Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ventilatory failure occurs when alveolar ventilation becomes too low to maintain normal arterial blood gas partial pressures.
There are many causes, involving the respiratory centre, the respiratory muscles or their nerve supply and abnormalities of the chest wall, lung or airways.
Modest increases in the inspired oxygen concentration will correct hypoxia because of ventilatory failure but may worsen hypercapnia.
Respiratory failure is defined as a failure of maintenance of normal arterial blood gas partial pressures. Hypoxia as a result of cardiac and other extrapulmonary forms of shunting are excluded from this definition. Respiratory failure may be subdivided according to whether the arterial P co 2 is normal or low (type 1) or elevated (type 2). The mean normal arterial P co 2 is 5.1 kPa (38.3 mmHg), with 95% limits (2 standard deviations [SD]) of ± 1.0 kPa (7.5 mmHg). The normal arterial P o 2 is more difficult to define because it decreases with age (page 148) and is strongly influenced by the concentration of oxygen in the inspired gas. Mechanisms that contribute to respiratory failure include ventilatory failure (reduced alveolar ventilation) and venous admixture as a result of either pure intrapulmonary shunt or ventilation perfusion mismatch ( Chapter 7 ).
Ventilatory failure is defined as a pathological reduction of the alveolar ventilation below the level required for the maintenance of normal alveolar gas partial pressures. Because arterial P o 2 (unlike arterial P co 2 ) is so strongly influenced by shunting, the adequacy of ventilation is conveniently defined by the arterial P co 2 , although it is also reflected in end-expiratory P co 2 and P o 2 . This chapter is concerned mainly with pure ventilatory failure; other causes of respiratory failure are described in Chapter 28 , Chapter 29 , Chapter 30 , Chapter 31 .
Figure 27.1 shows, on a P o 2 / P co 2 diagram, the typical patterns of deterioration of arterial blood gases in respiratory failure. The pale blue area indicates the normal range of values with increasing age corresponding to a leftward shift. Pure ventilatory failure in a young person with otherwise normal lungs would result in changes along the broken line. Chronic obstructive pulmonary disease (COPD), the most common cause of predominantly ventilatory failure, occurs in older people, and the observed pattern of change is shown within the upper orange arrow in Figure 27.1 . The limit of survival, while breathing air, is reached at a P o 2 of about 2.7 kPa (20 mmHg) and P co 2 of 11 kPa (83 mmHg). The limiting factor is not P co 2 but P o 2 . This prevents the rise of P co 2 to higher levels, except when the patient’s inspired oxygen concentration is increased. It may also be raised above 11 kPa by the inhalation of carbon dioxide. In either event, a P co 2 in excess of 11 kPa may be considered an iatrogenic disorder. The green arrow in Figure 27.1 shows the pattern of blood gas changes caused by shunting or pulmonary venous admixture ( Chapter 7 ).

In general, the arterial P o 2 indicates the severity of respiratory failure (assuming that the patient is breathing air), whereas the P co 2 indicates the differential diagnosis between ventilatory failure and shunting, as shown in Figure 27.1 . In respiratory disease it is, of course, common for ventilatory failure and shunting to coexist, and the relative contribution of each mechanism will determine whether type 1 or 2 respiratory failure develops.
Although the upper arrow in Figure 27.1 shows the effect of established ventilatory failure on arterial blood gases, short-term deviations from this pattern occur in acute ventilatory failure. This is because the time courses of changes of P o 2 and P co 2 in response to acute changes in ventilation are quite different.
Body stores of oxygen are small, amounting to about 1550 mL while breathing air. Therefore, following a step change in the level of alveolar ventilation, the alveolar and arterial P o 2 rapidly reach the new value and the half-time for the change is only 30 seconds (see page 157 and Fig. 10.18 ). In contrast, the body stores of carbon dioxide are very large: of the order of 120 L. Therefore, following a step change in the level of alveolar ventilation, the alveolar and arterial P co 2 only slowly attain the value determined by the new alveolar ventilation. Furthermore, the time course is slower following a reduction of ventilation than an increase (see Fig. 9.11 ), and the half-time of rise of P co 2 following a step reduction of ventilation is of the order of 16 minutes.
The practical point is that, during the early phase of acute hypoventilation, there may be a low P o 2 while the P co 2 is increasing but is still within the normal range. Thus the pulse oximeter may, under certain circumstances such as when breathing air, give an earlier warning of hypoventilation than the capnograph. This breaks the rule that the P co 2 is the essential index of alveolar ventilation, and it may be erroneously believed that the diagnosis is shunting rather than hypoventilation.
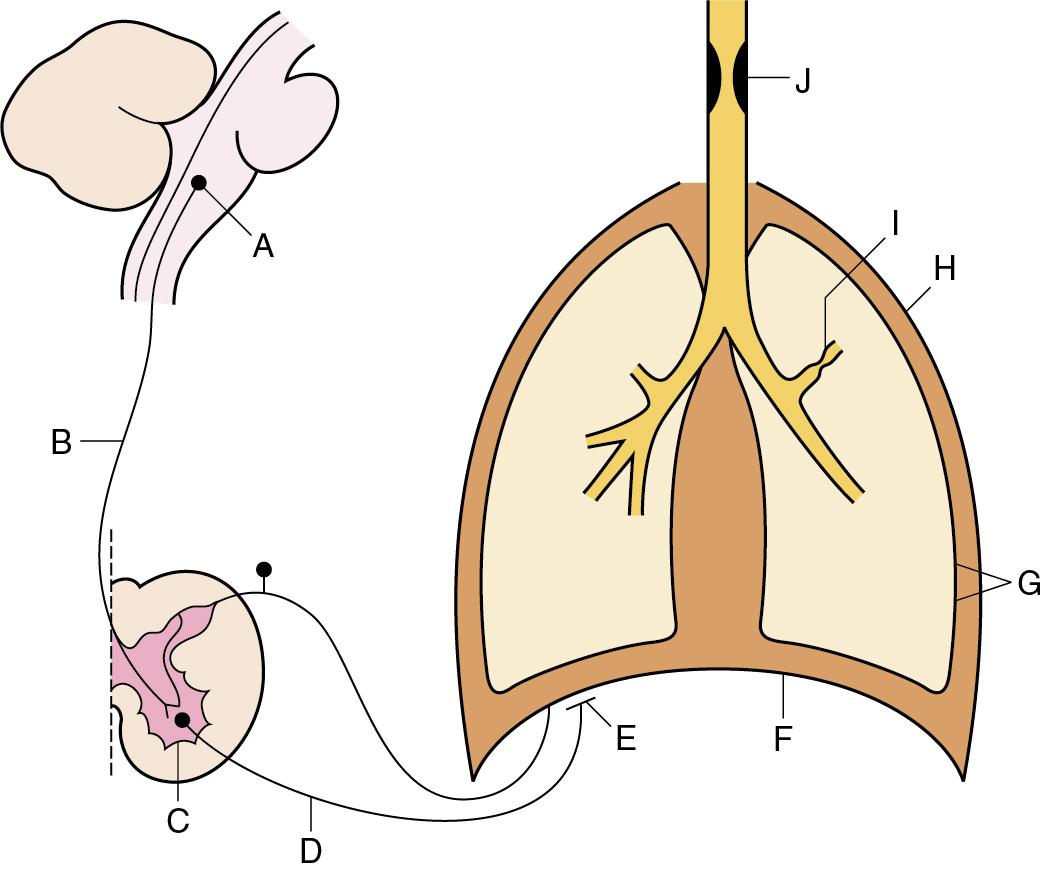
The causes of ventilatory failure may be conveniently considered under the headings of the anatomical sites where they arise. These sites are indicated in Figure 27.2 . Lesions or malfunctions at sites A to E result in a reduction of input to the respiratory muscles. Dyspnoea may not be apparent, and the diagnosis of ventilatory failure may be easily overlooked. Lesions or malfunctions at sites G to J result in evident dyspnoea, and no one is likely to miss the diagnosis of hypoventilation. The various sites will now be considered individually.
The respiratory neurones of the medulla are depressed by hypoxia and also by very high levels of P co 2 , probably of the order of 40 kPa (300 mmHg), but at a lower P co 2 in the presence of some drugs (see later). Reduction of P co 2 below the apnoeic threshold results in apnoea in the unconscious subject, but usually not in the conscious subject. Loss of respiratory sensitivity to carbon dioxide occurs in various types of long-term ventilatory failure, particularly COPD, and this is discussed further on page 333. A wide variety of drugs may cause central apnoea or respiratory depression (page 55), including opioids, barbiturates and most anaesthetic agents, whether intravenous or inhalational. The respiratory neurones may also be affected by a variety of neurological conditions such as raised intracranial pressure, stroke, trauma or neoplasm.
The upper motoneurones serving the respiratory muscles are most likely to be interrupted by trauma. Only complete lesions above the third or fourth cervical vertebrae will affect the phrenic nerve and result in total apnoea. However, fracture dislocations of the lower cervical vertebrae are relatively common, and result in loss of action of the intercostal and expiratory muscles, while sparing the diaphragm. Upper motoneurones may be involved in various disease processes, including tumours, demyelination and, occasionally, syringomyelia.
The anterior horn cell may be affected by various diseases, of which the most important is poliomyelitis. Fortunately this condition is now rare in the developed world, but it can produce any degree of respiratory involvement up to total paralysis of all respiratory muscles.
Lower motoneurones supplying the respiratory muscles are prone to normal traumatic risks, and in former times the phrenic nerves were surgically interrupted for the treatment of pulmonary tuberculosis. Today the most common causes of phrenic nerve damage are iatrogenic injury following surgery in the chest or compression by intrathoracic tumours. The later stages of motoneurone disease may cause ventilatory failure at this level. Idiopathic polyneuritis (Guillain–Barré syndrome) remains a relatively common neurological cause of ventilatory failure. The syndrome results from an immune-mediated aetiology and is characterized by a rapidly ascending motor nerve paralysis, which in 20% to 30% of patients progresses to quadriplegia and respiratory muscle paralysis. With modern ventilatory support and immunotherapy around 75% of sufferers make a complete neurological recovery, but unfortunately 3% to 7% of patients still die of the condition or its complications.
Neuromuscular junction function is impaired by several causes, including botulism, neuromuscular blocking drugs used in anaesthesia, organophosphorus compounds and nerve gases. However, myasthenia gravis is by far the most common cause of ventilatory failure at this site; marked respiratory muscle weakness occurs in 15% to 20% of cases. Myasthenia gravis is an autoimmune disease in which the acetylcholine receptors on the neuromuscular junction are destroyed, leading to progressive weakness. Administration of an anticholinesterase drug such as edrophonium increases acetylcholine concentration at the neuromuscular junction and causes an immediate improvement in symptoms. Plasma exchange, intravenous immunoglobulins or thymectomy are effective current therapies.
The respiratory muscles are rarely entirely responsible for ventilatory failure, but they often contribute to reduced alveolar ventilation in a variety of respiratory diseases. For example, the efficiency of contraction of the respiratory muscles is severely impaired by the hyperinflation that normally accompanies COPD. In these patients, although the curvature of the diaphragm may remain normal, the zone of apposition is reduced (see Figs 5.1 and 5.2 ), and the resultant shortening of diaphragmatic muscle fibres significantly impairs their function. Unilateral diaphragmatic paralysis is usually asymptomatic, but bilateral paralysis leads to significant dyspnoea, particularly when supine, when the diaphragmatic contribution to breathing is greater (page 64). The respiratory muscles may also become fatigued as a result of working against excessive impedance, but this is not thought to occur until very late in the course of most acute respiratory problems. Patients who require critical care commonly develop a polyneuropathy or myopathy of the respiratory muscles, particularly if sepsis is the underlying cause of their multiorgan failure. Activation of cytokines and malnutrition are believed to be contributing mechanisms. Furthermore, following a long period of artificial ventilation, respiratory muscles develop ‘disuse atrophy’. These factors all make weaning from ventilation difficult (page 385). Cardiac failure may result in respiratory muscle weakness because of reduced blood supply, often coupled with low-compliance lungs because of pulmonary oedema ( Chapter 29 ).
Assessment of respiratory muscle strength is described on page 71.
Loss of elasticity of the lungs or chest wall is a potent cause of ventilatory failure. It may arise within the lungs (e.g., pulmonary fibrosis or acute lung injury), in the pleura (e.g., empyema; page 363), in the chest wall (e.g., kyphoscoliosis) or in the skin (e.g., contracted burn scars in children). It is frequently forgotten that seemingly mild pressures applied to the outside of the chest may seriously embarrass the breathing and even result in total apnoea. A sustained pressure of only 6 kPa (45 mmHg or a depth of 2 ft of water) is sufficient to prevent breathing. This can occur when crowds get out of control and people fall on top of one another, or when either children or adults become accidentally buried under sand or other heavy materials.
Loss of structural integrity of the chest wall may result in ventilatory failure, for example, from multiple fractured ribs. A condition known as flail chest arises when multiple ribs are broken in two places, allowing the middle, ‘flail’, rib section to move independently of the anterior and posterior ‘fixed’ sections. Movement of the flail segment is then determined by changes in intrathoracic pressure; with spontaneous breathing, a paradoxical respiratory movement of the flail segment develops, which if large enough will compromise tidal volume. Flail chest may need to be treated by artificial ventilation, although conservative treatment with good analgesia, sometimes assisted by rib fixation, is becoming more common.
Closed pneumothorax causes interference with ventilation in proportion to the quantity of air in the chest and is described on page 361.
Small airway resistance remains the commonest and most important cause of ventilatory failure. The physiology of diseases affecting airway resistance is described in Chapter 28 , and will not be further discussed here. However, the relationship between airway resistance and ventilatory failure is a complex subject, which is considered later. In the clinical field, airway resistance is less frequently measured, but is most often inferred from measurement of ventilatory capacity.
Upper airway obstruction occurs in a wide range of conditions such as airway and pharyngeal tumours, upper respiratory tract infections, inhaled foreign bodies and tumour or bleeding in the neck causing external compression of the airway. Stridor is common and should quickly alert the clinician to the cause of respiratory distress. A smaller airway diameter in babies and children makes them more susceptible than adults to upper airway obstruction, as airway oedema from infections such as croup or epiglottitis quickly causes dramatic stridor. The excellent ability of the respiratory system to overcome increased airway resistance (page 37) is such that ventilatory failure is normally a late development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here