Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Edema represents expansion of the interstitial volume and is a common phenotypic presentation of multiple pathologic processes. The word diuretic is derived from the Greek roots dia “thoroughly” and ourein “urine.” Consequently, the term diuretic is defined by its ability to amplify urine output and treat edema. Historically, “dropsy” was coincidentally alleviated by various plant and mineral compounds, including mercurial agents such as calomel and novasurol. Subsequently, the primacy of salt retention’s role in edema formation led to the development of agents that generated urinary sodium loss. Chlorothiazide, which became available in 1958, ushered in the modern era of targeted diuretic therapy, initially as therapy of edematous states and shortly afterward as treatment of essential hypertension. These agents’ capacity to reduce blood pressure (BP) and thus decrease the morbidity and mortality associated with uncontrolled hypertension makes diuretics one of several first-line therapies for treatment of hypertension. Diuretics are also requisite treatment tools in volume overload states, including chronic kidney disease (CKD), nephrotic syndrome, cirrhosis, and heart failure (HF) where they facilitate decongestion. This chapter reviews the various diuretic classes, respective mechanisms of action, and physiologic adaptations that ensue from prolonged use.
The predominant nephron sites of action of the various diuretic classes are illustrated in Fig. 9.1 . The diuretic classes include inhibitors of sodium reabsorption in the proximal tubule (PT) where approximately 65% occurs, thick ascending loop of Henle (TALH) where up to 25% occurs, distal convoluted tubule (DCT) where approximately 5% to 10% occurs, and epithelial sodium channel (ENaC) that are, along with mineralocorticoid receptor antagonists (MRAs), potassium-sparing agents that act in the collecting tubule. Osmotic diuretics exert their effect at several points in the nephron that are highly water permeable. SGLT2 inhibitors have a diuretic effect, which may be a major contributor to their other benefits, as discussed in Chapter 26 .
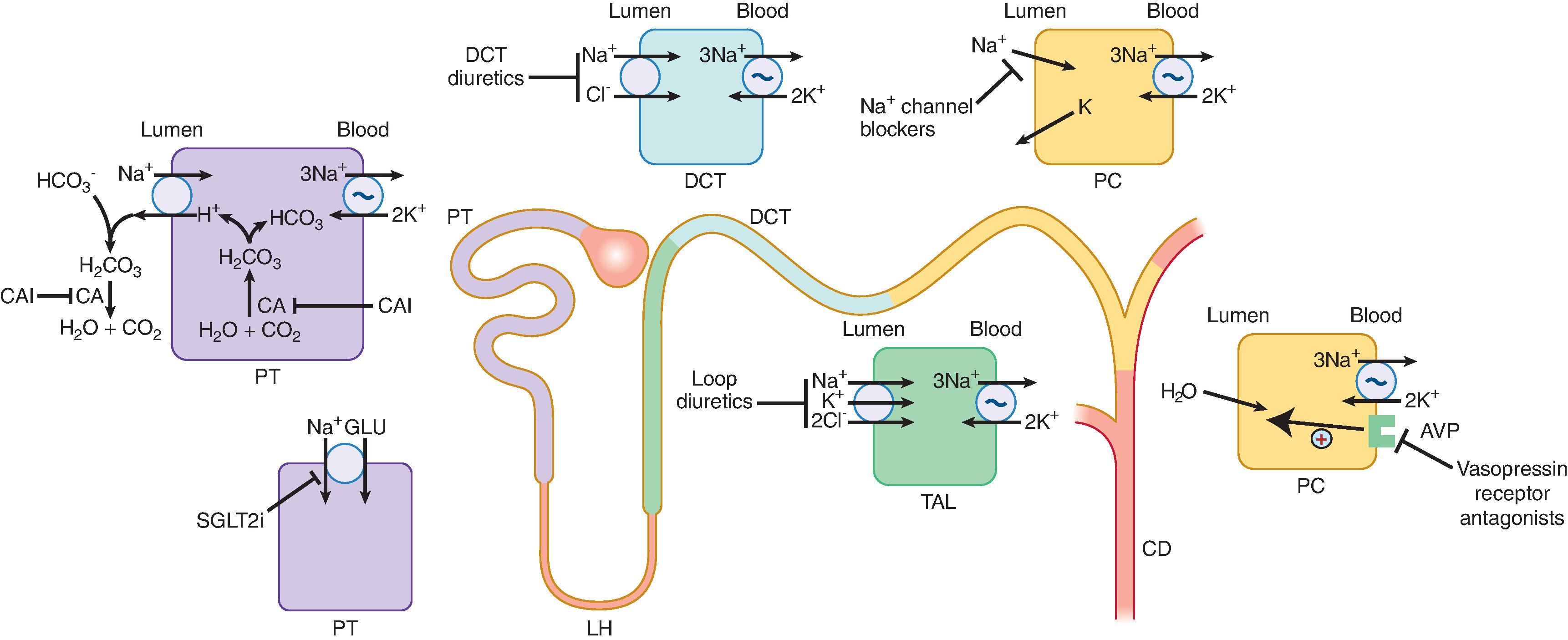
Carbonic anhydrase inhibitors (CAIs), represented by acetazolamide (ACZ), produce a brisk alkaline diuresis, impairing sodium reabsorption by decreasing generation of intracellular hydrogen ion (see Fig. 9.1 ). As a result, there is diminution of sodium reabsorption via the apical PT sodium-hydrogen exchanger (NHE-3). Although NHE3 accounts for nearly one-third of PT sodium reabsorption, CAIs do not produce a proportional loss of sodium because downstream sodium reabsorption increases at the TALH and collecting duct (CD), and glomerular filtration is reduced by tubuloglomerular feedback with a reduction in filtered sodium load. ACZ also facilitates diuresis by inducing kidney vasodilation and inhibiting the bicarbonate-chloride exchanger pendrin, localized to type B-interacted cells of the distal nephron.
ACZ is currently the only CAI used as a diuretic; other CAIs are used as topical formulations for glaucoma therapy. ACZ is readily absorbed and eliminated by tubular secretion (≈50%). However, its use is constrained by its transient action and because prolonged use produces hypokalemia and metabolic acidosis, among other side effects. These side effects can be used to therapeutic advantage: ACZ (daily dosage range, 250–500 mg) corrects the metabolic alkalosis that occurs with thiazide or loop diuretic therapy. ACZ can also attenuate lithium-induced nephrogenic diabetes insipidus. By inhibiting carbonic anhydrase (CA), ACZ decreases intracellular lithium content, resulting in greater urine osmolality with reduced urine volumes. ACZ must be employed cautiously in advanced CKD because systemic accumulation may lead to neurologic side effects. The anticonvulsant topiramate inhibits CA and may cause metabolic acidosis, high urine pH, and kidney phosphate stone formation. Administration of topiramate to patients with a history of kidney stone disease or renal tubular acidosis must be done with caution, if at all, and only when other treatment options are exhausted.
Glucose filtered through glomeruli is primarily reabsorbed by the PT sodium-glucose cotransporter-2 (SGLT2) (see Fig 9.1 ). Blockade by SGLT2 inhibitors (SGLT2i) or “flozins,” including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, reduce glycemia in type 2 diabetes by enhancing glucose excretion, which produces natriuresis via osmotic diuresis. Resultant natriuresis with reduction in extracellular fluid volume (ECFV) is accompanied by modest BP reduction and restoration of tubuloglomerular feedback that is frequently impaired in diabetic kidney disease. The diuretic effect of SGLT2i is heterogeneous. An initial polyuria is transient, with subsequent reduction of urine output attributable to upregulated sodium, water, and urea reabsorption along the remaining nephron and by renin-angiotensin-aldosterone system (RAAS) activation.
Several trials of SGLT2i demonstrated reductions in cardiovascular (CV) events and rate of progression of CKD, and are discussed in Chapter 26 . Sodium accumulation in extravascular tissues such as skin and muscle is associated with volume expanded states, and SGLT2i were shown to reduce cutaneous sodium in patients with type 2 diabetes, supporting the thesis that SGLT2 inhibitors reduce cardiovascular disease (CVD) by decreasing interstitial fluid sodium and volume.
The major site of action of thiazide and thiazide-like diuretics is the early DCT. Here, these agents inhibit coupled, electroneutral sodium and chloride reabsorption by the sodium chloride cotransporter (NCC). Water-soluble thiazides like hydrochlorothiazide (HCTZ) inhibit CA at high doses, which augments sodium excretion. Thiazides also inhibit sodium chloride and water reabsorption in the medullary CD, thereby impairing maximal urinary dilution. Additional drug effects include reductions of uric acid and calcium excretion, elevated urinary magnesium excretion, and impairment of potassium-mediated insulin secretion. The most widely prescribed agent in this class is HCTZ, with an onset of diuresis by 2 hours and peak effect within 3 to 6 hours of administration that dose-dependently continues for up to 12 hours. The half-life of HCTZ is prolonged in decompensated HF and kidney failure, and dosages from 100 to 200 mg daily are required for adequate diuresis of CKD patients. Cumulative natriuresis is arbitrated by the filtered sodium load. Historically, thiazides were eschewed at glomerular filtration rates (GFRs) less than 30 mL/min because of lower efficacy, but more recent data support the use of thiazide diuretics as antihypertensive agents in more advanced stages of CKD.
The concept of “class effect” among thiazide (HCTZ) and thiazide-like diuretics (chlorthalidone [CTD]) has been debated relative to BP reduction and CV outcomes. Although structurally similar, CTD and HCTZ differ pharmacokinetically. CTD has a longer half-life of 40 to 60 hours versus 3.2 to 13.1 hours for HCTZ and larger volume of distribution due to extensive partitioning into red blood cells that serve as a drug depot. The prolonged plasma half-life of CTD likely accounts for its greater potency on a milligram-per-milligram basis compared to HCTZ.
Loop diuretics act predominantly at the luminal TALH membrane where they compete with chloride ion binding to a kidney-specific sodium-potassium-2-chloride cotransporter (NKCC2). Consequently, loop agents inhibit sodium chloride reabsorption. Loop agents exert qualitatively minor effects on sodium reabsorption at other nephron segments. Clinically relevant effects of loop diuretics are a decrease in free water excretion and absorption during water loading and dehydration, respectively; a 30% increase in fractional calcium excretion; increased magnesuria; and a brief increase followed by a more long-lived decrease in uric acid excretion. Loop agents stimulate kidney prostaglandin (PG) synthesis, especially the vasodilator PG E2. Angiotensin II stimulation by loop agents in synergy with augmented PG E2 production are the probable causes for the shift of renal blood flow (RBF) from the inner to outer kidney cortex. Nonetheless, RBF and GFR are typically maintained when loop diuretics are administered to normal individuals.
Available loop diuretics include bumetanide, furosemide, torsemide, and ethacrynic acid (the last preferred for those with a true sulfa allergy). These compounds are highly protein bound, mainly to albumin. Therefore, to gain access to their site of action, loop agents undergo PT secretion, as do thiazide diuretics, via an organic anion transporter-dependent process. Tubular secretion of loop diuretics is impeded by elevated levels of endogenous organic acids, some of which increase in CKD, and agents that utilize the same transporter, for example, salicylates and nonsteroidal antiinflammatory drugs (NSAIDs). Uremic toxins and fatty acids can decrease loop diuretic protein binding and alter pharmacokinetics.
Diuretic excretion rates and natriuretic response approximate drug delivery to the medullary TALH. This relationship is exemplified by a sigmoidal curve. A normal dose-response relationship (typically observed in untreated hypertension patients) may be shifted (downward and rightward) by a variety of clinical conditions, ranging from ECFV depletion to HF or nephrotic syndrome and drug therapy ( Fig. 9.2 ). One example of the latter is the blunting of diuretic effect via inhibition of prostaglandin synthesis by NSAIDs.
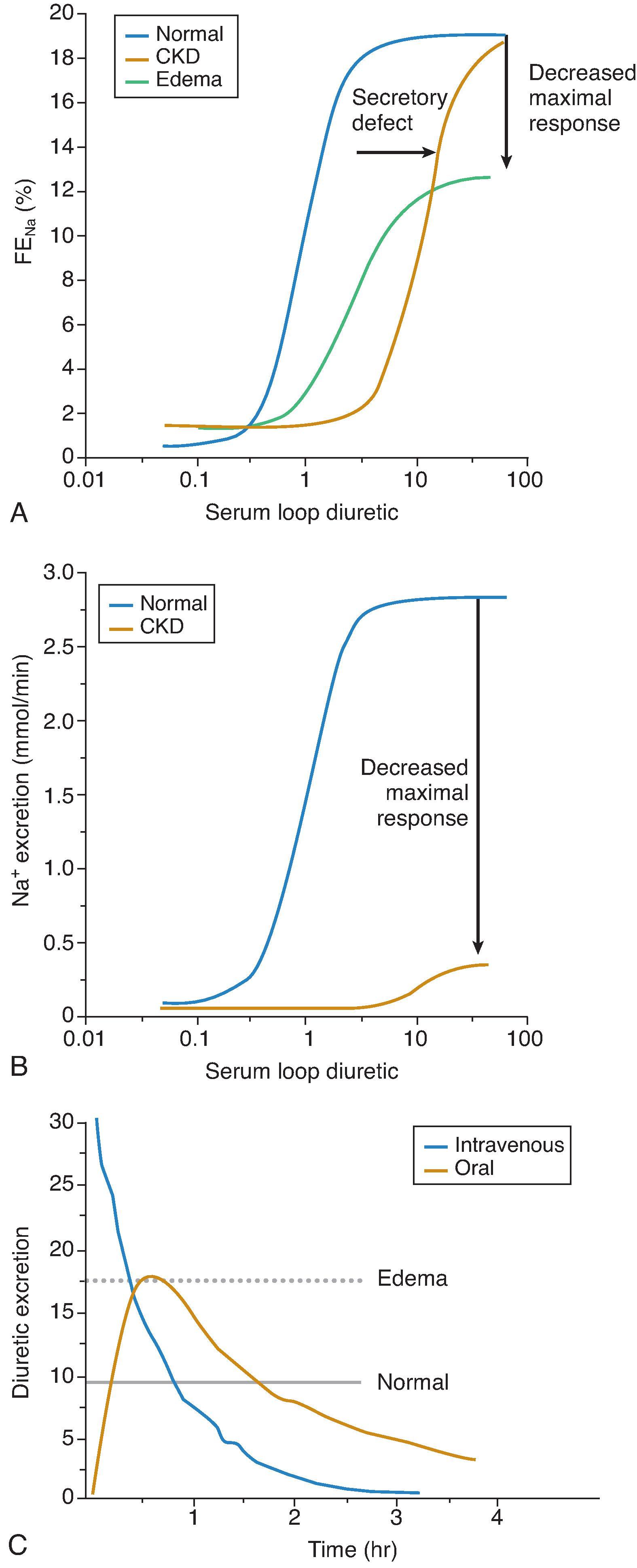
Furosemide is the most widely used loop diuretic. Efficacy is complicated by its variable absorption, with bioavailability ranging from 12% to 112%. The coefficient of variation for absorption varies from 25% to 43% for different furosemide products; exchanging one furosemide formulation for another will not standardize patient absorption and/or response to oral furosemide. Bumetanide and torsemide have superior gut absorption compared to furosemide. The consistency of torsemide absorption and its longer duration of action are features to consider when loop diuretic therapy is required for chronic HF patients and/or CKD patients. Loop diuretics are common therapy in CKD, with the kidney drug clearance reduced in parallel with declining kidney function. Generally, furosemide pharmacokinetics are more significantly changed in CKD than the other loop diuretics because its kidney metabolism and intact clearance is reduced in CKD. Alternatively, bumetanide and torsemide undergo significant hepatic metabolism that is marginally reduced by CKD. In CKD, pharmacokinetic profiles change only as the result of decreased kidney drug clearance.
Potassium-sparing diuretics are divided into two subclasses: MRAs that target the apical mineralocorticoid receptor and ENaC inhibitors that inhibit the luminal namesake channel, resulting in more downstream terminal effects.
The ENaC inhibitors, amiloride and triamterene, diminish sodium reabsorption at the late DCT and CD, thereby reducing basolateral sodium-potassium ATPase activity with consequent reduction in the intracellular potassium concentration. The net effect is reduction of potassium secretion into the tubular lumen. ENaC-directed potassium-sparing diuretics are modestly natriuretic; thus, clinical utility resides primarily in their ability to counterbalance potassium loss, particularly when more proximally acting diuretics increase distal sodium delivery. As a result, ENaC inhibitors are often marketed in combination with thiazide diuretics to avoid the need for potassium supplementation. These drugs are actively secreted by PT cationic transporters and possess modest natriuretic effect. These cationic compounds interfere with the PT secretion of creatinine. Thus, mild serum creatinine elevations may accompany their administration. Amiloride and triamterene undergo extensive kidney clearance and will accumulate with repetitive dosing in settings of reduced GFR.
The MRAs, spironolactone, eplerenone, and finerenone, differ from ENaC inhibitors not only in their site and mechanism of action, but also in clinical use as adjunctive therapy for congestive heart failure. The RAAS is pathologically upregulated in patients with chronic HF with reduced ejection fraction (HFrEF). The addition of MRAs to other approved HF medications has improved clinical outcomes in patients with HFrEF with mild to severe symptoms and also in patients with left ventricular dysfunction after myocardial infarction. Spironolactone is a well-absorbed, highly protein-bound, lipid-soluble MRA with a 20-hour half-life. Its onset of action is slow, with peak response occurring 48 hours or more after initial dosing. 7α-thiomethylspirolactone and canrenone are the two main metabolites of spironolactone and account for most of the drug’s effect. Spironolactone, unlike amiloride and triamterene, remains biologically active as a diuretic and antihypertensive agent at low GFRs because active drug enters the basolateral space before engaging the mineralocorticoid receptor to blockade aldosterone. Eplerenone is a highly selective MRA. Thus, its much lower affinity for “off target” androgen and progesterone receptors produces undesirable effects such as gynecomastia less often compared to spironolactone. Typically, eplerenone is at best a very mild diuretic; its antihypertensive effect originates from nondiuretic aspects of its action. Finerenone is also an MRA, but it has enhanced receptor affinity compared to eplerenone in vivo. Consequently, this agent has potent antiproteinuric effects without significant BP lowering or hyperkalemia.
Mannitol is a low-molecular-weight, uncharged polysaccharide, administered by the intravenous route. It undergoes unimpeded glomerular filtration and acts as an osmotic diuretic. Essentially non-reabsorbed along the nephron, mannitol exerts a dose-dependent effect, effectively trapping water and solutes in the tubular fluid, with augmented excretion of sodium, potassium, and chloride. Its onset of action is relatively rapid, between 30 and 60 minutes, and its diuretic action is brief. Although mannitol has been tried to prevent AKI by delivery pre-cardiopulmonary bypass, during rhabdomyolysis, or after radiocontrast media exposure, the literature does not support these practices. Because mannitol expands the ECFV en route to its diuretic site of action, it may precipitate pulmonary edema in HF patients and must be used cautiously in this circumstance, if at all. The receipt of excessive mannitol, especially in settings of reduced GFR, can cause dilutional hyponatremia, hyperkalemia, and/or AKI. The last adverse effect is attributable to dose-dependent afferent arteriolar vasoconstriction that is correctable with extracorporeal mannitol elimination of the surfeit, that is, hemodialysis.
Kidney urea transporters (UTs) reside in the descending limb of the loop of Henle (UT-A2), inner medullary CD (UT-A1 and UT-A3), and descending vasa recta (UT-B1). UTs contribute to maintenance of the corticomedullary osmotic gradient, and UT-A1 is regulated by arginine vasopressin. Knockout of mouse urea transporter genes diminishes urinary concentrating capacity and diuresis. Extrapolation of these observations has provoked development of UT inhibitors. Currently, none is available for clinical application.
Diuretic-induced inhibition of sodium reabsorption in one nephron segment elicits counter-regulatory adaptations by other segments. This adaptation not only limits the antihypertensive effect and ECFV depletion of diuretics but also contributes to development of side effects. Although some degree of diuretic resistance is associated with prolonged use, intractable disease state-related diuretic resistance may occur with HF, cirrhosis, or nephrotic syndrome.
The initial dose of a diuretic typically produces a brisk increase of urine output succeeded by a new equilibrium state with body weight stabilization. Daily fluid and electrolyte excretion either matches or is less than intake. In nonedematous patients treated with thiazide or loop diuretic, this adaptation, known as the “braking phenomenon,” occurs within 1 to 2 days and limits net weight loss to 1 to 2 kg. This event is most evident in normal subjects given a loop diuretic. For example, furosemide administered orally to subjects ingesting a high daily sodium diet of 270 mmol produces rapid natriuresis, with negative sodium balance after 6 hours. During the next 18 hours, positive sodium balance occurs with no net weight reduction. This phenomenon has been replicated for 3 consecutive days ( Fig. 9.3 ) and is reproducible even after a 1-month hiatus from drug exposure. Thus, single, daily furosemide dosing will generally not achieve negative sodium balance, unless a most stringent regimen of sodium restriction is followed.
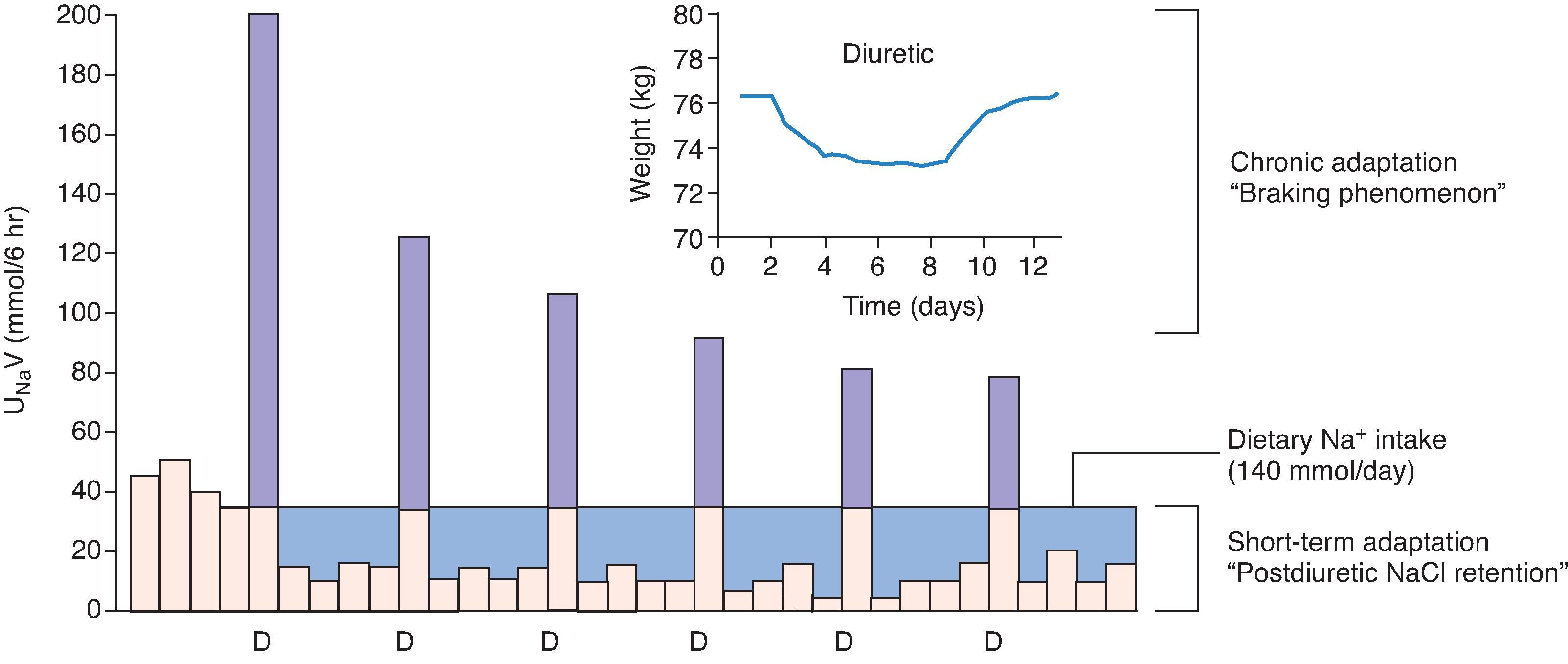
The mechanistic basis of the braking phenomenon is complex. The sigmoidal relationship between natriuresis and furosemide excretion rate is shifted rightward in subjects ingesting a low sodium diet, denoting blunted tubular response. The importance of ECFV depletion in the process of postdiuretic sodium retention has been clearly demonstrated. However, there is an ECFV-independent component to this process unrelated to aldosterone; that is, no effect on sodium balance following spironolactone administration. In rats, cellular hypertrophy of the distal nephron follows prolonged exposure to loop diuretic infusions and is marked by aldosterone-independent processes of amplified sodium chloride reabsorption and enhanced potassium secretion. These biological adaptations manifest as postdiuretic sodium retention and diuretic tolerance in humans, plausibly explaining why sodium retention may persist for up to 2 weeks following cessation of loop diuretic agents.
Plasma renin activity and aldosterone concentrations rise within minutes of receipt of an intravenous diuretic, a process independent of volume loss and/or sympathetic nervous system (SNS) activation. This elevation of plasma renin activity is generated by inhibition of NaCl reabsorption at the macula densa, in conjunction with loop diuretic stimulation of kidney prostaglandin release. This first wave of neurohumoral effects transiently increases afterload and may lessen the antihypertensive efficacy of a loop diuretic briefly. Shortly after this initial rise in renin activity, diuretics induce a more sustained increase in renin activity and aldosterone, attributable to an increase in SNS activity (β-agonism) and decline of ECFV. An increase in kidney prostaglandin production and nitric oxide is the likely explanation for the preload reduction and decrease in ventricular filling pressures that occur within 15 minutes of loop diuretic administration.
The pathophysiology of sodium and water retention in edematous patients is characterized by a complex interchange of hemodynamic and neurohumoral factors. For example, systemically perceived arterial underfilling sets into motion related sodium and water retention in HF patients. The level of neurohormonal activation, degree of kidney vasoconstriction, and extent to which kidney perfusion pressure is reduced modulate this process. In other instances, such as in the patient with a reduced GFR and/or nephrotic syndrome, sodium and water retention derive from a more primary set of kidney processes. In each circumstance, efforts should be directed toward correction of the underlying disease state as diuretic use is contemplated.
Two important considerations that must always accompany diuretic therapy at both initiation and during continuation are dietary sodium restriction (2 g sodium per day) and dose reduction/elimination of drugs that foster sodium retention, including NSAIDs, nonspecific vasodilators such as hydralazine and minoxidil, and high-dose β-blockers or central α-agonists. After diuretic initiation, choice of drug, dosage, and dosing frequency are subjective processes that focus on etiology and extent of ECFV overload. There is a treatment hierarchy among the thiazide diuretics; longer-acting compounds such as chlorthalidone or metolazone are preferred in edematous patients. CTD can be quite effective in the setting of mild-to-moderate edema given once or twice daily in the 25- to 50-mg/day range. When the underlying disease state worsens and/or dietary sodium restriction is not adequately maintained, conversion to a loop diuretic-based regimen is the more practiced approach. Combination diuretic therapy can be subsequently considered because severity of edema requires “sequential nephron blockade” or the underlying disease state is exceptionally responsive to diuretics other than loop agents, such as spironolactone (range, 50–400 mg/day) in patients with advanced liver disease.
Determining the minimally effective dose for a diuretic effect is a necessary clinical exercise, specifically for individuals with reduced GFR (see Fig. 9.2 ). Gradually increasing a diuretic dose until an adequate diuretic response occurs establishes the “threshold” dose; thereafter, dosing frequency is defined by clinical circumstances. The initial dose from which dose titration proceeds is influenced by level of kidney function and severity of edema. If kidney function is reduced, the diuretic dose-response curve shifts rightward and maximal effectiveness, based on absolute sodium excretion rate, is significantly reduced. Therefore, dietary sodium restriction becomes even more important. In a patient with CKD and edema, initial loop agent doses are furosemide 40 mg, torsemide 10 mg, and bumetanide 1 to 2 mg. Each agent should be administered twice daily, and the starting dose is titrated upward until desired efficacy is attained. Often, the dose that elicits an increase in urine output can be continued indefinitely unless the underlying disease state worsens and/or dietary sodium intake supervenes diuretic effect. Conversely, a diuretic dose that establishes “euvolemia” may occasionally be lowered due to strict dietary adherence to sodium intake. Diuretic dose reduction must always be contemplated because this maneuver minimizes loss of potassium, magnesium, and, in the case of loop diuretics, calcium.
ECFV regulation in most edematous patients is achievable with organized application of diuretic therapy principles. “Diuretic-resistant” patients abound in ambulatory and inpatient settings. Among hospitalized individuals, diuretic resistance is frequently linked to the complex nature of the sodium-retentive state, with multiple organ systems at play and acuity of illness as the major determinant. In diuretic-resistant ambulatory patients, excessive sodium intake is a key pathogenic factor often overlooked. Several features are important when determining the “dry” or “target” weight in edematous patients, including symptom relief that incorporates patient input into the treatment equation, extent of comorbid illnesses, and realistic appreciation of the limits of treatment. A systematic approach to safe and maximally effective diuretic treatment is outlined ( Fig. 9.4 ).
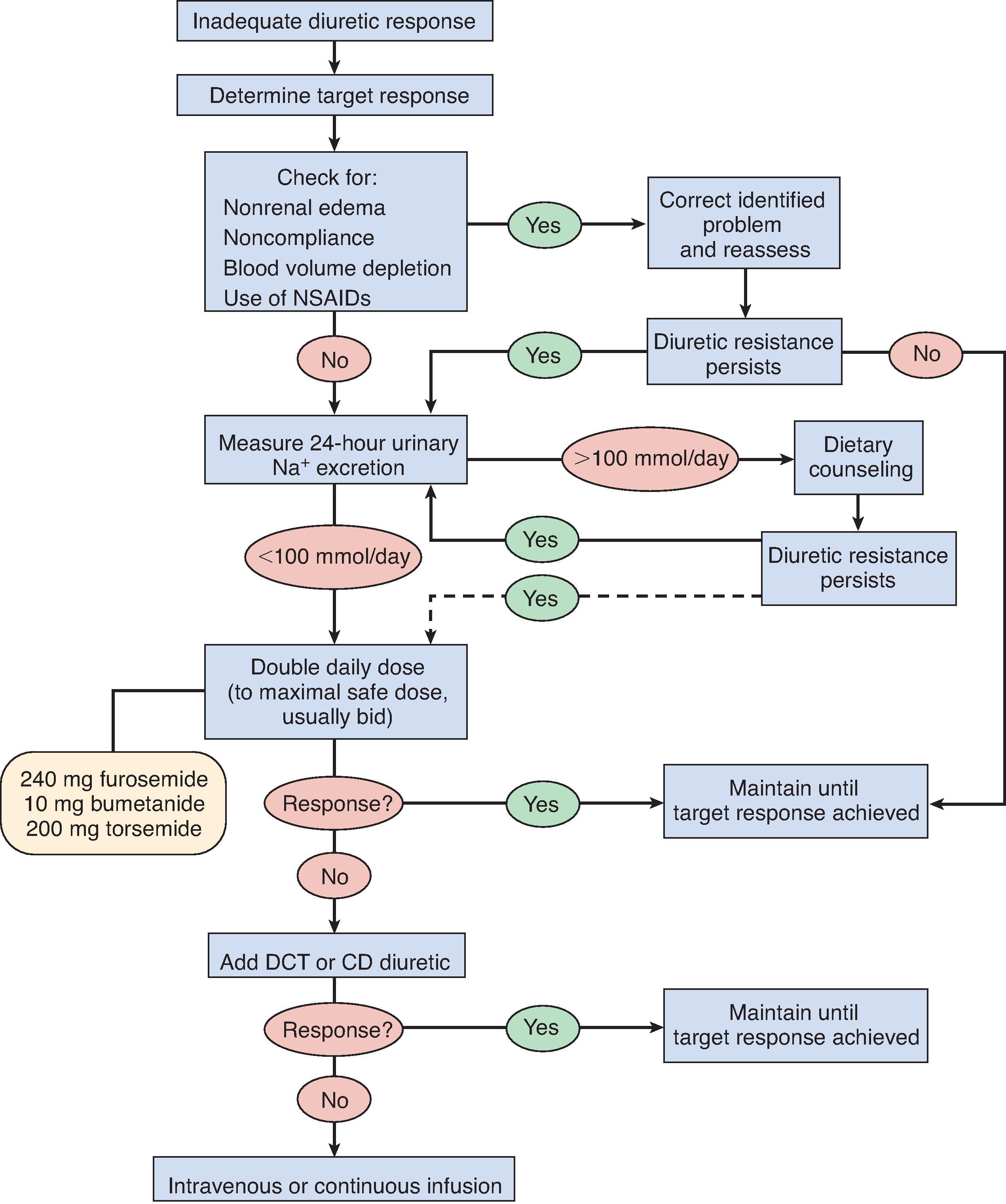
Poorly regulated sodium intake can scuttle the net negative sodium balance that characterizes a well-planned dietary and diuretic regimen. A 24-hour urine sodium excretion that exceeds 100 mmol is a reasonable marker of adequate diuretic action; however, obtaining a complete 24-hour urine collection may be problematic. An alternative approach that evaluates diuretic adequacy is calculation of the fractional sodium excretion 1–2 hours after administration of a well-absorbed diuretic. True diuretic resistance is unlikely if the fractional excretion of sodium exceeds 2%. However, diuretic resistance may be misconstrued when there is inconsistent drug absorption, especially with respect to furosemide—a problem circumventable by intravenous delivery of 80 to 120 mg, typically.
In CKD, reduced diuretic clearance mitigates efficacy; tubular secretion of loop agents is slowed, so larger doses must be provided to achieve the drug threshold. Conversion from the intravenous to oral route is often arbitrary. The oral agent has a wide coefficient of variation for gut absorption; however, oral furosemide, with mean gut absorption of 50%, should be prescribed at twice the intravenous dose.
The pathophysiology of edema formation in nephrotic syndrome is complex and incompletely understood. The “underfill” theory posits that low plasma oncotic pressure from hypoalbuminemia promotes egress of fluid from the vascular space to the interstitial space, with consequent kidney retention of salt and water via activation of the sympathetic nervous system, vasopressin, and RAAS. However, the alternative “overfill” theory is now considered the principal mechanism for edema formation. In this schema, the kidneys retain sodium independently of circulating plasma volume. Data from experimental animals with unilateral nephrotic syndrome or glomerulonephritis suggests that primary sodium retention in these disorders is from augmented distal CD sodium reabsorption by the CD. Recently, the serine protease plasminogen has been identified as a pathogenetic factor. Its aberrant appearance in urine in experimental and human nephrotic syndrome and subsequent activation to plasmin by urokinase-type plasminogen activator (uPA) amplifies ENaC-regulated sodium. Thus, amiloride may attenuate a portion of the excessive sodium reabsorption in nephrotic syndrome.
Nephrotic syndrome often presents as a diuretic-resistant state. Alterations in the pharmacokinetics and pharmacodynamics of loop diuretics account for this tempering effect. Loop diuretic delivery is impaired in hypoalbuminemic individuals as diuretic secretion is highly contingent on prevailing plasma albumin concentrations. In decompensated nephrotic syndrome, the diuretic dose-response relationship shifts rightward (higher threshold for effect) and downward (reduced maximal response or decreased sensitivity). Diuretics binding to albumin in tubular fluid reduce the proportion of unbound, active drug. At urine albumin concentrations exceeding 4 g/L, up to 65% of diuretic may be albumin bound. Accordingly, starting doses that are two- to threefold greater than normal oral starting doses are recommended.
Administration of anti-RAAS agents to reduce albuminuria are always a consideration in the edema-reduction strategy of nephrotic syndrome. Nonetheless, the cornerstone of therapy remains restriction of sodium intake, which typically is accompanied by diuretic therapy. However, the “reduced” diuretic responsiveness inherent to nephrotic syndrome typically mandates more frequent loop diuretic dosing. Another therapeutic option for treatment of ECFV overload includes diuretic combination regimens of 2 or 3 agents.
Hemodynamic abnormalities are frequent in diuretic-resistant patients, especially if HF and/or reduced ejection fraction is present. Accordingly, inotropic agents such as dobutamine may augment RBF and diuretic action in systolic HF. Permissive elevation of BP and/or the strategic withholding of RAAS inhibitors may restore diuretic efficacy as these agents may lower BP below a critical threshold for diuretic effect, particularly when small or large vessel disease is present in kidney vascular beds. In addition, diuretics should not be administered in temporal proximity to anti-RAAS agents because of the latter’s potential to abruptly lower BP.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here