Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
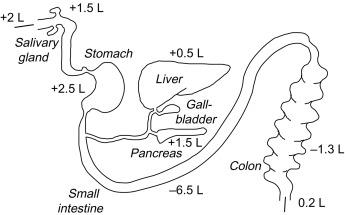
Large quantities of fluid are transported across epithelial barriers in the gastrointestinal (GI) tract for secretion of saliva, gastric juice, bile and pancreatic fluid, and for fluid absorption in the intestine. The quantity of fluid transported in the GI tract is second only to kidney, where ~ 180 L of fluid per day are filtered by the glomerulus in humans and processed by various nephron segments. In the human GI tract, the salivary glands produce ~ 1.5 L of fluid per day, the stomach secretes 2.5 L of gastric juice, the liver produces 0.5 L of bile, the pancreas produces 1.5 L of enzyme and bicarbonate-rich fluid, the small intestine absorbs 7.5 L of fluid, and the colon absorbs 1.3 L of fluid against significant osmotic gradients ( Fig. 55.1 ). The fluids transported across epithelial and endothelial barriers contain salts (~ 150 mM) and water (~ 55,000 mM). As discussed elsewhere in this volume, there is a considerable body of data on the molecular identities of the major salt transporters in epithelial cells of the GI tract and their role in transcellular ion transport. The molecular pathways for water transport in the GI tract have been identified relatively recently and remain an evolving and in some cases controversial subject. As in other organ systems, the general paradigm in the GI tract is that water movement occurs secondary to osmotic driving forces created by active salt transport and to hydrostatic pressure differences. Based on a substantial body of evidence in the kidney and other epithelia carrying out active near-isosmolar fluid secretion or absorption, greater cell membrane water permeability produces greater net fluid movement. A description of current concepts in fluid transporting mechanisms is described in the section, “Epithelial fluid transporting mechanisms” to follow.
Aquaporin (AQP) water channels provide the molecular pathway for cell membrane water transport in many cell types. The AQPs are a family of small, integral membrane proteins that transport water and in some cases water and small solutes such as glycerol and hydrogen peroxide (H 2 O 2 ). AQPs are expressed widely in cell plasma membranes in epithelial, endothelial, and other cell types in the GI tract and elsewhere. As described in this chapter, there is compelling evidence for physiological importance of some AQPs in some tissues based on studies in humans with AQP deficiency/mutations, and phenotype analysis of transgenic mouse models of AQP deletion. However, the role of AQPs in the GI tract remains to some extent uncertain despite a considerable body of data on the expression pattern, cellular processing and regulation of AQPs in various GI cell types. The available data are reviewed in this chapter, and major unresolved questions are identified.
The mechanism of how water is absorbed and secreted across epithelia has puzzled physiologists for decades. Although large volumes of water cross the various epithelia of the GI tract as diagrammed in Fig. 55.1 , there remains a lack of consensus about the mechanisms by which water is transported across epithelia both generally and in specific regions of the GI tract. However, the general paradigm of water transport following active solute movement has remained the basis of most models of fluid transport.
The original observation that fluid can be transported in the apparent absence of an osmotic gradient was made in a series of elegant studies by a few pioneering physiologists in the late 19th century. It was demonstrated that fluid could be transported across an epithelial sheet such as rabbit ileum in the absence of an external osmotic pressure difference, and that this transport occurred only as long as the tissue remained viable, implying that “active” metabolism within the tissue is required. In the middle of the 20th century, Ussing demonstrated that sodium is actively transported across epithelia, providing the basis for a model of fluid transport. Curran was the first to show that water transport was linearly related to Na + transport, and he and McIntosh thereafter proposed a “three-compartment model” ( Fig. 55.2 A ) of fluid transport that accounted for the ability of epithelia to transport water in the absence or against (“uphill”) an osmotic gradient. The model requires active solute transport from compartments I to II across membrane barrier A. Compartment II is then hypertonic to compartment I. If the reflection coefficient for the actively transported solute on membrane A is greater than that on membrane B, fluid will be transferred from compartments I to III in the absence of an osmotic pressure difference between compartments I and III.
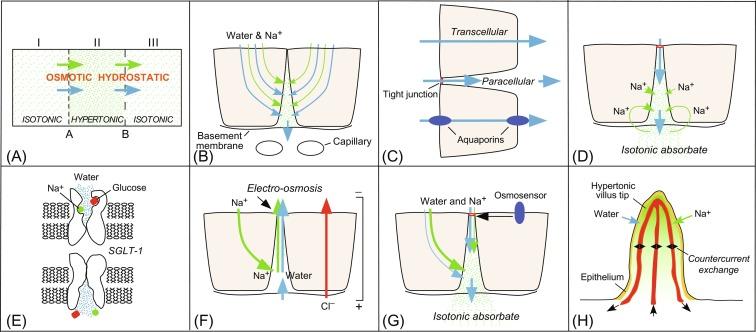
Following the anatomical observations of Diamond and Whitlock and Wheeler, Diamond and Bossert extended the Curran/McIntosh model by delineating the biological structures responsible for the different compartments and membranes within the epithelium, resulting in the “standing- gradient” model ( Fig. 55.2 B). The model suggested that the lateral intercellular space (LIS) between adjacent epithelial cells was the central hypertonic compartment in the Curran-McIntosh model. The basic feature of the model as illustrated in Fig. 55.2 B is that the driving solute (Na + in most cases) is actively transported from the lumen into the LIS, resulting in a steady-state (standing) osmotic gradient decreasing from the tight junction to the basolateral membrane. The osmotic gradient drives water flux through the cells into the LIS and across the basolateral membrane, resulting in an isosmolar or near-isosmolar absorbate.
As originally conceived, the model predicted a number of important characteristics for an epithelium to absorb water in the absence of an osmotic gradient between the luminal and basolateral solutions, which include a water impermeable tight junction between cells, relatively low water permeability for membranes facing the LIS, and clustering of Na + /K + ATPase pumps near the tight junction. However, subsequent experimental data indicated that many of the original requirements of the model were incorrect, leading to revision of the original model and the development of alternative models. Hydraulic conductivity (Lp) measurements of various intestinal membranes have generally shown relatively high water permeabilities for both apical and basolateral membranes. This observation, together with a series of optical studies of water and solute dynamics in the LIS, led to a revised model in which the osmotic gradient localized in the LIS is predicted to be so small (< 10 mOsm) as to be essentially unmeasurable. Another tenet of the revised model is that there is a much greater magnitude of water transport through the cell than through tight junctions.
The validity of the revised “standing-gradient” model has remained uncertain, with an increasing number of theoretical inconsistencies and problems related to the difficulty in measuring many of the key parameters in physiologically relevant epithelial cells in vitro or in vivo. Reported water permeabilities of apical and basolateral membranes have differed over orders of magnitude, introducing considerable uncertainty about osmotic gradients required to drive fluid absorption. Also, the methodologies used to measure membrane water permeability have been criticized as has the physiological relevance of the model systems. The high water permeabilities measured by Spring and coworkers in Necturus gallbladder and other systems form the basis for predicting a small osmotic gradients in leaky epithelia. Earlier reported values of low water permeability have been criticized because of neglect of unstirred layer effects, though the validity of this criticism has also been questioned. Also, the ultrastructural techniques used to identify the putative Curran and McIntosh hypertonic middle compartment as the LIS have been criticized.
Notwithstanding these criticisms, the revised “standing gradient” model of fluid movement by local osmotic gradients still remains the most widely accepted description of water transport across intestinal epithelia.
Debate on the mechanism of solute-driven water transport in near-isotonic fluid transport in leaky epithelia (small intestine and gallbladder in vertebrate GI tract) has largely focused on whether water passes through cells (transcellular) or between cells (paracellular). The revised standing-gradient model assumes that water movement is predominantly transcellular. Other models based on fluid transport secondary to solute transfer, where fluid transfer is largely paracellular, include the osmosensor feedback mechanism proposed by Hill and coworkers, and the electroosmotic model proposed by Fischbarg and coworkers (see also Ref. ). A completely different, nonosmotic mechanism, in which water is cotransported within solute transporters has been proposed by Zeuthen, Wright and coworkers. Central to the debate on the pathway of water transfer across the GI tract is the role of AQPs in fluid absorption and secretion. As discussed below, studies of AQP knockout mice have, in certain organs systems such as kidney proximal tubule, clarified the contribution of the transcellular pathway. In systems such as the small and large intestine where no role has yet been found for AQPs in physiological fluid movement, there is ongoing debate about the mechanism(s) responsible for fluid absorption and secretion ( Fig. 55.2 C).
The idea of “solute recirculation” was proposed initially in the early 1990s by Ussing et al. and further explored in experiments in toad intestine. Subsequent studies confirmed and extended the initial experimental data, leading to a mathematical model of fluid absorption in which sodium recirculation accounts for absorbate isotonicity. In the proposed scheme ( Fig. 55.2 D), sodium entering into the LIS from pumping through cells or across the tight junction results in water movement. A hypertonic fluid is then extruded across the basolateral membrane of the LIS, providing the vectorial water movement component for fluid absorption. Isotonicity of the resulting absorbate is created by reuptake of sodium, putatively by the Na + /K + /2Cl − cotransporter, into cells and consequent “recirculation” back into the LIS.
The compartmental model of Larsen et al. used their data from toad intestine along with other experimental data to model transepithelial solute fluxes, initially for electroneutral absorption, and subsequently for electrogenic absorption. Their conclusions of interepithelial water flux and basolateral Na + recirculation were supported by experiments using cesium as a paracellular probe to estimate the magnitude of solvent drag in the LIS and the movement of solute from the basolateral compartment. However, the use of cesium as a paracellular tracer has been criticized, so that definitive testing of the recirculation hypothesis remains to be done. The conflicting evidence from experimental studies and modeling about whether solute movement in the LIS represents true solvent drag resulting from water flux across the tight junction, or pseudo-solvent drag resulting from solute “swept” away by water flux through the cell, emphasizes the need for further testing of solute/solvent dynamics across epithelia.
An interesting hypothesis by Wright, Zeuthen, and coworkers proposes that in the small intestine (and gallbladder) water transport is mediated by “active” transport by cotransporters such as the sodium/glucose cotransporter (SGLT-1) present at the brush-border membrane of enterocytes. The theory proposes that water is cotransported stoichiometrically along with glucose and sodium as a consequence of conformational changes that occur during the normal transport cycle of SGLT-1 ( Fig. 55.2 E).
The principal evidence for active water transport comes from studies in Xenopus oocytes expressing SGLT-1, in which sodium current generated by the cotransporter is measured by microelectrodes, and cell swelling (a proxy for water transport across the membrane) is measured optically. Oocytes overexpressing SGLT-1 swell rapidly and manifest a parallel inward sodium current when exposed to an isotonic glucose-containing solution, which is inhibited by the specific blocker phlorizin. The initial fast rate of cell swelling was interpreted as the result of direct coupling of sodium and glucose transport to water transport. Additional evidence in support of this interpretation was the relatively slow rate of swelling produced by equivalent inward sodium currents generated by ion channels or ionophores. Also, it was shown that rapid changes in Na + transport driven by applied voltage changes induced concomitant changes in the rate of cell swelling. SGLT-1 has an intrinsic passive water permeability and in the steady-state sodium glucose cotransport is expected to induce osmotic water flow by changing intracellular osmolarity. It was proposed that in the steady-state that direct (nonosmotic) water cotransport contributes ~ 35% of the total water transport across the membrane. Follow-up work by Zeuthen and coworkers has implicated active water transport by a variety of cotransporters, including the Na + /glutamate cotransporter in the nervous system, H + /lactate cotransporter in retinal pigment epithelium and Na + /dicarboxylate cotransporter in kidney.
The physiological relevance of SGLT-1 water cotransport has been questioned based on studies of water transport in the kidney. Water permeability and fluid absorption in kidney proximal tubule are largely dependent on AQP1 as shown from knockout mouse studies (see below). Also, according to the proposed stochiometry of coupling of water to sodium and glucose (210:2:1, hSGLT-1), the measured quantities of sodium and glucose reabsorbed by the kidney cannot account quantitatively for the quantity of water reabsorbed. While it seems likely that cotransporter-mediated active water transport is not important in the kidney, in the small intestine it is less clear. AQP-dependent water transport in small intestine has not been demonstrated to date (see below), so in the small intestine at least, the physiological relevance of water cotransport remains an open question.
Studies by Lapointe and coworkers have called into question the interpretation and accuracy of the experimental data of Wright, Zeuthen, and coworkers in studies that aimed to replicate their findings. Lapointe and coworkers did not find good agreement between membrane voltage-induced changes in sodium current and cell swelling, concluding negligible water cotransport in the steady state. They proposed that the phenomena seen in the oocyte experiments can be accounted for quantitatively by the generation of local osmotic gradients close to the membrane (in effect, an unstirred layer), and concluded that the existing model of passive water flow across membranes produced by compartmentalized osmotic gradients can explain water transport in intestinal epithelia. Thus, there remains significant debate on details of the experimental evidence supporting the hypothesis of water cotransport, and hence water cotransport is neither sufficiently proven nor accepted to replace the established paradigm of water transport secondary to local osmotic gradients.
An alternative hypothesis, in which water is transported largely via a paracellular pathway, has been proposed by Fischbarg and coworkers. Although most of the experimental work that underlies the model has been carried out in the cornea, proponents of the model have suggested that it is widely applicable to fluid transporting epithelia based on previous studies showing current-induced fluid movement. The observation that underlies the model is that current across an epithelium results in fluid flows that mirror the direction and magnitude of the electrical current. In the model, active ion transport and recirculation generate a circulating current and transepithelial potential difference. The solute counterion is concurrently driven across the tight-junctions between the epithelial cells. Fluid flow across the tight junctions is consequently generated by the electroosmotic coupling of water and the solute counterion, resulting in paracellular fluid transport and isotonic fluid transport across the epithelium, although the later is dependent on the exact coupling ratio between water and counterion ( Fig. 55.2 F). The experimental basis of this theory has largely hinged on a series of studies showing current-induced fluid flow from the corneal stroma across the corneal endothelium in isolated, in vitro rabbit corneal preparations. In addition, a computational model of the corneal endothelium was developed to predict solute and fluid transport responses under experimental conditions.
Although the idea of electroosmosis has been around for many years, its application to epithelial physiology is relatively recent. In general, the theory seeks to resolve some of the apparent experimental inconsistencies in local osmosis models of fluid transport, including the quantitative difference between the reduction in osmotic water permeability (40%–50%) and the reduction in fluid transport (20%) in certain leaky epithelia in AQP knockout mouse models. Theoretical objections to electroosmosis have centered on the explanation that ion flow also produces solute concentration gradients close to the membrane, and current-induced water flow is therefore governed by local osmosis. The main counter to this argument is the fact that some experimental data show that the onset of fluid flow generated by changing transepithelial current is very fast (< 3 s) and therefore too fast to be explained by establishment of a local solute concentration gradient. Another theoretical issue is fluid flow generated by nonionic, osmotically active impermeant solutes, which cannot be explained by electroosmosis alone. Lastly, the theoretical treatment of electroosmosis is crucially dependent on number of parameters that have been only ever been tested in the cornea or are not fully described in the model. These include most crucially the coupling ratio at the tight junction, and also the composition of the tight junction charges and hydrostatic pressure effects within different epithelial compartments.
The electroosmosis model represents an interesting departure from the largely transcellular models of fluid transport and highlights the largely underappreciated importance of the tight junction in transepithelial water transport. However, the electroosmosis model has not to date been validated experimentally in different epithelia nor have its predictions been quantitatively confirmed.
Hill and coworkers have proposed an interesting solution to the problem of isotonicity of transported fluid in epithelia, which relies on paracellular water flow and an osmosensor ( Fig. 55.2 G). The basis of the model is that fluid transport across an epithelium is a blend of transcellular and paracellular solute and water transport. Water is transported across the cell osmotically and is therefore largely hypertonic. Paracellular water flow is generated by forced convection of fluid through tight junctional channels that reflect solute over water, resulting in a largely hypotonic fluid. The magnitude and rate of this junctional fluid flow is proposed to be controlled by an osmosensor that samples the source solution. The fluid transported across the epithelia is osmotically clamped by the osmosensor via regulation of the rate of fluid traversing the tight junctions, resulting in a transported solution with a tonicity close to that of the source solution.
The osmosensor model as proposed relies on experimental data showing significant junctional fluid flow using dextrans as probe molecules. However, besides this indirect experimental evidence on paracellular fluid flow the model remains speculative. AQPs were proposed to be the candidate osmosensor crucial to the model, although the mechanism by which they function in this capacity has not been described nor is there any experimental evidence to support this conjecture. Further, the cellular signaling mechanisms and tight junctional apparatus involved in controlling junctional fluid transfer have not been experimentally described. Therefore, although an interesting idea to explain fluid transport in the absence of an osmotic gradient between source and sink solutions, the osmosensor model is at present a speculative, unsubstantiated theory.
An intriguing model of fluid absorption, involving countercurrent exchange in the small intestinal villus, was proposed by Lundren and coworkers about 40 years ago. The idea that a countercurrent system operates in the villus was based on observations on the vascular morphology of mammalian villi. The villus contains a central arterial vessel surrounded by a dense capillary network and neighboring venous vessels. The hairpin arrangement of the arterial and venous vessels, resembling the renal microvasculature, could produce a “countercurrent” flow system in which blood flow occurs in opposite directions. The evidence that this countercurrent flow could operate as an exchange system came from measurements of relative absorption rates of the inert gases 133 Xe and 85 Kr from the intestinal lumen into the villus. These experiments, along with measurements of villus hemodynamics, suggested the presence of a villus countercurrent exchanger in several species including man.
These observations led to the proposal that the putative villus countercurrent exchanger could function as an osmotic “multiplier” as in the renal medulla. The evidence to support a multiplier function comes mainly from measurements of villus osmolality using cryoscopic techniques and sodium-sensitive microelectrodes. The villus was reported to have a large gradient of osmolality from tip to base with tip osmolality as high as 700–900 mOsm. From these measurements, it was proposed that the villus interstitium was the hypertonic compartment described in the Curran-McIntosh model. Water absorption from the intestinal lumen could be driven by the large osmotic gradient/pressure head developed in the villus tip by the countercurrent multiplier system ( Fig. 55.2 H).
The theoretical basis of countercurrent exchange and its presence in intestinal villi has been criticized (for theoretical details see Ref. ). The accuracy and validity of the techniques used to measure inert gas absorption to demonstrate countercurrent exchange have been criticized and alternative explanations for the relative gas absorption rates have been proposed. Conflicting data have also emerged on the existence of countercurrent exchange in certain mammalian species but not others, based on differences in villus anatomy. The in vitro techniques used to show high villus tip osmolality are thought to produce highly variable results, which may be an artifact of the tissue preparation. It has been pointed out that the microvascular anatomy in small intestinal villi is quite different from that of the renal medulla, and in particular there is no equivalent pathway in villi to the ascending limb of Henle in kidney. Also it has been argued that the existence of a hypertonic compartment in the villus tip does not necessarily require a countercurrent mechanism but does require a relatively water impermeable epithelium.
Thus, convincing in vivo evidence for a hypertonic villus compartment is lacking. Modern optical and fluorescent techniques may be able to elucidate the solute/solvent dynamics within the villus, although experiments on the small intestine in situ remain a significant technical challenge due to its complex geometry. Additionally, the efficiency of the putative countercurrent exchange system is critically dependent on the water permeability of the microvasculature, suggesting that endothelial AQP1 might be involved in villus fluid absorption.
An interesting model for fluid absorption in small intestine was proposed by Lucas in which the driving force for water movement across the small intestinal mucosa is located at the brush border membrane of enterocytes. This model postulates that fluid transport is driven by the neutralization of bicarbonate ions contained within an unstirred layer at the luminal membrane of enterocytes. Sodium-proton exchange at the brush border membrane produces local luminal acidification resulting from proton transport out of the cell. Bicarbonate ions diffusing from the bulk solution neutralize the protons, resulting in the production of water and carbon dioxide. The combination of two osmotically active ions to form a single osmotically active carbon dioxide molecule produces a hypotonic compartment adjacent to the luminal membrane, which, together with an increase in intracellular osmolarity from sodium entry and carbon dioxide diffusion into the cell, drives water across the membrane. Evidence for this model comes from observations of increased fluid absorption for bicarbonate-containing balanced electrolyte solutions in the upper small intestine where luminal acidification is high, along with data from bacterial diarrheas. In Salmonella typhimurium infection, it was shown that the ileum, which normally exhibits robust electrogenic Na + /glucose absorption, exhibits jejenum-like characteristics with increased luminal acidification and elevated plasma pCO 2 . This was proposed as evidence of increased Na + /H + exchange leading to fluid absorption.
Although the model suggests that the brush border membrane is the key site of solute-solvent coupling, it does not ultimately contradict the original Curran/McIntosh idea that a basolateral or abluminal hypertonic compartment drives fluid absorption. In the case of electrogenic sodium absorption by sodium channels or sodium/glucose cotransport, or absorption in the absence of luminal acidification, sodium movement across the epithelium into a putative hypertonic compartment remains the primary driving force for fluid movement. In addition, the model does not adequately explain how an osmotic gradient across the apical membrane alone results in overall fluid transport from lumen to blood, and studies of bacterially induced fluid transport argue against a significant role. Notwithstanding these caveats, the concept of a luminal hypotonic compartment potentially explains a number of unexplained observations in the upper small intestine. It remains unlikely, however, that it can explain fluid transport in other parts of the GI tract where luminal acidification does not normally occur.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here