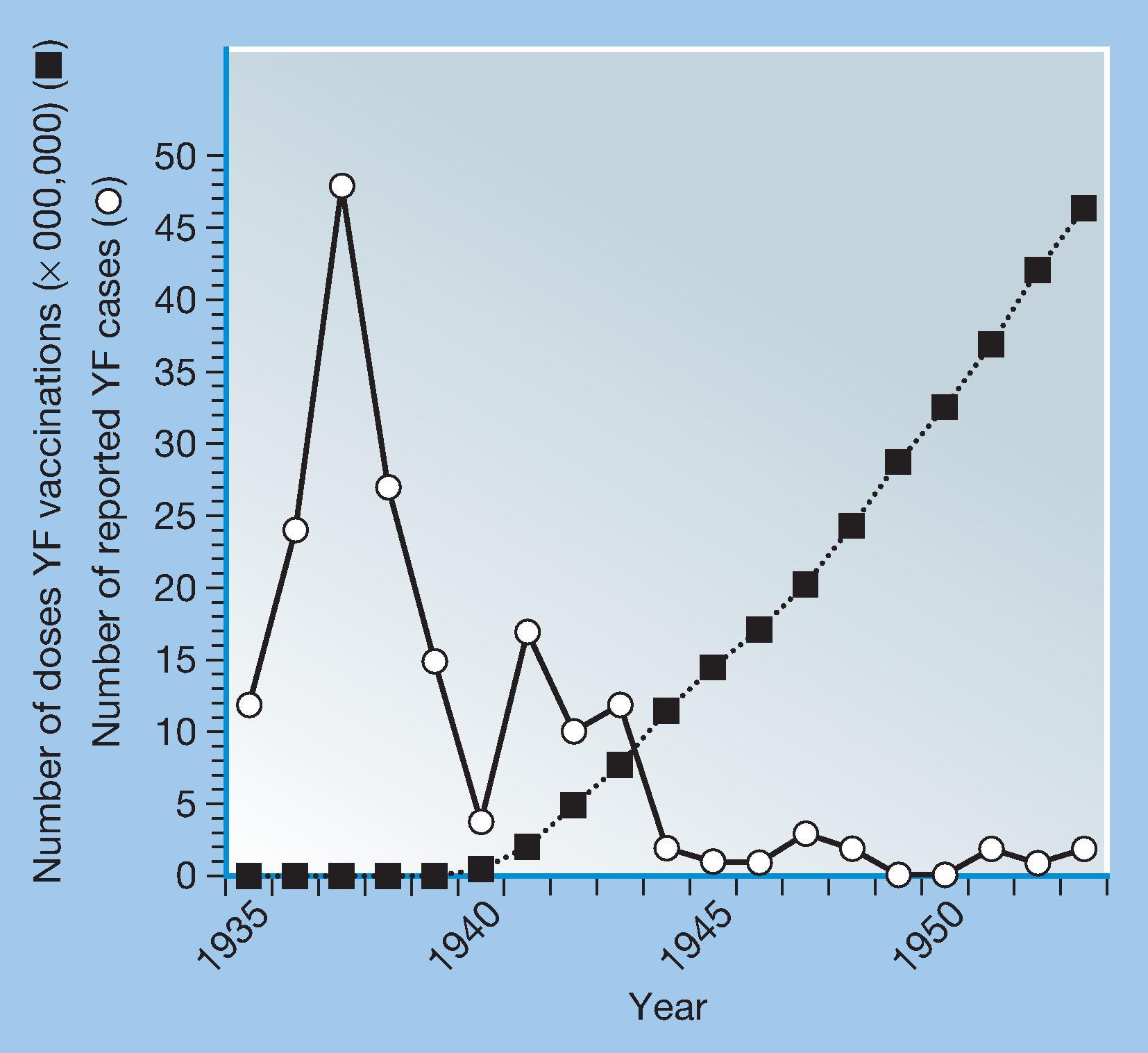
The safety of FNV was not carefully evaluated during the ramp-up to achieve full coverage. It was known that FNV caused viremia in two-thirds of subjects, that 10–15% experienced a mild reaction with fever, headache, and backache on days 4–6, and that rare cases of meningoencephalitis occurred 10–15 days after vaccination. , , The incidence of encephalitis was initially estimated at between 1 per 3000 and 1 per 10,000, but its importance was minimized because full recovery was the rule and because no serious reactions were noted during campaigns in French West Africa involving more than 40 million persons. , However, contemporary reports described outbreaks of encephalitis and deaths following YF immunization in French Equatorial Africa.
English and American workers considered FNV too dangerous for routine use, but when epidemics struck in Nigeria (1951–1952) and Central America (1950–1952), the danger of YF exceeded those of vaccine-associated risks. In Nigeria, use of FNV was followed by an outbreak of encephalitis principally in children, with an incidence of 3–4% and a case fatality of 40%. , Autopsies showed lesions of encephalitis consistent with direct viral injury, and YFV was isolated from brain tissue, confirming that FNV, and not an adventitious agent, was responsible. In Costa Rica and Honduras, 10 definite and five possible cases of postvaccination encephalitis occurred in children.
The increased recognition of severe reactions in children led to a change in the policy for use of FNV. In 1959–1960, vaccination was restricted to persons older than 10 years of age. The distribution of vaccine decreased from approximately eight million doses to four million doses per year. Within 5 years of cessation of routine immunization of children, epidemic YF reappeared in Senegal for the first time in 28 years. The 1965 epidemic at Diourbel was the largest on record in West Africa, affecting up to 20,000 persons. Because of the high incidence of YF in children and limited supplies of the safer 17D vaccine, the age limit for use of FNV was reduced to the original 2 years. Among 498,887 persons vaccinated with FNV, there were 231 cases of postvaccination encephalitis. The majority of cases occurred in children 2–11 years of age, in whom the incidence of encephalitis was approximately 1.4 per 1000 and the case-fatality rate was 9%. , Collomb and colleagues have described the clinical syndromes associated with acute encephalitis and the neuropsychiatric residua.
The high numbers of disease cases and vaccine adverse events in young children in West Africa confirmed the need to provide a high rate of coverage of the childhood population in Africa and the need to use a safer method of immunization. In 1966, the Pasteur Institute, with the assistance of the WHO, expanded production of 17D vaccine at Dakar, and by 1970 an official policy was established for use of 17D in persons younger than 5 years of age. Small amounts of FNV were used in regions with poor accessibility, where use of the more thermolabile 17D vaccine was problematic, but in 1982 production of the neurotropic vaccine was discontinued.
The live 17D vaccine is the only product currently approved for immunization against YF. Several recombinant or subunit vaccines using 17D virus have been and are being explored (see “Future YF Vaccines and YF-Vectored Vaccines” Section).
The original development of this vaccine, described by Lloyd and colleagues and Theiler and Smith, was achieved by empirical methods of sequential passage of the prototype wild-type Asibi virus in a substrate that was restrictive for multiplication. This process enhanced the selection of variants with altered biological properties. The first successful in vitro passages of Asibi virus were achieved in cultures of minced mouse embryo tissues. After 18 subcultures, the virus was passed to cultures of minced whole-chick embryo. After 58 passages, the virus, now designated subculture series 17D, was propagated by subcultures in minced chick embryo cultures from which the brain and spinal cord had been removed. The final passage prior to human inoculation was in embryonated eggs.
A reduction in monkey neurovirulence and loss of viscerotropism occurred between the 89th and 114th passages, and a reduction in mouse neurovirulence occurred between the 114th and 176th passages. Monkeys inoculated by peripheral routes did not develop encephalitis; those inoculated intracerebrally developed histopathologic changes, but only 5–10% succumbed to encephalitis. The animals developed antibodies and resisted challenge with Asibi virus. Preclinical safety and efficacy were deemed sufficient to permit human studies. The initial trials using virus at the 227th and 229th passage levels were conducted in 1936, first in YF immune and then in nonimmune volunteers. , The trials showed acceptable tolerability and development of neutralizing antibodies. In early 1937, 17D vaccine was taken to Brazil, where it was used in trials of increasing size, leading to the establishment of local manufacturing capacity and the initiation of a mass vaccination campaign in 1938. , , Between 1938 and 1941, more than two million persons were immunized in Brazil.
During the initial phase of YF vaccine production at the Rockefeller Foundation in NY and in Brazil between 1937 and 1941, a number of different substrains (viruses with different passage histories) were used, representing independent parallel subculture lineages originating at about the 200th passage of the original 17D line ( Fig. 64.6 ). Two main lineages (17D-204 and 17DD) are used for vaccine production (see “Substrains” Section).
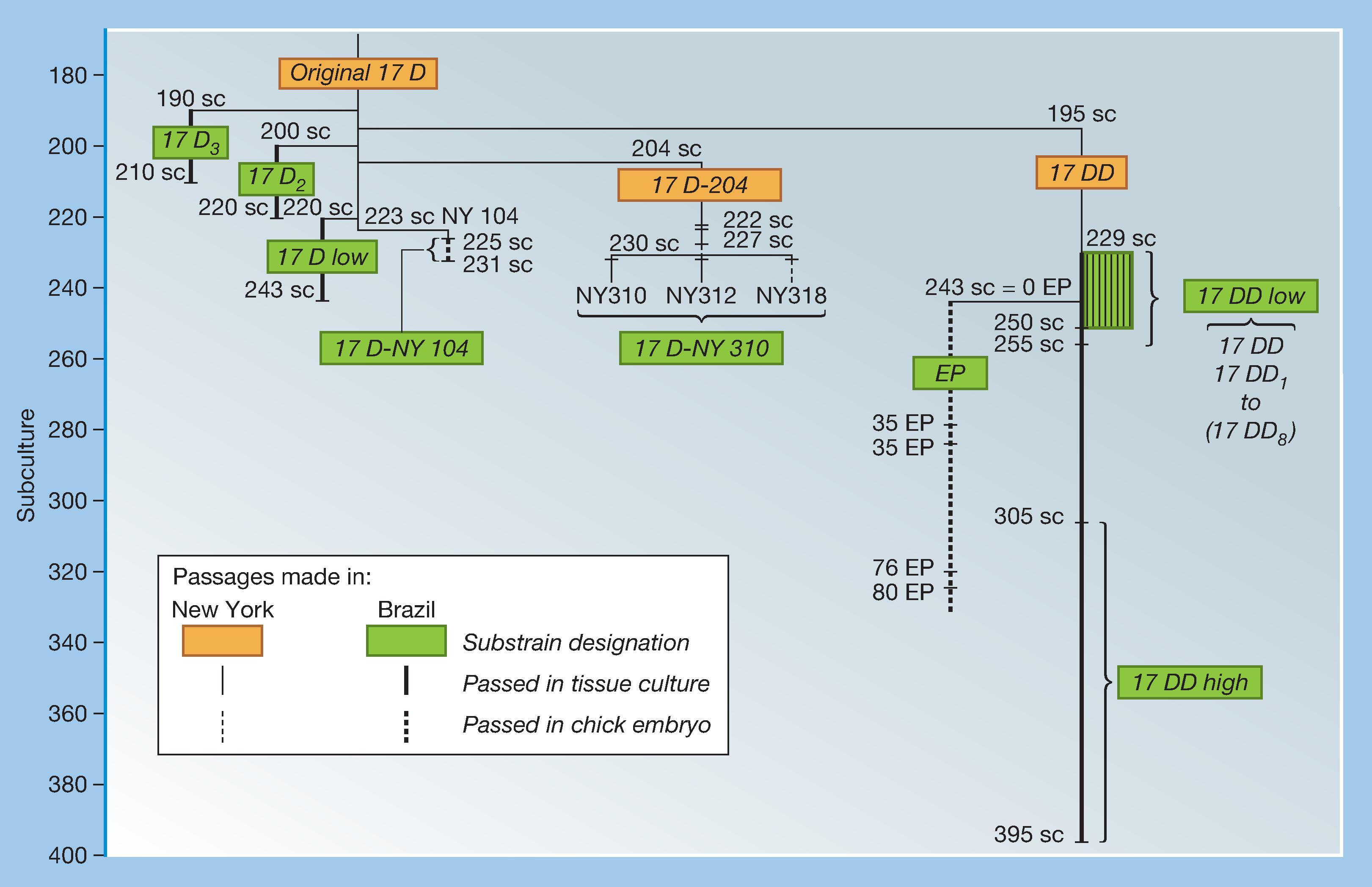
Between 1938 and 1941, field trials and experimental studies revealed the importance of controlling passage level and virus substrain. Substrains 17DD high (305th to 395th passage levels) and 17D 2 (passage 220) were found to be over attenuated, with poor seroconversion rates in humans and low levels of viremia and poor immunogenicity in monkeys, , indicating that loss of immunogenicity could occur in as few as 20 passages in minced chicken embryo tissue cultures. More important, some substrains were associated with the appearance of encephalitis. In 1941, following an outbreak of postvaccination encephalitis in Brazil, a survey of 55,073 persons who received different lots of the same substrain (NY17D-104) revealed the occurrence of 273 (0.5%) severe systemic reactions, including 199 (0.36%) with CNS signs and one fatal case of encephalitis.
A controlled study was performed, in which more than 19,000 subjects received different vaccine lots, including EP, 17D 3, 17D-NY 310, and 17D-NY 104 (see Fig. 64.6 ), that had been associated with the highest incidence of severe reactions or a control vaccine prepared from uninfected chick embryos. Children 5–14 years old sustained the highest incidence of encephalitis, with onset of CNS signs 9–12 days after immunization. The highest incidence of encephalitis (13/1000) was observed in recipients of the 17D-NY 104 vaccine. This substrain was shown also to produce the highest incidence of encephalitis in monkey neurovirulence tests. Other substrains (17D-NY 310 and 17D 3 ) that were associated with encephalitis in humans, but at a lower incidence than 17D-NY 104, caused early-onset and prolonged fever in monkeys. Unfortunately, these substrains and vaccine lots have not been retained and are not available for sequencing studies.
Recognizing that continued serial passage could lead to unwanted alterations in the biological properties of 17D vaccine, the Rio de Janeiro laboratory instituted a “seed-lot” system in 1941, in which primary seed and secondary seed lots were prepared, and the latter used to prepare multiple vaccine batches. The primary and secondary seeds were extensively characterized, and all vaccine lots were restricted to a single passage from the secondary seed. This system was used from 1942 onward by many manufacturers, and was formally established as a biological standard by UNRRA in 1945. However, appropriate seed lots were not distributed to all manufacturers, and preparation of vaccines by serial passage and without adequate neurovirulence testing continued in some countries in the 1950s. Several cases of encephalitis were reported following use of such vaccines at the Pasteur Institute during that time. In 1957, publication of the WHO Requirements for Yellow Fever Vaccine further standardized the seed lot and manufacturing procedures; this guidance has been retained with subsequent updates.
Currently there are six manufacturers of 17D vaccine ( Table 64.6 ). Four manufacturers in Brazil, France, Russia, and Senegal are WHO prequalified to supply vaccine for the Expanded Programme on Immunization (EPI) and mass vaccination campaigns during routine and emergency operations. Prior to 2002, roughly five million doses of vaccine were produced by prequalified manufacturers (namely Sanofi Pasteur) and distributed by United Nations Children’s Fund (UNICEF). Since 2002, there has been a concerted effort to increase the coverage of vaccine through large preventive campaigns being conducted in West Africa, as well as increasing demand through the EPI and establishment of a vaccine stockpile. In 2021, it is projected that the four prequalified manufacturers of YF vaccine will produce 85 million doses of vaccine to be used for routine childhood immunizations, reactive campaigns (i.e., emergency stockpile), and preventive mass vaccination campaigns in countries at high risk for YF. Vaccine supplies have been limited by a number of issues including availability of pathogen-free eggs and manufacturing equipment. Vaccine demand is anticipated to continue to outstrip vaccine supply for the immediate future.
| Country | Manufacturer | Trade Name | Prequalified by the WHO | Comment |
|---|---|---|---|---|
| Brazil | Bio-Manguinhos, Rio de Janeiro | — | Yes | Only 17DD vaccine in use; national use, exported |
| China | China National Biotech Group, Beijing | — | No | National use |
| France | Sanofi Pasteur, Marcy l’Etoile | Stamaril | Yes | National use, exported |
| Russia | Chumakov Institute, Moscow | — | Yes | National use, exported |
| Senegal | Institut Pasteur, Dakar | — | Yes | National use, exported |
| United States | Sanofi Pasteur, Swiftwater, PA | YF-Vax | No | National use, limited export market |
All YF vaccines are live-attenuated vaccines derived from the 17D strain and are produced in embryonated eggs. The WHO has established standards of safety and potency (see “Method of Manufacture” Section). The biological performance of all 17D vaccines is presumed to be similar or identical with respect to seroconversion rate, quality of the immune response, durability of immunity, safety, and tolerability. Vaccines in current production differ, however, with respect to 17D substrain, passage level, formulation with stabilizers, thermostability, salt and other excipients, and the diluent used for reconstitution. 17D vaccines are not biologically cloned and are heterogeneous mixtures of multiple virion subpopulations (genetic diversity/swarms). Unsurprisingly, minor differences have been found in the nucleotide sequences of these vaccines in current use. , , , , There is no evidence to suggest that such variations affect safety or efficacy.
Before the attenuation process was initiated, wild-type Asibi virus was passaged sequentially 54 times in monkeys, either by direct injection of blood from the previous animal or with an intervening passage in Ae. aegypti mosquitoes ( Fig. 64.7 ). Throughout this passage history the virus maintained its virulence for rhesus monkeys. Figs. 64.6 – 64.8 show the virus passage history during the original development of 17D vaccines. In vitro cultivation began in December 1933 with the passage of the virulent Asibi strain in mouse embryo tissue culture and subsequently in chick embryo tissue culture to produce attenuated 17D vaccines ( Fig. 64.8 ). Two 17D substrains—17DD and 17D-204—currently used for vaccine manufacture represent independent subcultures performed at the Rockefeller Foundation in NY (see Fig. 64.6 ). The 17DD and 17D-204 substrains were derived from passage levels 195 and 204, respectively, of the original 17D virus (see Figs. 64.6 and 64.8 ). 17DD virus was sent to Brazil at passage level 229, whereupon it was passed 14 times in minced chicken embryo tissue cultures and then, beginning at passage 243, in whole embryonated eggs (the EP lineage in Fig. 64.6 ). Primary and secondary seeds were prepared in Brazil at passages 284 and 285, respectively, and the current vaccine is at passage 286. One of two 17DD primary seeds was transferred to the Pasteur Institute in Dakar, Senegal, and was used for a time to prepare new seed stocks for vaccine manufacture; manufacture was subsequently switched to 17D.
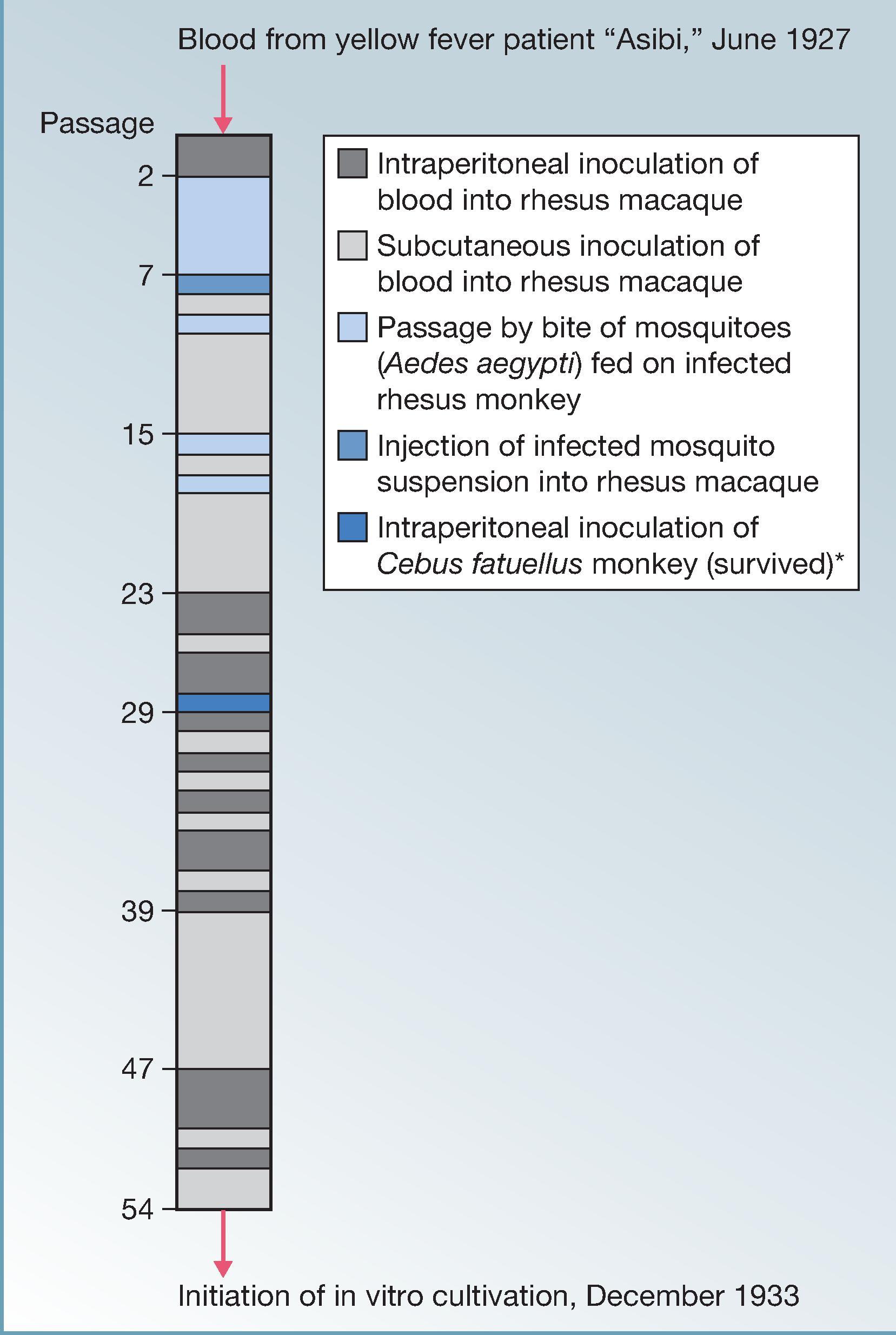
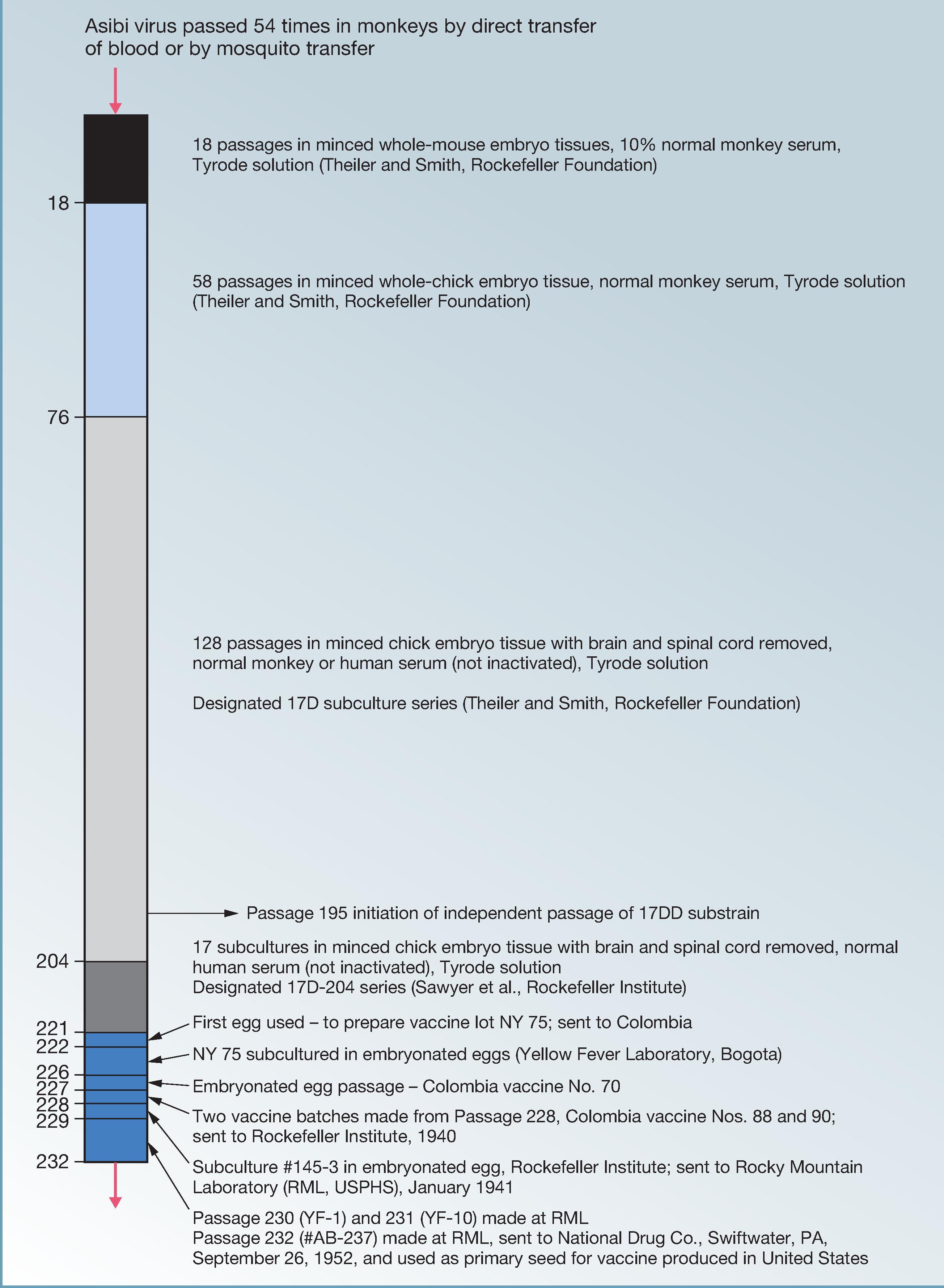
The 17D-204 substrain (and a derivative, 17D-213/77) has been used by all other manufacturers. At passage level 222, the virus was propagated in embryonated eggs to prepare vaccine lot NY 75 (see Fig. 64.8 ). This vaccine was transferred to the YF Laboratory in Bogota, Colombia, where additional egg passages were performed. Colombia No. 88 was returned to the Rockefeller Institute in 1940, whereupon a small number of passages in eggs were made at the Rockefeller Foundation or the Rocky Mountain Laboratory (National Institutes of Health) prior to the manufacture of primary seed stocks in France, the United States, Australia, The Netherlands, Germany, Colombia, South Africa, the United Kingdom, and India ( Fig. 64.9 ). Deep sequencing of the parental Asibi strain and 17D-204 (YF-Vax) found both viruses to contain quasispecies though Asibi actually contained more than the vaccine virus. Furthermore, the analysis indicated that none of the attenuating mutations in 17D-204 were present in Asibi virus quasispecies suggesting that the mutations in the vaccine were discrete and not due to selection of genomes in the wild-type population.
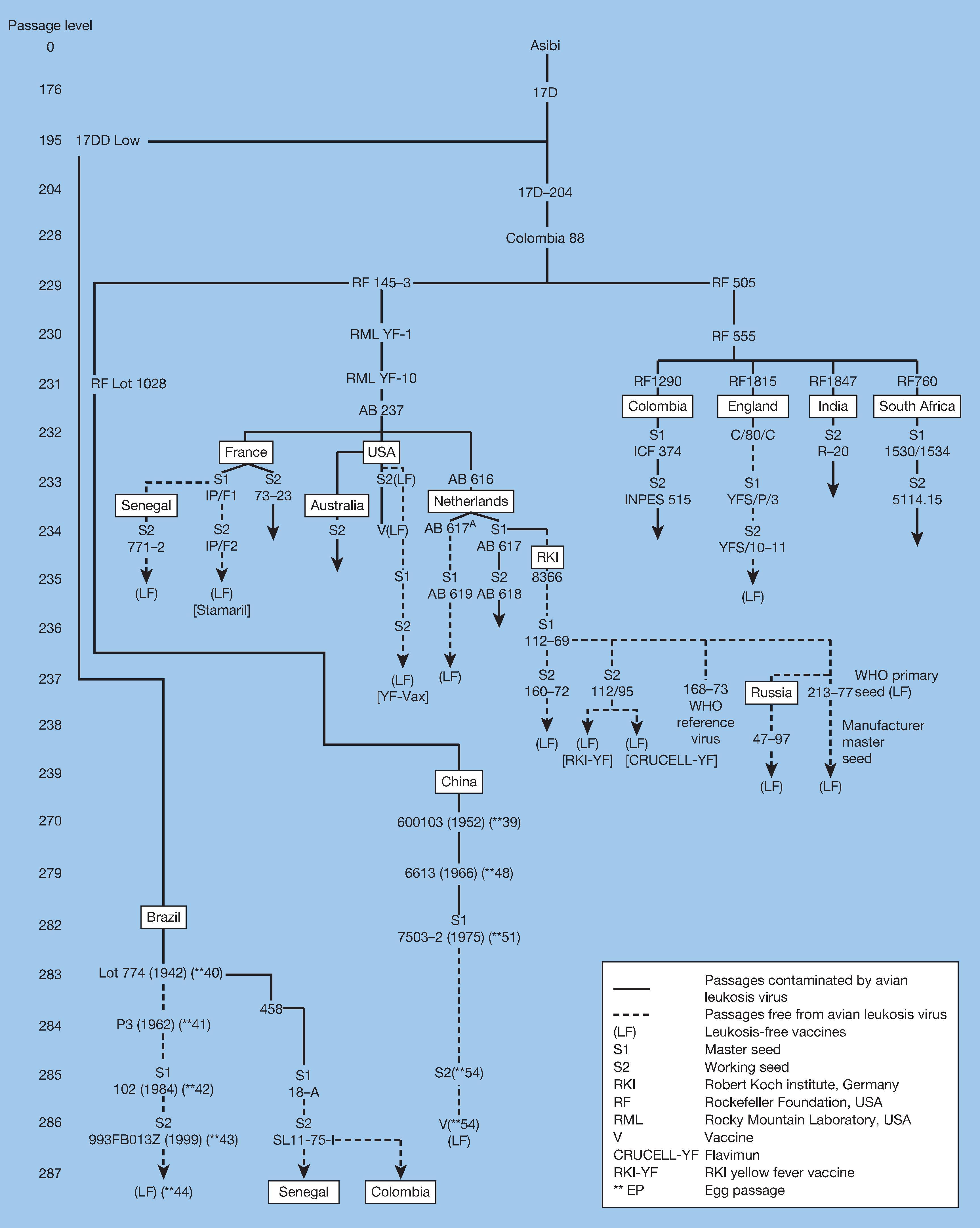
The Pasteur Institute in Dakar switched from 17DD to 17D-204 virus for preparation of secondary seed free from avian leukosis virus. In 1977, the Robert Koch Institute (Berlin) prepared for the WHO a primary seed free from avian leukosis virus, and it maintains this as a reference stock (designated 17D-213/77) available to new manufacturers and as a source for emergency production. Russia is the only manufacturing site using the 17D-213/77 virus for production. Current 17D-204 vaccines are produced at passage levels between 233 and 239. The 17DD vaccine is at higher passage, and the manufacturer in Brazil (Bio-Manguinhos) has on at least two occasions made new working virus seeds by making an additional passage in eggs rather than from the master virus seed, which was depleted. These additional passages were extensively characterized by sequencing and monkey neurovirulence testing.
The 17DD and 17D-204 substrains are distinguishable by MAb analysis, indicating variations in antigenic determinants on the E protein. , , , Antigenic differences have also been identified between vaccines produced from the 17D-204 substrain by different manufacturers. A comparison of the nucleotide and amino acid sequences of the 17DD substrain vaccine produced in Brazil, the WHO reference vaccine (17D-213), and 17D-204 strains manufactured in the United States, France, and the United Kingdom, revealed that 17DD vaccine had accumulated fewer nucleotide and amino acid changes per passage during the process of development and attenuation than 17D-204 vaccine viruses. , , NGS sequencing of multiple lots of 17DD vaccine has confirmed a high level of homology between multiple lots of 17DD vaccine.
Depending on the specific substrain, 17DD and 17D vaccines differ in up to 17 amino acid residues in the coding region. , , At 12 other amino acid residues, variability is observed between vaccines belonging to the 17D-204 substrain (including the WHO 17D-213/77 virus); 6 of these amino acid changes are unique to the China 17D-Tiantan substrain suggesting this is the most divergent. These differences among vaccines do not appear to be related to attenuation, because all vaccine strains are attenuated and one or more vaccine strains has an identical residue at each position to that found in parental (virulent) Asibi virus (refer to Table 64.1 for shared differences of all vaccine strains and Asibi virus that might play a role in attenuation).
The neurovirulence phenotypes of 17DD and 17D-204 substrains have been examined in mice and monkeys. , Vaccines were compared with respect to average survival time and mortality after intranasal inoculation of young adult mice. 17DD substrain vaccines were neuroinvasive after intranasal inoculation, whereas those derived from 17D-204 were not. In monkeys subjected to the WHO standard intracerebral neurovirulence test, higher clinical and histopathologic lesion scores were found in the brains of animals inoculated with 17DD than in those inoculated with 17D-204 vaccines. , A newer study found further differences in the neurovirulence of 17D-204 and 17D-213, with 17D-213 vaccine having lower neurovirulence in monkeys and mice than 17D-204. The difference in neurovirulence between the two substrain 17D vaccines was attributed to mutations in E envelope coding region and NS4B coding region. However, both 17DD and 17D vaccines passed WHO standards and were well within the limits of acceptable safety in the monkey test. Overall, the data suggest that 17DD vaccine may be somewhat more neurovirulent than 17D-204 substrain vaccines, as determined in animal models. In humans, however, this difference has not been appreciated as the rates of serious neurologic adverse events reported through passive surveillance have found slightly lower rates in Brazil (0.37 neurologic disease cases per 100,000 doses) where 17DD is used, compared to rates in the United States (0.8 per 100,000 doses) where 17D-204 is used. However, rates varied annually, particularly in Brazil, suggesting that the adverse event rates potentially were similar between the two vaccines. Given these data and the long history of use of both 17DD and 17D vaccines in many millions of persons, there is no reason to suspect that these vaccine substrains differ with respect to safety for humans.
The safety and immunogenicity of the 17DD and 17D-204 lineage (17D-213/77) vaccines have been compared in a clinical trial and had virtually identical safety profiles, seroconversion rates, geometric mean titers, and distribution of antibody titers.
Biological standards for YF vaccines have been established by the WHO, and all vaccines approved for international use must comply with these basic standards and be produced according to good manufacturing practices. National control authorities, such as the U.S. Food and Drug Administration (FDA), govern marketing approval, and thus biological standards differ somewhat from country to country. Manufacturers currently use seeds free from avian leukosis virus.
Primary (master) and secondary (manufacturer’s working) virus seeds are tested for bacteria, fungi, mycoplasma, and adventitious viruses and are subjected to a standard safety and immunogenicity test in rhesus or cynomolgus monkeys. A minimum of 10 monkeys are inoculated intracerebrally with 0.25 mL of virus containing 5000–50,000 MLD 50 , the equivalent in international units to 3.7–4.7 log 10 IU (see the discussion of potency measurements later in this section). Animals are monitored for clinical signs, viremia, antibody responses, and semiquantitative neuropathologic lesions at 30 days and are subjected to statistical analysis ( Table 64.7 ). , , For comparison, a reference control is inoculated into 10 other animals. Some authorities have advised using a reference vaccine with high neuropathologic scores that failed the safety test, which are hard to find, thus generally, when making a new virus seed, manufacturers are advised to use an internal reference control (e.g., a current working virus seed) that passed the test previously. , ,
| Criterion | Test | Result |
|---|---|---|
| Viscerotropism | Viremia level on days 2, 4, and 6 | Viremia <500 IU/0.03 mL in all samples, and not greater than 100 IU in more than one sample |
| Immunogenicity | Neutralizing antibody | >90% seroconversion on day 30 |
| Neurotropism | Clinical observation daily for 30 days | Frequency of encephalitis and semiquantitative clinical score less than reference seed |
| Histologic evaluation (day 30) | Mean lesion scores shall not be significantly greater than reference seed b |
a Ten nonimmune rhesus or cynomolgus monkeys are inoculated in the frontal lobe with 5000–50,000 MLD 50 and monitored for 30 days.
b Histologic grading performed on five levels of brain and six of cervical and lumbar spinal cord, using semiquantitative grading score of inflammation and neuronal degeneration. Areas of brain studied include target areas (areas that show more-severe lesions irrespective of degree of neurovirulence of virus tested), spared areas, and discriminator areas that distinguish between vaccines of low and high neurovirulence. Mean group scores for target and discriminator areas combined and for discriminator areas alone are calculated. The mean score for the test monkeys should not be significantly greater (at 5% significance level) than overall mean score for monkeys injected with reference virus. MLD 50 , mouse median lethal doses. World Health Organization. Recommendations to Assure the Quality, Safety and Efficacy of Live Attenuated Yellow Fever Vaccines. TRS 872, Annex 2. WHO Tech Rep. Ser. Geneva, Switzerland: WHO; 2010.
In a study of rhesus monkeys inoculated by either the intracerebral or subcutaneous route with 17DD vaccine, viremia was measured daily by plaque assay and RT-PCR. The proportion of animals developing viremia following intracerebral inoculation was higher than after subcutaneous inoculation, raising the question whether the neurovirulence test (intracerebral) is an appropriate way to measure viscerotropism. However, considering the combined groups (intracerebral and subcutaneous inoculation), the duration of viremia was only 2–6 days, and mean titers by plaque assay were 1.62 (standard deviation [SD], 0.76) log 10 PFU/mL.
The genomes of many seed viruses used for production have been sequenced by NGS, and this may become a future requirement. Minor differences and heterogeneities at individual nucleotides occur across vaccines and passage levels used by different manufacturers (see “Substrains” Section) but analysis of multiple vaccine lots by individual producers demonstrates a high degree of genetic homogeneity for a particular producer.
Vaccine is produced by aseptic inoculation of working virus seed into viable embryonated eggs. , Uninoculated control eggs (80 eggs per batch) are included and tested. All current manufacturers, as required, use eggs from closed, special pathogen-free flocks. The dose of seed virus inoculated into 7- to 9-day-old embryonated eggs is 2000–5000 MLD 50 or the equivalent in international units, in 0.1 mL (see the discussion of potency measurements later in this section). After incubation for 3–4 days, the peak titer of virus, infected embryos are aseptically harvested. Embryos must be less than 12 days of age at the time of harvest. A single harvest is the pool of embryos inoculated together in a single production run.
Methods for recovery of virus from infected embryos vary, but all include addition of sterile water for injection (typically 1–2 mL/embryo), homogenization to pulp, and clarification by centrifugation to yield supernatant fluid harvest. Individual harvests containing homogenate from a single pool of embryos are held frozen pending sterility tests.
The vaccine must be prepared aseptically, because terminal sterile filtration cannot be performed owing to high loss of virus. Sterility tests are performed at one or more steps prior to pooling single harvests into the final bulk, which may contain one or a pool of single harvests. The amount of final bulk prepared is determined principally by the volume capacity of the lyophilizer. The final bulk is diluted and blended with stabilizers (e.g., hydrolyzed porcine gelatin and sorbitol, but stabilizers vary by manufacturer) based on potency (infectivity titer) to produce the drug product, filled into glass vials, and lyophilized. Sterile filtration is not performed because the consistency of the product renders filtration difficult and results in significant loss of titer. The manufacturing process is thus performed aseptically under Class 100 (Class A) conditions.
The target infectivity titer is adjusted for losses expected across lyophilization and losses expected between release and end of shelf life. Consequently, the release titer must exceed the compendial minimum (3 log 10 IU). Data from current manufacturers indicate potencies range from 3.5 to 6.0 log 10 IU/0.5 mL dose. The excess at release is determined to provide for minimum potency at the end of shelf life (2 or 3 years, depending on the manufacturer). The yield per embryo is typically in the range of 100–300 doses.
The original WHO biological standard was tied to mouse MLD 50 (determined by intracerebral inoculation of mice 4–6 weeks of age) and is now based on the equivalent in a plaque assay. Because of the variability of the mouse and plaque assays and difficulty in establishing the PFU-to-MLD 50 ratio, and to assist manufacturers in the control of YF vaccines, the National Institute for Biological Standards and Control (NIBSC, United Kingdom) conducted a study with the aim of establishing an international standard. The study confirmed that the ratio of PFU to MLD 50 was approximately 10:1. An international standard (NIBSC code No. 99/616) preparation of 17D vaccine was established and was approved by the WHO for use by manufacturers and national control laboratories. , The standard for minimum potency is established at 3 log 10 IU, which is approximately equivalent to 3 log 10 MLD 50 and 4 log 10 PFU.
There has never been an upper limit established for potency, and some vaccine batches have titers up to 6 log 10 IU. The current view is that manufacturers should establish their own internal upper limit using trend analysis of multiple batches, and the upper limit should be approved by the national control authority. Interestingly, there are no clinical data comparing 17D vaccines at high versus standard dose but fractional dosing studies using one-fifth of a full dose found that fractional doses of vaccines from all four major vaccine manufactures met noninferiority criteria. In animals it is possible to show prozone effects, with lower immunogenicity at high doses (see “Vaccine Substrain or Dose” Section).
The final vaccine bulk is tested for absence of adventitious agents. Standards vary across national control authorities but include tests for bacteria (including Mycobacterium avium ), fungi, and mycoplasma. Because 17D vaccines were developed before the application of modern tests for adventitious viruses, the standards for testing may be less stringent than for new vaccines in some countries, but still meeting WHO requirements. Control (uninfected) eggs are routinely incubated along with eggs used for vaccine manufacture and are tested for viability, sterility, and hemagglutinating viruses.
After filling, the final product is tested for identity by neutralization test, potency, thermostability, sterility, general safety, residual moisture, residual ovalbumin, and endotoxin. Some countries require that a monkey safety test be performed on each vaccine lot. As specified by the WHO standards, potency must be at least 3 log 10 IU/mL. The dose in the final container exceeds the minimum specification by 50-fold to account for potential losses during storage and losses during thermostability testing (see “Vaccine Thermostability” Section).
There are no antibiotics or preservatives in 17D vaccines. Because of the risk of bacterial contamination and the thermal instability of the virus after reconstitution, single-dose and (especially) multidose vials must be used within a short time of opening, as specified in the package label: typically 1 hour, or up to 8 hours if maintained on ice. Salt, buffer, and stabilizer excipients vary by manufacturer. The stabilized vaccine manufactured in France by Sanofi Pasteur (Stamaril) contains no animal-derived excipients and is reconstituted in 0.4% saline. In contrast, the same manufacturer’s product made in the United States (YF-Vax) is stabilized with sorbitol and hydrolyzed porcine gelatin and is reconstituted with 0.9% saline. The amount of hydrolyzed porcine gelatin per dose in these stabilized vaccines is approximately 10 mg. Osmolality of 17D vaccines is slightly above normal serum levels.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here