Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The traditional concept of vasculogenesis in cancer maintains that malignant tumors prefer aerobic metabolism for growth. This theory posits that after continued proliferation, the tumor will “outgrow” its arterial vascular supply; as the resulting hypoxia ensues, the tumor will become dormant. The traditional vasculogenesis theory therefore states that tumors will reinitiate growth only after arteriogenesis occurs, allowing adequate blood flow to restore normoxia.
Unfortunately, tumor pathogenesis is not nearly this straightforward. Were the traditional vasculogenesis theory completely accurate, antiarterial tumor therapies would have profound success rates. Scientific research seems to support a newly proposed theory for tumor vasculogenesis, the ALPHA hypothesis ( A cidic L actate sequentially induces L ymphangiogenesis, PH lebogenesis, and A rteriogenesis). Because the published data exist in diverse subspecialties throughout all of the scientific literature, complementary findings that support the ALPHA hypothesis went overlooked. With the new ALPHA hypothesis for tumor vasculogenesis, many imaging findings associated with malignancies can now be explained. Using this new knowledge, there is the potential for new and diverse oncologic treatments.
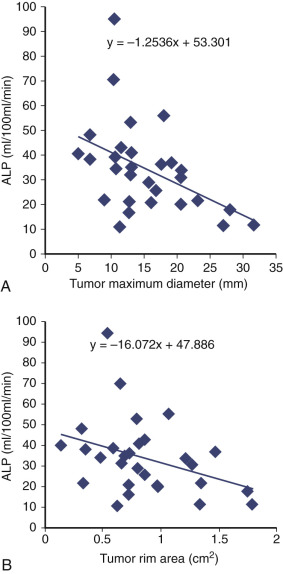
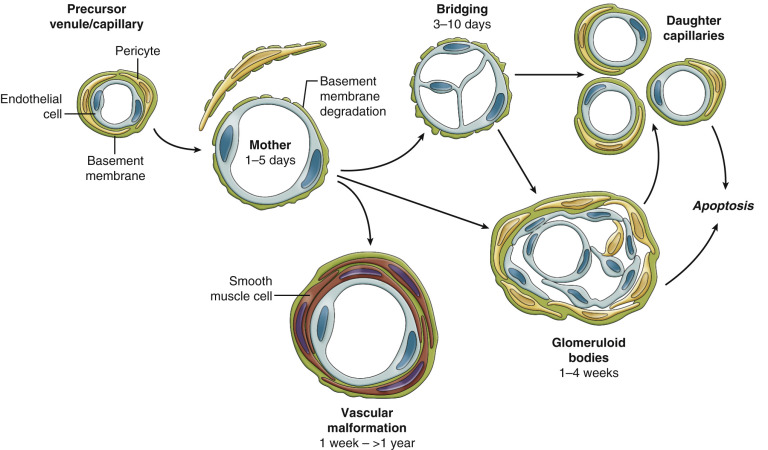
The traditional vasculogenesis theory for tumor development dates back to the 1970s. In a historical paper published by Gimbrone in 1972, it was concluded that implanted rabbit iris tumors increased in size in conjunction with increased vasculogenesis and subsequent perfusion. Because of these findings, Gimbrone et al. concluded that “neovascularization is a necessary condition for malignant growth of a solid tumor.” However, their analysis did not consider the presence of lymphatics or veins. Additionally, reevaluation of Gimbrone's statistics suggests that the tumor growth likely preceded arterial development by up to a day (Vera Chankung, PhD, Professor, and Norman Tien, PhD, Dean and Professor, Department of Engineering, Case Western Reserve University, personal communication, 2009). If this is true, it is impossible for arteriogenesis to be the catalyst for tumor growth, and therefore tumor growth must be dependent on other factors ( Table 70-1 , Fig. 70-1 ).
| Experiment Number | Prevascular Growth Curve Slope (a) | Vascular Growth Curve Slope (b) | Late Growth Curve Slope (c) | Primary Inflection (day) | Vascularization (day) |
|---|---|---|---|---|---|
| 1 | 0.04 | 0.49 | 0.23 | 5-7 | 7 |
| 2 | 0.05 | 0.68 | 0.14 | 5-6 | 6 |
| 3 | 0.08 | 0.58 | — | 5-6 | 6 |
| 4 | 0.03 | 0.96 | 0.14 | 6-7 | 7 |
| 5 | 0.04 | 0.87 | 0.25 | 6-7 | 7 |
| 6 | 0.16 | 0.54 | 0.13 | 4-6 | 6 |
| 7 | 0.08 | 0.51 | 0.21 | 6-7 | 7 |
| 8 | 0.19 | 0.70 | 0.15 | 5-6 | 6 |
| 9 | 0.06 | 0.64 | 0.12 | 5-6 | 6 |
| 10 | 0.12 | 0.56 | 0.12 | 5-7 | 7 |
| Mean ± SD | 0.085 ± 0.055 | 0.65 ± 0.155 | 0.16 ± 0.051 |
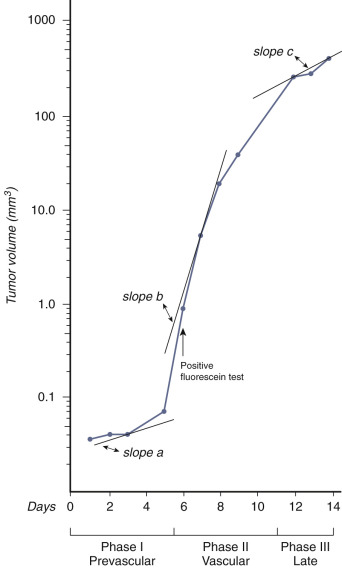
The traditional vasculogenesis theory also holds that cancers prefer aerobic metabolism. After a certain level of growth, typically described as beyond 1 to 2 mm or 10 5 cells, hypoxia occurs as the tumor outgrows its arterial supply. Arteriogenesis then follows in order to restore normoxia for continued tumor growth. Were this true, it would be expected that antiarterial therapies for cancer would have prolific success rates. Unfortunately, this has not been the case. Surgical arterial ligature has repeatedly failed to result in improved survival outcomes. Similarly, bland particle embolization has been shown to have an effect on slowing tumor growth but without improving patient survival. Drugs (e.g., bevacizumab) targeting vascular endothelial growth factor (VEGF) have had only limited success. Even more harrowing than treatment failure, studies have shown that anti-VEGF treatment failure may result in increased tumor aggressiveness related to increase in lactate levels (likely a byproduct of glycolysis) despite a significant reduction in blood volume and reduction in blood vessels ( Figs. 70-2 and 70-3 ). Other studies of anti-VEGF therapies have shown that although primary tumor growth may be inhibited, anti-VEGF therapies were associated with an increase in satellite tumors surrounding the initial mass.
If arteriogenesis is not the catalyst behind tumor growth and development, clearly something else must be responsible. The traditional vasculogenesis theory held that cancers were aerobic, using oxidative phosphorylation to convert a molecule each of glucose and oxygen to create 38 molecules of adenosine triphosphate (ATP). Instead it has been theorized that cancer prefers to use glycolysis for energy production. For the same glucose cost, glycolysis only results in 2 ATP (<10% of that produced by aerobic metabolism) as well as the waste product lactic acid. Although the glycolytic pathway appears to be less efficient compared to aerobic metabolism, cancer cells preferentially use glycolysis for energy production. The hypothesis that cancer prefers glycolysis is by no means new—in fact it was proposed by Warburg in the 1950s—but the glycolytic basis of tumor development has continued to confound scientists and researchers. Hypoxia has been shown to exist in multiple tumors. Even with oxygen readily available, cancer prefers a glycolytic method of metabolism. With these results in mind, the ALPHA hypothesis was proposed to help explain hypoxia and its relation to tumor growth and metastasis, the multiple roles of the glycolytic waste product lactate, and vasculogenesis, including the formation of lymphatics and veins.
#1: Complete vasculogenesis includes lymphangiogenesis, phlebogenesis, and arteriogenesis. Venous flow must precede arterial flow, but lymphangiogenesis can precede arterial flow.
Though its significance has previously been underappreciated, the development of lymphatics is now known to play a vital role in cancer development. Tumors are associated with a large volume of free interstitial fluid. Buildup of this fluid may result in a higher pressure gradient between the arterial and venous systems, ultimately leading to diminished or functionally obstructed arterial flow.
Cancer glycolysis results in accumulation of the waste product lactic acid within the extracellular spaces. This results in an increase in the concentration of the interstitial space. Simultaneously the expenditure of glucose during glycolysis decreases the concentration of the intracellular compartment. The resulting concentration difference between the intracellular and extracellular compartments is managed through the formation of aquaporins, a channel in the cellular membrane through which water can freely diffuse. Diffusion of water into the extracellular space restores the osmotic balance. However, it is not the osmotic disturbances themselves that stimulate the production of aquaporins. Lactic acid has been shown to be a catalyst for the development of aquaporins, and a higher number of aquaporins are detected within tumors, which we will see are highly correlated with lactate. Additionally the presence of aquaporins may be responsible for the cerebral edema associated with brain tumors. Therefore glycolysis results in a rise in interstitial fluid pressure due to its waste product lactate and eventual aquaporin formation.
Aquaporins may also help relieve intracellular lactic acid buildup. As the interstitial fluid (and therefore interstitial fluid pressure) increase, the interstitium requires tumoral lymphangiogenesis for adequate drainage for homeostatic control. Lymphatic proliferation has been identified in tumors even at very small sizes. It has also been demonstrated that lymphatic formation precedes metastasis in melanoma, reflecting the integral nature of early homeostasis in cancer development.
#2: Vasculogenesis occurs in small normoxic tumors before they outgrow their blood supply because of the angiogenic effects of lactate, similar to normal wound healing.
In the very early stages of cancer development, tumors may grow independently from the aid of neovasculature. When still at a very small size, the intercellular distances are short enough that oxygen can travel freely between cells. However, upon reaching a size of approximately 1 to 2 mm, or 10 5 to 10 6 cells, additional support is required for continued cancer growth. This support comes in the form of newly developed vasculature. Tumoral vasculogenesis occurs in two phases: an early incipient normoxic phase and a later hypoxic phase.
The initial angiogenic burst occurring in normoxic conditions is mediated by the release of VEGF and fibroblast growth factor (FGF). Angiogenesis and tumor growth have been shown to be induced in dormant MOLT-3 cells (a T-lymphoblast cell line) after pellets embedded with VEGF and FGF were injected, indicating a key role for angiogenesis in continued tumor growth. Additionally, previously dormant tumors, upon stimulation to reintroduce angiogenesis, return in a much more aggressive form. Following this initial phase, there is the hypoxic phase, which is multifactorial with various causes ranging from increased distances between tumor cells and arteries, elevated interstitial and capillary pressure, temporal and spatial variation in flow, blood viscosity, thrombosis, and vascular shunts. Hypoxia actually has very complex interplay with cancers, possibly resulting in growth inhibition and cell death while conversely also being a key component in tumor growth, local invasion, and metastatic progression. Phenotypic alterations as a consequence of hypoxia within the microenvironment are closely linked to metastasis. Irradiation of the tumor bed in animal models of melanoma has resulted in increased metastases, which reflects what is often seen clinically: patients with recurrent malignancy after radiation therapy tend to have increased local disease and distant metastases. The extent of metastasis is also proportional to the radiation dose. Not surprisingly, the pre-irradiated tumor beds also showed significant hypoxia compared with the unradiated beds.
As described by Vaupel et al., tumor angiogenesis first begins with vein formation, largely due to the need for waste removal ( Fig. 70-4 ). Although initially host venules may be incorporated into the tumor, continued growth of the tumor and lack of associated growth within the native vessels exhausts the host vasculature. Eventually the incorporated host veins become obliterated or otherwise inadequate for continued tumor growth. Neovascularization then begins, with veins being produced prior to arteries to maintain adequate outflow. Early on, multiple anastomoses between veins may actually result in veins being able to contribute blood inflow in the manner usually conducted by arteries.
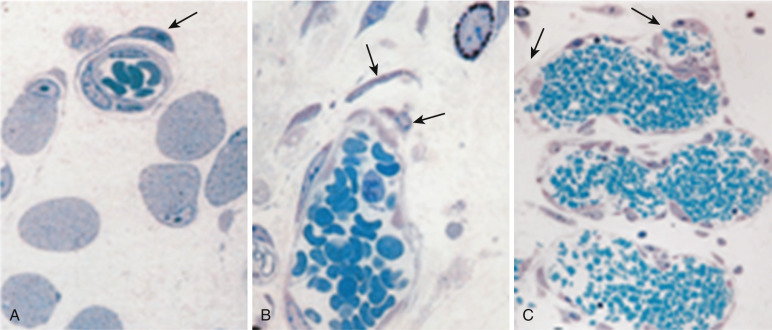
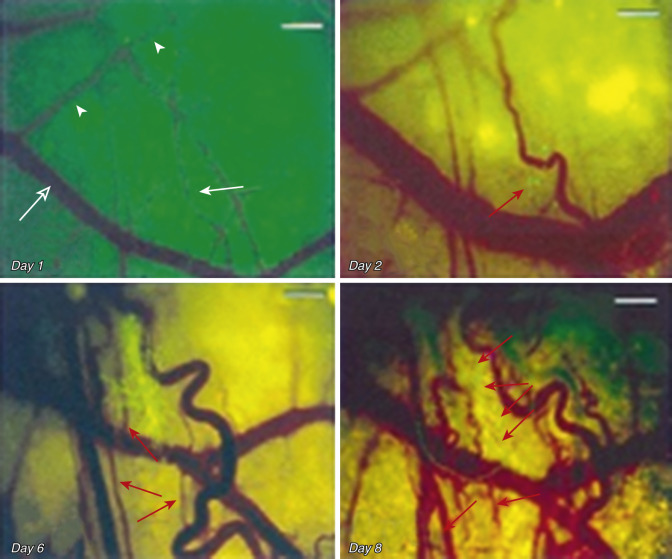
Visualizing tumor cells directly labeled with green fluorescence protein (GFP) and dorsal skin folds in a rodent model. Li et al. described the early phases of angiogenesis, beginning with malignant cells migrating toward the normal host vasculature ( Fig. 70-5 ). The next angiogenic phase seen involved dilatation and tortuosity of veins. This finding occurred after 6 days, as the tumor cells had proliferated from 20 to 50 cells to approximately 60 to 80 cells. No change in veins were noted in the nontumor cell line. Neovasculature within the tumor cell line was noted at day 8, approximately the 100- to 300-cell stage of growth. At this early stage of cell proliferation (and far below the 10 5 cell threshold previously alluded to), it can be assumed that this local microenvironment maintains normoxia at the onset of vasculogenesis.
The low extracellular pH seen in early tumor development, mediated by lactic acid, results in activation of phospholipase D and the subsequent expression of matrix metalloproteinase 9 (MMP-9). MMPs are important enzymes in tumor development, resulting in breakdown of the extracellular matrix, tumor cell attachment by cleavage of integrins, and migration by removal of E-cadherin. Lactate and MMPs are also essential for tumor migration. The acidic extracellular environment results in expression of transforming growth factor (TGF)-β2, which ultimately promotes MMP-2 activity. MMP-2 activity is directly correlated to the cell migration rate, with a significantly greater total distance migrated when compared to tumor cells undergoing MMP-2 inhibition. Conversely, small interfering RNA directed against lactate dehydrogenase type A (siLDHA) downregulates LDHA, lowering lactate levels, altering the extracellular environment, and ultimately reducing the ability of cancer cells to migrate. Cell locomotion is initiated by glycosaminoglycan hyaluronan and its interactions with its receptor for hyaluronan-mediated motility (RHAMM). This results in a tyrosine phosphorylation pathway, resulting in focal adhesion turnover and allowing for cell motility. High levels of RHAMM mRNA in breast cancer have been shown to be associated with worse patient prognosis.
Using the chemotherapeutic drug tirapazamine to selectively kill hypoxic cells, Cao et al. demonstrated no evidence of delayed angiogenesis, further supporting the notion that early angiogenesis occurs independently of a hypoxic environment. Other studies have similarly shown evidence of normoxia in early angiogenesis owing to the presence of VEGF.
In the aforementioned study by Li, implanted cells with fibroblastlike morphology were seen early on, prior to the dilatation of host venules or neoangiogenesis. This was likely due to what is now referred to as epithelial mesenchymal transition (EMT) in which epithelial cells gain properties of mesenchymal and fibroblastic cells, reducing cellular adhesion and allowing for cell locomotion. These changes actually precede those of neovascularity. Although EMT may be seen in normal development (e.g., neural crest cell formation), it also plays a key role in oncogenesis. The cell-cell adhesion protein E-cadherin permits cellular adhesion, thus inhibiting metastatic spread, and acts as a tumor inhibitor. In fact, reexpression of this protein in tumors has been shown to decrease their aggressiveness. TGF-β stimulates phosphatidylinositol 3-kinase (PI3K) phosphorylation of AKT, which downregulates E-cadherin expression. This allows EMT changes of poor cellular adhesion and subsequent cellular movement. EMT has also been shown to be decreased in cells lacking JNK1. Clinically, upregulation of AKT2 in ovarian carcinoma has been linked to more aggressive disease.
#3: Bioenergetic chemical reactions of oxidative phosphorylation and glycolysis indicate different vessel requirements for each.
From only one molecule of glucose and oxygen, the process of oxidative phosphorylation can produce 38 ATP and 2 CO 2 . This is a much more efficient means of energy production when compared to glycolysis, which would require nearly 20 times the number of glucose molecules for an equal amount of ATP production. Given the large amount of lactic acid also produced by glycolysis, it would seem on the surface that cancer would naturally prefer oxidative phosphorylation to glycolysis. A historical point of contention regarding cancer metabolism is the question of why cancer would prefer the less energy-efficient glycolysis. However, as noted by Warburg, cancer uses glycolysis even in the presence of ample oxygen; this has since been referred to as the Warburg effect. This of course contradicts the edicts of the traditional vasculogenesis concept, which held that neoangiogenesis is required in tumors to allow for and maintain oxygenation, as previously described.
The question of why cancer prefers glycolysis may have a few answers. A simple reason may simply be that glycolysis can produce energy very rapidly, roughly 100-fold faster compared to oxidative phosphorylation. Another potential reason is that too much glucose would be devoted solely to ATP production if oxidative phosphorylation were dominant, at the expense of other macromolecules from being developed. In addition to ATP, proliferating cells have multiple requirements including acetyl-CoA, amino acids, and ribose.
A key difference between the two metabolic mechanisms is that oxidative phosphorylation requires oxygen, necessitating arterial inflow. Oxygen diffuses quite poorly, so a distance of greater than approximately 100 micrometers from any arterial source will result in cellular hypoxia. In contradistinction, glycolysis functions without the use of oxygen and therefore an arterial inflow is much less important than venous and lymphatic outflow ( Fig. 70-6 ). Especially as a consequence of the significant buildup of lactic acid, lymphangiogenesis is a vital part of glycolysis given its ability for waste removal.
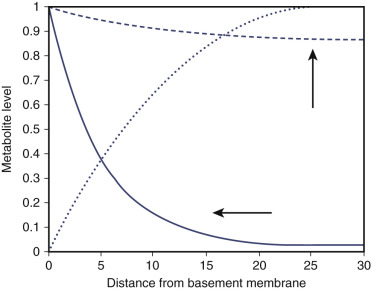
Another more complex and intriguing answer as to why glycolysis is preferred is that perhaps ATP is not the primary goal of metabolism as is often thought; rather, the key outcome may be production of the support waste product lactic acid. Lactic acid has multiple effects in oncogenesis. As already mentioned, local changes in the microenvironment pH are a necessary component for TGF-β expression. Other possible roles of lactate include hyaluronidase and hyaluronan expression, as well as an integral role in hypoxia inducible factor (HIF)-1α expression (which will be discussed in more detail later). However, the amount of lactate is tightly regulated by cancers because its overabundance may end up in tumor dormancy. Therefore early employment of an elegant outflow system for waste removal is a necessity for cancer development.
The most important factor in lactate removal is lymphatics. Carbonic anhydrase IX and XII can also help regulate the extracellular pH by conversion of acidic CO 2 into bicarbonate. MCT1/MCT2 are used by cancers for lactate absorption from the extracellular matrix. LDH1 is an enzyme used to convert lactate to pyruvate and has been noted to be upregulated in colon carcinomas. Pyruvate can then subsequently be used to fuel cancer cells or contribute to fibroblast proliferation.
#4: Although cancer uses both aerobic metabolism and glycolysis under various circumstances, glycolysis is the “preferred” pathway in normoxia or hypoxia because glycolysis and its byproduct lactate induce and support many procancer processes.
As previously mentioned, cancer preferentially uses a glycolytic method of metabolism, largely because of its production of lactate, which has been shown to have multiple subsequent effects in procancer processes. Even in the presence of sufficient oxygen, glycolysis is the preferred pathway and accounts for approximately 50% of all ATP production. Of course in the absence of arteries, glycolysis is responsible for 100% of the energy production.
This reliance on glycolysis confers a tremendous survival benefit to cancer cells, which tend to experience inconsistent levels of oxygenation. Intratumoral hypoxia is multifactorial. It is in part related to inadequate oxygen supply by arteries, related to a disorganized network of vasculature. Tortuous and dilated blood vessels in malignant tumors also decrease the efficiency of oxygen delivery and may result in stasis. As tumors grow and the distance from a vascular supply increases, cancer cells may experience hypoxia due to limitations related to the distance oxygen can freely diffuse between tissues. With the use of vascular mapping of tumor oxygenation, it has been demonstrated that the amount of oxygenation within the tumor can be quite heterogeneous as well as highly variable within a specific region over a given time period.
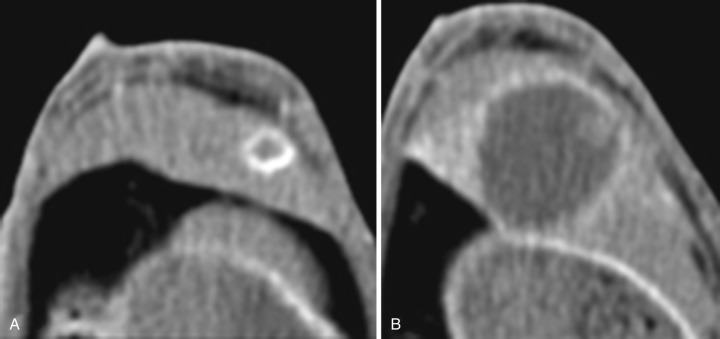
Cancer cells may experience increased interstitial fluid pressure. This may be a consequence of the higher permeability of the tumor vessels, or it may be related to osmotic effects from the buildup of lactate from glycolysis. Prior beliefs held that vessel permeability occurred primarily via capillaries, but research now shows that veins play a large part in permeability, with evidence of leakage of molecules as small as 17 nm ( Fig. 70-7 ). This leakage is most significant in the tumor periphery. Whatever the underlying cause, the resulting pressure changes have been shown to correlate with poor efficacy of chemotherapeutic regimens. Interstitial changes may also play a role in lymphangiogenesis and alterations in fibroblasts, including fibroblast motility.
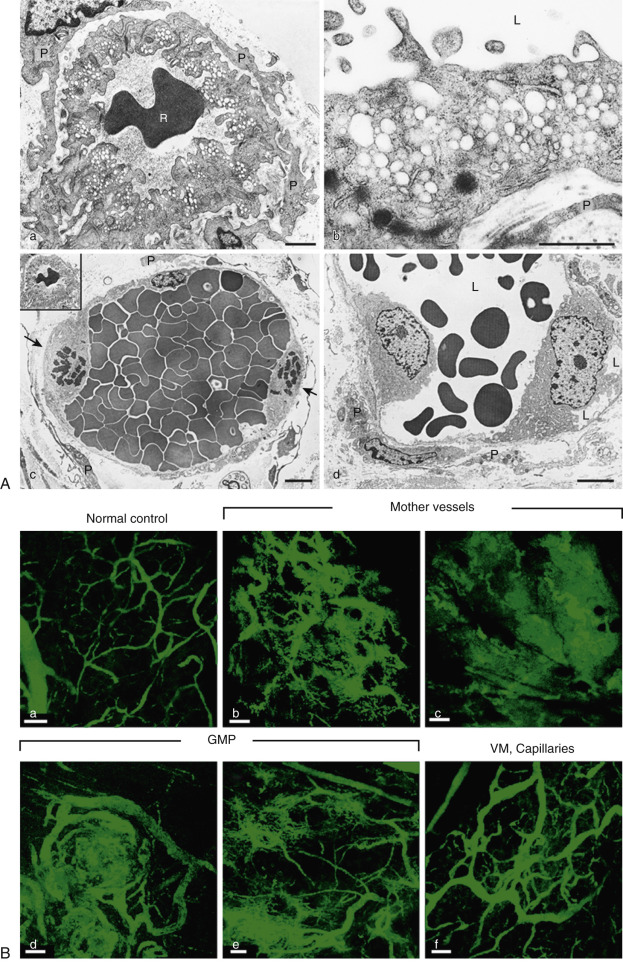
The use of glycolysis—and therefore independence from oxygen—permits cancer cells to survive and even metastasize in hypoxic environments. Glycolysis is therefore not merely a survival mechanism but a key contributor to oncogenic processes including cell motility and invasion. Lactic acid production from glycolysis renders the extracellular microenvironment of tumor cells acidic. This is advantageous for tumor cells because it results in apoptosis of normal nonmalignant cells via the p53 pathway. This low-pH environment also promotes release of interleukin (IL)-8 and VEGF, which result in angiogenesis. The extracellular matrix may also be degraded by enzyme release from fibroblasts and macrophages owing to the acidic environment. Lastly, acidosis can inhibit the host's immune response. Lactate itself has been shown to adversely affect the host's natural killer cells, macrophages, and T-lymphocytes.
Lactate and ketones result in the migration of MDA-MB-231 cells, which act as chemoattractants. Additionally, lactate and ketones may cause mutations resulting in cancer “stemness,” which corresponds clinically to poor patient survival because this trait is likely related to both tumor growth and metastasis ( Fig. 70-8 ).

As briefly alluded to already, lactate has a potential role in the expression of hyaluronan. In this way, lactate may have an indirect effect on metastasis. The hyaluronan receptor RHAMM mediates the motility of fibroblasts. When exposed to hypoxia or tumor growth factor, RHAMM becomes highly expressed. RHAMM is related to the invasive functions of cluster of differentiation (CD)44 by increasing its gene expression ( Fig. 70-9 ). CD44 can result in proliferation of tumor cells as well as promote cellular motility by acting on actin networks. RHAMM is also related to BRCA1 and affects centrosome and mitotic spindle alterations.
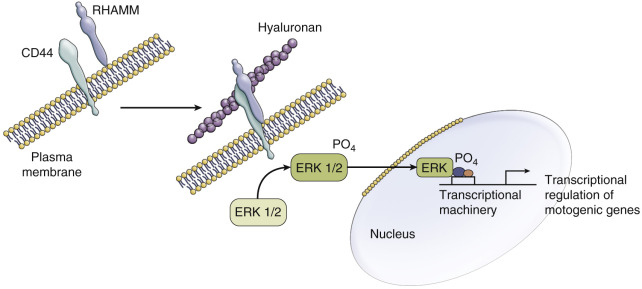
Lactate also plays an important role in the nuclear factor (NF)κB pathway. Lactate prompts IL-1 stimulation, which results in activation of NFκB. The NFκB pathway contributes to multiple procancer processes. Diffuse large B-cell lymphoma results in overexpression of the NFκB pathway. Suppressing this pathway indirectly with an IκB inhibitor results in apoptosis of lymphoma cells, demonstrating the indispensable nature of NFκB in oncogenic processes. This importance been shown not just in lymphoma but in most solid tumors. More specifically, NFκB has been shown to be integral in inhibiting apoptosis, which can help contribute to cell survival. Cell proliferation is also dependent on NFκB through interactions with cyclin D1, which is a necessary component of DNA synthesis. Tumor invasion and metastasis are also closely linked to NFκB and are inhibited with NFκB blockade.
#5: Considering the environmental factors of hypoxia and excessive lactate that might cause quiescence/dormancy, data indicate that excess lactate is more likely than hypoxia to cause dormancy.
Like tumor development and progression, tumor quiescence (also referred to as dormancy ) can be multifactorial, including genetic and cellular causes as well as environmental causes, such as hypoxia and elevated lactic acid. This dormancy is temporally variable but may last up to decades. By definition, tumor dormancy means that although asymptomatic, residual cancer cells remain present. This is a very common occurrence in cancer, occurring in 20% to 45% of breast and prostate cancers.
Based on the assumptions of the traditional vasculogenesis concept, it was originally believed that tumor dormancy occurred as a result of tumor outgrowing its arterial supply. However, as has already been mentioned, tumors are less dependent on a robust arterial supply because they are predominantly fueled via the glycolytic pathway, which works independently of oxygen ( Figs. 70-10 and 70-11 ). The ALPHA hypothesis posits that lymphatics, not arterial inflow and normoxia, are more important. Lymphatic outflow of acidic lactate decreases the concentration to a moderate level, which is the key factor in tumors emerging from quiescence.
One piece of evidence that contradicts the notion that tumor dormancy is secondary to hypoxia is the response of hypoxic tumors to hyperbaric oxygen. If dormant tumors were in a quiescent state because of cellular hypoxia, the return to normoxia as a result of hyperbaric oxygen should correspond to tumor progression and vasculogenesis. However, this has not been shown to be the case. Unlike in tumors, hyperbaric oxygen has been shown to have effects on increased rates of neoangiogenesis in wound healing. Curiously the converse is true for tumors, which tend to undergo neovascularization under hypoxic conditions. This may be related to hypoxia stimulating IL-8, which promotes angiogenesis.
When tumors are quiescent, they are resistant to radiation therapy. Previous attempts have been made to expose dormant tumors to hyperbaric oxygen under the assumption that tumor activity would resume after hypoxia resolved, and the tumor would therefore be radiosensitive. However, because hypoxia is not the driving factor for tumor dormancy, hyperbaric oxygen has no effect on tumor radiosensitivity. On the other hand, tumors affiliated with high levels of lactate show radioresistance, whereas those with more modest levels of lactic acid do not. Therefore although lactate has already been shown to be a key factor in tumor growth, invasion, and metastasis, it is only protumorigenic to a certain point. At very high concentrations, lactic acid pushes the tumor into dormancy through interference of the normal cell cycle. It is not until lymphangiogenesis occurs that the concentration of lactate is regulated to a more manageable level.
Based on the evidence, the authors postulate that a “bioenergetic quiescence/dormancy” is produced by increased levels of lactate, which causes:
End-product inhibition of glycolysis, which decreases energy production
Reduced availability of building substrates for cell growth and mitosis (i.e., carbohydrates, lipids, nucleotides, etc.)
Impaired growth of “mother cells” that precede mitosis
Prevention of apoptosis due to NFκB, which is modulated by lactate
Impaired response to mitogens because cells lapse into the G0 dormant state
Lactic acid has been shown to be a potent and versatile inhibitor of the enzyme phosphofructokinase, which is the rate-limiting step in glycolysis. By inhibiting glycolysis, the negative feedback mechanism perpetrated by lactate accomplishes what varying oxygen levels cannot: a method of preventing energy production in tumors.
In addition to glycolysis, lactate also has an inhibitory effect on glutaminolysis. With high lactate levels, ammonia and alanine concentrations have been proven to dramatically decrease. Other cell building blocks such as nucleotide precursors, a necessary component of DNA and RNA, are diminished as lactate levels rise. In vivo tumor cell cultures taken during the plateau phase of cancer growth have been shown to respond poorly to the addition of nutritional supplements such as glucose and glutamine, as well as to alterations in pH, indicating another etiology for tumor dormancy. Ultimately the effect of tumor inhibition was blamed on elevated lactate levels within the cellular media.
Cell growth is a key preliminary step prior to mitosis. For a mother cell to be able to divide in mitosis, it must first create enough building blocks before dividing into two daughter cells. It is theorized that glycolysis is the pathway by which the macromolecular constituents such as nucleotides, amino acids, and lipids are formed in cancer; therefore if glycolysis is blocked, mitosis cannot continue and cell division will not occur, ultimately resulting in tumor dormancy.
Lactic acid results in stimulation of the NFκB pathway. NFκB can help promote cancer cell survival by inducing Bcl-XL, which prevents apoptosis of premalignant cells. NFκB also acts on oncogenes called inhibitors of apoptosis, whose key function is self-explanatory. Many viral-induced cancers employ the NFκB pathway for tumor growth. Conversely if NFκB is suppressed with inhibition of IκB kinase β (IKKβ), apoptosis in cancer cells resumes.
TGF-β has been shown to be a potent inhibitor of growth arrest at the G1 step of the cell cycle. This corresponds to findings that TGF-β levels are elevated in quiescent tumors, contributing to antimitogenic effects, including inhibition of cyclin/cyclin-dependent kinase, which control the cell cycle.
#6: Acidic lactate releases and induces vascular growth factors and HIF-1α through pathways that function in normoxia and/or hypoxia.
Although a well-known and seemingly omnipresent compound, lactate has garnered very little attention in the research community. However, a careful analysis of available data suggests that the ubiquity of lactic acid in regard to cancer extends just beyond a mere curiosity; it is in fact a very important component in a number of oncogenic processes. When compared to oxygen, lactate is present and available in a relatively steady supply because glycolysis is being performed in both hypoxia and normoxia (see earlier discussion). Lactate, either directly or indirectly, has multiple influences on vascular growth factors by:
Releasing dormantly stored growth factors
Directly inducing factors from different cells
Attracting cells as active participants
Combining with other factors to stimulate vasculogenesis
Starting/participating in multistep signaling pathways such as HIF-1α and NFκB
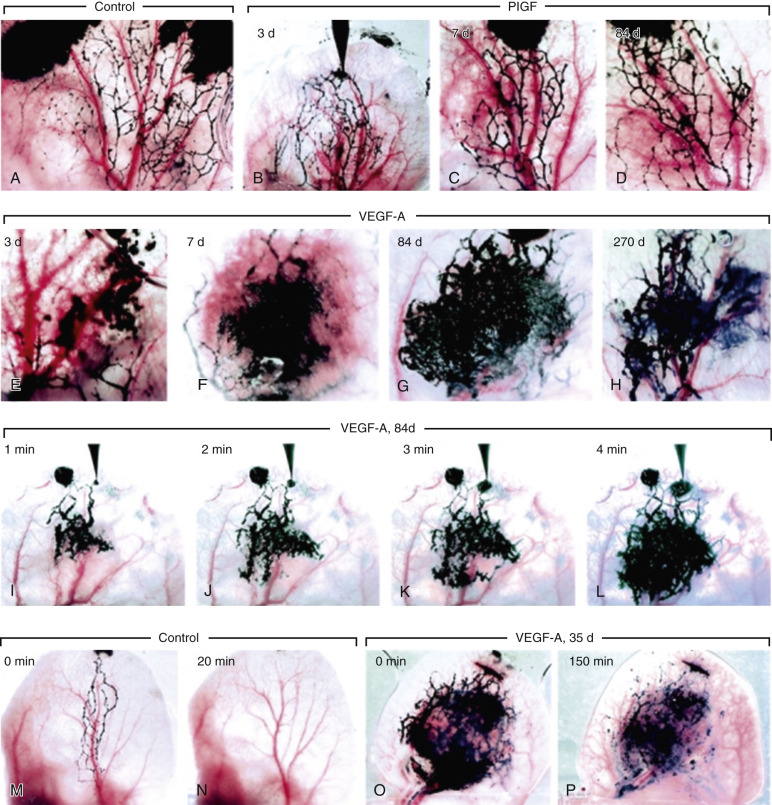
As noted earlier, tumor dormancy is interrupted by a so-called angiogenic burst, mostly as a result of two factors, FGF-β (also known as basic [b]FGF and FGF-2 ) and VEGF-A. Although it is a key component of early tumor growth, VEGF has been shown to be unnecessary for continued growth later on in cancer development. It is theorized that the role of VEGF is assumed by other factors such as FGF-β and TGF-α. VEGF and FGF are stored in the heparin sulfate matrix and are not released until stimulated by the release of lactic acid and subsequent decrease in pH. This alteration in the extracellular microenvironment allows even cells with weak angiogenic capabilities to cease quiescence and develop into cancers. As the initial normoxic angiogenic phase gives way to the hypoxic phase, HIF-1α is activated with additional support of proangiogenic processes. HIF-1α results in the activation of nearly three dozen genes, many of which are directly related to angiogenesis, tumor cell survival, and metastasis. SDF-1 and its receptor CXCR4 are regulated by HIF-1α and play a role in the chemotaxis of malignant cells. Cells expressing CXCR4 receptors are also attracted to the cancer site as a result of actions of SDF-1.
Of the cells that are attracted by SDF-1, some are involved in vasculogenesis and include angioblastic mesenchymal stem cells. Not only can blood vessels be developed, but their ultimate fate of developing into either artery or vein can be determined as well. The Notch signaling pathway results in stem cells forming arteries. Conversely the PI3k pathway results in vein formation.
The pathway for angiogenesis is complex and interconnected. Lactate contributes to expression of SDF-1, which attracts many cells, including macrophages and monocytes. These in turn also produce angiogenic factors such as TGF-β, IL-1, IL-8, VEGF-A, VEGF-C, and VEGF-D. The latter two VEGFs contribute to lymphatic formation through lymphangiogenesis. These complexities grow more daunting with multistep processes such as the HIF-1α and NFκB pathways. Potentially because of its significance in oncogenesis, the NFκB pathway can be activated by lactate in numerous ways, including IL-1, toll-like receptor (TLR)4 signaling activation, and monocarboxylate transporter (MCT) to name a few ( Fig. 70-12 ).
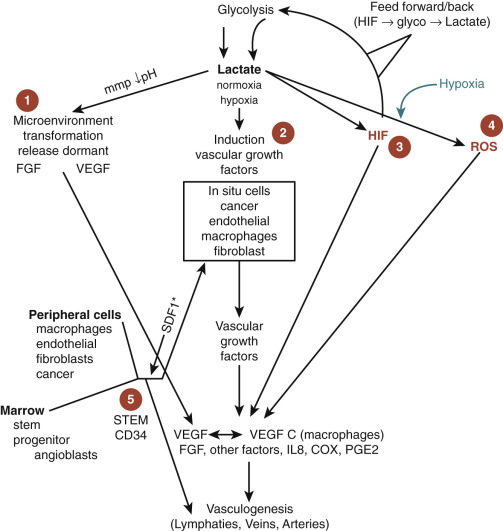
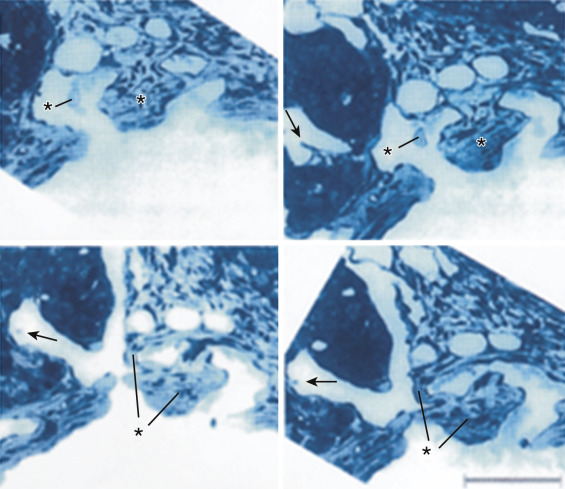
#7: Arteriogenesis is not the first morphologic change of vasculogenesis. The sequence of vasculogenic changes is first lymphatics, then veins, and finally arteries.
Before reliable biomarkers were known, investigators had to distinguish arteries from veins on the basis of blood flow direction and pressure of vessel fluid content. With the use of biomarkers, identification of vessels has become much more accurate. The distinction of arteries from veins has shown that contrary to the traditional belief, veins form before arteries in oncogenesis. Even before this, lymphatics form. After injection of VEGF, lymphatics were demonstrated to grow prior to veins. Previously the identification of lymphatics was challenging because of their transparency. With the use of LYVE-1, a marker specific for lymphatics that is not present in vasculature, lymphatics are now much more easily accounted for. Lymphatics and blood vessels play different roles in cancer. Whereas blood vessels are typically responsible for delivery of blood and oxygen to and from the tumor, the role of lymphatics is more based in homeostasis. Their role in maintaining a balanced level of lactate in which cancer can grow necessitates their early development ( Fig. 70-13 ).
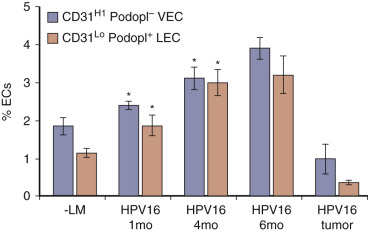
Multiple lab analyses have shown the effects of FGF-β and VEGF on tumor dormancy, with the angiogenic spike being triggered early on in oncogenesis. Again, these effects are more important for early growth, with less influence on the growth of more robust established tumors. Prior to vasculogenesis, FGF-2 and VEGF-A stimulate the subtype VEGF-C, which subsequently results in development and enlargement of lymphatics ( Figs. 70-14 and 70-15 ). This correlates with the previously discussed theory that lymphatics are a key regulatory component of oncogenesis and help maintain a modest lactic acid concentration that promotes tumor growth and proliferation while not being so high that it results in tumor dormancy. Other studies have clearly shown that vascular endothelial cells and lymphatic endothelial cells have distinctly different responses in tumor growth, further proving the two are separately promoted.
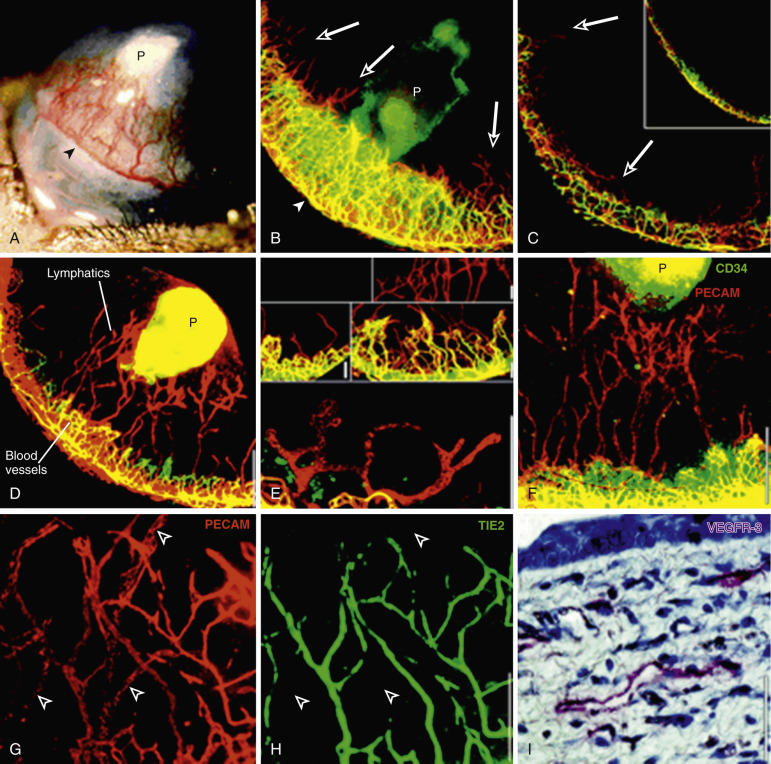
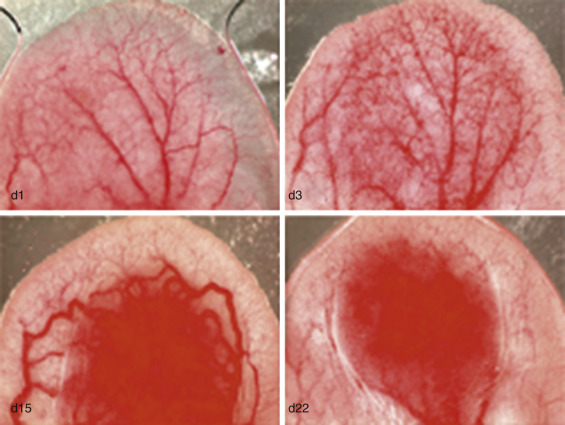
After lymphatic development, cancer results in the promotion of veins before arteries ( Fig. 70-16 ). Similar to lymphatics, this is likely a result of the importance of waste outflow compared to arterial inflow, insofar as tumors can function well anaerobically through glycolysis in the absence of arterial vasculature. Early on, arteries and veins arise from the same group of angioblastic progenitor cells. The enzyme PI3K promotes differentiation of these multipotent angioblasts to vein formation. COUP-TFII is also an essential regulator in the determination of a venous fate. Ephrin-B4 has been used in laboratory studies as a specific marker for venous structures. Conversely ERK (p42/44 MAP kinase) is a key factor in arterial specification. Additionally, zebrafish studies have shown that VEGF has a role in the Notch pathway, which helps promote arterial differentiation from stem cells. In research on mouse ears, Nagy et al. demonstrated that the first blood vessels to form are veins. It is not until after veins have formed, proliferated, and dilated that arteries begin to form ( Fig. 70-17 ). Similar findings after microdissections of thousands of rodent tumors showed an abundance of veins, with a marked paucity of arteries, again demonstrating early venous development being favored over arteriogenesis ( Fig. 70-18 ).
#8: ALPHA vasculogenesis serves the needs of glycolysis and therefore is complementary, supplemental, and synergistic with the traditional vasculogenesis concept based on oxygen/hypoxia.
As we have shown, cancer can utilize both aerobic and glycolytic metabolism, but for aerobic metabolism to occur, oxygen supply must be adequate. This necessitates a role of angiogenesis and arterial development. Conversely glycolysis occurs independently of an oxygen supply. The critical factor to favor continued glycolysis is therefore not the inflow of oxygen or other nutrients, but the need for a sufficient outflow mechanism to remove the glycolytic waste products. This comes in two forms: lymphangiogenesis and vein formation.
When an oxygen supply is readily available, aerobic metabolism and glycolysis may work in concert. However, a reduction in arterial supply—be it from tumor growth or physician intervention, including chemotherapy or embolization—will result in cancer shifting to glycolysis. If cancer were solely reliant on aerobic metabolism, arterial interruption would be a relatively elegant cure for cancer. Some studies of transarterial chemoembolization (TACE) have shown early partial response of hepatocellular carcinoma (HCC). However, follow-up at 2 and 4 years failed to show improvement in patient survival rates, likely because of the ability of tumors to convert to glycolysis. Similarly the chemotherapeutic drug bevacizumab, which works through VEGF inhibition, has been shown to be ineffective in the treatment of breast cancer, which is likely attributable to the use of glycolysis by cancer.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here