Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
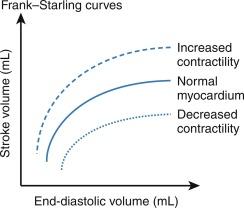
All major components governing the physiology of cardiac output, such as preload, afterload, inotropy, and chronotropy can be modulated by vasoactive agents. An underlying concept is the Frank-Starling principle, which states that increased myocardial fiber length, or preload, improves contractility up to an optimal state, then decreases with excess preload. Vasoactive agents, such as inotropes, can increase contractility, which can be conceptualized as a shift in the Frank-Starling curve ( Fig. 19.1 ). Other agents, such as vasopressors can increase or decrease arterial resistance (afterload) and venous compliance (preload). Other agents can increase or decrease heart rate (chronotropy), which affects cardiac output and coronary perfusion. Recall, blood pressure is the product of cardiac output and resistance, where cardiac output is the product of stroke volume and heart rate, and where stroke volume is determined by contractility, preload, and afterload. The anesthesiologist can modulate each of these physiologic variables using vasoactive agents to optimize the patient’s physiology.
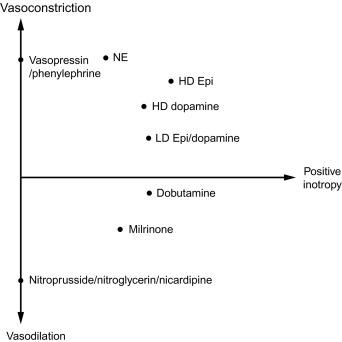
Trick question. It is both. Although some medications (e.g., phenylephrine) are pure vasopressors, several of the medications discussed in this chapter have mixed physiologic effects. Medication that have both vasopressor and inotrope characteristics are referred to as an inopressor (e.g., epinephrine) and agents that have both vasodilating and inotrope characteristics are referred to as an inodilator . See Fig. 19.2 as a general overview illustrating the various characteristics of the medications discussed in this chapter.
All inotropic, vasopressor, and vasodilating medications share one common denominator as a final endpoint: calcium. Both vascular smooth muscle and cardiac muscle require calcium as a cofactor to allow myosin and actin filaments to cross-bridge to facilitate muscle contraction. In the resting state, myosin and actin binding sites are blocked by tropomyosin. When intracellular calcium increases, calcium binds to troponin. Troponin, a small protein attached to tropomyosin, when bound to calcium causes tropomyosin to move away from the actin-myosin binding sites, allowing them to cross bridge and muscle contraction ensues.
Calcium administration can have a profound effect in improving cardiac contractility and increasing systemic vascular resistance in the setting of hypocalcemia. Hypocalcemia frequently occurs in the setting of massive blood transfusion as blood products often contain calcium chelating agents, such as citrate.
Patients with impaired contractility, such as in systolic heart failure may present with excess preload (i.e., hypervolemia) and afterload (i.e., increased systemic vascular resistance), which can decrease stroke volume and cardiac output. Vasodilators, in addition to diuretics, can help in “unloading the heart” by reducing both preload and afterload to a more optimal physiological state, thereby improving stroke volume or forward flow.
Because blood pressure is the product of cardiac output and systemic vascular resistance, inappropriate vasopressor administration to normalize the blood pressure can make the vitals “look good” at the expense of elevated systemic vascular resistance, and decreased cardiac output. Ultimately, the most important goal of the cardiovascular system is to deliver oxygen to tissue and a normal blood pressure itself does not guarantee an adequate cardiac output.
Vasopressors, such as phenylephrine, vasoconstrict both venous vasculature (increase preload) and arterial vasculature (increase afterload) through α 1 mediated vasoconstriction. Healthy patients with normal cardiac contractility generally tolerate the increase in afterload and, depending upon the situation, venoconstriction may improve preload and cardiac output. For most patients undergoing surgery with either general anesthesia or neuraxial anesthesia, phenylephrine is often a good choice to counteract the anesthetic induced vasodilation. However, in patients with decreased contractility, simultaneously increasing preload and afterload with vasopressors can “overload” the heart’s physiological reserve, leading to a decrease in cardiac output, therefore careful administration should be used to strike the appropriate balance.
Milrinone is an inotropic agent, classified as a phosphodiesterase (PDE) inhibitor, which decreases the degradation of cyclic adenosine monophosphate, leading to increased contractility and vasodilation. Right ventricular function, in particular, can be favorably impacted, as milrinone can decrease pulmonary vascular resistance to reduce right heart afterload.
Isoproterenol is a potent nonselective β agonist with no alpha stimulating properties. Isoproterenol administration will increase heart rate and contractility (β 1 ), while also decreasing afterload (β 2 ). Clinically, it is often used for provoking dysrhythmias in electrophysiology procedures and for the treatment of bradycardia in a denervated nonpaced transplanted heart.
Dobutamine acts principally on β-adrenergic receptors (β 1 > β 2 ), causing less vasodilation than isoproterenol. Dobutamine is often used as a first-line agent for cardiogenic shock because β 1 receptor agonism improves contractility and partial β 2 receptor agonism causes arterial vasodilation reducing afterload.
Both milrinone and dobutamine increase cardiac contractility (i.e., positive inotrope) and cause vasodilation (i.e., reduces afterload), which together increases cardiac output. Both are considered first-line agents to treat cardiogenic shock. Although both can cause hypotension and dysrhythmias, hypotension is more evident with milrinone, whereas dysrhythmias tend to occur more often with dobutamine. Dobutamine has a much shorter half-life (2–3 minutes) and is therefore easier to titrate than milrinone, which has a longer half-life (2–3 hours).
The effects of a low-dose epinephrine infusion (e.g., < 0.05 mcg/kg/min) are primarily limited to the stimulation of β 1 and β 2 adrenergic receptors in the heart and skeletal muscle, respectively. This results in positive β 1 -mediated chronotropy (heart rate), dromotropy (conduction velocity), inotropy (contractility), increased automaticity, and β 2 -mediated vasodilation in skeletal muscle. At higher doses (e.g., > 0.05 mcg/kg/min), epinephrine stimulates α 1 -adrenergic receptors. This increases overall systemic vascular resistance because the α 1 -mediated vasoconstriction is greater than the β 2 -mediated vasodilation.
Norepinephrine primarily stimulates α 1 adrenergic receptors and partially stimulates β 1 adrenergic receptors. It has very little β 2 receptor selectivity. This results in α 1 -mediated increased systemic vascular resistance. The partial β 1 -mediated effects help prevent reflex bradycardia that can be seen with pure α 1 agonists (e.g., phenylephrine) and helps preserve cardiac output despite an increase in afterload.
Dopamine, at high doses, is an indirect vasopressor facilitating the release of catecholamines, such as norepinephrine, and at low doses, a direct vasodilator, stimulating specific dopamine receptors in the renal, mesenteric, and coronary arterial beds. These dopaminergic effects occur at low doses (0.5–2.0 mcg/kg/min). At intermediate doses (5–10 mcg/kg/min), β 1 -adrenergic stimulation becomes evident. At higher doses (10–20 mcg/kg/min), α 1 -adrenergic stimulation predominates, overcoming the vasodilating dopaminergic effects, leading to an overall increased systemic vascular resistance. Dopamine has fallen out of favor as a vasoactive agent, as evidence to date shows increased mortality and a higher incidence of dysrhythmias in patients with septic or cardiogenic shock.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here