Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A benign polypoid growth that arises from the distinctive subepithelial stroma of the distal female genital tract.
Fibroepithelial–stromal polyps, which are hormonally sensitive, most commonly occur in the vulvovaginal region of reproductive age women, often during pregnancy. They may, however, also occur in postmenopausal women on hormonal replacement therapy. Often, the polyps are incidental findings discovered during routine gynecologic examination. Symptoms, when present, may include bleeding, discharge, or the sensation of a mass. These lesions are characteristically polypoid or pedunculated, varying in size but usually less than 5 cm, and are typically solitary, although multiple polyps may occur and are usually associated with pregnancy. Evidence supporting that these lesions are hormonally driven, benign, reactive proliferations include: (1) their occurrence during pregnancy, during which they can be multiple and after which they can spontaneously regress; (2) their association with hormonal replacement therapy in postmenopausal women; and (3) expression of estrogen and progesterone receptors by the constituent stromal cells. Incomplete excision or continued hormonal stimulation (e.g., pregnancy) may be associated with recurrence.
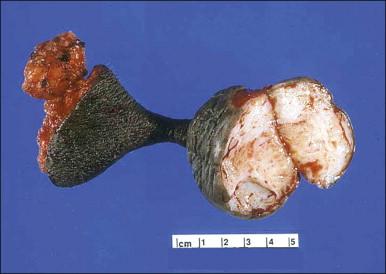
Gross examination typically reveals a polypoid mass with a central fibrovascular core covered by glistening squamous mucosa or skin ( Figure 6.1 ). On occasion, multiple finger-like projections, which may clinically mimic a condyloma, are present. Histologically, these lesions exhibit: (1) a variably cellular spindle cell stroma most often located close to the surface epithelium with scattered stellate and multinucleate stromal cells; (2) a central fibrovascular core; and (3) overlying squamous epithelium or skin, which may exhibit varying degrees of hyperplasia ( Figures 6.2 and 6.3 ).
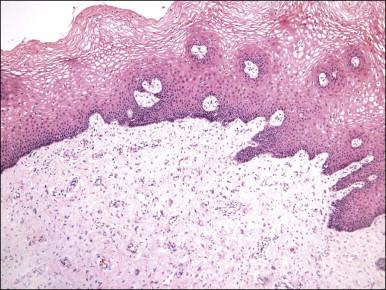
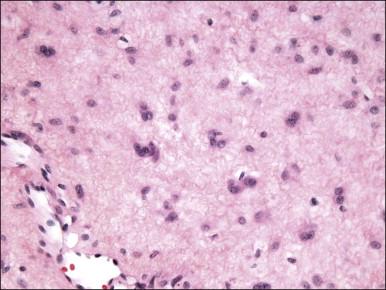
The stromal component has no clearly defined margin and extends up to the epithelial–submucosal interface. Similar to non-neoplastic vulvar stroma, the stromal cells of these polyps may be reactive for desmin, actin, vimentin, and estrogen and progesterone receptors. The most variable component of these lesions is the stroma, which may exhibit a significant degree of cellularity, nuclear pleomorphism, and mitotic activity, thereby mimicking a malignant process ( Figure 6.4 ). These worrisome histologic features are particularly, but not invariably, present in polyps that occur during pregnancy (and account for the historical term ‘pseudosarcoma botryoides’).
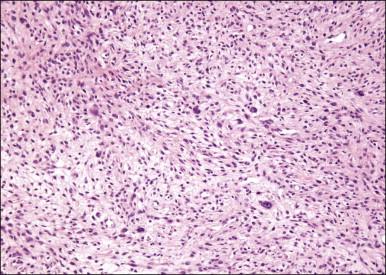
Pseudosarcomatous stromal polyps can be distinguished from a malignant process by the presence of stellate and multinucleate stromal cells near the epithelial–stromal interface, which are characteristically present in these polyps even in the most floridly pseudosarcomatous examples, and by the lack of an identifiable lesional margin. Fibroepithelial–stromal polyps are also readily distinguished from botryoid embryonal rhabdomyosarcoma as they are rare before puberty and lack both the characteristic hypercellular subepithelial (cambium) layer of sarcoma botryoides and specific markers of skeletal muscle differentiation.
A benign, self-limiting myofibroblastic neoplasm characterized by MYH9-USP6 gene fusion that shows rapid growth and spontaneous regression.
Nodular fasciitis typically occurs as a rapidly growing, painful, or tender subcutaneous mass in young adults. It most commonly involves the upper limbs, particularly the forearm; however, it occasionally occurs in the vulva. These lesions are benign and local marginal excision is adequate; if left untreated they will spontaneously regress over a period of months.
Similar to its counterparts elsewhere, nodular fasciitis involving the vulva is usually a well-circumscribed, unencapsulated mass that typically measures less than 3 cm in size. Histologically, it is a relatively well-circumscribed cellular proliferation of loosely arranged spindle cells set within a variably edematous or myxoid matrix that may exhibit microcystic change ( Figure 6.5 ). The spindle cells, which are arranged in short interconnecting fascicles, have bipolar eosinophilic cytoplasmic processes with indistinct borders and ovoid nuclei with occasional nucleoli, imparting an overall appearance to the cells that has been likened to tissue culture fibroblasts. Scattered inflammatory cells, particularly lymphocytes, and extravasated red blood cells are commonly present. Osteoclast-like giant cells are also quite common. Immunohistochemically, the spindle cells are typically reactive for smooth muscle actin and negative for desmin. Nodular fasciitis is distinguished from a sarcoma, particularly leiomyosarcoma at this site, by the following: (1) lack of nuclear hyperchromasia or pleomorphism, (2) lack of necrosis, and (3) characteristic reactive ‘tissue culture’-like myofibroblastic growth pattern.
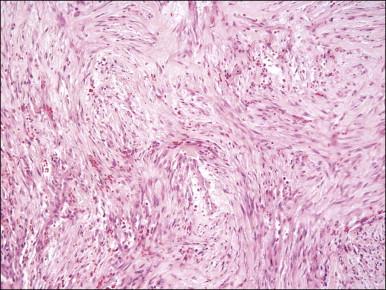
A benign, nonrecurring tumor composed of myofibroblasts and thin-walled capillaries, which principally occurs in vulvovaginal soft tissue.
Angiomyofibroblastoma is an uncommon tumor that occurs almost exclusively in the superficial soft tissue of the vulvovaginal region of reproductive age women; however, similar tumors also occur rarely in men in the inguinoscrotal region. Clinically, it is often mistaken for a cyst on examination, in particular a Bartholin gland cyst. Local excision is typically adequate treatment, as these tumors do not recur. Exceptionally, they undergo sarcomatous transformation. Rarely, they may have areas indistinguishable from aggressive angiomyxoma, suggesting that these two tumors may be related. In either of these unusual settings, excision with clear margins is recommended.
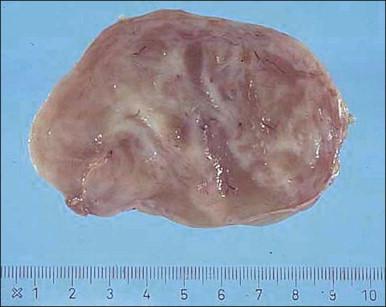
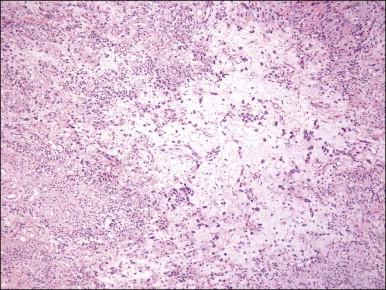
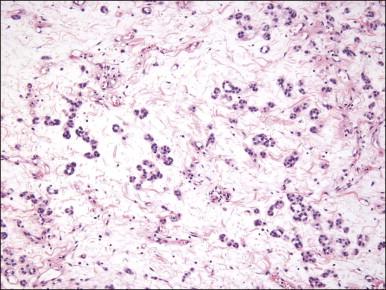
Tumors are typically small (usually <5 cm), well demarcated, tan/white and may have a rubbery consistency ( Figure 6.6 ). They are composed of plump, round to ovoid or spindle-shaped cells, set within a variably edematous to collagenous matrix with alternating zones of cellularity ( Figure 6.7 ). These cells, which have moderate amounts of eosinophilic cytoplasm and round nuclei with fine chromatin and inconspicuous nucleoli, characteristically cluster around the prominent vascular component, which is composed of numerous delicate, thin-walled capillaries ( Figure 6.8 ). Tumor cells may be binucleate or appear somewhat epithelioid. Mitotic activity is typically sparse and occasional cases may have an adipocytic component. The spindle cells are typically reactive for desmin and negative for actin. In postmenopausal patients, lesional cells may be desmin negative.
Angiomyofibroblastoma is distinguished from aggressive angiomyxoma by its well-circumscribed margin, its prominent vascular component (which is typically composed of smaller caliber vessels), and its alternating zones of cellularity. As both tumors share a similar immunophenotype, distinction is based on morphologic differences ( Table 6.1 ).
| Fibroepithelial–stromal Polyp | Angiomyofibroblastoma | Cellular Angiofibroma | Superficial Angiomyxoma | Deep Angiomyxoma | |
|---|---|---|---|---|---|
| Age at presentation | Reproductive age | Reproductive age | Reproductive age | Reproductive age | Reproductive age |
| Location/configuration | Typically polypoid, pedunculated | Subcutaneous | Subcutaneous | Superficial, subcutaneous, polypoid | Deep seated, not polypoid |
| Size | Variable | Usually <5 cm | Usually <3 cm | Usually <3 cm | Variable |
| Margins | Merges with normal | Well circumscribed | Usually well circumscribed; may show focal infiltration | Lobulated, well demarcated | Infiltrative, poorly demarcated |
| Cellularity | Variable | Alternating hypercellular and hypocellular zones | Uniformly cellular | Hypocellular | Hypocellular |
| Vessels | Variable, usually large, thick-walled and centrally located | Numerous, capillary sized | Abundant, small to medium sized, often thick walled and hyalinized | Delicate, elongated, thin-walled capillaries | Medium to large, thick-walled; perivascular collagen condensation and myoid bundles |
| Mitotic index | Variable | Typically low | Variable; may be brisk | Typically low | Rare |
| Clinical course | Benign, rare recurrences (e.g., during pregnancy) | Benign, no recurrent potential; rare sarcomatous transformation | Benign, no recurrent potential; rare sarcomatous transformation | 30% local nondestructive recurrence | 30% local, sometimes destructive, recurrence |
A benign tumor of vulvar mesenchyme composed of a prominent vascular network admixed with bland spindle cells resembling those of spindle cell lipoma.
Cellular angiofibroma is a rare benign stromal tumor that predominantly occurs in the vulva or perineum of middle-aged women (mean 54 years). Although initially described to occur exclusively at this site, they also occur in the inguinoscrotal region in men (so-called angiomyofibroblastoma-like tumor) and in extragenital sites (e.g., retroperitoneum, skin). In the vulva, they most commonly present as a relatively small (mean 2.7 cm) subcutaneous mass that is well circumscribed and painless. Cellular angiofibroma, if completely excised, behaves in a benign fashion with no recurrent potential. Incomplete excision may lead to regrowth of tumor; therefore, local excision with negative margins is adequate treatment. Very rare cases show sarcomatous transformation.
On gross examination, cellular angiofibroma typically is gray-white and has a firm, rubbery consistency ( Figure 6.9 ). Histologically, it is usually well circumscribed; however, focal infiltration into surrounding soft tissue may be present. These tumors are characteristically cellular, composed of the following: (1) short, intersecting fascicles of bland, spindle-shaped cells with ovoid nuclei and scant palely eosinophilic cytoplasm; (2) numerous small to medium sized, thick-walled, and often hyalinized blood vessels; and (3) admixed wispy collagen bundles ( Figure 6.10 ). In approximately 25% of cases, a usually minor component of adipose tissue is present. Mitotic activity, although brisk in some cases, is usually infrequent while necrosis and nuclear pleomorphism are typically absent. On occasion, atypia may be present, most commonly in the form of scattered hyperchromatic multinucleated cells ( Figure 6.11 ). Rarely, abrupt transition to a discrete sarcomatous component can occur, which may exhibit features of atypical lipomatous tumor, pleomorphic liposarcoma or pleomorphic sarcoma, not otherwise specified. The spindle cells of cellular angiofibroma are reactive for CD34 in 60% of cases, and less commonly reactive for smooth muscle actin (20%) and desmin (8%). Half of all cases show reactivity for estrogen and progesterone receptors. Keratin, epithelial membrane antigen, and S-100 protein are negative.
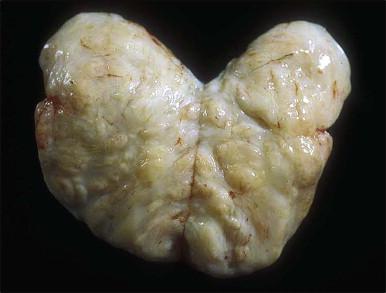
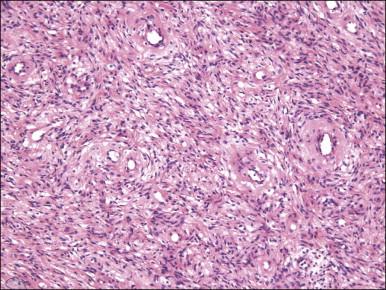

Cellular angiofibroma is distinguished from a smooth muscle tumor by its more prominent vascular component, shorter fascicular growth pattern, and relative lack of eosinophilic cytoplasm. Distinction from angiomyofibroblastoma is based on its uniform cellularity; larger, thick-walled, and hyalinized blood vessels; and spindle cells arranged in short intersecting fascicles ( Table 6.1 ). Mammary type myofibroblastoma and cellular angiofibroma are both well-circumscribed tumors composed of short intersecting fascicles of bland, CD34-positive spindle cells within a collagenous stroma; however, the vascular component of the former is typically not as prominent but can show a similar degree of hyalinization. These tumors both have similar genetic changes with loss of 13q14 ( FOX1A1 ) and thus likely represent tumors along a spectrum exhibiting varied cellularity and vascularity.
A poorly circumscribed, hypocellular lesion composed of bland spindle cells within a variably edematous, myxoid, or collagenous matrix that usually occurs in prepubertal girls.
Prepubertal vulvar fibroma most commonly involves the labia majora of girls and usually presents as a painless, gradual vulvar swelling or enlargement. Lesions typically are under 5 cm in size, unilateral, and ill-defined submucosal or subcutaneous masses. While these lesions have been considered neoplastic due to their ability to recur, it has also been proposed that they represent growth of a hormonally responsive vulvar mesenchyme to changes in the hormonal environment at the time of puberty and thus represent a hyperplastic rather than a neoplastic process. Although only a limited number of cases have been studied, the lesions appear to be benign, except for quite frequent local recurrence when incompletely excised.
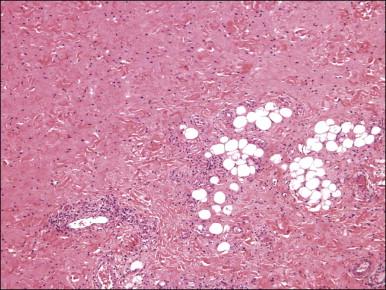
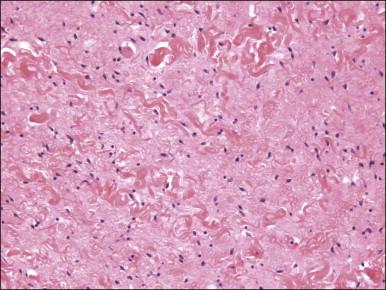
Histologically, these tumors, which are located in submucosal or subcutaneous tissue, are poorly marginated with infiltration into surrounding tissue, including around adnexal structures and nerves, and into adipose tissue ( Figure 6.12 ). In addition, there is no clear interface with the overlying epithelium. The lesion is hypocellular and composed of a patternless proliferation of bland, uniform spindle cells with ovoid nuclei and palely amphophilic cytoplasm set within a variably myxoid, edematous, or collagenous matrix ( Figure 6.13 ). Small to medium sized vessels, some with mural thickening, are present. Mitotic activity is sparse and nuclear pleomorphism is absent. Lesional spindle cells are typically reactive for CD34 but are negative for smooth muscle actin, desmin, and S-100 protein.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here