Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Papillary endothelial hyperplasia (PEH) may occur in any location, but most commonly presents in the superficial soft tissues of the extremities, head, and neck. The thumb is a common location. A history of trauma is occasionally present. PEH may be superimposed on a pre-existing benign vascular lesion, such as a cavernous hemangioma.
PEH typically presents as a small, firm, red to blue mass in the superficial soft tissues. Most cases are predominantly intravascular, and the lesion may be surrounded by the remnants of a vessel wall or by a fibrous pseudocapsule. In bone, multiple tumor nodules are typically present in the cortex and/or medulla, involving one to several bones.
Reactive proliferations of endothelial cells, sometimes with known infectious or paraneoplastic etiology
Rare
Typically cutaneous or mucosal
Pyogenic granulomas are entirely benign
Diffuse dermal angiomatosis is observed in patients with underlying medical conditions, sometimes with a poor prognosis (i.e., calciphylaxis)
Bacillary angiomatosis responds to antibiotics and treatment of underlying immunosuppression
Glomeruloid hemangioma is usually seen with POEMS syndrome, which has only a 60% 5-year survival
None for pyogenic granuloma and diffuse dermal angiomatosis
Bacillary angiomatosis is more common in HIV-positive men but may be seen in any immunosuppressed patient
Glomeruloid hemangioma occurs in older patients of either sex
Pyogenic granuloma presents as an ulcerated, polypoid mass
Diffuse dermal angiomatosis presents as a violaceous, sometime ulcerated, plaque
Bacillary angiomatosis presents as multiple, elevated, pink skin, or mucosal lesions
Glomeruloid hemangioma presents as small, red to violaceous cutaneous papules, usually on the trunk and proximal extremities
Excellent prognosis for pyogenic granulomas with simple excision
Prognosis for diffuse dermal angiomatosis depends on underlying medical conditions
Bacillary angiomatosis has excellent prognosis with antibiotic therapy, particularly if underlying immunosuppression can be corrected
Glomeruloid hemangioma is treated with simple excision; treatment of underlying cause of POEMS syndrome may be more difficult
Polypoid mucosal tumor or erythematous/violaceous/ulcerated skin lesion
Lobular proliferation of mitotically active, bland endothelial cells in association with ulceration and neutrophilic infiltrate in pyogenic granuloma
Infiltrative proliferation of well-formed capillaries in diffuse dermal angiomatosis, often with minute thrombi
Vaguely lobular growth pattern, capillary-sized vessels lined by epithelioid endothelial cells with clear cytoplasm, numerous stromal neutrophils, and amorphous, eosinophilic aggregates containing fibrin and bacilli in bacillary angiomatosis
Ectatic small vessels containing intraluminal nests of proliferating capillaries, resembling glomeruli, with large, vacuolated endothelial cells containing eosinophilic proteinaceous material in glomeruloid hemangioma
Expression of CD31, CD34, ERG/FLI-1 protein, and vWF
Pyogenic granuloma: angiosarcoma, Kaposi sarcoma, reactive vascular proliferations overlying other pathology
Diffuse dermal angiomatosis: angiosarcoma
Bacillary angiomatosis: granulation tissue, Kaposi sarcoma
Glomeruloid hemangioma: Dabska tumor, papillary endothelial hyperplasia
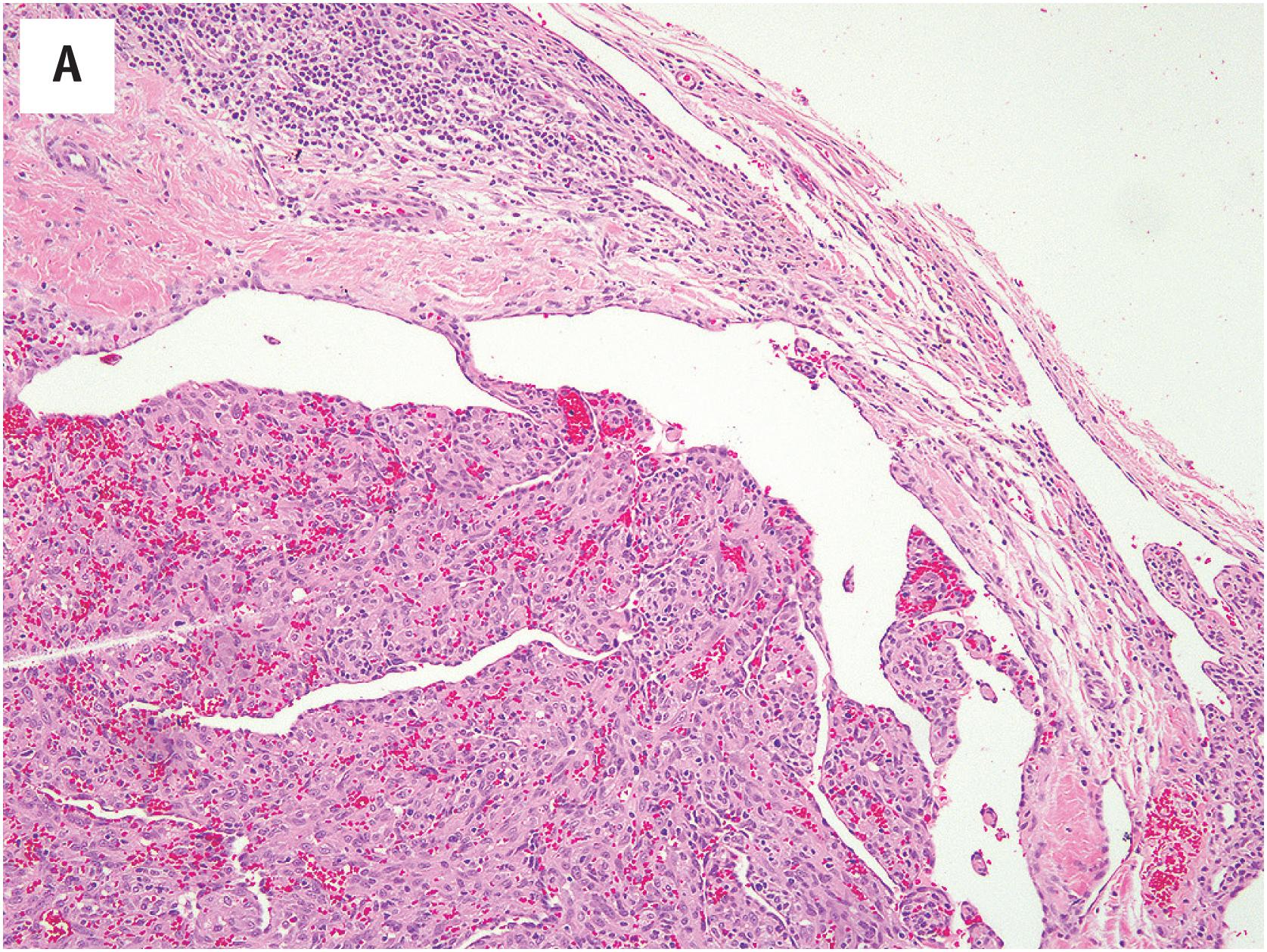
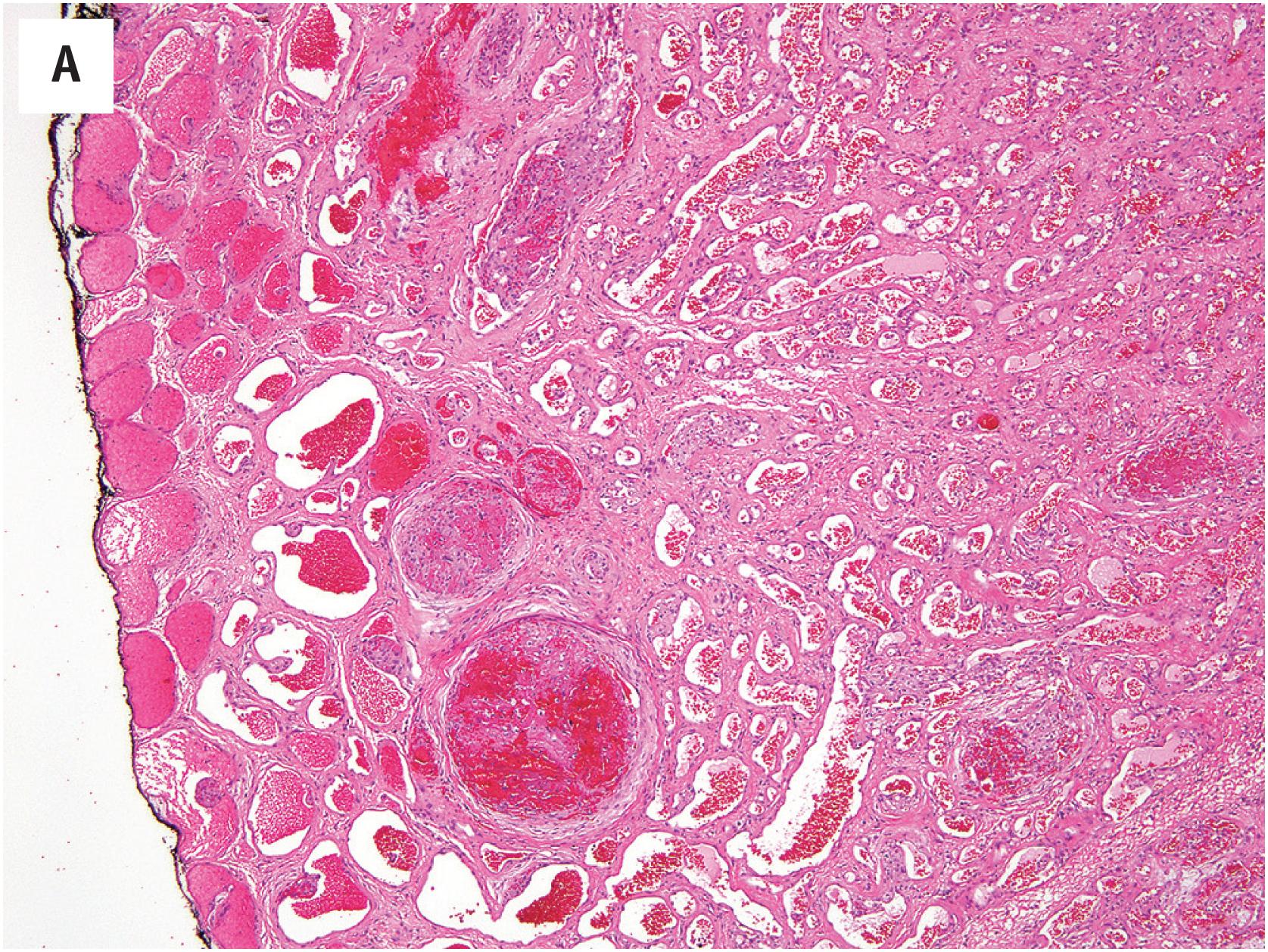
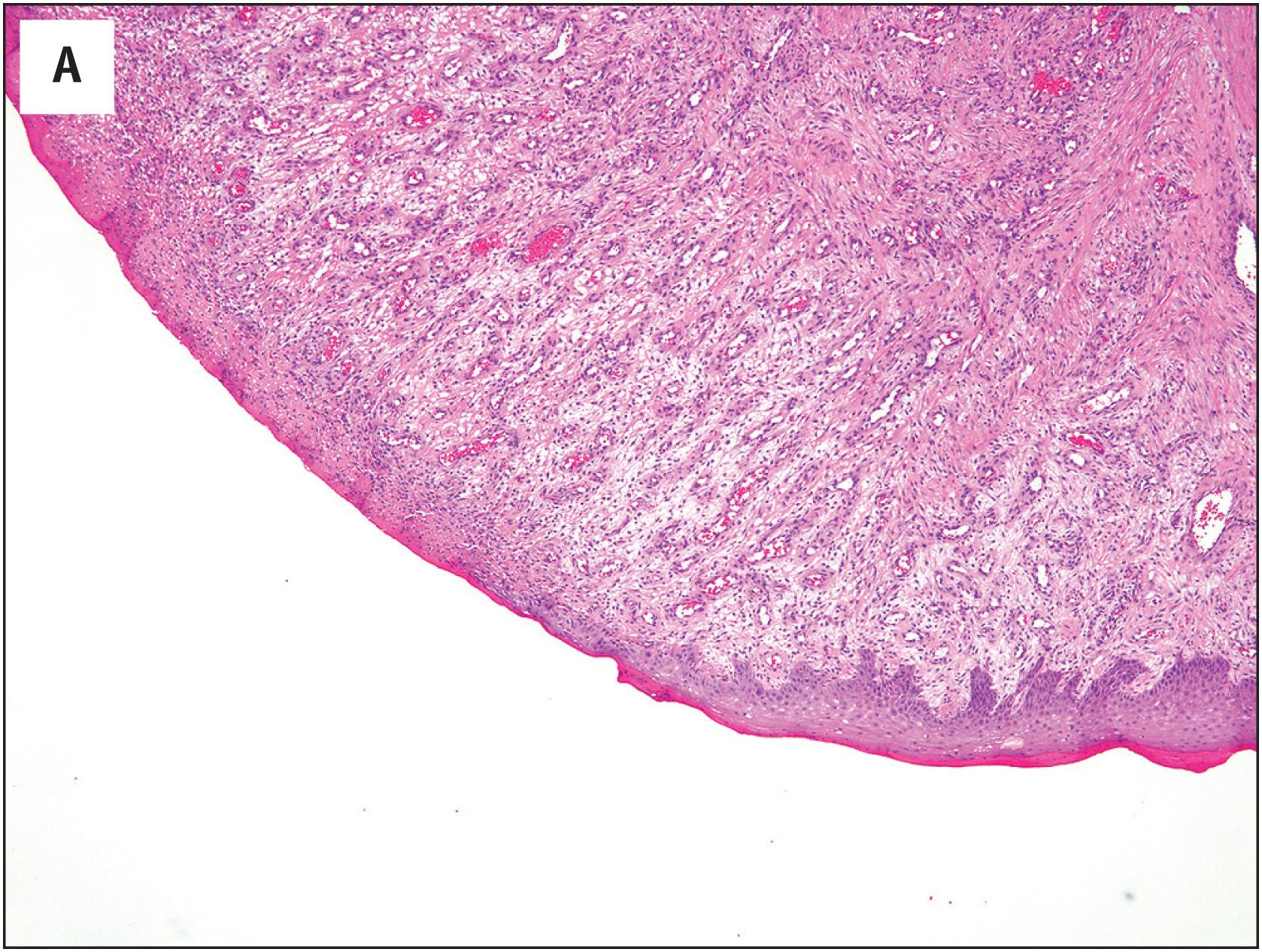
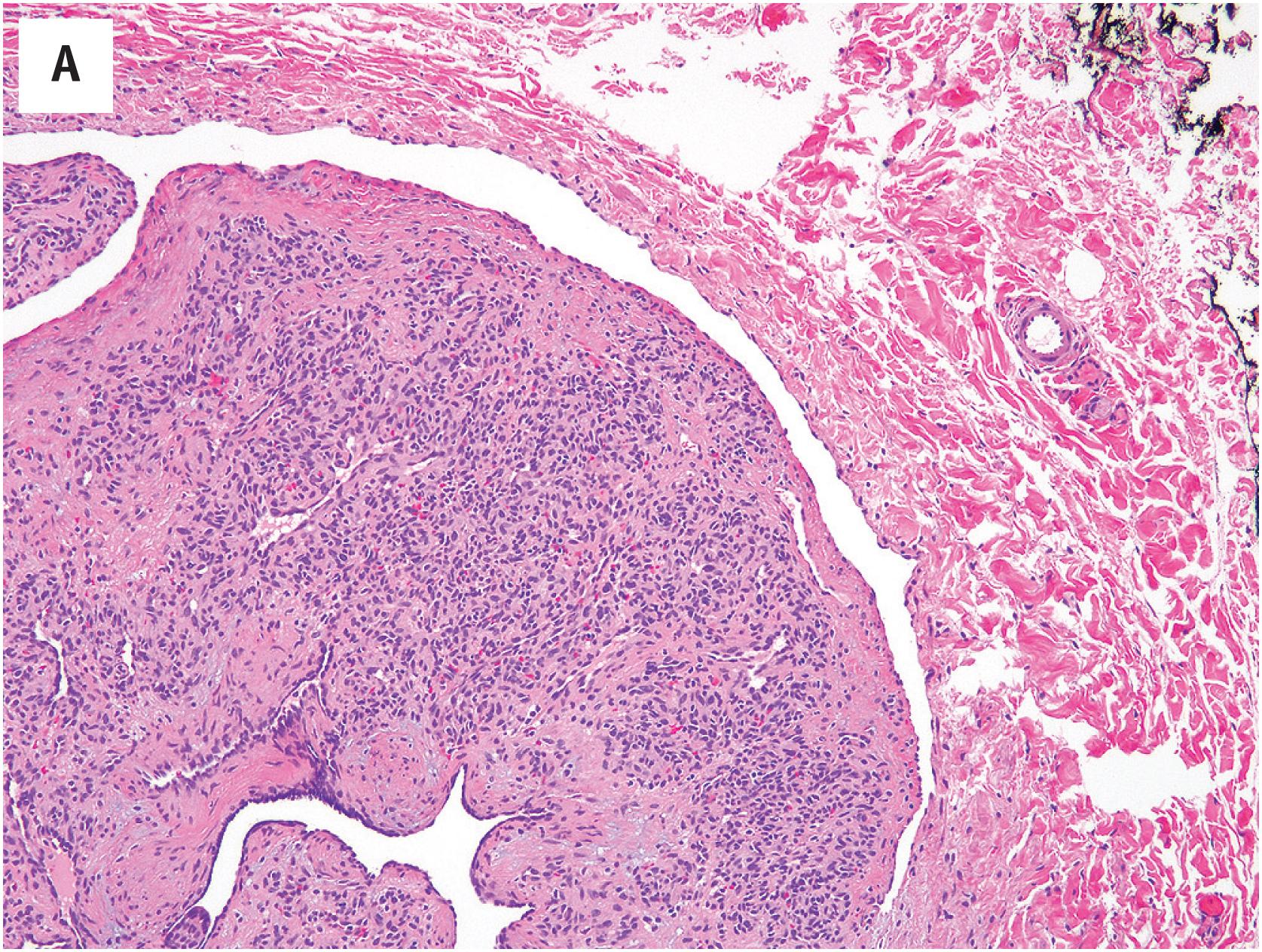
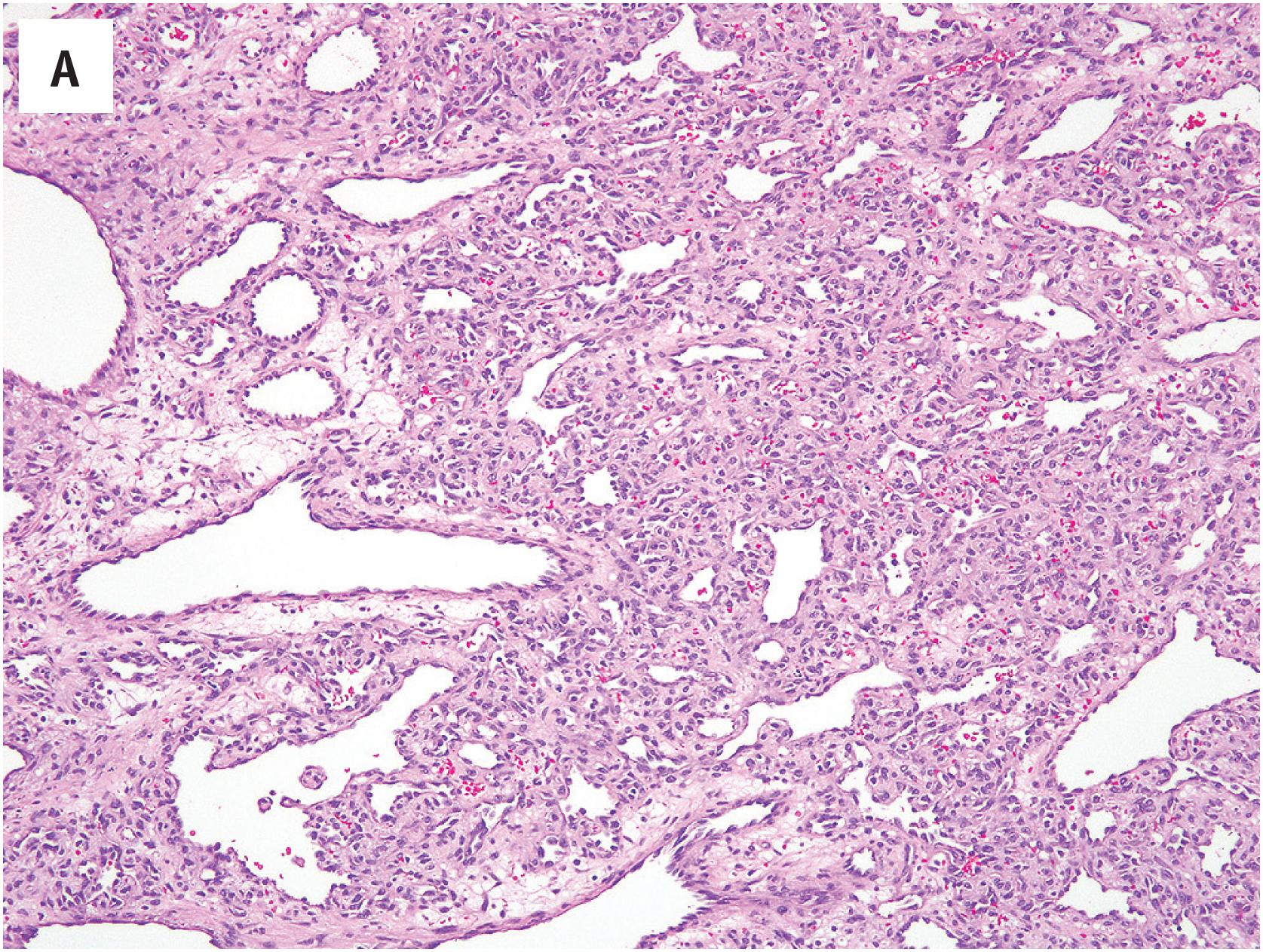
PEH usually begins within a thrombosed blood vessel, and abundant thrombotic material is typically present ( Fig. 10.1A ). On occasion, the process may extend beyond the vessel wall, and thrombotic material and proliferating endothelial cells may be observed in the adjacent soft tissues. Early lesions show ingrowth of endothelial cells into the thrombus, with the formation of pseudopapillae. These papillae are lined by plump, normochromatic endothelial cells, which grow in a single, non-stratified layer ( Fig. 10.1B ). Mitotic activity may rarely be focally present Fig. 10.1C ). The bland appearance of the endothelial cells, the overall circumscription of the lesion, and recognition of the typical features of PEH are the keys to avoiding this serious misdiagnosis. Papillary endothelial hyperplasia may also occur within other vascular tumors, such as hemangiomas ( Fig. 10.2A ). In more fully developed lesions, the endothelial proliferation may create the appearance of inter-anastomosing, sinusoidal vascular channels, simulating angiosarcoma ( Fig. 10.2B ).
The cells of PEH are differentiated endothelial cells, which express markers such as CD31, CD34, ERG/FLI1 proteins, and von Willebrand factor (vWF, factor VIII-related protein). Immunohistochemistry is seldom necessary in arriving at this diagnosis.
As noted above, the most important differential diagnostic consideration is angiosarcoma. The great majority of angiosarcomas will present as larger masses, with clear-cut nuclear atypia, frequent mitotic figures, and necrosis. In cases of angiosarcoma with foci resembling PEH, attention to the cytologic atypia of the cells lining the papillae and the presence of infiltrative growth at the periphery of the lesion should allow this distinction ( Fig. 10.3A and B ). Many cases of PEH arise within the blood vessels, a distinctly unusual feature in angiosarcoma. It is important to remember that PEH may engraft itself upon virtually any vascular tumor and onto areas of hemorrhage and thrombosis within other types of tumors. Thus, one should always hunt carefully for any evidence of a pre-existing neoplasm, particularly in deep locations.
PEH is a reactive lesion that requires only simple excision.
Diffuse dermal angiomatosis, also known as “reactive angioendotheliomatosis,” is a rare, reactive vascular proliferation which usually occurs in patients with underlying medical diseases (e.g., peripheral vascular disease, calciphylaxis, renal failure, collagen vascular disease, and arteriovenous fistulae ) . In the skin of the breast, identical vascular proliferation can be seen in women with macromastia, obesity, and a history of cigarette smoking.
Diffuse dermal angiomatosis often presents as a violaceous plaque, sometimes with associated ulceration.
This process consists of an exuberant, infiltrative proliferation of small, well-formed capillaries and other small vessels, occasionally with tiny fibrin thrombi ( Fig. 10.4A ). A lobular growth pattern is typically absent. Although the infiltrative growth pattern is worrisome, endothelial atypia and multilayering are absent, and mitotic figures are generally few in number and normal ( Fig. 10.4B ). A thin smooth muscle coat is present on close inspection, a reassuring feature ( Fig. 10.4C ).
Immunostains to smooth muscle actins can be helpful in identifying the vascular smooth muscle surrounding the infiltrative small vessels.
Diffuse dermal angiomatosis is most often mistaken for angiosarcoma. The well-formed nature of the proliferating vessels, the presence of smooth muscle surrounding them, and the presence of small thrombi suggest a benign lesion. Fat necrosis and calcium deposition in the vessels and fibroadipose tissue may be seen with associated calciphylaxis ( Fig. 10.4D ). Clinical correlation with regard to the presence of an underlying medical disorder is also of great assistance in excluding a more ominous vascular lesion.
Diffuse dermal angiomatosis is a reactive process. The treatment should be aimed at the underlying medical condition.
Pyogenic granulomas typically occur on the mucosal surfaces, particularly the mouth and the skin. The lesions may occasionally be multiple and arise following trauma in roughly one-third of cases. The tumors often have an initial period of rapid growth, followed by stabilization and occasionally regression. Identical mucosal lesions may arise spontaneously during pregnancy (granuloma gravidarum). Rarely this process may occur within a small vein, so-called “intravascular pyogenic granuloma.”
Pyogenic granulomas are typically small, polypoid lesions, which are often ulcerated and friable.
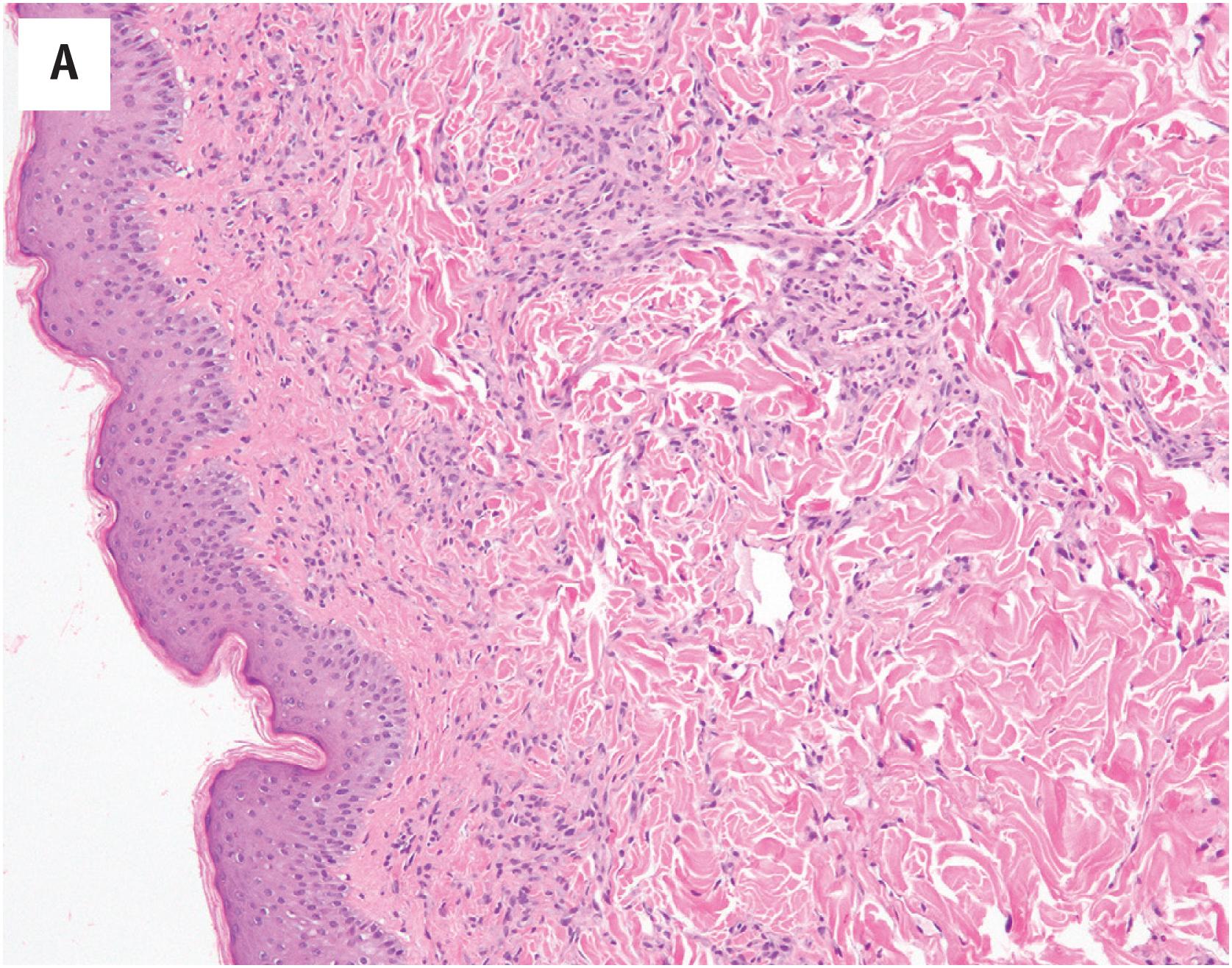
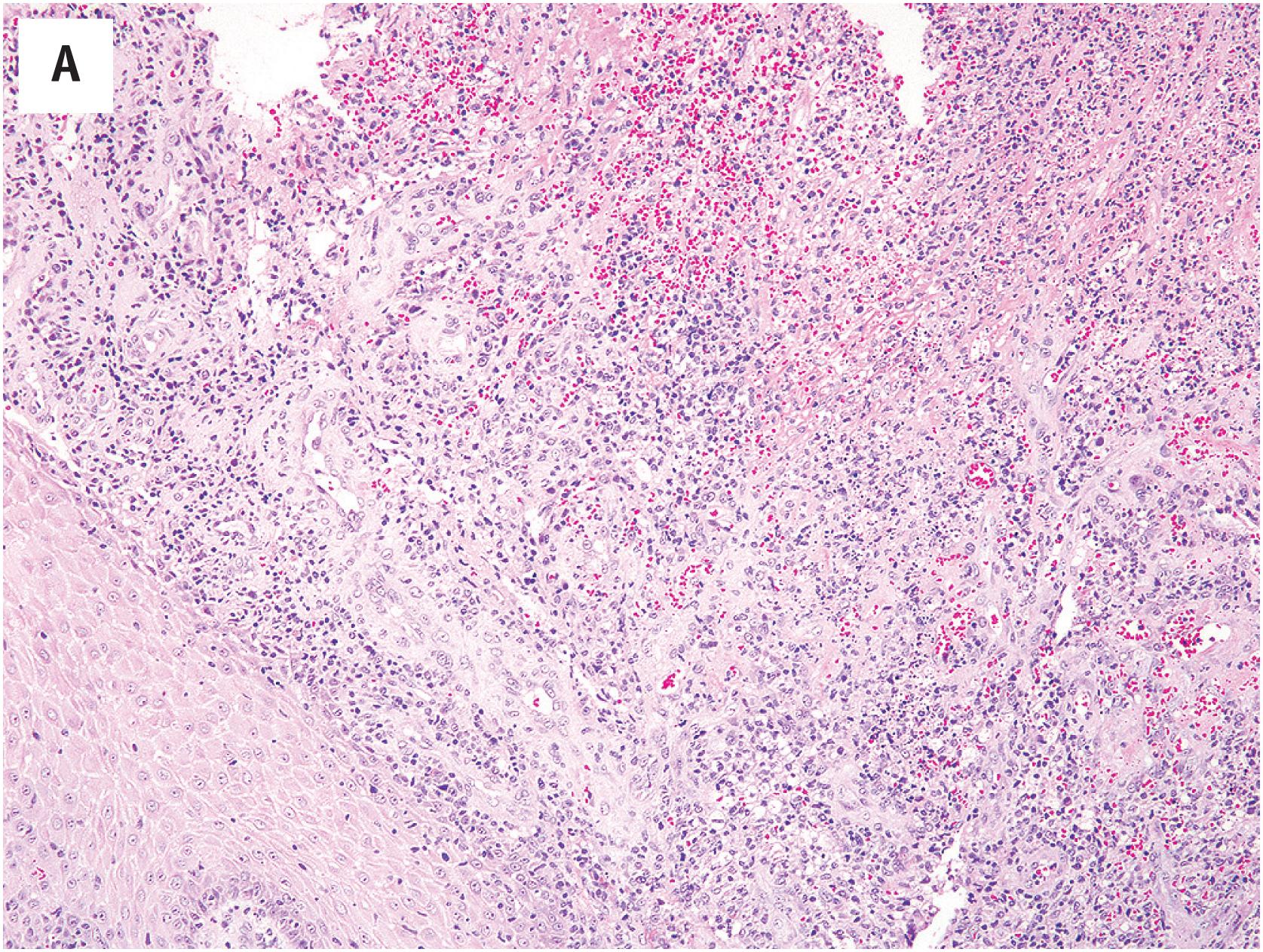
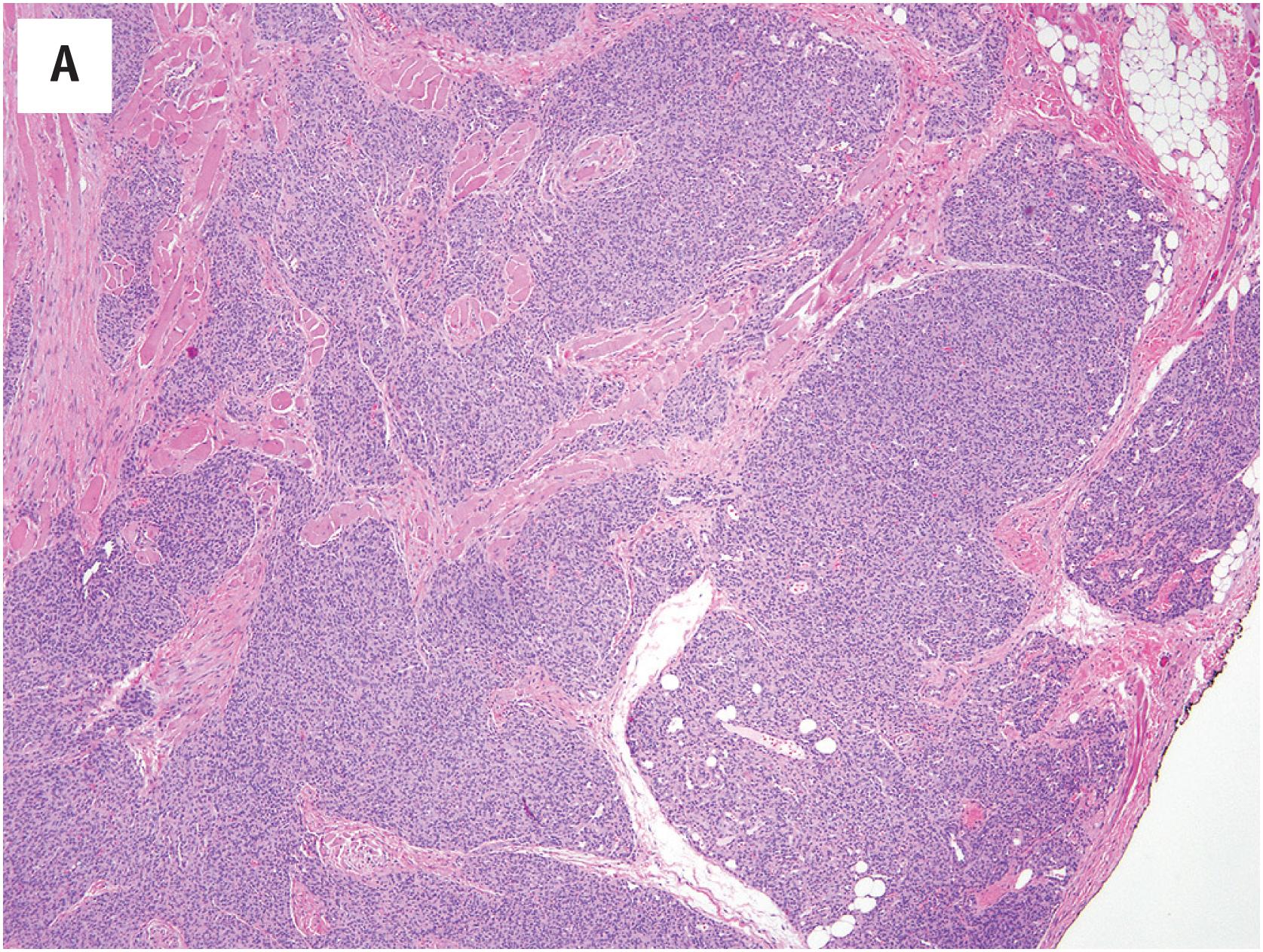
At low-power magnification, pyogenic granulomas are exophytic, polypoid masses which are often surrounded by an epidermal collarette ( Fig. 10.5A ). The lesions are well–demarcated and distinctly lobular, with central larger vessels and peripheral aggregates of well-formed capillaries. A mixed acute and chronic inflammatory cell infiltrate is almost always present, similar to that in granulation tissue ( Fig. 10.5B ). The mitotic activity is often brisk. Necrosis may be observed in association with surface ulceration. Lesions identical to pyogenic granuloma/lobular capillary hemangioma may also occasionally be observed in intravascular locations (intravascular pyogenic granuloma) ( Fig. 10.6A and B ). Intranasal pyogenic granulomas often exhibit extensive stromal hyalinization and myxoid change, and may be mistaken for angiosarcoma, nasal angiofibroma or “hemangiopericytoma” ( Fig. 10.7 ).
The cells of pyogenic granuloma express endothelial markers such as CD31, CD34, ERG/FLI1 protein, and vWf.
The sometimes high cellularity and brisk mitotic activity of pyogenic granuloma occasionally creates concern for an angiosarcoma. The most important features distinguishing these two entities are the circumscription and lobular growth pattern present in pyogenic granuloma and absent in angiosarcoma. Kaposi sarcoma may on occasion exhibit superficial zones with a vaguely lobular growth pattern, suggestive of pyogenic granuloma. However, the examination of deeper portions of the biopsy will invariably reveal infiltrative growth, with the formation of slit-like vascular spaces by hyperchromatic, vaguely myoid-appearing spindled cells. In difficult cases, immunohistochemistry for HHV-8 LANA protein (present in Kaposi sarcoma, absent in pyogenic granuloma) may be helpful. It is also important to recognize that areas reminiscent of pyogenic granuloma may be identified at the surface of almost any ulcerated lesion, including oral squamous cell carcinomas, for example, and one should examine the entire specimen, including the deepest portions, carefully before definitely making this diagnosis. The identification of more typical areas is the key to recognizing extensively hyalinized and/or myxoid nasal pyogenic granulomas.
Pyogenic granulomas are usually cured with simple excision. These lesions may recur locally in 10%–15% of cases, particularly when incompletely excised, and occasionally recur as multiple satellite lesions.
Bacillary angiomatosis is a reactive vascular proliferation caused by infection with the bacteria Bartonella henselae and B. quintana . It occurs almost exclusively in immunocompromised patients, typically men with HIV, and usually presents as multiple, elevated, pink skin or mucosal lesions. This process may also involve the visceral organs, such as the liver, where it produces peliosis.
Bacillary angiomatosis usually exhibits a vaguely lobular growth pattern, with capillary-sized vessels lined by epithelioid endothelial cells with clear cytoplasm ( Fig. 10.8A ). Numerous stromal neutrophils are observed, as are amorphous, eosinophilic aggregates containing fibrin and bacilli ( Fig. 10.8B ). The bacilli may be identified with a Warthin-Starry stain. In the liver, peliosis hepatis is identified, with surrounding aggregates of fibrin and bacilli.
The cells of bacillary angiomatosis have a typical endothelial immunophenotype.
Occasionally the vaguely lobular growth pattern and the clear endothelial cells may be relatively inapparent, and bacillary angiomatosis may closely resemble ordinary granulation tissue. Clinical correlation with regard to immunosuppression may be very valuable here. Careful inspection should reveal the characteristic clear endothelial cells and eosinophilic debris identified in bacillary angiomatosis. The clinical setting of bacillary angiomatosis also often raises the possibility of Kaposi sarcoma, a lesion which has as its hallmark spindled cells forming slit-like vascular spaces, rather than lobules of clear, epithelioid cells.
The therapy for bacillary angiomatosis is aimed at eradicating the underlying infectious etiology, most often with erythromycin.
Glomeruloid hemangioma is an extremely rare reactive vascular proliferation usually identified in patients with POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M-protein, skin lesions). Such lesions are seen in approximately 25%–45% of patients with this syndrome. Although patients with POEMS syndrome may present with other benign vascular tumors (e.g., microvenular hemangioma, cherry hemangioma), glomeruloid hemangiomas are the most characteristic vascular tumor in this syndrome. Occasional glomeruloid hemangiomas occur in patients without other features of POEMS syndrome.
Glomeruloid hemangiomas present as small, red to violaceous cutaneous papules, usually on the trunk and proximal extremities.
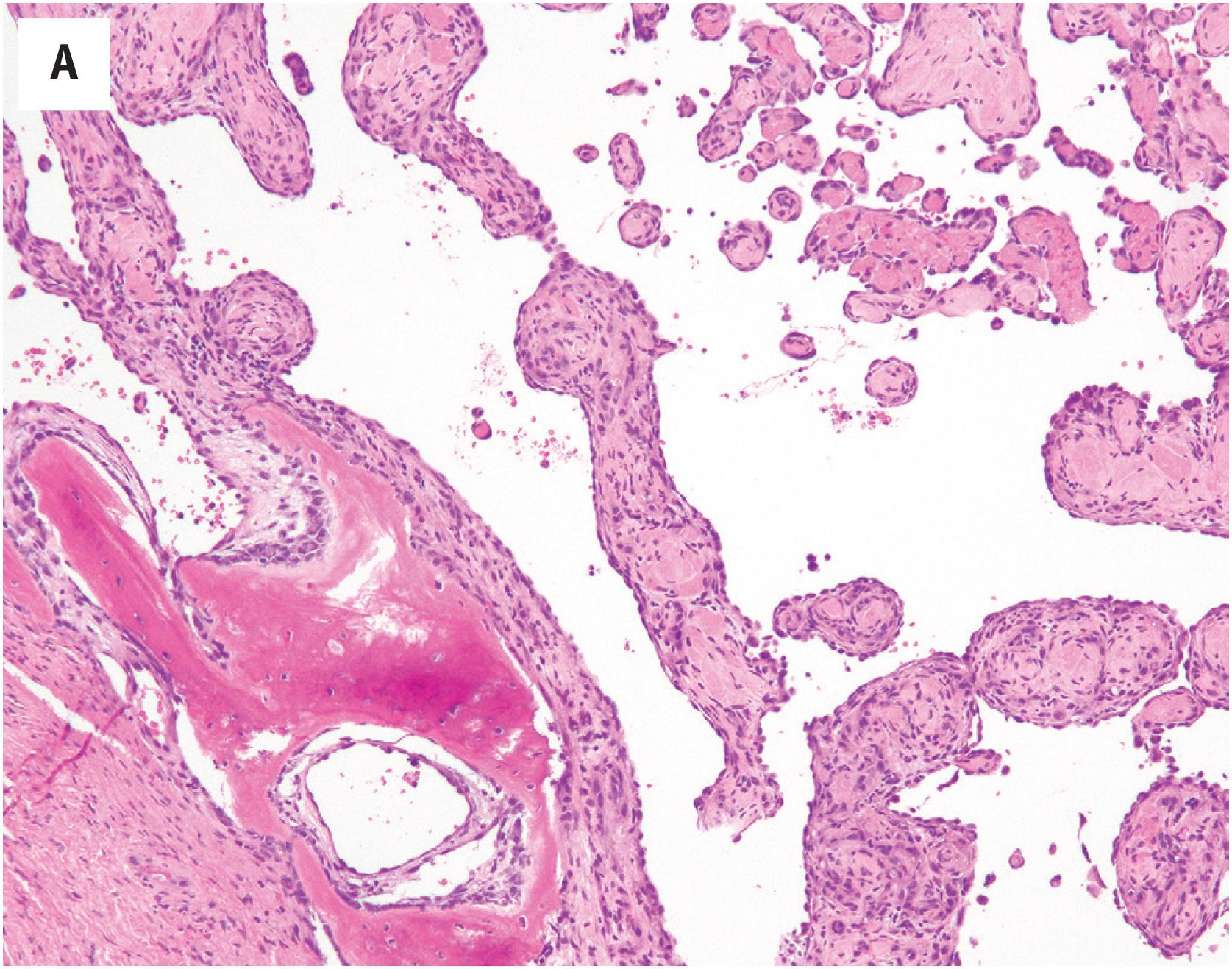
Glomeruloid hemangiomas are dermal lesions, consisting of collections of ectatic small vessels containing intraluminal nests of proliferating capillaries, resembling glomeruli ( Fig. 10.9A ). A distinctive feature is the presence of large, vacuolated endothelial cells containing eosinophilic proteinaceous material (polytypic immunoglobulin) ( Fig. 10.9B ), staining for PAS-D ( Fig. 10.9C ).
Glomeruloid hemangiomas express markers of normal endothelium, such as CD31, s, ERG/FLI1 proteins, and vWf.
The differential diagnosis of glomeruloid hemangioma principally includes other vascular tumors containing intraluminal papillations. Papillary endothelial hyperplasia contains thrombotic material, exhibits fibrous stalks lined by plump endothelial cells, and lacks vacuolated cells with eosinophilic inclusions. Papillary intralymphatic angioendothelioma (Dabska tumor, malignant endovascular papillary angioendothelioma) almost always occurs in young children and has dilated, lymphatic-like spaces, surrounding fibrosis, and hyalinized papillae lined by hyperchromatic, “hobnailed” endothelial cells. Intravascular pyogenic granuloma occurs within the larger vessels and has a distinctly lobular growth pattern.
Glomeruloid hemangioma is a reactive lesion that requires only simple excision. Patients with POEMS syndrome have a poor prognosis, with a 5-year survival of approximately 60%.
Capillary hemangiomas are the most common subtype of hemangioma, and are the most common subtype of soft tissue tumor in infants and children. Many capillary hemangiomas present either at birth or immediately thereafter and have an initial period of rapid growth, followed by stabilization and eventual involution. In approximately 15%–20% of infantile cases multiple hemangiomas are identified. Rare familial capillary hemangioma syndromes have been reported in association with mutations in chromosome 5. The skin and oral mucosa are by far the most common locations for capillary hemangiomas, although they may occur in essentially any location, including the viscera. In infants, extremely large capillary hemangiomas of the head and neck may be seen as part of the PHACES syndrome ( p osterior fossa abnormalities, h emangiomas of the cervicofacial region, a rterial, c ardiac and ocular abnormalities, s ternal clefting). Also in infants, lumbosacral hemangiomas have been associated with occult spinal malformations and other anomalies. Infantile hemangiomas present after birth, grow rapidly, and then regress over several years. Congenital hemangiomas, as the name would suggest, are present at birth and may rapidly involute (rapidly involuting capillary hemangioma, RICH) or not involute (non-involuting capillary hemangioma, NICH). The definitive diagnosis of childhood hemangiomas typically requires clinical correlation.
There is great variation in the gross appearance of capillary hemangiomas, depending on their size and depth. Small, superficial hemangiomas may appear as red macules, whereas larger, deeper hemangiomas may appear as firm, blue-violet, tumorous masses.
All capillary hemangiomas grow as multinodular, well-circumscribed, distinctly lobular proliferations of well-formed capillaries, lined by bland endothelial cells ( Fig. 10.10A and B ). In very young children these lesions may be highly cellular and appear solid, with only subtle lumen formation, due to protrusion of the plump endothelial cells into the lumens and the large numbers of pericytic cells (“infantile hemangioma”) ( Fig. 10.11A and B ). Mitotic figures may be present. Such lesions may be confused with a variety of pediatric round cell malignancies. In older patients (and older tumors) the lumen formation is typically much more obvious, as the endothelial cells flatten out and the individual vessels are surrounded by increasing amounts of fibrous connective tissue ( Fig. 10.12 A and B ). Very old lesions may be largely fibrotic, with only scattered thin-walled vessels arranged in a vaguely lobular pattern.
Capillary hemangiomas, particularly cellular infantile variants, typically contain both endothelial cells (CD31, CD34, ERG/FLI1 proteins, and vWf-positive) as well as pericytic cells (smooth muscle actin-positive). The glucose transporter protein Glut-1 is expressed in all infantile capillary hemangiomas, but not in other vascular malformations or neoplasms; this finding may suggest differentiation along the lines of placental capillary endothelium, rather than the normal adult capillary endothelium. Congenital hemangiomas lack Glut-1 expression.
Mutations in the GNAQ and GNA11 genes have been identified in NICH and RICH.
Highly cellular infantile capillary hemangiomas may be mistaken for a pediatric round cell malignant neoplasm, such as Ewing sarcoma or rhabdomyosarcoma. This is particularly true if one is evaluating overly thick sections. The careful evaluation of properly prepared sections from even the most cellular infantile hemangioma will reveal subtle lumen formation, often best observed at the periphery of the tumor. Additionally, infantile hemangiomas are well-circumscribed, unlike malignant round cell tumors, which are infiltrative. Immunohistochemical demonstration of endothelial marker expression may be very helpful in especially cellular cases. Large capillary hemangiomas may also be confused with kaposiform hemangioendothelioma, and vice versa. Kaposiform hemangioendotheliomas are usually larger than are capillary hemangiomas, and have infiltrative margins. Although kaposiform hemangioendotheliomas exhibit vaguely lobular zones composed of well-formed capillaries, they also contain areas composed of spindled cells forming slit-like vascular spaces, and glomeruloid structures containing fibrin microthrombi and fibrotic stroma, features not seen in capillary hemangioma. Kaposiform hemangioendotheliomas do not express Glut-1 protein, unlike cellular infantile capillary hemangiomas. Glut-1 is not helpful in the distinction of congenital hemangiomas from kaposiform hemangioendothelioma.
Treatment of capillary hemangiomas is based on the age of the patient and their location and size. Many tumors in children will spontaneously involute, and these can simply be followed clinically. Larger tumors or those affecting vital structures have been successful treated with glucocorticosteroids and/or interferon-alpha. A role for anti-angiogenesis agents in the treatment of unresectable tumors has also recently been proposed.
Benign, often-regressing tumor of endothelial cells
Most common soft tissue tumor of childhood
Arise before birth (NICH and RICH) and after birth (infantile hemangioma)
Most common in the skin; may involve any location
Benign, frequently regress spontaneously
Large capillary hemangiomas may be cosmetically deforming
Some are associated with various syndromes and congenital malformations
No gender or sex predilection
Infants and young children
Small, superficial hemangiomas appear as red macules
Larger, deeper hemangiomas appear as firm, blue-violet, tumorous masses
Many spontaneously regress
Interferon therapy effective for large tumors in potentially dangerous locations
Anti-angiogenic therapy may also be effective for some
Polypoid mucosal tumor or erythematous skin lesion
Multinodular, well-circumscribed, distinctly lobular proliferations of well-formed capillaries, lined by bland endothelial cells
May be highly cellular, mitotically active, and solid-appearing in young children
Old lesions may be largely fibrotic, with only scattered thin-walled vessels arranged in a vaguely lobular pattern
Express CD31, CD34, ERG/FLI-1 protein, and vWF
Glut-1 positive
Kaposiform hemangioendothelioma
Pediatric round cell tumors
Cavernous hemangiomas are now considered to represent a form of venous malformation, rather than a true neoplasm. However, the term “cavernous hemangioma” remains widely used and is understood by clinicians, and will thus be used here.
Cavernous hemangiomas also usually occur in young children, but are far less frequent than are capillary hemangiomas. Cavernous hemangiomas tend to be larger than are capillary hemangiomas and more frequently involve the deep structures, with the liver being a particularly common site of visceral involvement. They tend to be less well-circumscribed. Unlike capillary hemangiomas, cavernous hemangiomas do not regress. Cavernous hemangiomas may be associated with consumptive coagulopathy (Kasabach-Merritt syndrome). The association of cavernous hemangiomas of the skin and gastrointestinal tract has been referred to as the “blue rubber bleb nevus syndrome.”
Cavernous hemangiomas may appear as irregular reddish lesions when they involve the skin, or may appear blue or even non-pigmented when they present in deeper soft tissues. On cut section, a “sponge-like” appearance is common.
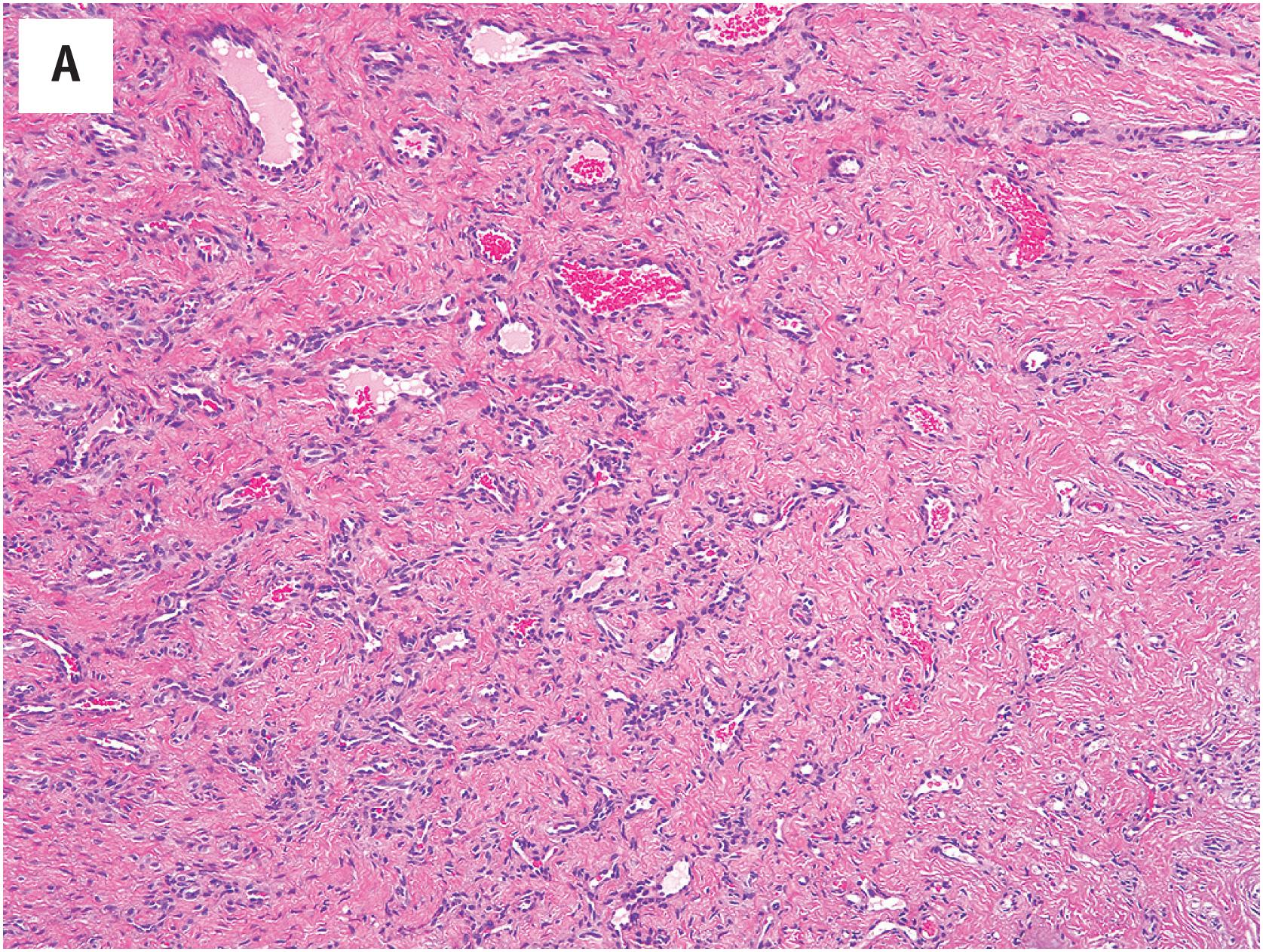
Cavernous hemangiomas consist of large, dilated, blood-filled spaces, often with fibrotic walls ( Fig. 10.13A ). The tumors may be vaguely lobular or consist of elaborately inter-anastomosing vascular spaces, resembling a sponge. Thrombosis and calcification are common findings, and osseous metaplasia may occasionally be seen. The lining endothelium is usually flattened ( Fig. 10.13B ). On occasion, thrombosis and recanalization may produce a sinusoidal pattern (so-called “sinusoidal hemangioma”).
The cells of cavernous hemangioma have a typical endothelial immunophenotype.
Most sporadic venous malformations are linked to somatic mutations in the TEK and PIK3CA genes.
Thrombosis, recanalization, and papillary endothelial hyperplasia may occur within a pre-existing cavernous hemangioma, and may impart the appearance of elaborately inter-anastomosing vascular channels, worrisome for angiosarcoma. Low-power examination, however, reveals these lesions to be well-circumscribed at the periphery, unlike the diffusely infiltrating growth seen in angiosarcoma. Angiosarcomas will also exhibit nuclear atypia, mitotic activity, and frequently necrosis. Spindle cell hemangioma typically displays areas identical to those in cavernous hemangioma, admixed with spindled zones reminiscent of Kaposi sarcoma, and distinctive vacuolated endothelial cells. The recognition of this characteristic admixture of patterns, as well as its frequent association with a damaged-appearing blood vessel is the key to the recognition of spindle cell hemangioma.
Cavernous hemangiomas do not regress and may cause local tissue destruction due to compressive effects. They require surgical removal and do not respond to medical therapy. Preoperative embolization may allow the resection of very large lesions involving locations such as the liver.
Benign non-regressing lesion, now considered malformative rather than neoplastic in nature
Less common than capillary hemangioma
More frequently involve the deep structures, particularly the liver
Benign, do not regress spontaneously
May be associated with consumptive coagulopathy (Kasabach-Merritt syndrome)
No gender or sex predilection
Infants and young children
Liver lesions are common in adults
Cutaneous tumors are irregular and reddish
May appear blue or even non-pigmented when they present in deeper soft tissues
Non-regressing
Do not respond to interferons or anti-angiogenic therapy
Require surgical treatment
Sponge-like appearance, bloody
Large, dilated, blood-filled spaces with flattened endothelial lining, often with fibrotic walls
May be vaguely lobular or consist of elaborately inter-anastomosing vascular spaces, resembling a sponge
Thrombosis and calcification commonly seen
Thrombosis and recanalization may produce a sinusoidal pattern
Express CD31, CD34, ERG/FLI-1 protein, and vWF
Glut-1 negative
Angiosarcoma
Spindle cell hemangioma
Capillary hemangioma
Spindle cell hemangioma was originally described as “spindle cell hemangioendothelioma” and was considered to represent an unusual form of low-grade angiosarcoma. Subsequent studies have convincingly identified it as a benign and possibly reactive lesion, and it has been reclassified by the World Health Organization as “spindle cell hemangioma.”
SCH typically occur in the dermis or subcutis of the distal extremities, in young adults. Very rare cases have been reported in deep soft tissue. In a significant subset of patients the lesions are reported to have been present for many years, without symptoms. Approximately 5% of SCH occur in patients with Maffucci syndrome (multiple enchondromas and vascular tumors), and one should always raise this possibility in a patient with a new diagnosis of spindle cell hemangioma.
SCH typically present as a slow-growing, violaceous, small, solitary, subcutaneous mass in the distal extremities. Over time, multiple lesions may develop in the same general location.
SCH typically grow in a well-circumscribed fashion and are frequently at least in part intravascular ( Fig. 10.14A ). Organizing thrombi, calcifications, and phleboliths are frequently present. A characteristic feature is the presence of two distinct zones; the first is characterized by thin-walled, dilated vessels, reminiscent of cavernous hemangioma, and the second by a proliferation of normochromatic, eosinophilic spindle cells, creating “slit-like” vascular spaces. Within these spindled areas, vacuolated, epithelioid cells are identified, a useful diagnostic feature of SCH ( Fig. 10.14B ). Mitotic figures are very rare and necrosis is absent. A chronic inflammatory cell infiltrate is absent, helping to distinguish SCH from Kaposi sarcoma (KS).
SCH contain a mixture of spindled and epithelioid endothelial cells, which almost always express the typical vascular markers such as CD31, CD34, and ERG, and smooth muscle/pericytic cells, which variably express smooth muscle actins and desmin. The HHV-8 LANA protein is not present.
Somatic mutations in IDH1 or less commonly IDH2 underlie the pathogenesis of SCH. These same genes are mutated in the multiple enchondromas seen in Mafucci syndrome and Ollier syndrome.
SCH is most often mistaken for KS or angiosarcoma. Although KS may on rare occasions grow in a nodular fashion, it lacks the superb circumscription of SCH, does not exhibit intravascular extension, and invariably displays infiltration of the spindled cells into the surrounding stroma. Additionally, KS lacks the admixture of spindled and vacuolated endothelial cells characteristic of SCH, and typically has an associated chronic inflammatory cell infiltrate. The cells of KS also display a greater degree of nuclear enlargement and hyperchromasia, contrasting with the bland spindled cells of SCH, and frequently contain intracytoplasmic hyaline globules.
Occasional angiosarcomas have predominantly spindled features and can be confused with SCH. Again, careful attention to the absence of circumscription and the presence of diffusely infiltrating growth should point one toward the correct diagnosis. The great majority of spindled angiosarcomas have focal areas of typical angiosarcoma, characterized by more epithelioid cells forming primitive vascular channels. Angiosarcomas also exhibit significant nuclear atypia, frequent mitoses, and often have foci of necrosis; these features are typically absent in SCH.
In a child, the differential diagnosis of SCH may also include kaposiform hemangioendothelioma (KHE). KHE is characterized by a distinctly lobular proliferation of spindled endothelial cells, surrounded by a fibrous stroma. Dilated blood vessels and lymphatics are often noted in association with the tumors. The features that allow the distinction of SCH from cutaneous KHE include circumscription of the lesion, intravascular growth, the presence of vacuolated epithelioid endothelial cells, and the absence of lobular growth or fibrosis.
SCH are benign tumors, without capacity for metastasis. A significant percentage of patients may have multiple lesions and “local recurrences.” The latter is most likely related to propagation of the process along adjacent, possibly abnormal blood vessels.
Benign or possibly reactive endothelial tumor, frequently associated with Maffucci syndrome
Rare
Dermis or subcutis, rarely deep
Benign
May propagate along damaged vessel with proximal local recurrences
No sex or race predilection
Young adults
Violaceous nodule in skin or subcutis
5% are associated with Mafucci syndrome (hemangiomas and enchondromas)
Benign
May recur locally through propagation along vessel
Surgical excision
Small, circumscribed
May be calcified
Organizing thrombi, calcifications, and phleboliths
Thin-walled, dilated vessels, reminiscent of cavernous hemangioma
Normochromatic, eosinophilic spindle cells, creating “slit-like” vascular spaces
Vacuolated, epithelioid endothelial cells
Rare mitoses and absent necrosis
Absent chronic inflammatory cell infiltrate
Vacuolated endothelial cells express CD31, CD34, ERG/FLI-1 protein, and vWF
Spindled cells are SMA-positive pericytes
IDH1 and IDH2 somatic mutations
Angiosarcoma
Kaposi sarcoma
Kaposiform hemangioendothelioma
Hemangioma is a benign slow-growing tumor composed of well-formed vessels, each lined by bland endothelial cells. Hemangioma of bone is common, with an estimated prevalence of ∼10% based on autopsy studies. The age of presentation is wide and patients can present with solitary or multiple hemangiomas of bone. The locations involved include most commonly the vertebrae and skull, and rarely the diaphysis of long bones and other sites. Most patients are asymptomatic and present incidentally during radiologic evaluations for unrelated conditions. Rarely, symptoms secondary to nerve root compression from spinal hemangiomas have been reported.
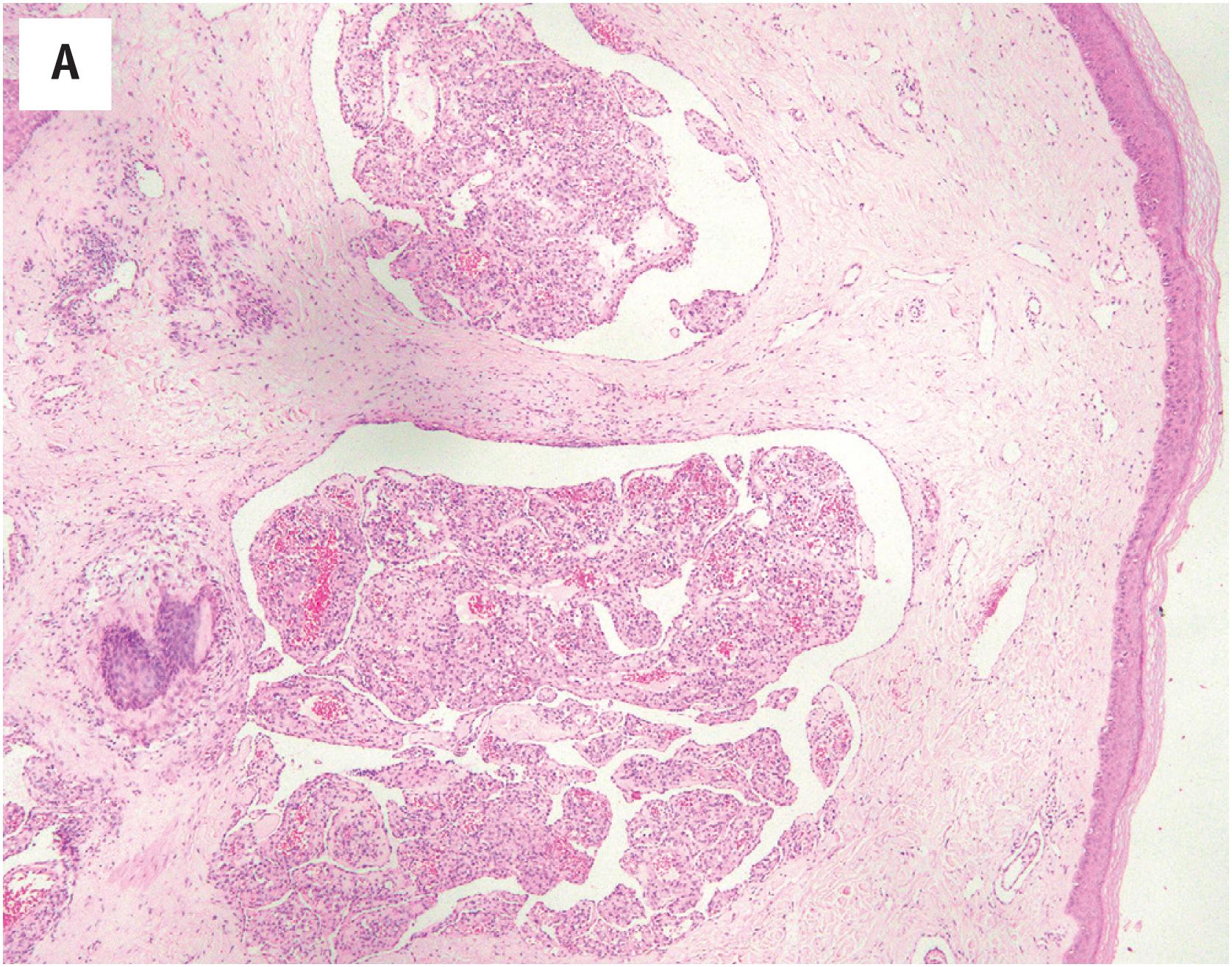
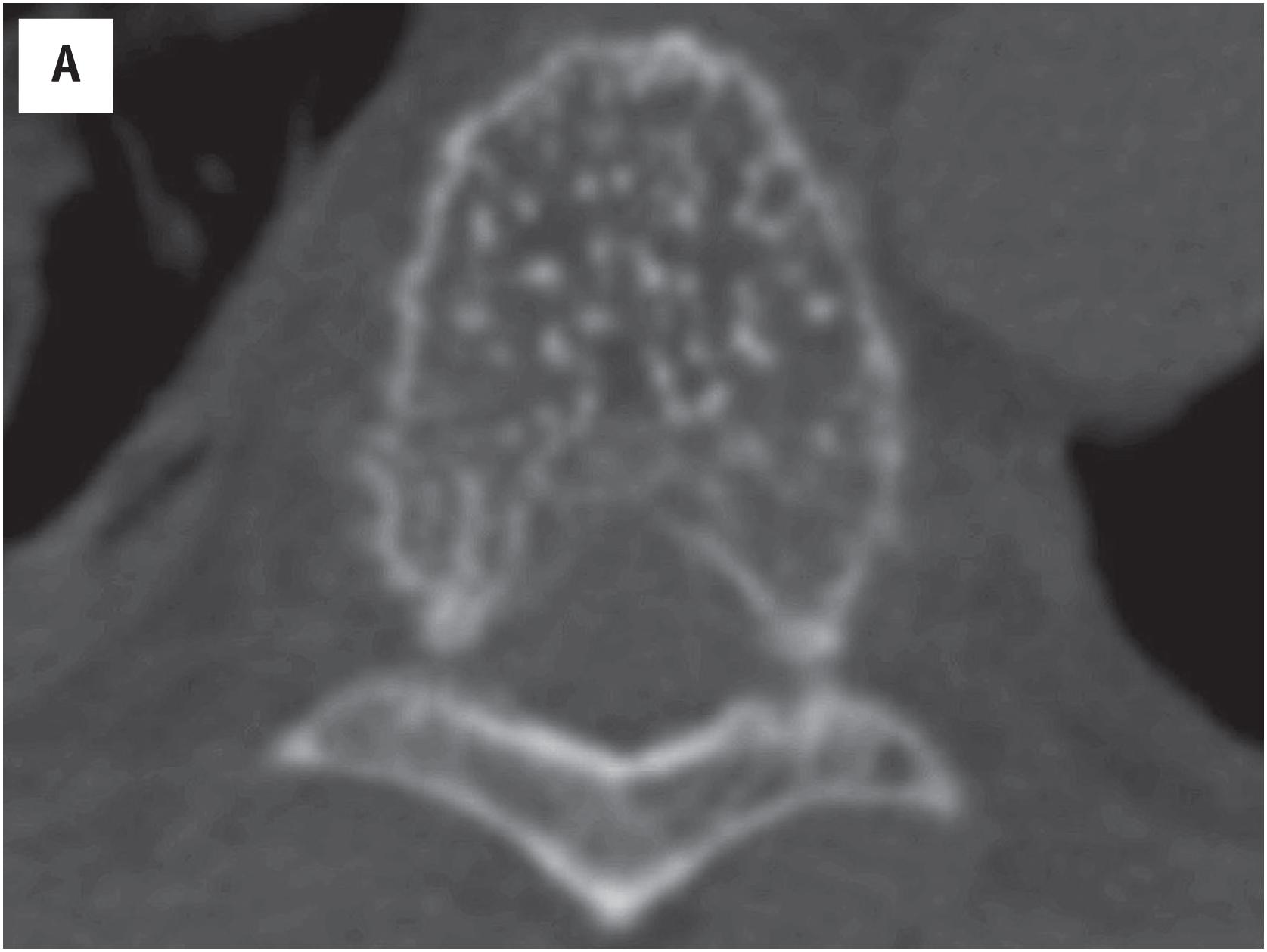
Hemangioma of bone is radiolucent, typically with a “corduroy” or “polka dots”-like appearance ( Fig. 10.5A ). On MR imaging, hemangioma of bone appears hyperintense on both T1- and T2-weighted sequences.
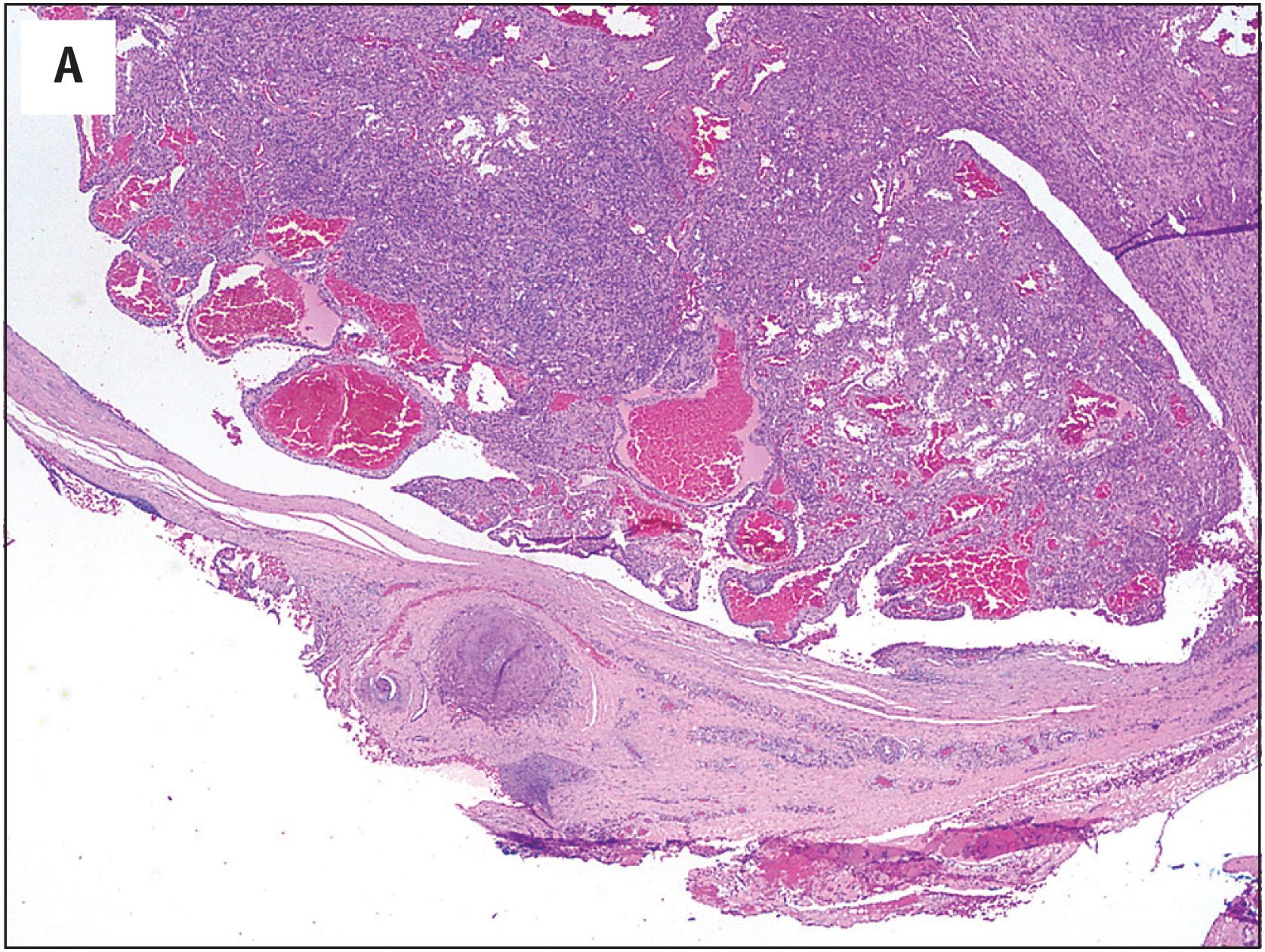
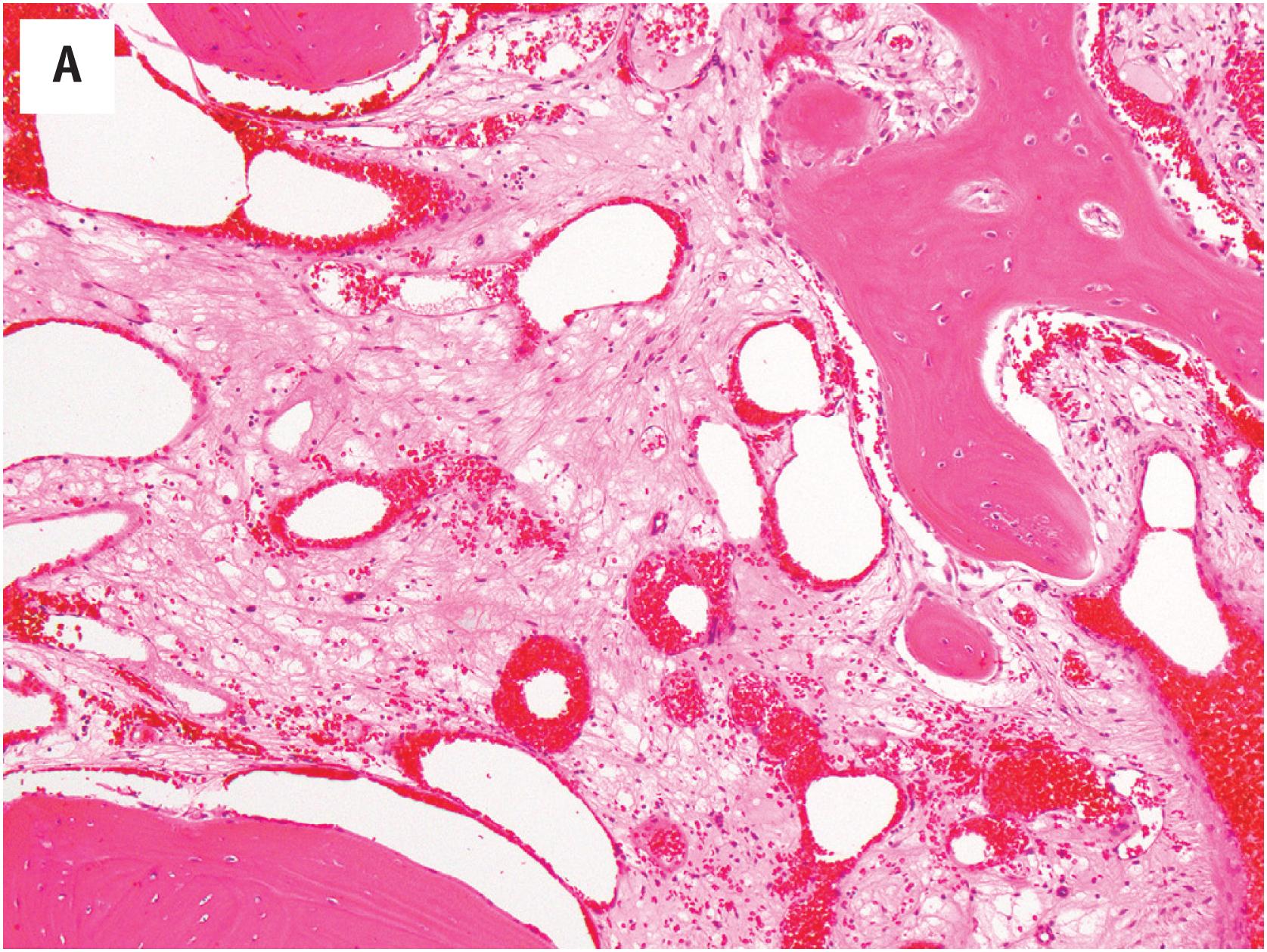
Hemangioma of bone is dark brown, with hemorrhage and a sponge-like appearance ( Fig. 10.15B ). Hemangioma of bone is centered typically in the medullary cavity and is seldom intracortical or periosteal.
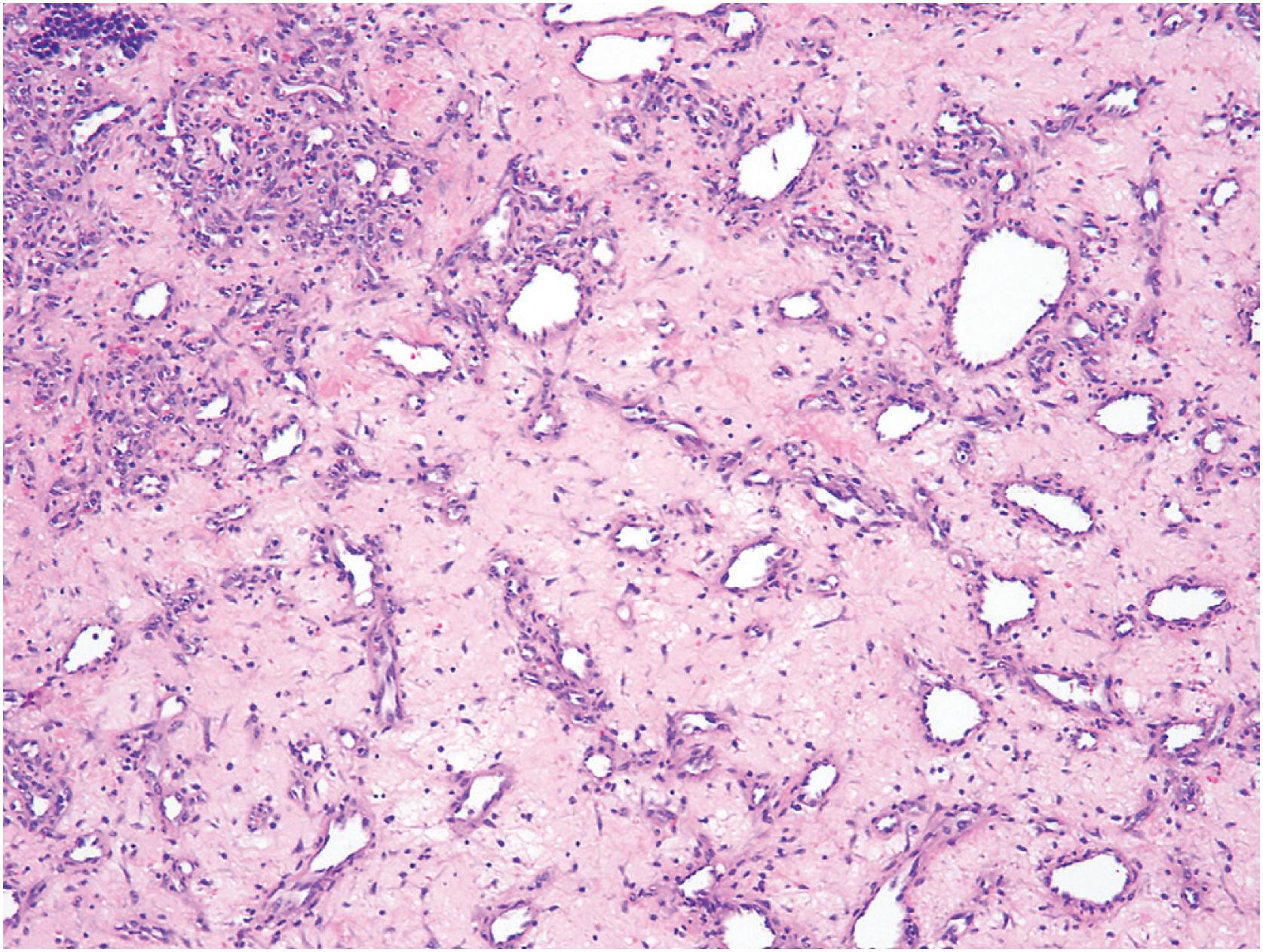
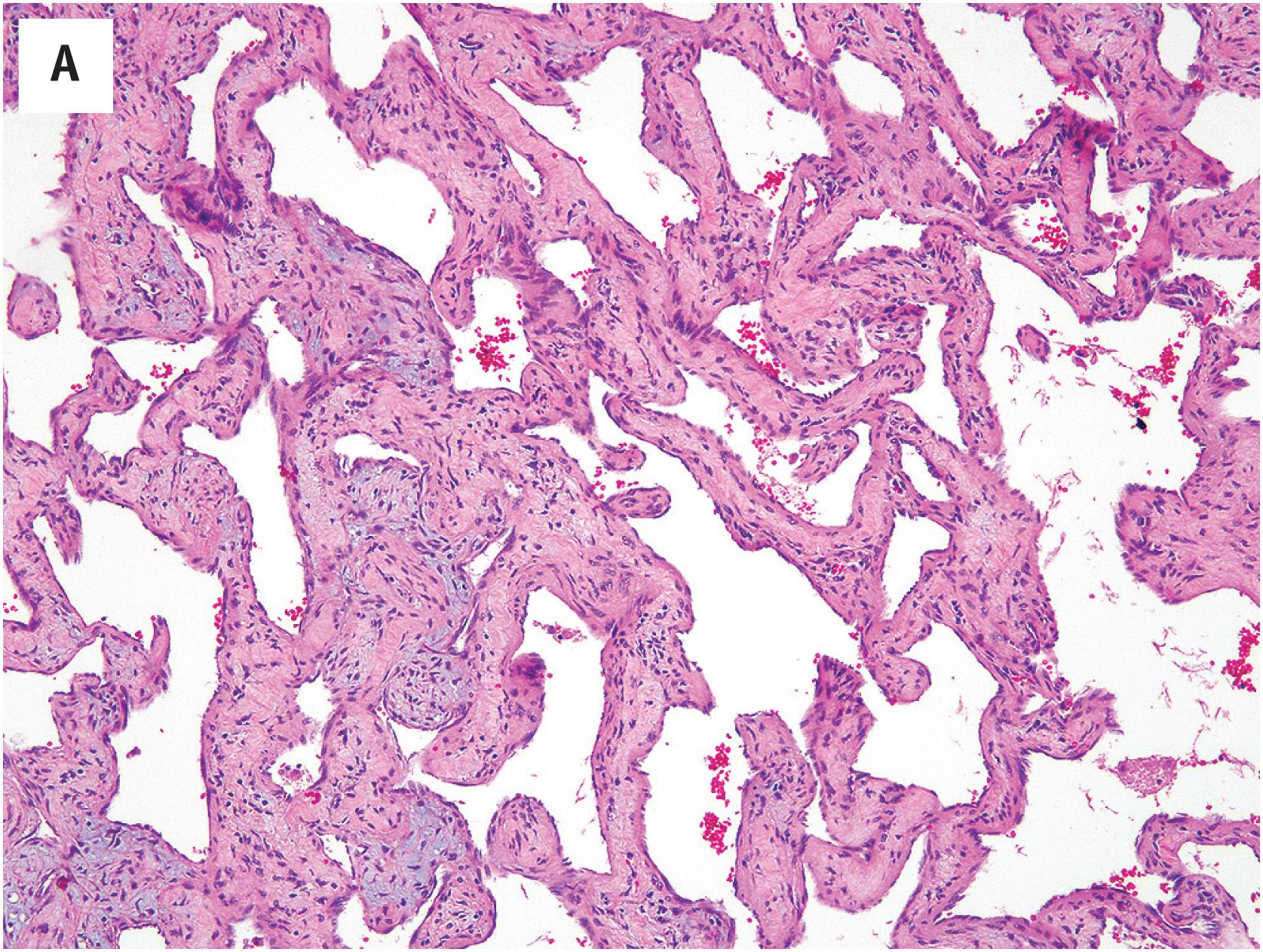
Hemangioma of bone can be subclassified into two histologic subtypes. Cavernous hemangioma is characterized by variably-sized and gaping vascular spaces amidst loose stromal connective tissue ( Fig. 10.16A ). Capillary hemangioma displays prominent lobular proliferation of capillaries and small vessels ( Fig. 10.16B ). Regardless of the histologic subtype, each vascular space is lined by a single layer of flat banal endothelial cells ( Fig. 10.16C ). The vascular spaces traverse among the bony trabeculae, with variable reactive sclerosis in the adjacent bone.
Although hemangioma of bone is straightforward to diagnose in resections, it can be difficult to recognize in curettages or biopsies; the vascular spaces can be subtle among fragments of bony spicules and fibroconnective tissue.
The endothelial cells in hemangioma of bone express vascular markers, including ERG, FLI1, CD31, CD34, and factor VIII. Ancillary studies are generally not needed for its diagnosis.
No specific recurrent genetic alterations have been described in hemangiomas of bone.
In curettages or biopsies, hemangioma of bone can be overlooked, and the tissue may be deemed non-diagnostic. Other differential diagnosis of hemangioma includes arteriovenous malformation, epithelioid hemangioma, and angiosarcoma. Given their microscopic resemblance, the distinction between hemangioma and arteriovenous malformation requires correlation with clinical, radiologic, and angiographic information. In hemangioma of bone, while the endothelial cells may adopt epithelioid cytomorphology, such a finding is limited in its degree and extent, whereas epithelioid hemangioma demonstrates pronounced epithelioid morphology in the majority of endothelial cells. Although well-formed vascular channels can be identified in both hemangioma and angiosarcoma, their distinction should be straightforward: hemangioma displays circumscription and lobulation, unlike in angiosarcoma. The endothelial cells in hemangioma are banal and arranged in a single layer, in contrast to the malignant cytology with hyperchromasia and frequent multilayering in angiosarcoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here