Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
American Academy of Pediatrics
Advisory Committee on Immunization Practices
alanine aminotransferase
Center for Disease Control and Prevention
cytomegalovirus
Epstein-Barr virus
enzyme-linked immunosorbent assay
ELISA-linked units
histologic activity index
hepatitis A virus
hepatitis B e antigen
hepatitis B surface antigen
hepatitis B virus
hepatocellular carcinoma
hepatitis C virus
hepatitis D virus
hepatitis E virus
human herpes virus 6
human immunodeficiency virus
interferon-alpha
immunoglobulin
immunoglobulin M
liver-kidney microsomal antibody type 1
polymerase chain reaction
Studies of Pediatric Liver Transplantation
viral capsid antigen
Many viruses can infect the liver during childhood. Some, such as hepatitis A and B viruses, are largely hepatotropic, whereas others, such as adenovirus, can cause multisystem disease. Some, such as herpes viruses 1 and 2, only cause acute infection whereas others, such as hepatitis B virus, can cause acute hepatitis, with or without liver failure, or result in chronic infection. Some may cause very mild or asymptomatic disease such as cytomegalovirus (CMV), and some can cause acute liver failure, such as parvovirus B19. Finding evidence of viral infection in a patient with “hepatitis,” meaning elevation of serum aminotransferases, does not necessarily prove that the hepatitis is related causally to the viral infection.
Similarly, the presence of elevated serum aminotransferases in a child does not necessarily indicate viral infection of the liver. There is a long list of differential diagnoses depending on the age of the child, whether the child is cholestatic or not, and whether or not extrahepatic symptoms are present. These conditions include m etabolic, autoimmune, genetic, and nonviral infections, such as those secondary to bacteria, fungi, or parasites. Cholangiopathies, such as biliary atresia, sclerosing cholangitis, or autoimmune cholangitis, are usually accompanied by elevation of serum aminotransferases. Drug-induced liver disease is increasingly diagnosed in children. As many as one quarter of children with gluten-sensitive enteropathy may exhibit elevated aminotransferases. Furthermore, a child with marked elevations of serum aminotransferases may have suffered nonaccidental trauma from child abuse and abdominal battering. Such children often have very high elevation of serum aminotransferases, up to 20,000 IU/L with prompt return to normal values over just a few days, a pattern which is not typical of viral hepatitis. Myopathies such as Duchenne muscular dystrophy are often accompanied by elevation of serum aminotransferases of muscular origin. In such children, serum creatine phosphokinase is usually dramatically elevated, whereas in viral hepatitis it is in the normal range. Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease have now become the most common liver diseases of children. Thus, when an obese child presents with clinical signs and symptoms of an infection such as fever, accompanied by elevation of serum aminotransferases, it may be a challenge to determine if the patient has viral hepatitis or underlying NASH, or both.
This chapter on viral hepatitis in children is organized according to the major clinical presentations of viral infections of the liver in childhood: acute hepatitis with possible progression to pediatric acute liver failure (PALF) and chronic hepatitis.
Acute hepatitis is a general term indicating an illness arising in a host without previous evidence of liver disease. The incidence of acute viral hepatitis in North America has decreased since the introduction of universal vaccines for hepatitis A and B viruses. The pediatric patient with acute viral hepatitis may present with nonspecific symptoms of a viral infection, including fever, malaise, and gastrointestinal symptoms with or without respiratory symptoms. Initially, these symptoms may be attributed to viral gastroenteritis or respiratory illness. It is only when the illness has lasted more than a few days that physicians tend to order laboratory testing for hepatic enzymes. The detection of viral hepatitis in a child may be more challenging than in an adult because young children with viral hepatitis are often anicteric. When the serum aminotransferases are elevated, viral hepatitis is suspected. If the patient develops signs of cholestasis, generalized icterus, acholic stools, and dark urine, hepatic involvement is obvious. Several viruses known to cause acute hepatitis have also been implicated as causes of pediatric acute liver failure (PALF). The Pediatric Acute Liver Failure Study Group, recognizing the difficulty of diagnosing encephalopathy in a frightened ill young child, has defined PALF as acute liver injury combined with either severe coagulopathy (International Normalized Ratio [INR] > 2.0 or prothrombin time [PT] > 20 seconds) or encephalopathy with moderate coagulopathy (INR ≥ 1.5 or PT ≥ 15 seconds).
In the developing world, viral infections, particularly hepatitis A, B and E, are the most common causes of PALF. PALF secondary to viral hepatitis in the developing world is associated with mortality rates of 54% to 85%. The Pediatric Acute Liver Failure Study Group recently studied the role of viruses in a group of 860 North American children with nonacetaminophen-induced liver failure. Twenty percent (166 of 820) had one or more positive tests for acute viral infection. The authors considered six viruses to be “causative viruses” (CV)—in other words, viruses which hepatologists and infectious disease specialists agreed were capable of causing PALF. These viruses included hepatitis A virus (HAV), hepatitis B virus (HBV) when HBV DNA was detected, herpes virus 1 or 2, adenovirus, parvovirus B19, and, in children less than 7 months of age, enterovirus. A CV was identified in 81 of 762 (10.6%) participants, whereas an “associated virus” (AV) was identified in 99 of 795 (12.5%) participants. An AV is a virus for which there was agreement amongst the aforementioned specialists that a positive test for the presence of the virus did not generally indicate that the PALF was secondary to infection of the host with that virus. Those viruses included HBV (when HBsAg/HBeAg were positive but HBV DNA was negative), hepatitis C virus (HCV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human immunodeficiency virus (HIV), and human herpes virus 6 (HHV6). The CVs and AVs of children enrolled in the PALF registry and the diagnostic tests for these viral infections are shown in Table 65-1 . The results of testing for CVs are shown in Table 65-2 and for AVs in Table 65-3 . Testing for viral infections and other etiologies of PALF were investigator-dependent so there were many subjects in whom testing for either CVs or AVs was not performed. Approximately one quarter of infants less than 7 months of age were positive for herpes simplex viruses 1 and 2 (HSV), or enterovirus. HSV was the most frequently diagnosed CV being found in 11.6% (39 of 235 tested). However, the majority of the group was not tested for this treatable viral infection. The three most frequently identified AVs relative to the diagnostic tests performed were HHV6 (14.3 %), EBV (7.8 %), and CMV (5.0%). Overall, only approximately 10% of the children were diagnosed by the investigators as having a viral cause of PALF despite the fact that approximately twice that many had objective evidence of an acute viral infection. The authors concluded that children with nonacetaminophen PALF should be aggressively tested for CVs, particularly HSV, which is treatable, and that a systemic search for AVs might also lead to improved understanding of the role of viral triggers in this life-threatening liver disease of childhood.
| Clinical Features | Acute or Chronic | Diagnostic Tests | |
|---|---|---|---|
| Causative of PALF | |||
| Adenovirus | Respiratory, GI | A | Adenovirus DNA |
| Enterovirus (EV) | GI, ALF only < 7 months | A | EV RNA or EV IgM antibody (age 0-6 months old) |
| Hepatitis A virus (HAV) | Jaundice except in neonates, gastrointestinal symptoms | A | HAV IgM |
| Hepatitis B virus (HBV) | In neonates born to HBeAg negative mothers | A, C | HBV anticore IgM, HBV DNA |
| Herpes simplex virus (HSV) 1 or 2 | Rapidly progressive in the neonate with “blueberry muffin” rash, but occurs at all ages; treatable with acyclovir | A | HSV IgM antibody, HSV DNA, HSV culture from blood, tracheal or nasopharyngeal aspirate, liver tissue, and/or cerebrospinal fluid |
| Parvovirus (PV) B19 | Aplastic anemia | A | PV IgM antibody or PV DNA |
| Associated With PALF | |||
| Cytomegalovirus (CMV) | Asymptomatic | A, C | CMV DNA, CMV IgM antibody, or CMV culture from liver tissue and/or tracheal aspirate |
| Epstein-Barr virus (EBV) | Lymphadenopathy, splenomegaly | A, C | EBV IgM, EBV DNA |
| Hepatitis B (HBV) | Asymptomatic | A, C | HBsAg, HBeAg |
| Hepatitis C (HCV) | Asymptomatic | C | HCV RNA |
| Human herpes virus (HHV) 6 | High fever for several days, followed by self-limited dermatologic condition | A, C | HHV6 DNA from blood or liver |
| Human immunodeficiency virus 9 (HIV-9) | Immune deficiency | A, C | HIV IgM antibody |
| Viral Pathogens | PREVALENCE OF TESTING AND RESULTS OBTAINED | ||
|---|---|---|---|
| Total ( N = 860) | < 7 Months Old ( N = 220) | ≥ 7 Months Old ( N = 640) | |
| n (%) | n (%) | n (%) | |
| HAV | |||
| Not tested | 250 | 111 | 139 |
| Negative | 595 (97.5) | 106 (97.3) | 489 (97.6) |
| Positive | 15 (2.5) | 3 (2.8) | 12 (2.4) |
| HBV | |||
| Not tested | 578 | 173 | 405 |
| Negative | 279 (98.9) | 47 (100.0) | 232 (98.7) |
| Positive | 3 (1.1) | 0 (0.0) | 3 (1.3) |
| HSV | |||
| Not tested | 525 | 117 | 408 |
| Negative | 296 (88.4) | 77 (74.8) | 219 (94.4) |
| Positive | 39 (11.6) | 26 (25.2) | 13 (5.6) |
| Adenovirus | |||
| Not tested | 661 | 175 | 485 |
| Negative | 191 (96.0) | 44 (97.8) | 147 (95.5) |
| Positive | 8 (4.0) | 1 (2.2) | 7 (4.6) |
| Parvovirus | |||
| Not tested | 600 | 177 | 423 |
| Negative | 248 (95.4) | 42 (97.7) | 206 (94.9) |
| Positive | 12 (4.6) | 1 (2.3) | 11 (5.1) |
| Enterovirus * | |||
| Not tested | 189 | ||
| Negative | 24 (77.4) | ||
| Positive | 7 (22.6) | ||
* Note: Enterovirus was only identified among participants less than 7 months old.
| Viral Pathogen | PREVALENCE OF TESTING AND RESULTS OBTAINED | ||
|---|---|---|---|
| Total ( N = 860) | < 7 Months Old ( N = 220) | ≥ 7 Months Old ( N = 640) | |
| n (%) | n (%) | n (%) | |
| HBV | |||
| Not tested | 197 | 80 | 117 |
| Negative | 656 (98.9) | 140 (100.0) | 516 (98.7) |
| Positive | 7 (1.1) | 0 (0.0) | 7 (1.3) |
| HCV | |||
| Not tested | 768 | 211 | 557 |
| Negative | 91 (98.9) | 9 (100.0) | 82 (98.8) |
| Positive | 1 (1.1) | 0 (0.0) | 1 (1.2) |
| CMV | |||
| Not tested | 218 | 73 | 145 |
| Negative | 610 (95.0) | 138 (93.9) | 472 (95.4) |
| Positive | 32 (5.0) | 9 (6.1) | 23 (4.7) |
| EBV | |||
| Not tested | 297 | 120 | 177 |
| Negative | 519 (92.2) | 96 (96.0) | 423 (91.4) |
| Positive | 44 (7.8) | 4 (4.0) | 40 (8.6) |
| HHV6 | |||
| Not tested | 692 | 193 | 499 |
| Negative | 144 (85.7) | 26 (96.3) | 118 (83.7) |
| Positive | 24 (14.3) | 1 (3.7) | 23 (16.3) |
| HIV | |||
| Not tested | 417 | 143 | 274 |
| Negative | 440 (99.3) | 77 (100.0) | 363 (99.2) |
| Positive | 3 (0.7) | 0 (0.0) | 3 (0.8) |
The diagnostic tests for these agents are described in Table 65-1 and possible treatments are shown in Table 65-4 .
| Virus | Agent | Comments |
|---|---|---|
| Adenovirus | Cidofovir (not FDA-approved for adenovirus but the expert's choice) | Usually used only in immunosuppressed individuals |
| Cytomegalovirus | Gancyclovir, valgancyclovir Broad spectrum T cells in the immunosuppressed host |
For use after reactivation after hematopoietic stem cell transplant |
| Epstein-Barr virus | Broad spectrum T cells | For use after reactivation after hematopoietic stem cell transplant |
| Enterovirus | Intravenous Immune Globulin | Effective for severe neonatal infection when given within 3 days of symptom onset |
| Hepatitis A virus | None | |
| Hepatitis E virus | Ribavirin | May be effective in solid-organ transplant recipients if reduction of immunosuppression is ineffective |
| Human herpes virus 6 | Broad spectrum T cells | |
| Parvovirus B19 | Intravenous immune globulin | May be effective in some cases but have a paradoxical exacerbating effect in others |
| Varicella-zoster | Acyclovir |
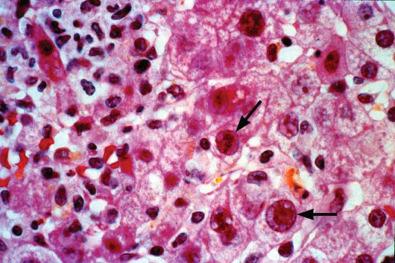
Adenovirus (AD) is a ubiquitous DNA virus which most often causes mild multisystem disease with a predilection towards the lung. Children who are immunocompromised are particularly vulnerable. On rare occasions AD can cause PALF, most often in immunosuppressed individuals such as bone marrow transplant recipients. Fig. 65-1 demonstrates characteristic hepatic histopathology with the arrows indicating intranuclear inclusion bodies. Cidofovir is considered the drug of choice for severe AD infections, but not all patients require treatment.
Congenital cytomegalovirus (CMV) infection is often asymptomatic, but a minority of infected newborns will develop hepatitis, hepatosplenomegaly, conjugated hyperbilirubinemia, and/or thrombocytopenia and neurologic involvement. The diagnosis of CMV infection may be made by liver biopsy, urine CMV culture, or IgM antibody to CMV. CMV does not cause chronic infection in immunocompetent children. Acute CMV hepatitis is usually not a disorder of healthy children or adolescents, but a common disease in immunocompromised hosts. Treatment with ganciclovir or valganciclovir is indicated.
Enteroviruses such as coxsackie B virus or echovirus 11 may cause PALF in infants less than 7 months of age, as well as myocarditis and meningoencephalitis. The infectious signs and symptoms in older children are usually much milder and gastrointestinal symptoms predominate. The age-related difference in pathogenesis may be related to the age-related decline in the coxsackie virus-adenovirus receptor. Antibodies to this receptor have been shown to abrogate the severe hepatitis in mice infected with coxsackie B virus. Early intravenous immunoglobulin (IVIG) therapy may be effective for severe neonatal infection when given within 3 days of symptom onset.
Clinical symptoms of infectious mononucleosis caused by Epstein-Barr virus (EBV) infection include fever, pharyngitis, lymphadenopathy, splenomegaly, and hepatitis with jaundice. Abnormal aminotransferases are present in up to 80% of patients. Acute liver failure and EBV-associated hemophagocytic syndrome are rare complications. Serum IgM to the EB viral capsid antigen (VCA) and/or EBV DNA by polymerase chain reaction (PCR) are the diagnostic tests of choice. Hepatitis associated with infectious mononucleosis usually resolves spontaneously and fully over 2 to 3 weeks.
Hepatitis A virus (HAV) is an RNA virus which causes acute hepatitis and, rarely, acute liver failure in children and young adults. The infection is spread by the fecal-oral route. It has an incubation period of 15 to 40 days followed by an abrupt onset of symptoms, often gastrointestinal in nature. Anorexia is severe. Seldom, the infected child may suffer from arthritis, arthralgias, or skin rash. The illness usually lasts weeks and, in the rare cases of relapsing HAV, the symptoms can last months. However, the infection is always self-limited. Postinfectious immunity is life-long. Postexposure prophylaxis is with the active vaccine and passive (immune serum globulin) immunization. Children with HAV can be completely asymptomatic and thus serve as an infectious reservoir. HAV is a vaccine preventable disease and the American Academy of Pediatrics (AAP) recommends HAV vaccine at 12 to 23 months of age. There are two vaccines approved for use in children: Havrix (Glaxo Smith Kline) and Vaqta (Merck). Twinrix (Glaxo Smith Kline) vaccine is approved for age 18 years and older and protects recipients against both HAV and HBV. Other indications for HAV vaccine include catch-up immunization of unimmunized children 2 to 18 years of age, travelers to endemic areas, close contacts of newly arrived international adoptees, patients with chronic liver disease or clotting factor disorders, and users of injections and illicit drugs. Preexposure prophylaxis for travelers to endemic areas is via immune serum globulin for infants younger than 12 months of age and active vaccine for children older than 12 months of age, providing the immunization is given 1 month or more before travel. Household and other close contacts should receive immunoprophylaxis (active vaccine if within 8 days of exposure or passive immune serum globulin within 2 weeks of exposure). Fecal shedding usually decreases rapidly after the onset of jaundice and for this reason the child may return to school soon after becoming jaundiced, provided he or she feels well. There is no specific treatment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here