Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The diagnosis and classification of pulmonary vasculitis is a challenging topic because pulmonary vasculitides represent pulmonary manifestations of systemic diseases. Extrapulmonary involvement is, in some cases, a significant source of morbidity and mortality. To further complicate matters, there is considerable overlap in the histologic patterns of manifestations from different systemic vasculitis syndromes, as well as secondary inflammatory processes that affect blood vessels as seen in infectious granulomatous diseases and sarcoidosis.
To the pathologist, the difficulty in the interpretation of a surgical biopsy for the diagnosis of pulmonary vasculitis is contributed to by the fact that careful correlation between clinical, radiographic, and pathologic features, rather than histologic interpretation alone, is necessary to reach the correct diagnosis. In many cases, the biopsy is obtained early in the process when not all of the classical histologic criteria are present in the specimen, or the biopsy is taken after some course of treatment, which can modify the histologic appearance of lesions. In these two circumstances, a strong clinical suspicion can help lead to the correct diagnosis. In addition, the rarity of pulmonary vasculitis syndromes prevents most pathologists from acquiring critical experience with the subtle criteria that are helpful in the distinction of these pathologic entities.
Three major idiopathic vasculitis syndromes commonly affect the lungs: granulomatosis with polyangiitis (Wegener’s granulomatosis), eosinophilic granulomatosis with polyangiitis (EGPA; Churg-Strauss syndrome), and microscopic polyangiitis (MPA). Their pathologic, clinical, and radiographic features will be the focus of this chapter. Other less common vasculitides and secondary conditions that affect the pulmonary blood vessels will be discussed briefly, mainly as they relate to the differential diagnosis of the three main syndromes named previously.
Systemic necrotizing granulomatous vasculitis of unknown etiology
Rare (estimated incidence in the United States is 0.3 per million persons)
Most common systemic vasculitis to involve the lung (90% of cases show pulmonary involvement)
Fatal disease if untreated
Most patients die of renal or pulmonary insufficiency
Peak incidence is between the fourth and sixth decades (mean age, 50 years)
Rare in children
No sex predominance
More common in Caucasians
Classical triad includes sinusitis, pneumonia, and glomerulonephritis
Most common signs and symptoms: epistaxis (sinusitis), cough, chest pain, dyspnea (pneumonia), hypertension and edema (glomerulonephritis), arthralgias, fever, cutaneous lesions, and weight loss
Ninety percent of patients have a positive c-ANCA test result
Multifocal pulmonary nodular opacities that wax and wane and can be associated with a feeding vessel
More frequent in the lower lobes
Cavitation occurs in approximately 25% to 50% of cases
Diffuse airspace disease may be seen, corresponding to pulmonary hemorrhage
A solitary nodule is rare
Treatment includes immunosuppressive drugs (corticosteroids and cyclophosphamide are the most commonly used drugs)
Ninety percent of affected patients respond to treatment
Seventy-five percent of patients have a complete remission
Relapse rate is approximately 50%
Lung can be dark red due to pulmonary hemorrhage
Solid nodular zones of consolidation with punctuate or geographic necrosis are often seen
Classical triad includes vasculitis, necrosis, and inflammatory background
Vasculitis usually involves medium- and small-sized vessels
Poorly formed granulomas and/or mixed inflammatory cell infiltrates permeate blood vessel walls
Capillaritis (neutrophilic infiltrate within the alveolar wall) is often seen and accompanied by hemorrhage
Necrosis is geographic, basophilic, and contains neutrophils
The inflammatory background is extensive and composed of macrophages, plasma cells, lymphocytes, neutrophils, eosinophils, and multinucleated giant cells
Other abnormalities can include bronchitis, bronchiolitis, endogenous lipoid pneumonia, and organizing pneumonia
There is a continuum between the areas of granulomatous reaction and the inflammatory background, and there is no sharp demarcation between normal and affected lung
Posttreatment changes include interstitial fibrosis with scattered giant cells, bronchial/bronchiolar scarring, and cicatricial vascular changes
Elastic stain will highlight the angiocentric nature of the lesions
Special stains to rule out microorganisms must be done with any granulomatous inflammation; no microorganisms are seen in GPA
Lymphomatoid granulomatosis
Eosinophilic granulomatosis with polyangiitis
Microscopic polyangiitis
Necrotizing sarcoid granulomatosis
Infectious granulomatous inflammation
Rheumatoid nodules
Bronchocentric granulomatosis
Other pulmonary hemorrhage syndromes
In an attempt to replace eponyms with disease-descriptive terms, three medical societies—namely, the American College of Rheumatology, the American Society of Nephrology, and the European League Against Rheumatism—recommended new terminologies to be used in some rheumatologic diseases. Therefore Wegener’s granulomatosis is now known as granulomatosis with polyangiitis (GPA). GPA, as the name indicates, is a systemic process characterized by necrotizing granulomatous vasculitis that primarily affects the upper and lower respiratory tract and the kidney. The pathogenesis of GPA has not been fully elucidated, but there is evidence for an immunologically mediated process involving a T helper 1–like reaction. The presence of antineutrophil cytoplasmic antibodies (ANCAs) in the serum from patients suffering from this condition raises the possibility of an autoimmune process. The pathogenesis is characterized by endothelial cell injury leading to activation of inflammatory mediators and accumulation of inflammatory cells (monocytes, neutrophils, eosinophils), producing vasculitis and other inflammatory lesions.
GPA is a rare condition that seems to have a peak incidence between the fourth and sixth decades of life, although it has been reported in any age group. The disease tends to affect both sexes equally, and it is more commonly seen in Caucasians.
The disease frequently affects the upper and lower respiratory tracts and the kidneys, although it can involve any part of the body. Skin manifestations include leukocytoclastic angiitis that is more commonly seen in the lower extremities; other relatively less common manifestations include involvement of the eye and central nervous system, and rarely, the gastrointestinal and genitourinary tracts are involved.
The classical clinical triad of GPA includes sinusitis, lung disease, and glomerulonephritis. Most patients complain of constitutional symptoms such as fever, malaise, arthralgia, and weight loss, and the constitutional symptoms may precede organ-specific symptoms.
In the classical clinical triad, patients present with epistaxis and lower respiratory tract symptoms such as cough, chest pain, hemoptysis, or dyspnea. In many patients, the clinical presentation also includes hypertension and edema, which are indications of renal involvement characterized by glomerulonephritis.
Laboratory tests are not specific, with alterations consistent with a chronic process, such as normochromic normocytic anemia, elevated sedimentation rates, and elevated C-reactive protein. Urinalysis frequently reveals microhematuria and proteinuria.
The laboratory diagnosis of vasculitis has been revolutionized by the advent of the ANCA test. The two most common patterns of ANCA reactivity are based on the immunofluorescence patterns of cytoplasmic staining (c-ANCA) or perinuclear staining (p-ANCA). Proteinase 3 is the usual target antigen in c-ANCA reactivity, and myeloperoxidase is the usual target antigen in p-ANCA reactivity. In GPA, c-ANCA is found in approximately 90% of cases. However, c-ANCA is not specific. Cases of GPA with a positive p-ANCA or even a negative ANCA test have been described. The latter is more commonly seen in indolent, previously treated disease or in limited disease (upper respiratory tract and lung involvement only). Although ANCA testing should be performed in any patient with symptoms suggestive of vasculitis, the test result must be considered in light of the clinical context for a given patient. A positive test result should not be considered diagnostic for GPA without appropriate clinical features because the serologic marker can be positive in other diseases such as infective endocarditis.
Most patients affected by GPA present with multiple bilateral opacities easily seen on computed tomography (CT) scan of the chest, with well-marginated nodules. The lower lobes are involved most frequently. The nodules may have spiculated margins mimicking a neoplasm and very often show cavitation with thick walls. Careful examination of the CT scan can reveal an association with a feeding vessel. Lesions can wax and wane over time.
In some patients, pulmonary hemorrhage can be the only manifestation and appear radiographically as diffuse infiltrates or airspace opacities. Another common finding in GPA is the presence of a wedge-shaped peripheral opacity mimicking a pulmonary infarct. Rarely, the disease manifests as a solitary cavitary nodule. The diagnosis of GPA in this setting should be made with caution because most solitary cavitary nodules are infectious in etiology. A strong clinical suspicion, as well as histologic support, is necessary to establish the diagnosis of GPA in this context.
The histologic manifestations of GPA are varied but are composed of three main elements that are very helpful in the histologic diagnosis of this entity. They include vasculitis, necrosis, and inflammatory background.
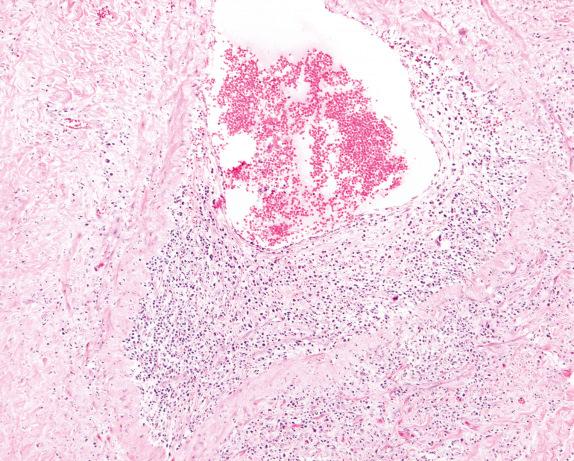
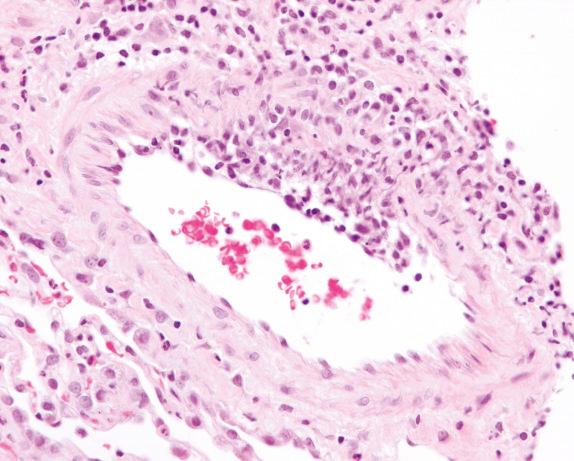
The vasculitis involves medium-sized and small veins and arteries. Capillaritis is also frequently present and an important diagnostic feature. In general, vasculitis is best appreciated in the parenchyma away from the other inflammatory lesions. The vasculitis is often focal with eccentric involvement of the vessel wall ( Fig. 8.1 ), or it can be limited to the endothelium and subendothelial spaces ( Fig. 8.2 ). The inflammatory cells that produce the vasculitis often include lymphocytes, macrophages, plasma cells, and occasionally eosinophils. A granulomatous reaction is often present within the vessel wall, and in some cases it can obscure and destroy the vessel. An elastic stain can be helpful for identification of the vessel wall within the granulomatous inflammation ( Fig. 8.3 ).

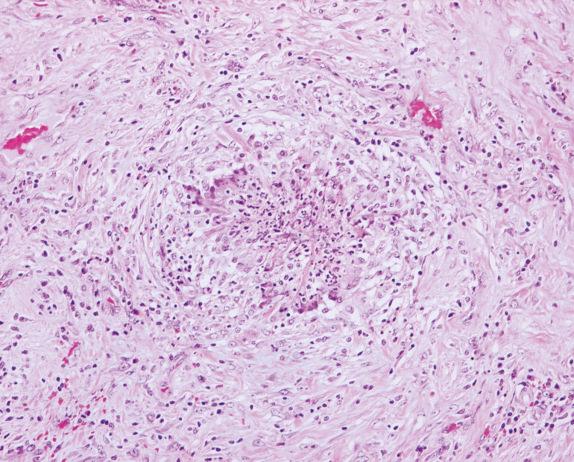
In GPA, granulomas are generally poorly formed or may show palisading histiocytes ( Fig. 8.4 ). Well-formed sarcoidlike granulomas are uncommon in GPA, and their presence should stimulate consideration of other diagnoses. In these cases, an infectious process or necrotizing sarcoidosis should be considered in the differential diagnosis. In contrast to an infectious or sarcoid granuloma, the lesions in GPA do not have a clear demarcation from the adjacent uninvolved pulmonary parenchyma, which is an important criterion for the differential diagnosis of these pathologic entities. In GPA, an inflammatory overflow always extends beyond the area of granuloma ( Fig. 8.5 ).
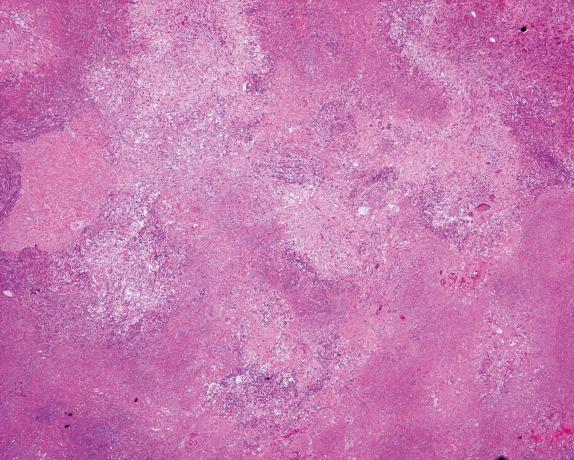
The necrosis in GPA is very often described as geographic and basophilic ( Figs. 8.5 and 8.6 , [CR] ). It is considered geographic due to its irregular border. The necrosis is thought to represent progressive collagenous necrosis and not infarctlike necrosis. It is considered basophilic because, contrary to the caseating necrosis of infectious granulomatous diseases, the area of necrosis in GPA is rich in cellular debris and neutrophils, which imparts a “dirty” appearance.
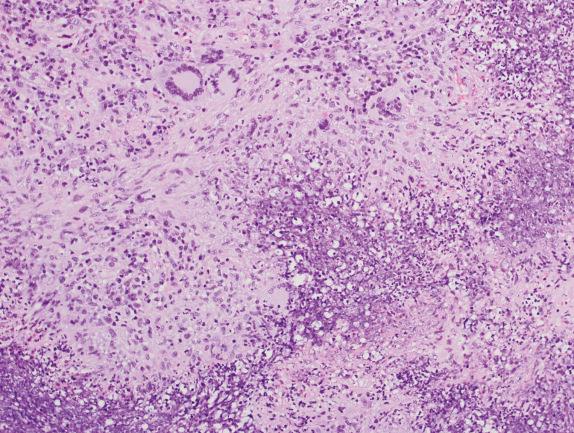
Small foci of collagen necrosis can be seen around involved blood vessels or within the pulmonary parenchyma. The necrosis is characterized by a small collection of neutrophils (microabscess) around an area of dense eosinophilic collagen fibers ( Fig. 8.7 ).
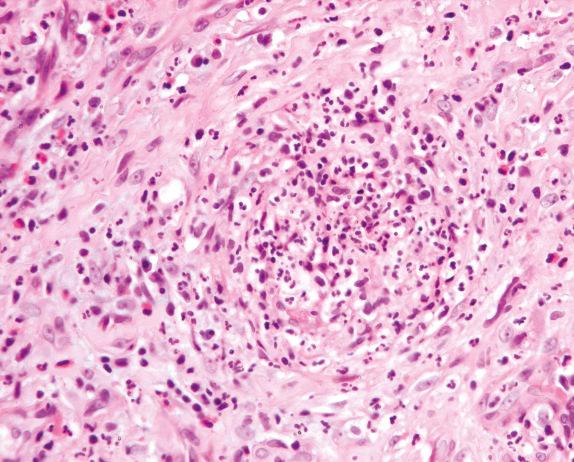
Another helpful characteristic of the inflammatory infiltrate in GPA is the presence of scattered multinucleated giant cells ( Fig. 8.8 ). They can be seen within the vasculitic process or within the background inflammatory infiltrate.
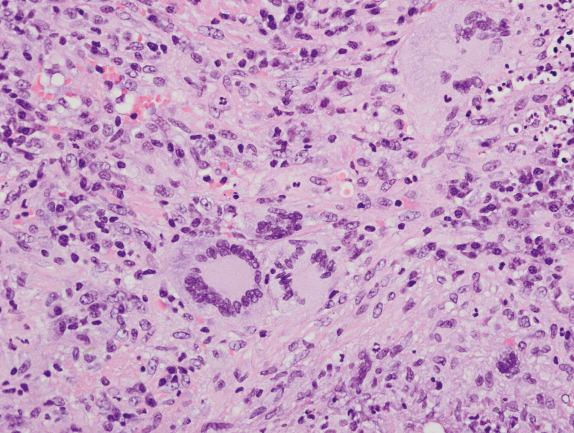
Within the inflammatory background, areas of organizing pneumonia and hemosiderin-laden macrophages can also be found. The latter is an indication of chronic hemorrhage due to the vasculitic process ( Fig. 8.9 ). Bronchitis, bronchiolitis, and endogenous lipoid pneumonia can be observed.
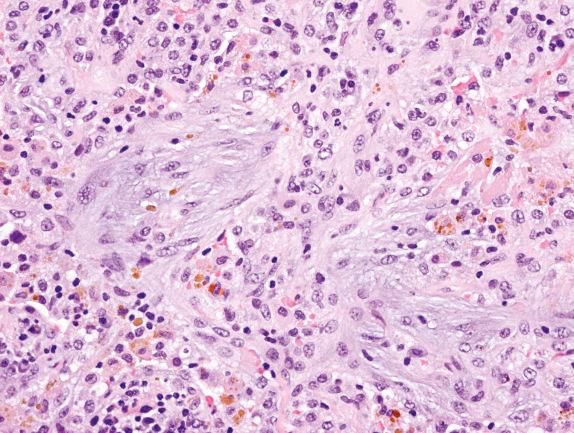
The lung biopsy findings from patients with GPA may not show the classical histologic findings, especially if the biopsy is taken very early in the course of the disease or after treatment. The presence of capillaritis with microabscess formation in a small biopsy should raise the possibility of vasculitis, including GPA. Interstitial fibrosis, sometimes with scattered giant cells, or bronchial and bronchiolar scarring or cicatricial vascular changes, can be seen in biopsies of patients who received treatment.
Lymphomatoid granulomatosis (LYG) is an important consideration in the differential diagnosis of GPA. The two entities have similar clinical and demographic features. Histologically, LYG is a form of diffuse large B-cell lymphoma characterized by a polymorphic lymphoid population with a T-cell–rich lymphocytic vasculitis, which is secondary. Granulomatous inflammation is characteristically absent. The difference between LYG and GPA is in the cellular composition of the infiltrate. The former is composed of a mixture of small and large atypical lymphocytes, whereas in GPA the infiltrate is composed of a mixture of granulomatous and acute and chronic inflammatory cells with neutrophil microabscesses. Also, in GPA, the inflammation causes necrosis of the vascular wall, whereas in LYG, the lymphocytes infiltrate the walls of the blood vessels. Immunohistochemical stains for lymphoid markers usually reveal a B-cell lymphoma phenotype in LYG with a reactive T-cell–rich infiltrate and vasculitis, discussed more extensively in another chapter.
EGPA (Churg-Strauss syndrome) and MPA are two ANCA-related diseases where the vasculitic component is almost impossible to distinguish from GPA in small biopsies. The two diseases, however, differ from GPA in their clinical and laboratory features, as well as their histology. These disorders are discussed later in this chapter. Bronchocentric granulomatosis (BCG) also enters into the differential diagnosis of GPA, because the latter may have a bronchocentric pattern of lung involvement, and BCG may show secondary involvement of blood vessels. However, systemic involvement and positive ANCA tests are seen in GPA but not BCG.
Necrotizing sarcoid granulomatosis (NSG) is characterized by confluent areas of nonnecrotizing granulomatous inflammation with extensive coagulative necrosis. Contrary to GPA, the granulomas in this disease are well formed (sarcoidlike), there is a clear demarcation between granulomatous and normal pulmonary parenchyma, and the ANCA test is negative.
The differential diagnosis of GPA and infectious granulomatous inflammation has been already discussed. An important point to keep in mind, however, is that whenever a granulomatous inflammatory process is identified in a biopsy, special stains to rule out the presence of microorganisms must be performed, even if GPA is considered clinically and histologically.
Rheumatoid nodules also form part of the differential diagnosis but usually occur in patients with a known diagnosis of rheumatoid arthritis and lack the geographic necrosis and microabscess formation seen in Wegener’s granulomatosis.
Other causes of diffuse pulmonary hemorrhage with small-vessel vasculitis (capillaritis) may also be considered in the differential diagnosis. These include drug-induced vasculitis, collagen vascular disease–associated vasculitis, cryoglobulinemia, and antiphospholipid antibody syndrome. These processes lack the inflammatory lesions of GPA and often have other associated laboratory abnormalities that can help suggest the correct etiology.
The use of corticosteroid and other immunosuppressive drugs such cyclophosphamide has revolutionized the natural course of GPA. Before the advent of these drugs, GPA was a fatal disease, with 90% of affected patients dying within 2 years of diagnosis. With therapy, approximately 90% of patients respond to treatment. Seventy-five percent of these patients experience complete remission of the disease within a year of therapy. Even with a good response to therapy, however, most patients sustain sequelae of the disease or treatment. The relapse rate is high; for approximately 50% of affected patients, a second course of treatment is required.
The addition of trimethoprim-sulfamethoxazole to the treatment regimen has been suggested to reduce the relapse rate of patients in remission.
Systemic disorder characterized by asthma, peripheral blood eosinophilia, and systemic vasculitis
Occurs almost exclusively in patients with asthma or allergic rhinitis
The true incidence of EGPA is unclear
Cardiac involvement is the most common cause of death
Early recognition of the syndrome with early treatment is important to prevent irreversible organ injury
Peak incidence is between the fourth and fifth decades of life
The diagnosis is clinical and must include at least four of the following parameters: asthma, peripheral blood eosinophilia (10% of white blood cells), neuropathy, radiographic pulmonary infiltrate, sinusitis, evidence of extravasated eosinophils in tissues, and systemic vasculitis
Common organs involved include the lungs, heart, central nervous system, kidney, gastrointestinal tract, and skin; renal involvement is less common than in GPA and microscopic polyangiitis
Three clinical phases: prodromic, eosinophilic, and vasculitic
Symptoms depend on the organ system(s) involved and the phase of the disease
Patients usually show a positive p-ANCA test result (70%)
Multifocal parenchymal consolidation that changes in location and appearance
Very often the consolidation shows a peripheral distribution
Cavitation is rare
Generally has a good prognosis, but cardiac and severe gastrointestinal involvement are associated with a poor prognosis
Most patients with EGPA show a good response to corticosteroids
Cyclophosphamide is added in cases refractory to corticosteroids or with involvement of more than one organ system
The relapse rate is approximately 30%
Peripheral patchy nodular consolidations
Cavitation is extremely rare
Microscopic findings depend on the phase of the disease
Eosinophilic pneumonia, gastroenteritis, or lymphadenitis are typical of the eosinophilic phase
Asthmatic bronchitis is usually present
Granulomas with palisading borders, multinucleated giant cells, and necrotic centers containing eosinophils are frequent
Vasculitis with fibrinoid necrosis containing eosinophils that affects small arteries, venules, and capillaries, is typical
Diffuse pulmonary hemorrhage with small-vessel vasculitis (capillaritis) can be seen
Samples obtained after corticosteroid therapy may lack well-preserved eosinophils
Bronchoalveolar lavage fluid contains eosinophils
Pleural fluid often contains eosinophils
Special stains to rule out microorganisms must be done in settings of granulomatous inflammation; no microorganisms are seen in EGPA
Eosinophilic pneumonia associated with drugs, infections, etc. (see Chapter 17 ).
Allergic bronchopulmonary fungal disease
Wegener’s granulomatosis (granulomatosis with polyangiitis)
Microscopic polyangiitis
EGPA, previously known as Churg-Strauss syndrome, is a systemic disorder characterized by asthma, peripheral eosinophilia, and systemic vasculitis. EGPA is basically a clinical entity, and its diagnosis is established in most cases based on clinical and laboratory findings. The parameters for the clinical diagnosis of EGPA are as follows:
Prodromic phase: asthma or history of allergy, including allergic rhinitis.
Eosinophilic phase: peripheral eosinophilia greater than 10% of white blood cell count, nonfixed radiographic pulmonary infiltrates, and a biopsy containing extravascular eosinophils in any or multiple organs.
Vasculitic phase: presence of vasculitis of medium and small vessels associated with granulomatosis and eosinophils. The vasculitis phase is life threatening if not recognized and treated.
Although the true incidence of EGPA is unclear, it most commonly affects patients between the third and fifth decades of life. It is usually seen in patients with asthma, some of whom have inadequate use of corticosteroids or a history of corticosteroid tapering. EGPA progresses through three clinical phases: prodromic, eosinophilic, and vasculitic. In the eosinophilic phase, the patient experiences peripheral blood eosinophilia and infiltration of eosinophils in the tissues. In the lung, this phase is characterized by the presence of eosinophilic pneumonia, with infiltrates of eosinophils in alveolar spaces. Another common site for eosinophilic tissue infiltration is the gastrointestinal tract. The vasculitic phase is characterized by systemic signs and symptoms such as neuropathy and cutaneous leukocytoclastic vasculitis. A postvasculitic phase is often characterized by neuropathy. Cardiac involvement is seen in approximately 47% of patients with EGPA, a striking difference from other systemic vasculitides that involve the lung. The cardiac involvement can manifest as cardiac failure, pericarditis, and myocardial infarction. Contrary to GPA, renal involvement in EGPA is less frequent and less severe.
No laboratory test is specific for EGPA. Apart from peripheral eosinophilia and bronchoalveolar lavage fluid containing eosinophils, patients show nonspecific laboratory abnormalities such as chronic normocytic-normochromic anemia and elevated sedimentation rate. The ANCA test is often positive in the vasculitic phase and will usually show the p-ANCA pattern (70% of the cases), although a c-ANCA pattern has also been demonstrated.
The radiologic findings in EGPA are nonspecific. The disease can manifest with multifocal parenchymal consolidation or nodules that wax and wane or change location. The consolidation is generally in the periphery of the lung but can be widespread.
The histopathologic findings in biopsies of patients with EGPA depend on the phase of the disease. In the prodromic/eosinophilic phase, eosinophilic pneumonia is the most common finding ( Fig. 8.10 ). Extravasation of eosinophils in other anatomic sites or eosinophilic bronchitis can also be seen.
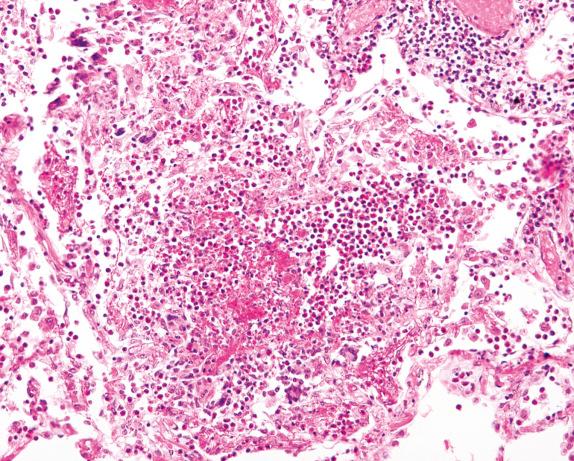
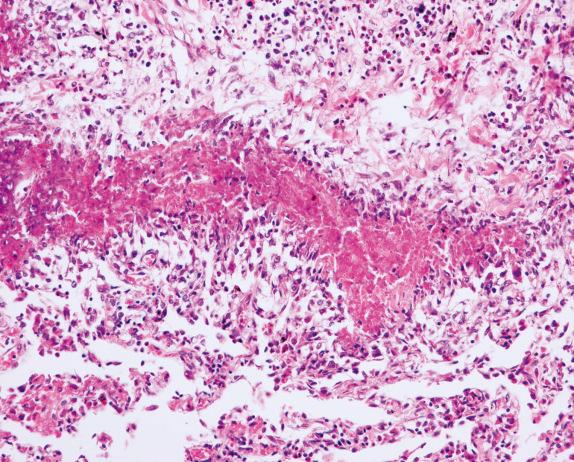
In the vasculitic phase, besides eosinophilic pneumonia, eosinophilic granulomas (allergic granulomas) and eosinophilic vasculitis can be seen. The granulomas are extravascular and consist of a necrotic center with eosinophils surrounded by palisading histiocytes ( Fig. 8.11 ). Multinucleated giant cells can be present. The vasculitis involves arteries, veins, and capillaries ( Fig. 8.12 ). The inflammatory infiltrate includes lymphocytes, neutrophils, plasma cells, and epithelioid macrophages in addition to eosinophils. Diffuse pulmonary hemorrhage can occur in association with eosinophilic capillaritis.
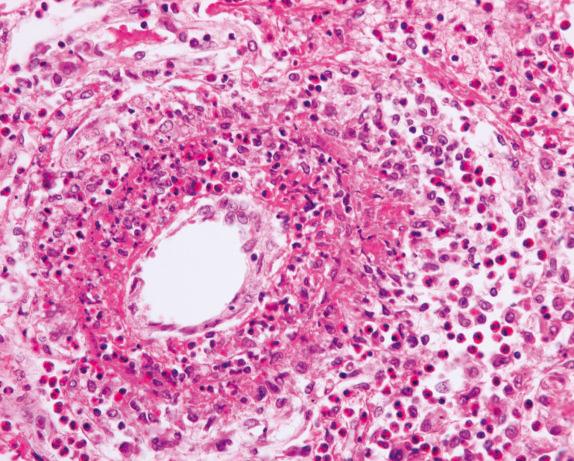
Eosinophils are very sensitive to corticosteroid therapy. Therefore in patients who have been treated with corticosteroids, the characteristic eosinophils can be absent or reduced in numbers and may appear fragmented. These degenerative changes can make histologic interpretation more difficult, and it is possible to be misled into interpreting degenerated eosinophils as neutrophils. However, the larger size of the nuclear lobes in eosinophils compared with neutrophils is a helpful clue, as well as the more eosinophilic appearance of the background compared with the more basophilic appearance of the necrotic/inflammatory background with degenerating neutrophils.
The differential diagnosis of EGPA includes other vasculitides and other pathologic entities characterized by the presence of eosinophils.
EGPA is distinguished from GPA by the clinical presentation, histopathology, and usual presence of c-ANCA (as opposed to p-ANCA in most patients with EGPA). Although eosinophils can be seen in GPA, they are not the predominant cells of the inflammatory infiltrate, and the range of histologic findings differs, as discussed earlier. The ANCA test, although not specific, can be helpful in the differentiation between the two entities. Clinically, EGPA is characterized by a spectrum of manifestations that differ from those of GPA, and most patients have peripheral blood eosinophilia. In contrast, renal involvement is much more common in GPA.
Eosinophilic pneumonia caused by other entities such as parasitic infections or drugs must be differentiated from EGPA. Clinical information indicating a systemic process (prodromic phase–asthma and allergic rhinitis) is helpful for making the correct diagnosis. Eosinophilic pneumonia can be an early manifestation of EGPA (eosinophilic phase). Observation of vasculitis is also helpful for supporting a diagnosis.
Allergic bronchopulmonary fungal disease (ABPFD) can show eosinophilic pneumonia and bronchocentric granulomas. Unlike EGPA, in ABPFD signs and symptoms of systemic vasculitis are absent.
Patients with EGPA can usually be treated successfully with corticosteroids. Other immunosuppressive agents such as cyclophosphamide can also be used to prevent irreversible organ injury in patients who do not respond to corticosteroids.
If untreated, EGPA is a fatal disease. Most patients who die of the disease have cardiac complications such as myocardial infarction and cardiac insufficiency. Other less common causes of death include renal failure, cerebral hemorrhage, respiratory failure, and gastrointestinal perforation.
Necrotizing vasculitis that affects small vessels (arterioles, venules, and capillaries) with few or no immune complex deposits
Rare (estimated incidence in the United States is 0.1 per million persons)
Causes of death include multiorgan system vasculitis and complications of treatment with immunosuppressive drugs
Approximately 25% of patients have persistent pulmonary abnormalities after complete recovery
Peak incidence is between the fourth and sixth decades (mean age 56 years)
Slight female predominance (1.5:1)
No racial predilection
Affects predominantly the lungs and kidneys and is the most common cause of pulmonary-renal syndrome
50% of cases show pulmonary involvement
Most patients have a rapid onset of symptoms
Most common clinical manifestation is glomerulonephritis
Most common pulmonary finding is alveolar hemorrhage
Systemic symptoms include arthralgias, myalgias, fever, weight loss, dyspnea, hemoptysis, cough, and chest pain
80% of patients have a positive p-ANCA test result
Bilateral alveolar opacities (pulmonary hemorrhage) without nodules
The lower lobes are affected more frequently
Treatment includes immunosuppressive drugs (corticosteroids and cyclophosphamide are the most commonly used drugs)
Complete recovery is seen in approximately 70% of patients
Relapse rate is approximately 40%
Lungs are dark due to pulmonary hemorrhage
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here