Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Epstein-Barr virus (EBV) is a ubiquitous herpesvirus with tropism for B cells. More than 90% of humans are infected with EBV, and the infection persists for life. Usually primary infection is asymptomatic and occurs early in life, and when symptomatic is usually a self-limited disease occurring in adolescents or young adults manifested as acute infectious mononucleosis (IM). IM is characterized by a polyclonal expansion of infected B cells and a cytotoxic T-cell response composed of a transient, antigen-driven oligoclonal expansion of CD8-positive T cells. Both the quantity and quality of the CD8-positive T-cell response to EBV are critical to control the infection. In vivo EBV is capable of infecting in addition to B cells, T cells and natural killer (NK) cells, as well as epithelial and mesenchymal cells. The infection of T cells and NK cells may lead to several EBV-related lymphoproliferative diseases, with disease manifestations generally depending on the type of EBV-infected cells and the state of host immunity. EBV-positive T-cell and NK-cell lymphoproliferative disorders encompass disease entities with a broad clinicopathologic spectrum.
Chronic active EBV infection (CAEBV) was originally described as a disease related to chronic or persistent EBV infection lasting longer than 6 months after acute EBV infection, with severe IM-like symptoms, elevated titers against EBV, and evidence of organ damage without evidence of an underlying immunodeficiency (see Chapter 29 ). Although originally considered as an EBV infection targeting B cells, subsequent studies demonstrated that this disorder is more often associated with infection of T cells and less often of NK cells. The term T/NK-cell chronic active EBV infection has been used in the literature to encompass a broad spectrum of diseases comprising a systemic polyclonal or monoclonal form (T cells; αβ or γδ cells, and NK-cells) and a cutaneous form including hydroa vacciniforme–like T/NK lymphoproliferative disorder (usually of T-cell origin) and severe mosquito bite allergy (usually of NK-cell origin). In the revised fourth edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, hydroa vacciniforme–like T/NK lymphoproliferative disorder was the proposed term that included both hydroa vacciniforme (an indolent form) and hydroa vacciniforme–like T-cell lymphoma. They are considered to represent a continuum of the same disease and cannot be separated reliably based on clinical or morphologic features.
Systemic EBV-positive T-cell lymphoproliferative disorder of childhood represented the fulminant form of a clonal EBV-infected T-cell proliferative disease occurring after primary EBV infection. The fourth edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues recognized systemic T-cell lymphoproliferative disorder of childhood as a neoplasm; to clarify the aggressive nature of this disease, the term was changed to systemic EBV-positive T-cell lymphoma in the revised fourth edition. Systemic EBV-positive T-cell lymphoma shares clinical and pathologic features with aggressive NK-cell leukemia, but they differ in the lineage of origin; the former is of T-cell origin, whereas the latter is of NK-cell origin. A rather difficult differential diagnosis and often overlapping disease is EBV-associated hemophagocytic lymphohistiocytosis (HLH) in childhood, which is a hyperinflammatory syndrome induced by dysregulated immune reaction secondary to EBV infection. Although clonal EBV-infected T cells have been demonstrated in some cases, patients usually respond to treatment, which can be limited in some cases, and recover completely after the acute disease.
Aggressive NK-cell leukemia and extranodal NK/T-cell lymphoma are recognized as the prototypes of EBV-positive T-cell or NK-cell lymphoma/leukemia in the 2008 WHO classification. Extranodal NK/T-cell lymphoma, nasal-type primarily involves extranodal sites mainly the upper aerodigestive tract. EBV-positive cytotoxic T-cell or NK-cell lymphomas involving primarily the lymph nodes are uncommon, and are discussed under the name of nodal T/NK-cell lymphoma. This chapter details the clinicopathologic features of all these disease entities ( Box 30-1 ).
EBV-associated hemophagocytic lymphohistiocytosis
Systemic CAEBV
Cutaneous forms of CAEBV
Severe mosquito bite allergy
Hydroa vacciniforme–like T/NK-cell LPD
Systemic EBV + T-cell lymphoma of childhood (neoplastic and fulminant, T cell)
Aggressive NK-cell leukemia (neoplastic and fulminant, NK cell)
Extranodal NK/T-cell lymphoma, nasal-type
Nodal T/NK-cell lymphoma (provisional)
CAEBV, chronic active EBV infection; EBV, Epstein-Barr virus; LPD, lymphoproliferative disorder; NK, natural killer.
Hemophagocytic lymphohistiocytosis (HLH) is a clinicopathologic syndrome encompassing a markedly dysregulated immune response and hypercytokinemia. HLH is characterized clinically by fever, splenomegaly, and cytopenias, and is accompanied by histologic evidence of hemophagocytosis, which causes extremely high serum levels of ferritin, lactate dehydrogenase, and soluble CD25. The disease is classified into a primary (genetic) form and a secondary (acquired) form. Secondary HLH occurs in the setting of infection or underlying rheumatologic disorders or malignancy. Epstein-Barr virus (EBV)-associated HLH accounts for about 40% of all HLHs and is the most common type of secondary HLH. The diagnosis is established when EBV is documented in the blood or tissue in addition to fulfilling the criteria in the HLH-2004 guidelines. EBV-associated HLH can occur in association with underlying diseases such as primary HLH, T/NK-cell lymphoma/leukemia, chronic active EBV infection, or systemic EBV-positive T-cell lymphoma of childhood. However, EBV-associated HLH in childhood is usually encountered in the context of primary EBV infection without underlying disease ( Box 30-2 ).
A hyperinflammatory syndrome induced by dysregulated immune reaction secondary to EBV infection
Demonstration of high EBV load in the blood or tissue
Fulfilment of HLH 2004 guideline: fever; splenomegaly; cytopenias affecting at least 2 of 3 lineages in the peripheral blood; hyperferritinemia; hyperglyceridemia and/or hypofibrinogenemia; hemophagocytosis in bone marrow, spleen or lymph node; low or absent NK-cell activity; high level of CD25. Five of these 8 criteria are required for diagnosis.
Prevalent in Asia
Usually encountered in the context of primary EBV infection in children (median age, 3.9 years) without underlying disease.
Often fatal but can be effectively controlled in 90% of children by HLH 2004 protocol
Poor prognostic factor includes hyperbilirubinemia, hyperferritinemia, or cytogenetic abnormality
Hemophagocytic histiocytosis in bone marrow, liver, and spleen
Infiltration of EBER-positive T cells without atypia in the bone marrow and hepatic sinusoids.
EBV found mainly in T cell.
Infiltrating T cells express CD8 and granzyme B
The clonality of EBV-infected T cells is often observed
EBER, EBV-encoded RNA; EBV, Epstein-Barr virus; HLH, Hemophagocytic lymphohistiocytosis; NK, natural killer.
EBV-associated HLH in childhood is rare. Most reports of EBV-associated HLH have arisen from East Asian countries including Taiwan, Japan, and Korea, but it has also been described in Western countries, and more commonly in indigenous populations of Mexico, Central America, and South America. In Japan, 25 cases of EBV-associated HLH are diagnosed in children each year. There is a female predominance and a peak incidence between 1 and 2 years of age. The median age of patients is 3.9 years. The epidemiology for adult cases is not known.
Primary infection with EBV is usually asymptomatic except for infectious mononucleosis, in which EBV infection in B cells triggers a cytotoxic T-cell response. In EBV-associated HLH, EBV infects primarily CD8-positive T cells and results in a cytokine storm that involves the release of proinflammatory and Th1-type cytokines including TNF-alpha and IFN-gamma, and leads to secondary activation of histiocytes and macrophages. IFN-gamma plays a critical role in macrophage activation and hemophagocytosis. TNF-alpha can induce a pathologic process similar to that seen in HLH, including fatty liver, hyperglyceridemia, and bone marrow suppression. The particular prevalence of EBV-associated HLH in Asian children suggests that underlying genetic factors contribute to the EBV-related dysregulated immune responses. A recent study of cytokine gene polymorphisms showed that the frequency of TGF-beta1 codon 10C allele was significantly higher in patients with EBV-associated HLH.
EBV-associated HLH is a systemic illness that is usually first detected as a persistent fever that is unresponsive to antibiotics. Patients with EBV-associated HLH exhibit cytopenias, liver dysfunction, hepatosplenomegaly, and hemophagocytosis in the bone marrow, lymph node, liver, or spleen. Coagulopathy, pleural effusions/ascites, and CNS disease can also occur. Elevated triglyceride or ferritin level, low fibrinogen level, low or absent NK-cell cytotoxicity, and elevated soluble CD25 are sometimes seen. EBV viral load in peripheral blood is high and correlates well with disease activity. Serology shows that some patients have elevated VCA-IgM, indicating primary EBV infection, although the other patients have viral antibodies indicative of past infection or reactivation.
In patients with EBV-associated HLH, the bone marrow shows hemophagocytic histiocytes and a variable number of EBV-positive T cells in addition to myeloid and erythroid cells ( Fig. 30-1 ). The liver biopsy shows Kupffer cell hyperplasia, mild infiltration of small T cells in the portal tract and sinusoids, and intrasinusoidal infiltration of hemophagocytic histiocytes. Because of minimal histologic changes in the early stage, diagnostic abnormalities may not be detected with hematoxylin-eosin staining. EBER in situ hybridization highlights EBER-positive cells in the bone marrow and hepatic sinusoids.
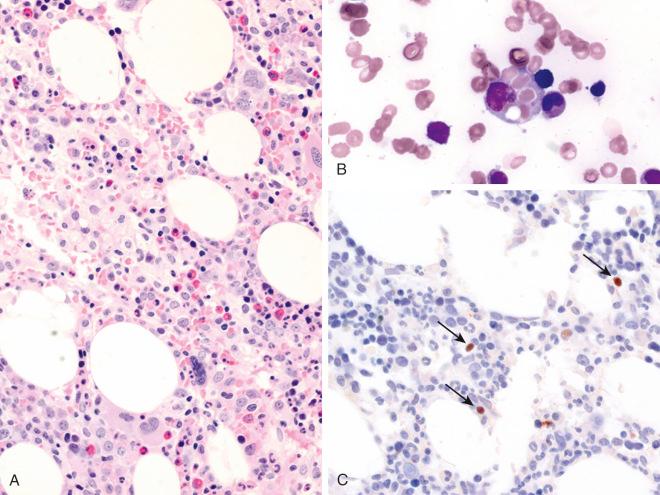
Cells infiltrating the bone marrow and liver are cytotoxic T cells that express CD8 and granzyme B or, uncommonly, NK cells. In peripheral blood, flow-cytometric analysis may show a significant increase in a subpopulation of CD8-positive T cells that exhibit downregulation of CD5. EBV is best determined by molecular methods that can detect the presence of EBV genomic DNA or EBV-encoded RNA (EBER) in biologic specimens such as serum, bone marrow, or lymph nodes. The clonality of EBV-infected T cells can be observed.
The postulated cells of origin are EBV-infected CD8-positive cytotoxic T cells and, rarely, NK cells.
EBV-associated HLH is a heterogeneous disorder with various symptoms that can range from mild to severe. In the past, a significant proportion of patients died because of cytokine storm or disease progression to chronic active EBV infection or EBV-positive systemic T-cell lymphoma of childhood. With the development of combination treatment including etoposide, dexamethasone, and cyclosporine A, EBV-associated HLH in children without underlying disease can be effectively controlled in more than 90% of patients. However, the other 10% often die of fulminant disease. Patients with hyperbilirubinemia, hyperferritinemia, or cytogenetic abnormality at the time of diagnosis have significantly poorer outcomes. The presence of clonality at the time of diagnosis is not associated with a poor outcome, but a change in clonality may be a good marker of disease activity in childhood EBV-associated HLH.
After the diagnosis of EBV-associated HLH, underlying immunodeficiency disorders associated with EBV-associated HLH should be excluded (see Chapter 54 ). T/NK-cell leukemia/lymphomas show obvious atypia of infiltrating EBV-positive cells. EBV-positive cells in chronic active EBV infection are deceptively benign. An abnormal increase in anti-EBV antibody titers and a long clinical history compatible with chronic EBV infection are helpful in the differential diagnosis. EBV-associated HLH and systemic EBV-positive T-cell lymphoma of childhood share a significant overlap in clinical presentation and pathologic changes. Because EBV-HLH can be associated with clonal T-cell populations, the distinction between these two diseases is often difficult. Primary HLH that is associated with EBV infection can be excluded by genetic testing and family history.
Chronic active EBV infection (CAEBV) is a systemic EBV-positive polyclonal, oligoclonal, or often monoclonal T-cell or NK-cell lymphoproliferative disorder that exhibits varying degrees of clinical severity depending on the host immunity and EBV factor. Chronic active EBV infection was initially defined as a severe illness of greater than 6 months' duration that (1) begins as a primary EBV infection or is associated with markedly abnormal EBV antibody titers (e.g., anti-EBV viral capsid antigen [VCA] immunoglobulin [Ig]G ≥5120, anti-EBV early antigen IgG ≥640, or anti–Epstein-Barr nuclear antigen [EBNA] <2); (2) shows histologic evidence of major organ involvement, such as interstitial pneumonia, hypoplasia of the bone marrow, uveitis, lymphadenitis, persistent hepatitis, or splenomegaly; and (3) exhibits increased EBV RNA or proteins in affected tissues. Kimura and colleagues proposed revised diagnostic criteria for CAEBV stipulating that patients have an EBV-related illness or symptoms lasting more than 3 months' duration and increased EBV DNA (>10 2.5 copies/mg EBV DNA) in peripheral blood mononuclear cells or RNA in the tissue, or grossly abnormal levels of EBV antibodies. The initial description of CAEBV did not specify the lineage of the EBV-infected cell, but since then the syndrome has almost always been associated with a proliferation of EBV-infected T cells or NK cells. Most cases consist of a systemic EBV-positive polyclonal, oligoclonal, or monoclonal T-cell or NK-cell lymphoproliferative disorder characterized by a high viral load in peripheral blood and tissues and intermittent or chronic infectious mononucleosis–like features such as fever, lymphadenopathy, and hepatosplenomegaly at least 3 months after primary virus infection in patients with no known immunodeficiency. CAEBV infection of B-cell derivation occurs rarely. Chronic persistent infectious mononucleosis, which is somewhat more common, represents an illness in which EBV-positive B cells persist in significant numbers beyond the acute illness, accompanied by continued clinical symptoms associated with infectious mononucleosis. Because the term CAEBV does not specify the lineage of the EBV-infected cell, a recent international report recommended that the term be modified to include the cellular lineage affected: T cell, NK cell, or B cell ( Box 30-3 ).
CAEBV-T/NK is a systemic EBV + polyclonal, oligoclonal, or often monoclonal T-cell or NK-cell lymphoproliferative disorder
High viral load in peripheral blood or tissues
Intermittent or persistent infectious mononucleosis–like symptoms such as fever, lymphadenopathy, and hepatosplenomegaly for at least 3 months
No known immunodeficiency
Prevalent in Asia and Latin America
Most patients are children or young adults (median age, 11.3 years; range, 9 months to 53 years)
Often accompanied by hydroa vacciniforme or severe mosquito bite allergy
High antibody titers against EBV VCA IgG and early antigen IgG
Poor prognostic factors: late onset of disease (older than 8 years), thrombocytopenia, EBV infection in T cells
Cause of death: hemophagocytic syndrome, multiple organ failure, T-cell or NK-cell malignancy
Polymorphic infiltrates of inflammatory cells with granuloma and focal necrosis
No significant atypia in infiltrating lymphocytes
Sinusoidal infiltration by small lymphocytes without atypia in liver
EBV found mainly in T cells and NK cells
EBV terminal repeat: polyclonal, oligoclonal, or monoclonal
T-cell receptor gene rearrangement: polyclonal, oligoclonal, or often monoclonal
CAEBV, Chronic active EBV infection; EBV, Epstein-Barr virus; Ig, immunoglobulin; NK, natural killer; VCA, viral capsid antigen.
CAEBV-T/NK has a strong racial predisposition, with most cases reported from Asia (including Japan, Korea, and China ) and Latin America. It occurs rarely in whites and blacks.
Primary EBV infection in childhood may cause infectious mononucleosis in adolescence and young adulthood. In the primary infection, which is normally controlled by an EBV-specific cytotoxic T-lymphocyte (CTL) response, EBV infects B cells via the cell surface receptor CD21.
Abnormal activation and replication of EBV, together with the proliferation and clonal expansion of infected cells, play a key role in the pathogenesis of CAEBV-T/NK. In this condition, unlike classic infectious mononucleosis, T cells or NK cells are the main targets of EBV and proliferate to involve multiple organ systems. T cells and NK cells usually lack the EBV receptor CD21, but EBV-infected T cells and NK cells are occasionally observed in pharyngeal tonsils obtained from patients with infectious mononucleosis. Normal peripheral T cells express CD21 at low levels, and NK cells can acquire CD21 by synaptic transfer from B cells, allowing EBV to bind to T cell or NK cells during the primary infection. Most patients with CAEBV-T/NK have no consistent immunologic abnormality, although both reduced NK activity and impaired EBV-specific CTL activity have been reported in some patients with CAEBV-T/NK. Other reports suggested low numbers of cytomegalovirus-specific CTLs, and an overall T-cell dysfunction has also been observed.
In patients with CAEBV-T/NK, EBV-infected T cells or NK cells express a limited number of EBV-related antigens, including EBNA1, latent membrane protein-1 (LMP-1), and LMP-2, but not EBNA2, 3A, 3B, or LP. Interestingly, EBNA1 and LMP-1 are less antigenic than other EBNA proteins. These findings suggest that EBV-infected T cells and NK cells evade the immune system through decreased antigen presentation and possibly other immunomodulatory factors. The host factors that allow the development of CAEBV-T/NK are not clear, but the strong racial predisposition in CAEBV-T/NK and related diseases suggests that genetic polymorphisms in genes related to the immune response are likely responsible for the development of this disease.
CAEBV-T/NK is almost always accompanied by varying degrees of lymphoproliferation. Proliferation and transformation of EBV-infected T cells or NK cells depend on the EBV factors and host immunity.
CAEBV-T/NK is a disease of children, but it is also detected in young adults and, more rarely, in middle-age and older adults (see Box 30-3 ). The mean age of disease onset is 11.3 years, with a range of 9 months to 53 years, and the male-to-female ratio is 1 : 1. The symptoms generally consist of prolonged or intermittent fever (93% of patients), hepatomegaly (79%), splenomegaly (73%), thrombocytopenia (45%), anemia (44%), and lymphadenopathy (40%). Cutaneous manifestations are common and include severe mosquito bite allergy (33%), rash (26%), and HV (10%). Life-threatening complications include hemophagocytic syndrome, interstitial pneumonia, malignant lymphoma, coronary aneurysm, central nervous system involvement, and bowel perforation. Most patients have high antibody titers of EBV VCA IgG and early antigen IgG, and they often have IgA antibodies against VCA and early antigen. All patients have elevated levels of EBV DNA in their blood, which is well correlated with clinical severity.
In general, patients with CAEBV-T/NK do not exhibit changes suggestive of a neoplastic lymphoproliferative process in affected tissues ( Fig. 30-2 ). The lymph nodes show variable histologic changes with paracortical hyperplasia, a polymorphic and polyclonal lymphoid proliferation, large numbers of EBV-encoded RNA (EBER)-positive cells, and infiltration with many other inflammatory cells, including plasma cells and histiocytes. Granulomas associated with necrosis may be present. The liver shows portal or sinusoidal infiltration by small lymphocytes without atypia. In cases complicated by hemophagocytic syndrome, histiocytic hyperplasia with erythrophagocytosis can be seen in the bone marrow, liver, and skin. In cases with a monoclonal CAEBV-T/NK, the infiltrating cells tend to have slight cytologic atypia and include a higher proportion of EBV-positive cells.
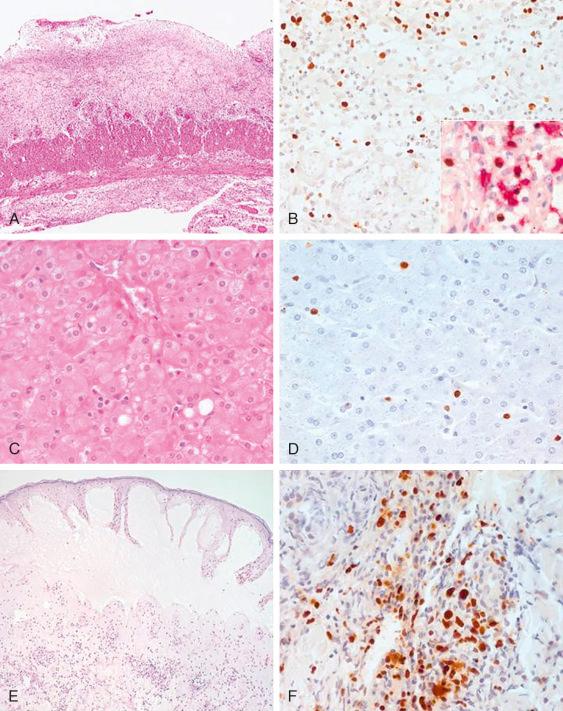
The immunophenotype of the EBV-positive cells infiltrating the tissue and circulating in the peripheral blood varies from case to case and includes alpha-beta T cells, gamma-delta T cells, CD4-positive T cells, CD8-positive T cells, NK cells, or mixtures of these cells ( Fig. 30-2 ). Many cells express cytotoxic molecules such as perforin, TIA-1, and granzyme B. Rarely, EBV-infected B cells are also present
EBV is polyclonal, oligoclonal, or monoclonal, as confirmed by terminal repeat analysis. T-cell receptor gene rearrangement is also polyclonal, oligoclonal, or monoclonal. No specific chromosomal abnormality has consistently been shown in CAEBV-T/NK to date, but cases with progression to monoclonal T- or NK-cell lymphoproliferative disorders show complex chromosomal aberrations. Mutation of the perforin gene was reported in one case.
The proliferating cells in cases of CAEBV frequently lack histologic evidence of malignancy and can be polyclonal, oligoclonal, or monoclonal according to the stage of transformation. Ohshima and colleagues proposed a three-tier classification for CAEBV diseases. Category A1 is polymorphic lymphoproliferative disorder with polyclonal proliferation of EBV-infected T cells or NK cells. Category A2 is polymorphic lymphoproliferative disorder with proliferation of monoclonal T cells or NK cells. Category A3 is monomorphic lymphoproliferative disorder of monoclonal T cells or NK cells. According to the 2008 WHO classification, category A3 would be classified as EBV-positive systemic T-cell lymphoma, extranodal NK/T-cell lymphoma, or aggressive NK-cell leukemia.
The postulated cells of origin are cytotoxic T cells or NK cells.
The prognosis of CAEBV-T/NK is variable. Some patients experience an indolent clinical course, but many patients die of the disease. The process may evolve from a polyclonal to a monoclonal proliferation of T cells or NK cells and eventually progress to overt lymphoid malignancy. The main causes of death are hemophagocytic syndrome, multiple organ failure, and T-cell or NK-cell malignancy. The median survival is 78 months. Patients with a late onset of CAEBV-T/NK (older than 8 years), thrombocytopenia, and T-cell infection have poorer outcomes. The monoclonality of EBV alone does not correlate with an increased risk for mortality. Patients with T-cell CAEBV often present with high fever, lymphadenopathy, hepatosplenomegaly, and high titers of EBV-specific antibodies, and they experience rapid disease progression. Patients with NK-cell disease, in contrast, often have hypersensitivity to mosquito bites, rash, and high levels of IgE but do not necessarily have elevated EBV-specific antibody titers. The 5-year survival rate of patients with T-cell CAEBV is 59%, whereas that for NK-cell disease is 87%. Hodgkin's lymphoma–like lymphoproliferative disease can occur rarely.
Because the infiltrating cells in CAEBV-T/NK are not atypical, it is easy to overlook the diagnosis. In situ hybridization for EBER is a valuable tool for recognizing the disease in the appropriate clinical setting. Systemic EBV-positive T-cell lymphoma or other EBV-positive T-cell and NK-cell lymphomas must be distinguished from CAEBV-T/NK. Systemic EBV-positive T-cell lymphoma is an acute and fulminant disease. CAEBV T/NK with monoclonal population is usually a protracted disease in which patients are diagnosed after months and years of EBV-related symptoms. Clinical history is important for the differential diagnosis ( Box 30-4 ).
In situ hybridization for EBER
Immunostains for CD3, CD20, CD4, CD8, and CD56
Immunostains for cytotoxic markers: TIA-1, granzyme B, perforin
Viral load in peripheral blood
EBV antibody titers
EBV terminal repeat analysis
T-cell receptor gene rearrangement
EBER, EBV-encoded RNA; EBV, Epstein-Barr virus; NK, natural killer.
Severe mosquito bite allergy is a cutaneous manifestation of chronic EBV infection characterized by intense local skin symptoms, including erythema, bullae, ulcers, and scar formation, and by systemic symptoms such as fever, lymphadenopathy, and liver dysfunction after mosquito bites, vaccination, or injection. It has a close association with CAEBV-T/NK and aggressive NK-cell leukemia occurring in children ( Box 30-5 ).
Cutaneous manifestation of chronic EBV infection activated by mosquito bite or injection
Intense local skin symptoms, including erythema, bulla, ulcer, and scar formation, associated with systemic symptoms such as fever, lymphadenopathy, and liver dysfunction after mosquito bites
Most patients are in the first two decades of life, with a median age of 6.7 years
Often high EBV load and NK-cell lymphocytosis
Variable clinical course; some patients may develop hydroa vacciniforme, systemic symptoms of CAEBV, or NK-cell lymphoma/leukemia
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here