Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vulvar and vaginal exfoliative cytology, although not a substitute for biopsy, may provide valuable information about the nature of a lesion without causing significant discomfort to a patient. Although unsuspected cancer is rarely identified by this approach, a wide variety of infectious and inflammatory diseases, dermatologic diseases, and benign and malignant tumors have characteristic cytologic features. A biopsy remains the standard management for any suspicious lesion. Colposcopy of the vulva and vagina is an important component of this process.
Technical success in procuring an optimal specimen from the vulva depends on the method used. Scrapings are best obtained by means of physical force using various instruments such as the edge of a glass slide, a scalpel blade, or vulva brush. Generally, the scalpel blade yields the best suitable preparation. Employing a spatula, in a manner similar to a cervical Papanicolaou (Pap) test, has resulted in poor correlation with the tissue diagnosis and is not recommended. A warm, moist saline towel or cloth placed over the lesion to be sampled softens the superficial keratinized layer, resulting in a more cellular specimen with reduction in the amount of drying artifact. Vigorous scraping may be required to dislodge cellular material covered by a thick and dry layer of keratin, which should be discarded prior to obtaining a more cellular second scrape. Moist or ulcerated lesions may be sampled by touching a glass slide directly against the lesion or by swabbing the edge of the ulcer and spreading the material on a slide. All slides should be immediately fixed with spray fixative or 95% ethanol for optimal preservation. Employing ThinPrep, while lessening air drying, did not improve accuracy, as it did not offer improvement in cell procurement, using a spatula however. Pigmented lesions are perhaps best sampled by biopsy unless the surface is ulcerated or the specimen can easily be obtained through non-aggressive methods. An unusual artifact representing silver nitrate crystal contamination used as a hemostatic agent to control bleeding, following a biopsy procedure, has been described as a contaminant in SurePath vulvar cytology specimens. Fine-needle aspiration (FNA) cytology is particularly useful in assessing palpable subcutaneous or submucosal lesions of the vulva and vagina.
The vulva is composed of the mons pubis, the labia majora, the labia minora, the clitoris, the vestibule, and Bartholin's glands. Except for the Bartholin's glands, these structures are covered by stratified squamous epithelium. Bartholin's glands are paired mucus-secreting glands located in the posterior aspect of the labia majora. Their main excretory ducts are lined by stratified squamous epithelium, as are the ducts of the minor vestibular gland.
A number of infectious and inflammatory diseases may involve the vulva. Proper specimen collection and culturing are often necessary for complete identification of a causative agent. Many venereal and non-venereal processes have characteristic morphologic appearances that on Pap smears suggest the causative agent. Viral infections such as herpes simplex and human papillomavirus (HPV) have readily identifiable cytopathic effects. Other viral infections such as molluscum contagiosum ( Fig. 11-1 ), cytomegalovirus, varicella, herpes zoster ( Fig. 11-2 ), variola, Epstein–Barr virus, and vaccinia may less commonly involve the vulva. Mycotic vulvar infections are usually secondary to Candida albicans or Candida tropicalis . Unusual fungal organisms include Torulopsis glabrata and the superficial dermatophytic mycoses, including tinea cruris, tinea versicolor, and tinea circinata. Deep mycotic infections of the vulva (blastomycosis, sporotrichosis, coccidioidomycosis, and actinomycosis) are rare. Parasitic infections, such as trichomoniasis, amebiasis, and schistosomiasis, usually involve the vagina as well as the vulva. The diagnosis can easily be made by identifying organisms on a cytologic preparation or in a biopsy specimen.
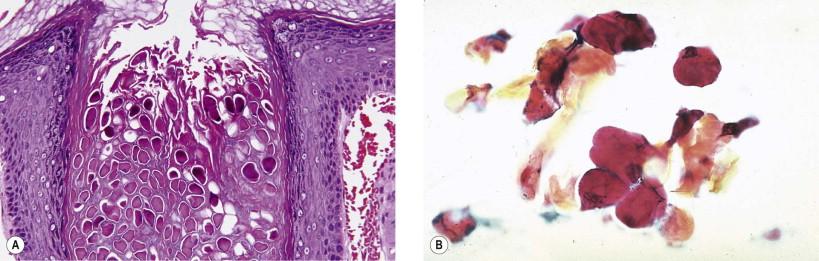
Bartholin's gland infections are caused by various anaerobic and aerobic bacterial organisms. Both ductal and acinar elements may be infected, resulting in a blockage of secretion and causing the formation of an abscess that may be diagnosed by FNA with appropriate cultures.
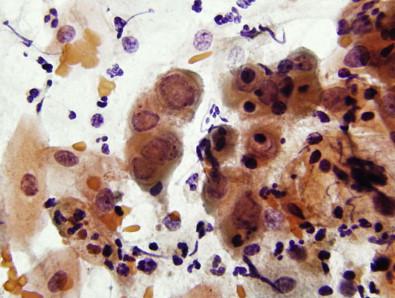
Inflammatory and dermatologic diseases of the vulva include vulvar vestibulitis syndrome, Behçet's syndrome, Crohn's disease, malacoplakia, contact dermatitis, psoriasis, pemphigus vulgaris, bullous pemphigoid, lichen planus, and erythema multiforme. Vulvar lesions are the second most frequent site of mucous membrane involvement in pemphigus vulgaris. Scraping the base of a vulvar or vaginal lesion in a patient with pemphigus vulgaris may yield intermediate squamous cells, and isolated or loose aggregates of acantholytic cells, characterized by round-to-oval, central, large nuclei; with regularly distributed, coarsely granular chromatin; and prominent nucleoli ( Fig. 11-3 ). These cells may mimic dysplastic or malignant cells. Pemphigus vulgaris may also rarely involve the uterine cervix and manifest similar findings, and differential interpretation on the Pap test. The definitive diagnosis of pemphigus vulgaris involving the vulva and vagina, necessitates a biopsy and immunofluorescence studies.
Intermediate squamous cells isolated or in loose aggregates
Acantholytic cells
Round-to-oval central nuclei
Regularly distributed chromatin with prominent nucleoli
Biopsy and immunofluorescence studies are necessary for diagnosis.
Non-neoplastic epithelial disorders of the vulvar skin have traditionally been subdivided into lichen sclerosus, and other dermatoses (including lichen simplex chronicus, candidiasis, lichen planus, psoriasis, seborrheic dermatitis, and allergic contact dermatitis). The variable clinical appearances of the lesions, degree of hyperplasia, and extent of disease process offer little indication about whether an underlying vulvar intraepithelial neoplasia (VIN) is present. A biopsy is necessary for a definitive diagnosis.
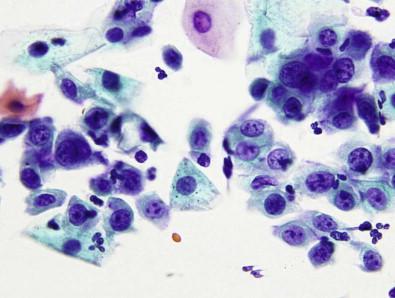
Many of these dermatoses have been previously grouped under the term “squamous cell hyperplasia.” While this term may be an appropriate histopathologic descriptor, more specific subtyping better reflects the clinicopathologic and management implications. Although these lesions are generally considered “non-neoplastic,” they may be associated or coexistent with neoplastic lesions. The cytologic findings from vulvar scrapings of any of the above non-infectious skin disorders of the vulva are indistinguishable. These scrapes yield varying degrees of nucleated and anucleated squames, parakeratotic cells, and hyperkeratosis ( Fig. 11-4 ). The squamous cells retaining their nuclei may demonstrate reactive changes such as slight nuclear enlargement; however, significant degrees of nuclear atypia and pleomorphism are not observed unless an underlying VIN is present. Caution should be exercised in coming to a conclusion about the pathologic significance of squames, parakeratosis, and hyperkeratosis as they may similarly be obtained from normal skin.
VINs had been traditionally graded according to their severity and their potential to progress into an invasive process as follows: VIN 1 (mild dysplasia); VIN 2 (moderate dysplasia); and VIN 3 (severe dysplasia and carcinoma in situ). In 2004, this division was revised and the term VIN should only apply to histopathologically high-grade squamous lesions (previous VIN 2–3). VIN 1 is no longer diagnosed, as it was believed that there was no convincing evidence that a VIN 1–3 morphologic spectrum reflects biological continuum, and VIN 1 is not proven to be a cancer precursor. However, this concept has been further modified in the recently published 2012 guidelines for terminology in the Lower Anogenital Squamous Terminology (LAST) project. The LAST project recommended a two-tier classification system for HPV-associated lesions across all lower anogenital tract sites in both females and males: low-grade squamous intraepithelial lesion (LSIL) (previous – IN1) and high-grade squamous intraepithelial lesion (HSIL) (previous – IN2 and – IN3) in a manner similar to that of The Bethesda System of cytology reporting. The aim of this terminologic change was to enable better interobserver reproducibility, improved communication with both clinicians and pathology colleagues, and to streamline clinical management protocol development and implementation.
Histopathologically, VIN lesions are subdivided into two major morphologic types: classic (usual) and differentiated (simplex). The classic (usual) VIN encompasses former VIN 2–3 of warty, basaloid, and mixed types. It also encompasses the previously used terms of Bowen's disease, Bowenoid VIN, HPV-positive VIN, and undifferentiated VIN. Classic VIN cases are characterized by disordered cell maturation, cellular crowding and nuclear hyperchromasia and pleomorphism ( Fig. 11-5 ). The warty type of classic VIN displays koilocytotic atypia toward the surface with cellular eosinophilia, multinucleation, and hyperkeratosis and/or parakeratosis. The basaloid type of classic VIN is composed of more uniform and crowded basaloid cells with scanty cytoplasm and no maturation near the surface. VIN differentiated (simplex) type only refers to simplex VIN 3 since simplex VIN 1–2 cannot be recognized. The differentiated (simplex) type of VIN has some features that overlap with a hyperplastic or reactive process ( Fig. 11-6 ). However, there are certain morphologic features that are helpful in differentiating simplex VIN from squamous hyperplasia. At the surface, simplex VIN often shows parakeratosis, whereas squamous hyperplasia often displays hyperkeratosis. At the parabasal zone, the presence of enlarged cells that have prominent eosinophilic cytoplasm and large pleomorphic nuclei with prominent nucleoli is the feature characteristic of simplex VIN. There may be prominent dyskeratosis even with early keratin pearl formation in the parabasal zone, a phenomenon often described as “paradoxical maturation.”
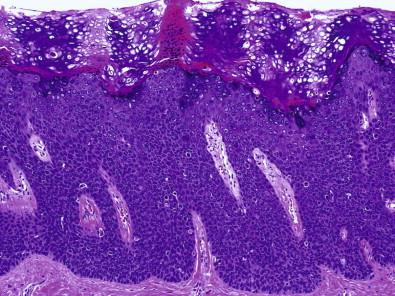
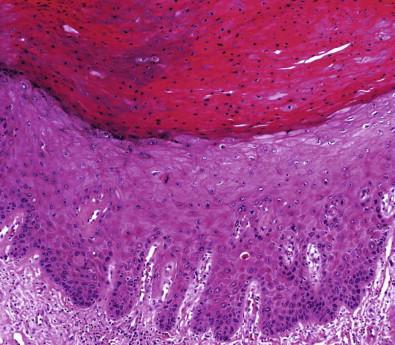
The classic VIN constitutes approximately 80–90%, the differentiated (simplex) 5–10%, and the mixed type around 1% of all VINs. The mean age of classic VIN is 35–45 years and there is usually a history of condyloma, sexually transmitted disease, abnormal Pap tests, and CIN lesions or human immunodeficiency virus (HIV) positivity. The risk factors are similar to those of cervical cancer. The lesions tend to be multicentric pigmented or focal papules, or warty lesions of vulva and perineum. Differentiated (simplex) VIN, on the other hand, is mostly seen in older women (mean 67 years) and is often associated with a background of lichen sclerosus (>62%).The lesions are usually flat, thin, and thinning with discoloration. Skin thickening and focal ulceration may develop at a later stage.
Distinct pathogenic pathways exist for classic VIN and differentiated (simplex) VIN. Classic VINs are associated with HPV infection. High-risk HPV types, such as HPV 16 and HPV 18, have been identified in most of the VIN 2–3 cases. The distribution of low-risk HPV types in VIN 1 lesions generally supports the notion that VIN I is synonymous with flat condyloma. p16 protein is indirectly increased due to decrease of pRB when high-risk HPV is integrated into the host genome. Overexpression of p16 is seen in >97% in classic VIN 2–3 lesions. Approximately two-thirds of differentiated (simplex) VIN overexpress p53 protein, which is indicative of a p53 mutation. The p53 positivity should be in >90% of basal cell layers, with positive cells reaching suprabasal layers. Approximately one-third of differentiated VIN is p53 negative; some of which are due to p53 allelic deletion. In addition, scattered p53 staining can be seen in benign reparative reactions. Therefore, caution in rendering a simplex VIN 3 diagnosis is warranted when there is a discrepancy between the morphology and p53 immunostain.
Classic VIN is associated with local recurrence (7–30%) and infrequent progression to invasive carcinoma. The warty and basaloid types of VIN serve as precursor lesions for the warty and basaloid subtypes of squamous cell carcinomas (SCCs). Although it represents the most commonly seen VIN (90%), less than 18% progress to invasive SCC, accounting for less than 30% of all invasive SCCs. The potential to progress to invasive SCC increases in patients older than 40 years and in immunocompromised individuals. In contrast, differentiated (simplex) VIN, which represents one of the most underdiagnosed lesions in vulvar pathology, frequently (70%) progresses to invasive keratinizing SCC. Factors that contribute to underdiagnosis of differentiated (simplex) VIN include subtle presentation without mass or raised lesions, a relatively short intraepithelial phase before progression to invasive SCC, and the subtle and difficult to recognize morphologic features that often overlap with squamous hyperplasia.
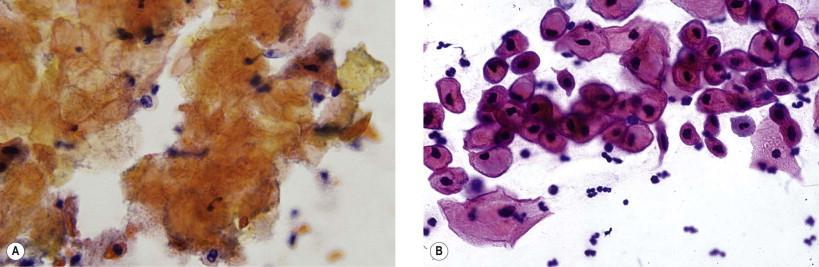
Cytologically, the abnormal cells in low-grade vulvar lesions are usually polygonal ( Fig. 11-7 ), whereas those from high-grade lesions tend to be round or oval ( Fig. 11-8 ). The nuclear to cytoplasmic (N : C) ratio increases with the increasing severity of the lesion. Koilocytotic change is frequently noted within and adjacent to the lesions. Hyperkeratosis, parakeratosis, and squamous pearl-like formations may also be present. In most cases, the number of abnormal cells and extent of cytologic abnormality make it possible to separate low- from high-grade intraepithelial lesions. Low-grade lesions, however, may be clinically indistinct and the cellular changes subtle.
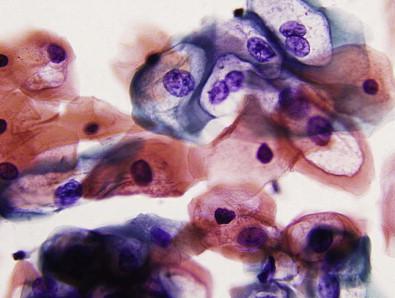
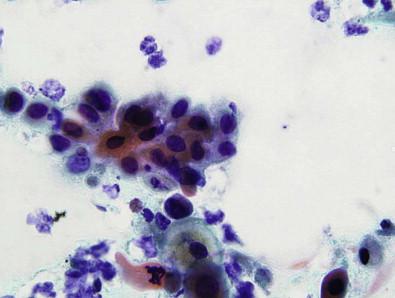
Cytologic scraping of high-grade lesions reveals abnormal cells occurring singly or in loose aggregates. The cells are polygonal and have well-defined cytoplasmic borders. Variation in size and shape of the cells is usually noted; however, lesions may mimic either a basal cell carcinoma having small uniform cells or a large cell squamous carcinoma having marked variability. The nuclei are enlarged and hyperchromatic and have granular chromatin. Anucleated squames and parakeratotic cells are usually observed in the background.
The above cytologic features are best applicable to the classic VIN (warty and basaloid) as dysplastic cells are present in the superficial layers. A scrape of the differentiated (simplex) VIN is likely to yield cells derived from surface reaction and differentiated squamous cells that lack dysplastic changes.
Abnormal cells in loose aggregates or as single cells
Round to oval cells
Enlarged hyperchromatic nuclei
Anucleated squamous and parakeratotic cells (usually).
Still, some have reported that vulvar cytological evaluation does not correlate well with tissue diagnosis for all types of VIN and they do not support the use of this method in the clinical management of VIN. Therefore, the biopsy remains the criterion standard diagnostic modality which will determine the management for any suspicious vulvar lesion.
Papillary hidradenoma is a benign tumor composed of complex branching papillae lined by an inner layer of secretory cells and an outer layer of myoepithelial cells. The lesion is usually diagnosed by biopsy; however, occasionally an FNA may be obtained. The aspirates reveal variable combinations of three cell types: uniform columnar, myoepithelial, and metaplastic apocrine. The differential diagnosis includes rare cases of mammary-like tumors, endometriosis involving the vulva, and tumors of skin adnexal origin.
Malignant tumors of the vulva make up approximately 5% of all gynecologic malignancies. The majority of vulvar malignancies are squamous cell carcinomas (90%), followed by malignant melanoma, Paget's disease basal cell carcinoma, Bartholin gland malignancies and soft tissue malignancies. Most vulvar carcinomas occur in an older population.
Invasive SCC of the vulva is generally divided into three main groups: keratinizing, warty, and basaloid carcinoma. Keratinizing SCC constitutes approximately 70% of the vulvar carcinomas and occurs at a mean age of 77 years ( Fig. 11-9A ). Differentiated (simplex) type of VIN is believed to be the precursor lesion for the most and the risk factors for CIN are absent (HPV-unrelated cancer). The warty/basaloid carcinomas constitute 30% of vulvar carcinomas and occur at a mean age of 55 years. The warty and basaloid VIN lesions serve as precursors of these carcinomas, respectively. The recognized cervical cancer risk factors are present (HPV related). Using a polymerase chain reaction-based method HPV 16 followed by HPV 33 are the most common types of HPV detected in invasive squamous vulvar cancer. Immunohistochemical staining for p16 is a reliable marker for HPV-positive vulvar SCC and its associated precursor lesions.
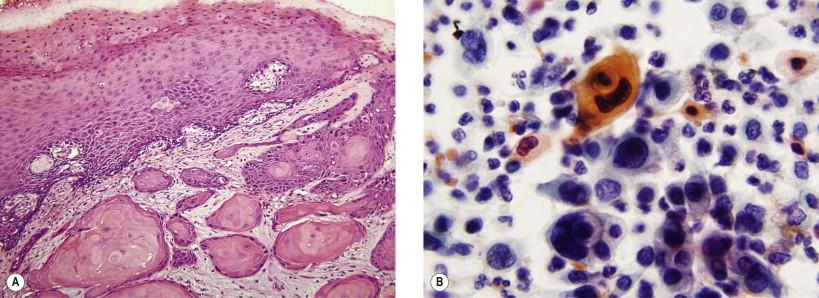
The cytologic characteristics resemble, for the most part, their cervical counterparts. The abnormal cells may occur in aggregates or singly, depending on the tumor type and method of preparation. Owing to the presence of increased numbers of desmosomal junctions, those tumors showing evidence of keratinization are more frequently found in aggregates. The cells are polygonal and have well-defined cell borders and eosinophilic cytoplasm. Variation in the size and shape of cells may be noted; occasional bizarre forms have cytoplasmic processes that create elongated or tadpole forms. The finding of abnormally keratinized cells (dyskeratotic cells) should always raise concern for malignancy. The nuclei are reactively uniform although usually enlarged and hyperchromatic. Anucleated squames or parakeratotic cells are also frequently noted ( Fig. 11-9B ).
Aggregates or single abnormal cells
Cellular and nuclear pleomorphism; polygonal, elongate, and tadpole forms
Abnormally keratinized cells
Enlarged hyperchromatic nuclei.
These are large, cauliflower-like tumors that have a well-demarcated base. Histologically, the tumors are composed of exophytic papillary fronds with hyperkeratosis or parakeratosis on the surface. The tumor is well demarcated from the underlying stroma, and the rete pegs have blunt, pushing borders. Nuclear pleomorphism is mild. Keratin pearls are usually present. In cytologic smears from verrucous carcinomas, the cells usually occur in aggregates that retain their cytoplasmic processes. Sheets of hyperkeratotic and parakeratotic cells are usually found. Little cellular or nuclear pleomorphism is noted. Owing to the marked histologic and cytologic similarity to pseudoepitheliomatous hyperplasia and condyloma acuminatum, verrucous carcinomas are difficult to diagnose, and a biopsy that includes the base of the lesion is necessary for a definitive diagnosis.
Basal cell carcinoma is a low-grade neoplasm that predominately occurs in postmenopausal women of an average age of 65 years. It constitutes 2–3% of all vulvar malignancies. It most frequently involves the labia majora. Grossly, the lesion can appear as nodules, polyps, ulcers, or flat areas of hyper- or hypopigmentation. Histologically, vulvar basal cell carcinomas are similar in appearance to basal cell carcinomas at other sites. Basal cell carcinoma is generally regarded as non-fatal. Metastases to lymph nodes are rare. Care should be observed with perirectal lesions to distinguish basal cell carcinomas of the skin from basaloid cloacogenic carcinoma of the rectum and adenoid cystic carcinoma. Cytologically, few cases of basal cell carcinoma have been described. The cells are small and uniform and have a scant amount of poorly defined cytoplasm. The nuclei are enlarged and uniform and contain hyperchromatic chromatin. Nucleoli may be observed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here