Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The centrality of vascular smooth muscle cells (VSMCs) to normal functioning of the cardiovascular system has led to decades of intensive research into the physical, cellular, and molecular mechanisms regulating VSMC biology. VSMCs are the major cell type within the enclosed vascular continuum; they are primarily responsible for adaptations necessary for repeated cycles of contraction and relaxation resulting from cardiac-driven pulsatile blood flow. VSMCs maintain contractile tone by a highly organized architecture of contractile/cytoskeletal proteins and associated regulatory components within the cell cytoplasm and establish distensibility by the synthesis, secretion, and organization of extracellular matrix (ECM) components with elastic recoil and resilience properties. The ability of VSMCs to adapt the expression of proteins involved in contraction and ECM synthesis, according to extrinsic and intrinsic cues during different developmental stages and in disease or response to injury, results from a phenomenon known as VSMC phenotypic modulation, or plasticity, and is a major feature distinguishing VSMCs from terminally differentiated cells.
Just as VSMCs are central to normal physiology, they are also important mediators in cardiovascular disease (CVD) and response to injury. Data from recent clinical trials and advanced technologies have led to reappraisals of VSMC origin, plasticity, gene regulation, and contribution to CVD. Advances in developmental biology and genetic approaches have identified regions of the vascular tree with very distinct boundaries and distinct susceptibilities to CVD. These data suggest that intrinsic epigenetic marks, established early in development and maintained into adulthood, determine localized disease susceptibility and that environmental cues such as shear stress are not the only determinants of disease location. Similar fate-mapping studies have shown that, in addition to the well-studied VSMC phenotypic switch from contractile to synthetic (dedifferentiated) in atherosclerotic lesions and response to injury, resident VSMCs in the vascular wall can also transition to macrophage-like and osteoblast-like phenotypes, resulting in inflammation and calcification and leading to unstable atherosclerotic plaques. The gene sequencing and “omics” revolution has identified multiple noncoding RNA regulatory molecules, including microRNAs (miRs) and long noncoding RNA (lncRNA), which impact the VSMC phenotype in CVD progression. As a result, current research is focusing on the roles of VSMCs in inflammation and inflammatory processes, calcification, and aging. Finally, there is an increasing appreciation for a more holistic view of the cross talk not only in the physical and chemical communication between vascular wall cells but also among the networks of signaling and molecular processes within cells, such as the redundancy among cellular processes relating to inflammation and aging.
This chapter highlights how these reappraisals of VSMC biology and shifting paradigms on VSMC origins, lineage, plasticity, and regulation of gene expression have positioned VSMCs at center stage in normal and pathological blood vessel function and how the canonical view of atherosclerosis as a disease of lipid dysfunction has been expanded to include inflammation and inflammation-related processes. The discussion centers on the complex webs of signaling networks generated by diverse extrinsic and intrinsic factors and how these networks are regulated and integrated at multiple transcriptional and posttranslational levels to mediate the diverse functions of multiple VSMC phenotypes in normal physiology and disease/injury pathology. Finally, studies of vascular cells and CVD using novel technologies, including omics and C lustered R egularly I nterspaced S hort P alindromic R epeats (CRISPRs)/Cas9-mediated genome modification, are discussed as promising tools for a more comprehensive understanding of CVD.
Initially in embryonic vasculature development, endothelial precursor cells form a common progenitor vessel, which then gives rise to the first artery (dorsal aorta) and vein (cardinal vein) by selective sprouting and subsequent arterial-venous cell segregation. The distinct molecular identities of arteries and veins are regulated by complex interactions of several signaling pathways, including Sonic hedgehog (Shh), a member of the Hedgehog (Hh) family of secreted morphogens; secreted growth factors in the vascular endothelial growth factor family (VEGFs); Notch receptors (Notch 1-4) and Notch ligands (Jagged1,2), and transmembrane proteins that can transduce cell-cell interactions into signals determining cell fates. Interactions of these signals induce differential expression of VEGF receptors, ephrin ligands, and tyrosine kinase Eph receptors on the segregating arterial/venous cells, with ephrin B2 and EphB4 as markers expressed in arteries and veins, respectively. Endothelial cells (ECs) within these primordial vascular networks, in response to VEGF signaling, recruit mural cells, including nascent VSMCs.
Nascent VSMCs derive from at least eight independent embryonic origins, including the neural crest of the ectoderm, the lateral plate mesoderm, and the somites of the paraxial mesoderm. These developmental regions are controlled by signaling through bone-morphogenetic protein (BMP), Wnt, and growth factors (fibroblast growth factor [FGF] and transforming growth factor β [TGF-β]) pathways. These embryonic origins are reflected in different anatomical locations within the adult. Ectodermal cardiac neural crest cells give rise to the large elastic arteries, such as the ascending and arch portions of the aorta, the ductus arteriosus, and the branches of the common carotid arteries; proepicardium mesothelial cells produce the coronary arteries; lateral plate mesodermal cells are origins for the abdominal aorta and small muscular arteries; paraxial mesoderm forms the descending aorta; secondary heart field cells form the base of the aorta and pulmonary trunk; and satellite-like mesoangioblasts give rise to the medial layers of arteries.
There is increasing evidence that these lineage-diverse VSMCs exhibit morphologically and functionally distinct properties and respond differently to soluble factors in vitro and to morphogenetic cues in vivo, suggesting that the major determinants of VSMC responses to signals in vascular development and disease are principally lineage-dependent rather than environment-dependent. For example, in vitro–derived VSMC subtypes produced from human pluripotent stem cells (hPSCs) that had initially been induced to form neuroectoderm, lateral plate mesoderm, or paraxial mesoderm showed that origin-specific VSMCs exhibited differential activation of matrix metalloproteinases (MMPs) 9 and tissue inhibitors of MMP (TIMPs) 1 in response to the inflammatory mediator IL-1β. Given that MMPs degrade ECM and induce VSMC migration in pathological remodeling in CVD, these results suggest that site-specific disease development could be due to hard-wired intrinsic differences in the VSMC’s proteolytic ability and promigratory responses in disease settings.
Genetic fate-mapping techniques and the use of a triple-transgenic mouse model have shown a new paradigm for the origins and fates of VSMCs. Roostalu et al. used novel lineage tracking to follow the fate of immature VSMCs throughout the life of the animal. The earliest VSMCs formed in the mouse embryo dorsal aorta at E10.5 are uniquely identified by the coexpression of NG2 (neural/glial antigen 2) and CD146 (cell adhesion molecule and receptor for α4 chain of laminin). Aortic VSMCs exhibit a transient expression of CD146, whereas CD146 expression is retained into adulthood in smaller-caliber arteries such as mesenteric and superficial femoral arteries (SFAs). Using this mouse model―with fluorescence expression allowing for the identification of progenitor cells expressing two fluorescent colors and progeny expressing one fluorescent color―CD146/NG2 double-positive VSMCs, indicating immature cells, were found at arterial branch points and flow dividers in the adult. The authors speculate that if these “immature,” double-labeled VSMCs at branch points are predisposed to clonal expansion and if equivalent cells exist in human arteries, this finding could support a monoclonal theory of VSMC accumulation in atherosclerotic plaque, as proposed by Benditt and Benditt in 1973. Also expressed at these branching sites is YAP1 (yes-associated protein 1), a transcriptional regulator of CD146. YAP1 is involved in VSMC phenotypic switching and is downregulated in mature VSMCs. In additional studies using the same genetic tools, the authors examined the origin of intimal cells after different degrees of injury in adult mice. They found that after moderate injury to the SFA, most intimal cells came from double-labeled medial VSMCs. However, after severe injury, cells repopulating the injured site came from pluripotent Sca1 + progenitor cells in the adventitia. These data indicate the presence of multiple subsets of immature VSMCs and progenitor cells with a division of labor in normal physiology and pathological remodeling after disease or injury.
The Hox genes, a family of conserved developmental control genes specifying positional identity at a given location, function as topographical ZIP codes for localization of VSMCs from different lineages to specific segments within the vascular tree during development, with implications for differential responses and different susceptibility to injury and inflammation in the adult. For example, VSMCs in the descending aorta (derived from paraxial mesoderm) versus atherogenic ascending thoracic and transverse aorta (AA) (derived from neural crest) express different levels of HoxA9 , a repressor of NF-κB subunit p65; therefore, there are differences in responses in these arterial segments to the proinflammatory and proatherogenic tumor necrosis factor-α (TNF-α)–induced NF-κB activation. These anatomical differences are reflected in regional susceptibility to lesion development. Thus there are intrinsic phenotypic differences among VSMCs, defined by distinct differences in embryonic origin, that are maintained in the adult and determine responses to inflammatory mediators (e.g., interplay between HoxA9 and NF-κB), establishing atherosclerosis-resistant and atherosclerosis-susceptible regions in the vasculature independent of hemodynamic factors. Therefore environmental cues such as shear stress are not the only important determinant of areas of disease progression.
Given the multiple origins and distinct subpopulations of VSMCs, a compelling central question for understanding VSMC biology is how cells from these diverse embryonic origins, initially expressing lineage-specific pathways, differentiate to express the same marker genes specifically characteristic of VSMCs and, additionally, how these same VSMCs, responding to both extrinsic and intrinsic cues, can alter expression of these genes and thus molecular pathways. VSMCs are one of the most plastic cells in the body, but recent studies suggest that there is far more plasticity in these cells than previously acknowledged. Evidence suggests that not only can VSMCs transition to a synthetic phenotype during disease/response to injury, a transition supported by decades of research, but also that VSMCs can transition to macrophage-like cells―assuming macrophage-like characteristics such as phagocytosis, efferocytosis, and reverse cholesterol transport―and to an osteoblast-like phenotype with calcification function ( Fig. 3.1 ). These phenotypic transitions are regulated by multiple different mechanisms: transcriptional, posttranscriptional, and epigenetic. Factors regulating the plasticity of VSMCs include master regulators of contractile genes, such as serum response factor (SRF) and myocardin; ligand-receptor interactions; additional cofactors and signaling pathways such as Kruppel-like factor 4 (KLF4), platelet-derived growth factor (PDGF)-BB, Notch pathway, and TGF-β; epigenetic factors controlling chromatin remodeling and thus the transcription of contractile genes; multiple miRs; and lncRNAs.
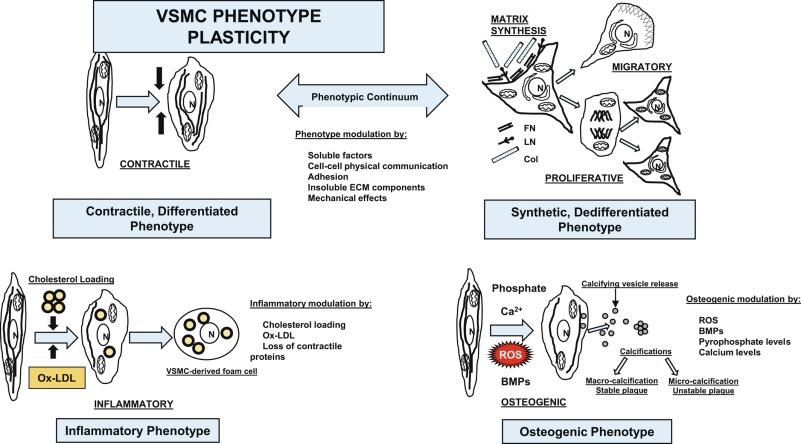
Contractile, or differentiated, VSMCs are characterized by a repertoire of contractile proteins, contractile-regulating proteins, contractile agonist receptors, and signaling proteins responsible for contraction and maintenance of vascular tone. Of the VSMC “marker” proteins expressed in the contractile phenotype repertoire (see Fig. 3.1 ), the most discriminating markers are smooth muscle myosin heavy chain (SM-MHC) in conjunction with SM (smooth muscle) α-actin, smoothelin, SM-22α, h1-calponin, and h-caldesmon. In addition to expression of these proteins associated with the contractile function, contractile VSMCs exhibit differential levels of ECM components (increased collagen types 1 and IV) and of matrix-modifying enzymes (decreased MMPs and increased TIMPs). Contractile VSMCs are further characterized by an elongated, spindle-shaped morphology in culture; a low proliferative rate; expression of α1β1, α7β1 integrins; and the dystrophin-glycoprotein complex (DGPC).
Synthetic, or dedifferentiated, VSMCs have decreased expression of SMC-related genes for contractile proteins, such as SM-MHC, with concomitantly increased osteopontin, l-caldesmon, nonmuscle myosin heavy chain B (NM-B MHC aka MYH10 ), vimentin, tropomyosin 4, and cellular-retinal binding-protein-1 (CRBP1) . “Positive” marker genes, such as MYH10 or SM MHC embryonic (SMemb), expressed specifically in embryonic or phenotypically modified VSMCs, are characteristic of dedifferentiated VSMCs in association with vascular injury. Other characteristics of synthetic VSMCs include a decreased number of actin filaments, an increase in secretory vesicles, increased rates of proliferation and migration, extensive ECM synthesis/degradation capabilities, increased cell size and “hill and valley” morphology in culture, a high proliferative rate, and increased expression of α4β1 integrin.
In addition to the phenotypic continuum between contractile and synthetic phenotypes, recent genetic inducible fate-mapping studies in ApoE -/- mice have shown that medial VSMCs can undergo clonal expansion in plaques, lose classic VSMC marker expression, and express the macrophage markers MAC-2 and CD68. Quantitative assessments of these cells in plaques in murine models showed that 16% of CD68 + cells were derived from mature VSMCs and not from myeloid sources. In further lineage tracing studies, Shankman et al. showed that more than 80% of VSMCs in lesions were undetectable using conventional SM α-actin staining and undergo a phenotypic transition to a macrophage-like cell that expresses markers of macrophages. Importantly, VSMC-specific conditional knockout of KLF4 in ApoE -/- mice resulted in reduced plaque size and numbers of macrophage-like cells and increased fibrous cap thickness, providing further evidence that VSMC contributions to plaques have been underestimated and indicating an important role for VSMCs in foam cell formation and plaque progression. However, VSMC-derived macrophage-like cells, established by cholesterol loading and downregulation of the miR143/145 myocardin axis, exhibited a dysfunctional macrophage-like phenotype, suggesting that these cells are not “classical” macrophages. In humans, a recent report stated that more than 40% of CD68-expressing cells in atherosclerosis of the human coronary artery were derived from resident VSMCs and not monocytes. These data are contrary to the paradigm that VSMCs, having undergone transition from a contractile to a synthetic/proliferative phenotype in the plaque, stabilize the plaque by secreting ECM components that contribute to protective fibrous cap formation. Instead these findings support a deleterious, proatherogenic role for VSMCs in plaque pathology.
A major environmental factor that contributes to the maintenance of the VSMC proinflammatory phenotype is the matrix milieu in which cells exist. In atherosclerotic plaques, VSMCs begin to secrete collagens I and III but they also, as a result of NF-κB activation, express the metalloproteinases MMP-1, MMP-3, and MMP-9, which degrade collagen fibrils to the monomeric form, thus promoting an inflammatory phenotype, as evidenced by an increase in the expression of vascular cell adhesion molecule-1 (VCAM-1). A similar response is seen with regard to osteopontin, which is also increased in atherosclerosis. The effects of these matrix proteins on VSMCs are mediated by binding to specific integrins, most likely α5β1 or αvβ3. The nonintegrin matrix receptor CD44, which binds to hyaluronic acid in the matrix, has also been implicated in the transition to the proinflammatory phenotype, as shown by its ability to stimulate VCAM-1 expression.
One of the primary stimuli for the development of the inflammatory phenotype is oxidized low-density lipoprotein (LDL), but ECs activated by disturbed flow also contribute to inflammatory changes in VSMC by secreting proinflammatory cytokines. Oxidized LDL (oxLDL) and other cytokines like interleukin 1β (IL-1β) and TNF-α stimulate the VSMC expression of chemokines such as monocyte chemoattractant protein 1 (MCP-1), TNF-α, and chemokine (C-X-C motif) ligand 1 (CXCL1) as well as adhesion molecules such as VCAM-1, ICAM-1, and CCR-2, the receptors for MCP-1. Because many of these molecules activate NF-κB, with exposure to one often induces the expression of others, which results in the propagation of a positive feedback signaling mechanism to enhance the local inflammatory response. The end result is the recruitment and adhesion of T cells and monocytes to smooth muscle cells in the vessel wall.
Vascular calcification is a hallmark and one of the major risk factors for CVD. One possible mechanism is the switch of VSMCs from a contractile to an osteoblastic phenotype. In the presence of proinflammatory mediators such as TNF-α and BMPs, procalcifying levels of phosphate, or oxidized forms of cholesterol, conditions endemic to plaque, VSMCs lose contractile markers and start to express bone-related genes, including BMPs, runt-related transcription factor 2 (Runx2), Msx, and osteocalcin. Concomitant with this phenotypic switch are other cellular responses facilitating the calcification process. Responses on the cellular level include increased oxidation and stress on the endoplasmic reticulum (ER); the resultant stress repair processes, including DNA damage response signaling, in turn induce senescence and autophagy and inhibit matrix vesicle release.
Osteogenic-like VSMCs deposit calcifying matrix vesicles (extracellular vesicles [EVs]), which serve as initial nucleation sites of calcium and phosphate mineralization. These VSMC-derived calcifying EVs, originating in the endosomal pathway via multivesicular bodies (MVBs) that dock at the plasma membrane and release membrane-bounded exosomes, are enriched in alkaline phosphatase (ALP), annexins, MMP-2, and specific Rab GTPases implicated in vesicular trafficking. Matrix vesicles contain at least 79 proteins implicated in a wide range of functions including calcification, oxidant- and ER stress–related proteins, and matrix-modifying enzymes for both ECM biogenesis and degradation, contributing to disruption of the vessel wall. In addition to proteins in matrix vesicles, there is selective loading of miRNAs, many of which target osteogenic marker genes in osteogenic differentiation.
Released EVs become entrapped within the ECM in the plaque, acting as multifoci for microcalcifications. Contrary to the original paradigm that calcification stabilizes plaque, new evidence using computational modeling suggests that microcalcifications in the fibrous cap are plaque-destabilizing by serving as foci for high levels of local stress within the cap, making the cap prone to rupture, whereas large calcification areas stabilize plaque by reducing deformation of the fibrous cap during systole. Importantly, microcalcifications in the fibrous cap correlate with cardiovascular adverse events. In fact, the calcium score is a better indicator of future acute events than the lipid scores. Therefore the mechanisms for calcification, including the cell types involved, are of great interest owing to their considerable clinical impact, as there are no therapies for the treatment of cardiovascular calcification.
A recent genome-wide association study (GWAS) indicated an association of the Sort1 gene with coronary artery calcification. Sortilin, a multiligand sorting receptor with additional functions in lipid metabolism and inflammation, is a risk gene for hypercholesterolemia and myocardial infarction. Of interest, sortilin is also a high-affinity receptor for PCSK9 (proprotein convertase subtilisin/kexin type 9), a potent regulator of LDL-C via its ability to shift LDLR traffic from recycling to degradation in the lysosome of hepatocytes, thus leading to excessive accumulation of LDL-C. PCSK9 is constitutively expressed in cultured VSMCs and is found in VSMC regions in human atherosclerotic plaque. In Sort -/- mice, vascular calcification was reduced by a single injection of a gain-of-function mutant PCSK9 adeno-associated virus vector into mice on a high-fat, high-cholesterol diet.
In addition to a role for VSMC-derived EVs in calcification, VSMC-derived EVs loaded with Gla-containing coagulation factors have phosphatidylserine (PS) on their external surface, which can bind the vitamin K–dependent coagulation protein prothrombin (PT) and induce thrombogenesis, providing evidence for a dual role for VSMC-derived EVs in calcification and coagulation. Manipulation of exosome biogenesis/inhibition or cargo holds promise for either facilitating vascular repair or preventing excessive thrombosis and calcification.
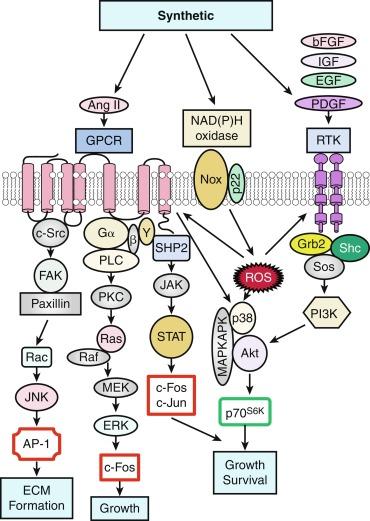
Soluble factors, including growth factors, hormones, and reactive oxygen species (ROS), serve as upstream mediators of the phenotypic switch from contractile to synthetic VSMCs, which results in large part from the coordinate activation/repression of VSMC marker genes important in the contractile response ( Fig. 3.2 ). Some of the most important growth-inducing factors include PDGF, epidermal growth factor (EGF), insulin-like growth factor (IGF), and basic fibroblast growth factor (bFGF). Growth factors bind to surface membrane receptor tyrosine kinases (RTKs), triggering sequential downstream signaling pathways mediated through the complex formation of activated RTKs with adaptor and signaling proteins Grb2/Shc/Sos, and activation of intracellular kinases, including phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinases (MAPKs: extracellular signal regulated kinase, ERK1/2, p38MAPK, and c-jun NH 2 -terminal kinase, JNK), Akt, MAPK activated protein kinases 2 (MAPKAPK2), and p70 S6 kinase (p70 S6K ). These signals not only transcriptionally mediate the switch to the synthetic phenotype but also serve to promote growth and survival. In addition, ROS such as hydrogen peroxide (H 2 O 2 ), produced by activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a multimeric enzyme containing p22phox and other subunits depending upon the specific isoform, can act as second messengers for canonical G protein–coupled receptor (GPCR) and RTK pathways.
In contrast to growth factor–stimulated proliferation, the cytokine TGF-β and members of the BMP subgroup of this family promote the differentiated contractile phenotype in VSMCs by inducing expression of the VSMC contractile genes SM α-actin (ACTA2) and calponin (CNN1) ( Fig. 3.3 ). TGF-β binds to a tetrameric complex consisting of two type I and two type II receptors, resulting in the phosphorylation of Smads, transcription factors (TFs) named for Caenorhabditis elegans Sma and Drosophila melanogaster Mad (mothers against decapentaplegic). Within the TGF-β signaling pathway itself, different Smads control expression of different markers. For example, Smad3 transactivates the SM22α ( TAGLN ; also known as transgelin) promoter, whereas Smad2 activates the ACTA2 gene. Other soluble factors that inhibit proliferation and increase differentiation include heparin and retinoic acid. Most smooth muscle differentiation markers share additional common transcriptional pathways, discussed in more detail further on. For example, both TGF-β–induced phosphorylated Smads and ECM-induced activation of integrins―mediated through focal adhesion components vinculin, talin, and tensin in concert with changes in cytoskeletal F/G actin dynamics―result in myocardin-related transcription factor (MRTF) induction of cytoskeletal/contractile genes (see Fig. 3.3 ).
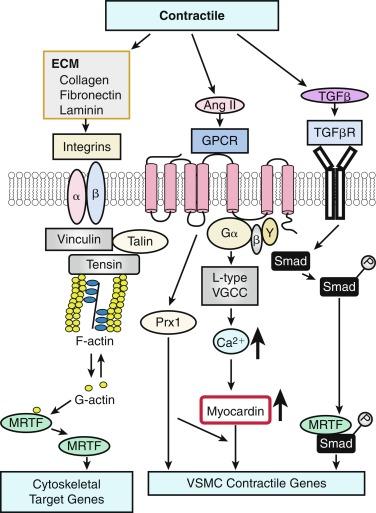
One factor with a potential dual role, depending on initial phenotype/ developmental stage, is the octapeptide hormone angiotensin II (Ang II), the effector molecule of the renin-angiotensin II system. Ang II can induce either contractile or synthetic phenotypes, with differential responses depending on cell context and locations within the artery (see Figs. 3.2 and 3.3 ). Ang II binding to its GPCR AT 1 R activates VSMC marker gene expression indicative of the contractile phenotype through L-type voltage-gated Ca 2 + channel–induced elevations in intracellular Ca 2 + concentrations and subsequent increased myocardin transcription coactivator expression. This coactivator expression is dependent upon Prx1, a homeodomain protein that promotes SRF binding to conserved elements in VSMC marker gene promoters. In addition, Ang II binding to AT 1 R can induce signatures of the synthetic phenotype by activating multiple kinase and enzyme pathways that are interconnected in signaling networks (see Fig. 3.2 ). These include the (1) MAPKs; (2) RTKs, including ROS-sensitive transactivation of EGFR; (3) nonreceptor tyrosine kinases (c-Src/focal adhesion kinase [FAK]/paxillin/Rac/JNK/AP-1) and tyrosine phosphatase; (4) SHP2/Janus kinase and signal transducers and activators of transcription (JAK/STAT); and (5) GPCR classic signaling cascades (phospholipase C [PLC]/protein kinase C [PKC]/Ras/Raf/mitogen extracellular signal regulated kinase kinase (MEK)/ERK leading to stimulation of early growth response genes ( c-fos, c-jun ), survival pathways (e.g., Akt) and ECM formation (JNK/AP-1).
In addition to its critical function in development, Notch signaling is also important in defining VSMC differentiation. Downstream Notch effector gene activation results in the activation of “master regulators” of VSMC differentiation (myocardin, MRTFs, or SRF) or direct induction of contractile proteins SM-MHC and SM α-actin as well as the VSMC-specific differentiation marker SM22α. The data regarding Notch signaling on VSMC differentiation, however, are conflicting, with some studies supporting a repressive effect, whereas others indicate a promoting effect on the expression of VSMC marker genes MYH11 and ACTA2 . These discrepancies may be due to the antagonistic roles of Notch and the Notch effector Hairy-related transcription factor 1 (HRT1) on markers of VSMC differentiation. HRT1 inhibits Notch/RBP-Jκ binding to the ACTA2 promoter in a histone deacetylase-independent manner. The context-dependent roles of Notch and HRT1 on markers of VSMC differentiation may serve to fine tune VSMC phenotypic modulation during vascular development, injury, and disease.
There is considerable cross talk between Notch and other signaling pathways. Notch and TGF-β cooperatively induce a functional contractile, differentiated phenotype through parallel signaling axes, whereas HRT factors block VSMC differentiation in both pathways. Other examples of cross talk among key signaling pathways for morphogenesis (Hh, Notch) and mitogenesis (VEGF-A, PDGF) include a Shh/VEGF-A/Notch signaling axis in VSMCs in the neointima to increase growth and survival and Notch-induced upregulation of PDGF receptor β (PDGFR-β) to mediate growth and migration.
Homotypic VSMC-VSMC Notch-mediated signaling pathways are also apparent in adult vascular pathologies and response to injury. After injury, Notch receptors are increased along with elevated levels of HRT. Negative feedback between HRT and Notch may account for the adaptive response to injury in which initial Notch/HRT-induced suppression of the contractile phenotype is followed by arterial remodeling. As Notch/HRT signaling decreases, the contractile phenotype is reestablished.
The complex web of signaling pathways induced by these external signals―whether they are soluble, insoluble, structural, or mechanical―converge on a network of TFs that coordinately regulate gene expression and act as “masters switches” for growth and differentiation ( Fig. 3.4 ). Transcription of VSMC-specific differentiation or proliferative genes is regulated by cooperative interaction of TFs and their coregulators, including SRF, myocardin and myocardin-related TFs (MRTF-A and –B), Ets domain TFs known as ternary complex factors (TCFs), zinc finger factors, GATA6 and PRISM ( PR domain I n S mooth M uscle)/PRDM6, and KLF.
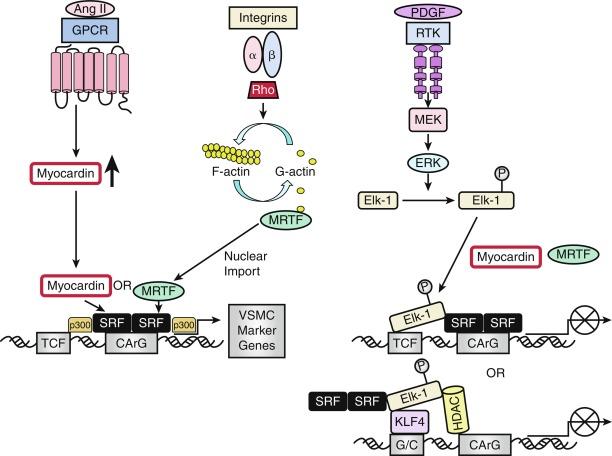
The discovery of the cell-restricted SRF transcriptional coactivator myocardin, which is expressed specifically in cardiac and VSMCs, resolved the paradoxical observations that SRF can regulate mutually exclusive gene expression programs for growth or differentiation. In VSMCs, myocardin is a master regulator of marker gene expression and is sufficient for the smooth muscle–like contractile phenotype because conversion of cells to a VSMC-like phenotype involves transient ectopic expression of just one factor: myocardin.
SRF, a widely expressed member of the MADS (MCM1, Agamous, Deficien, SRF) box of TFs, is a nodal point linking signaling pathways to differential gene expression related either to growth or differentiation, depending on which transcriptional partner is bound to SRF. SRF self-dimerizes and binds with high affinity and specificity to a consensus DNA sequence CArG box found in the promoters of cytocontractile genes. More than half of the VSMC “marker” genes that define the VSMC molecular signature contain CArG boxes. Included in these genes are three categories modulating actin filament dynamics: (1) structural (e.g., ACTA2, TAGLN, CALD1, MYH11 ); (2) effectors of actin turnover (e.g., CFL1, GSN ); and (3) regulators of actin dynamics (four and a half LIM domains proteins [FHL1 and 2], MMP9, and myosin light chain kinase [MYCK]).
To study the function of CArG boxes, precision-guided genome editing with CRISPR technology was used to edit the consensus intronic CArG box in the SMC-specific calponin ( Cnn1 ) gene to drastically reduce calponin expression in the vessel wall without affecting other SMC-specific marker genes. Near elimination of calponin increased DNA synthesis, suggesting a role for calponin in VSMC quiescence. These studies represent the first use of CRISPR-Cas9 technology for genome editing of a regulatory element in the control of an SRF target gene and show the importance of the CArG box for calponin-1 expression.
SRF itself is a weak activator of CArG-dependent genes. Potent SRF-dependent transcriptional activation is therefore dependent upon regulation at several levels: by interaction with different signal-regulated or tissue-specific regulatory SRF transcription cofactors/corepressors; by postranslational phosphorylation, acetylation, and sumoylation, modifications that affect these interactions; and by epigenetic alterations in chromatin structure in which myocardin serves as a scaffold for recruitment of chromatin-remodeling enzymes that enable SRF and its cofactors to gain access to SRF target genes. Myocardin association with histone acetyltransferases (HATs), including p300, enhances transcription of VSMC-restricted genes, whereas association with class II histone deacetylases (HDACs) suppresses myocardin-induced transcription of VSMC marker genes (see Fig. 3.4 ).
SRF interacts with cofactors in two principal families: the TCF family of Ets-domain proteins (Elk, SAP-1, and Net) that are activated by the MAPK pathway and lead to SRF binding to immediate early growth factor–inducible genes such as c-fos, and the myocardin/MRTF-A/MRTF-B family that promotes activation of VSMC-specific marker genes, most of which code for filamentous proteins that function in contractile activities or proteins that function in cell-matrix adhesions. These alternative pathways provide the “plasticity” associated with VSMC phenotypic modulation, ranging from contractile functions to maintain vascular tone to synthetic or proliferative functions in response to vascular injury.
Myocardin competes with Elk-1 for direct binding to SRF in VSMCs; thus, myocardin and Elk-1 can act as binary transcriptional switches that may regulate contractile versus synthetic VSMC phenotypes (see Fig. 3.4 ). In addition, myocardin transduction leads to lower levels of the cell cycle–associated gene cyclin D1, resulting in repression of growth. Therefore myocardin is a nodal point for two features indicative of SMC differentiation: expression of the contractile apparatus and suppression of growth.
Although myocardin functions exclusively as a transcriptional coactivator, additional proteins function to regulate the transcriptional activity of myocardin. Positive regulators include myocyte-specific enhancer factor 2 (Mef2), TEA domain transcription factor 2 (Tead2), and forkhead transcription factor 4 (Foxo4), epigenetic markers such as ten-eleven translocation-2 (TET2) and TGF-β and Notch signaling pathways. Negative regulation of myocardin can occur by interaction of an upstream repressor region (URR) within the myocardin promoter (PrmM) with binding sites for KLF4 or PDGF-BB within the PrmM, mediating transcriptional repression of myocardin expression.
In addition, miRNAs and lncRNAs regulate myocardin expression. The miR-143/145 cluster positively regulates myocardin expression by targeting inhibition of KLF4/5, itself an inhibitor of myocardin, regulating the phenotypic shift in VSMCs. miR-146a targets KLF4 to promote VSMC proliferation in vitro and neointimal hyperplasia in vivo. Knockdown of the cytoplasmic lncRNAs SENCR (smooth muscle and endothelial cell–enriched migration/differentiation/associated long noncoding RNA) attenuates the expression of myocardin, decreases expression of VSMC contractile-associated genes, and increases expression of promigratory genes. Thus, SENCR seems to function in maintenance of the contractile, nonmotile phenotype.
HRT-2 and GATA factors repress or enhance myocardin-induced transcriptional activity depending upon cell context. In addition, activation of Notch receptors by Jagged1 endogenous ligand induces translocation of Notch intracellular domain (ICD) to the nucleus, where it inhibits myocardin-induced SMC gene expression. Ang II stimulation, as well as activation of L-type voltage-gated Ca 2 + channels, activates SMC marker genes by inducing myocardin expression, and, in the case of Ang II, increasing SRF binding to CArG elements in the promoter regions of VSMC marker genes such as ACTA2 .
SRF transcriptional activity is also controlled by Rho-induced actin dynamics that facilitate the movement of MRTFs into or out of the nucleus (see Fig. 3.4 ). In most cell types, MRTFs form a stable complex with monomeric G-actin and remain sequestered in the cytoplasm. MRTFs in VSMCs, however, are localized in the nucleus, where binding to SRF in the basal state promotes contractile gene expression and the differentiated phenotype. In response to growth factors or vascular injury, extracellular signals transduced through the Rho-actin pathway result in nuclear export of MRTF, downregulation of SRF/MRTF-induced VSMC contractile gene expression, and promotion of mitogen-induced ERK1/2 phosphorylation of TCFs, resulting in TCF displacement of MRTFs and SRF/TCF-mediated activation of growth responsive genes. These differential pathways provide a switch in which SRF target genes are differentially regulated through growth factor–induced signaling for growth (active TCF, MRTF blocked) or Rho-actin signaling for differentiation (inactive TCF, MRTF active) (see Fig. 3-4 ).
Myocardin has important roles beyond its role in activating transcription of VSMC-specific marker genes. A recent study showed that myocardin is also a negative regulator of VSMC inflammation in CVD and after vessel injury. Therefore, myocardin is a possible therapeutic target owing to its antiinflammatory properties. Furthermore, myocardin has been shown to suppress VSMC dedifferentiation and proliferation. Myocardin inhibits VSMC proliferation by interaction with the NF-κB subunit p65 to suppress NF-κB activity independently of SRF. Finally, myocardin has been shown to drive the formation of caveolae, or plasma membrane sphingolipid/cholesterol-rich domains, also in an SRF-independent manner, suggesting that myocardin has a role in lipid metabolism. These antiinflammatory and lipid-regulatory roles of myocardin extend beyond its role in activating the transcription of VSMC-specific marker genes for a contractile phenotype.
The Hippo kinase pathway has been shown to modulate the myocardin/SRF pathway by inhibiting the phosphorylation of YAP, which, when phosphorylated, remains located in the cytosol and therefore cannot repress VSMC marker gene expression by interacting with myocardin and disrupting SRF binding. In rat carotid artery injury, YAP is induced in VSMCs in transition from contractile to synthetic phenotype; its expression promotes VSMC proliferation and migration. Deletion of YAP protects against neointimal formation after injury. Another kinase shown to regulate VSMC phenotypic switching by acting through MRTF-A is p38 MAPKα ( Mapk14 ). Both the Hippo and the p38 MAPKα pathways are activated by extracellular cell-cell or cell-matrix contacts, which then modulate the cytoskeleton and cell adherence necessary for VSMC survival.
GATA6, a zinc finger TF expressed in VSMCs, induces growth arrest by increasing expression of the general cyclin-dependent kinase inhibitor (CDKI) p21 CIP1 and inhibiting S-phase entry. PRISM is a smooth muscle–restricted member of zinc finger proteins belonging to the PRDM family and acts as a transcriptional repressor by interacting with class I HDACs and G9a histone methyltransferases (HMTs). PRISM induces the proliferative phenotype while repressing differentiation regulators myocardin and GATA6.
One of the most intensely studied zinc finger transcriptional regulators in VSMCs is the KLF subfamily, which bind to the TGF-β control element (TCE) in the regulatory sequences of target genes. VSMCs express four KLFs (KLF4, KLF5, KLF13, and KLF15), each with individual biological functions implicated in regulating a range of processes in both growth and differentiation. Individual KLFs may have opposing functions, depending upon temporal and developmental expression patterns and interactions with other factors. For example, KLF4 inhibits, whereas KLF5 and KLF13 induce, VSMC marker gene expression. Mechanisms that may account for these opposing functions of KLF factors include posttranslational modifications, interaction with specific cofactors, differential expression by growth factors, cytokines and differentiation state, or regulation by another KLF.
KLF4 functions as both a VSMC growth repressor and as a repressor for VSMC differentiation, although the data on the effect of KLF4 on VSMC differentiation are conflicting (see Fig. 3.4 ). As a growth repressor, KLF4 inhibits PDGF-BB–induced mitogenic signaling and induces expression of the negative cell cycle regulator p53 and its target gene p21 CIP1 . As a differentiation repressor, KLF4 prevents SRF from binding to the TCE in the promoters of VSMC marker genes, suppresses expression of myocardin, inhibits myocardin-induced activation of SMC marker genes, reduces SRF binding to CArG elements in SMC contractile gene promoters, and induces histone hypoacetylation at SMC CArG regions associated with gene silencing. On the other hand, there is evidence that KLF4 promotes VSMC differentiation by directly activating VSMC marker gene transcription of SM22α and SM α-actin. KLF4 thus functions as a bifunctional TF or “molecular switch” that can both activate and repress VSMC marker genes, depending upon regulation of KLF4.
Even though the closely homologous KLF4 and KLF5 TFs share similar developmental and tissue pattern expression, they exert different, often opposing, effects on gene regulation and proliferation/differentiation. Whereas KLF4 is associated with growth arrest, KLF5 exerts proproliferative effects, particularly in vascular remodeling in response to injury. KLF5 expression, which is abundant in fetal VSMCs but downregulated in the adult, is induced after vascular injury by activation of immediate early response genes by Ang II and ROS. KLF5 in turns mediates the reexpression of SMemb/NMHC-B, a marker for the dedifferentiated phenotype and activates other critical injury response genes involved in remodeling, such as PDGF-A/B, Egr-1, plasminogen activator inhibitor-1 (PAI-1), inducible NO synthase (iNOS) and VEGFR, implicating KLF5 as a key regulator for VSMC response to injury. In additional injury responses, KLF5 increases cyclin D1 expression and inhibits the cyclin kinase inhibitor p21, thus leading to vascular remodeling by increased cell proliferation. Similar to KLF4 regulation, KLF5 expression, and activity are regulated at multiple levels, including upstream Ras/MAPK, PKC, and TGF-β signaling pathways, downstream interactions with TFs―including retinoic acid receptor (RARα), NF-κB, and peroxisome proliferator-activated receptor gamma (PPARγ)―as well as posttranslational modifications that can positively or negatively regulate KLF activity. In addition, KLF5 activity is regulated in the nucleus by chromatin-remodeling factors such as SET, a histone chaperone that inhibits the DNA-binding activity of KLF5 ; p300, a coactivator/acetylase that coactivates KLF5 transcription; and HDAC1, which inhibits KLF5 binding to DNA.
Two additional KLFs have been identified in VSMCs: KLF13 and KLF15. After vascular injury, KLF13 is induced and activates the promoter for the VSMC differentiation marker TAGLN , whereas KLF15 expression is downregulated, implicating KLF15 as a negative regulator of VSMC proliferation and a counterbalance to the growth promoting effects of KLF5 in vascular injury response.
The landscape for understanding protein-based regulation dramatically changed with the discovery of noncoding RNAs, previously referred to as “junk” DNA but now known to comprise 98% of the human genome. These noncoding RNAs include the highly conserved miRs and the more recently described lncRNAs. These molecular rheostats are responsible for important regulatory control over the transcriptome or proteome in mediating VSMC phenotypic diversity.
Upstream signaling and downstream transcriptional pathways in VSMCs are intertwined with a multitude of miRs that act as “rheostats” and “switches” in regulating protein activity in development, function, and disease. miRs are small noncoding RNAs, 20 to 25 nucleotides (nt) in length, that associate with an miRNA-induced silencing complex (miRISC) of regulatory proteins, including Argonaute family proteins, Argonaute interacting proteins of the GW182 family, eukaryotic initiation factors (eIFs), polyA-binding complexes, decapping enzymes/activators, and deadenylases to induce posttranscriptional silencing of their target genes. These multiple components are assembled and interact in a multistep process with components of the translational machinery to inhibit translation initiation, to mark mRNAs for degradation through deadenylation, and to sequester targets into cytoplasmic P bodies. Multiple mechanistic models for miRNA-induced gene silencing have been proposed that provide insight into the molecular mechanisms of translational inhibition, deadenylation, and mRNA decay, but questions remain concerning the kinetics and ordering of these translational events and whether these events are coupled or are independent. A recent unifying model for miRNA-regulated gene repression is an attempt to reconcile the often conflicting existing data and proposes that recruitment of Argonaute and associated GW182 proteins to miRNA induces binding to the mRNA 5′m cap, thus blocking translation initiation, potentially by mRNA deadenylation. Subsequent to miRNA-mediated deadenylation, mRNA is degraded through recruitment of decapping proteins. In this model, inhibition of translation initiation is linked to subsequent rapid mRNA decay in a coupled process. Because miRs, which in general are negative regulators of gene expression, may be almost as important as TFs in controlling gene expression in the pathogenesis of human diseases, insights into the functions of this class of noncoding RNAs are important in evaluating their potential use as therapeutic targets.
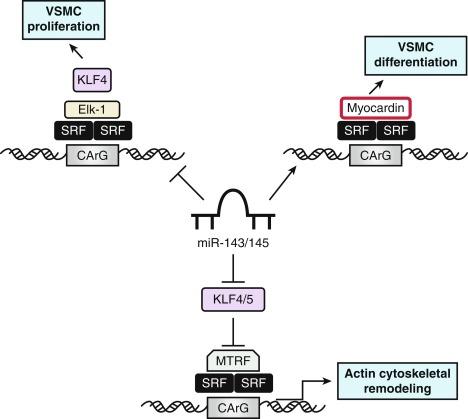
Cardiovascular-specific, highly conserved miRNAs miR-143 and miR-145, the most abundant miRNAs in the vascular wall, are key players in programming of VSMC fate from multipotent progenitors in embryonic development and in the reprogramming of VSMCs during phenotypic modulation in the adult ( Fig. 3.5 ). miR-143 and miR-145 have distinct sequences but are clustered together and transcribed as a bicistronic unit. Upstream in the genomic sequence of the miR143/145 is a conserved SRF-binding CArG box site, indicating control by SRF and myocardin. These miRNAs cooperatively feed back to modulate the actions of SRF through targeting a network of TFs/coactivators/corepressors. This network includes miR-145-induced repression of KLF4, a positive regulator of proliferation and myocardin repressor; miR-143–induced repression of Elk-1, a myocardin competitor and positive regulator of proliferation; and, contrary to the usual inhibitory role of miRNA, miR-145–induced stimulation of myocardin, a positive regulator of differentiation. Thus miR-145 is necessary and sufficient for VSMC differentiation and the miR-143/miR-145 cluster acts as an integrated signaling node to promote differentiation while concurrently repressing proliferation. Although mice with genetic deletions for miR-143/145 show no obvious abnormalities in early development, VSMCs in the adult exhibit both structural and phenotypic differences in injury- or stress-induced vascular remodeling. Ultrastructural analysis of arteries from miR-143/145 knockout mice shows reduced numbers of medial VSMCs with a contractile appearance and an increase in synthetic VSMCs. These results suggest that miR-143 and miR-145 modulate cytoskeletal structure, actin dynamics, and modulation to a dedifferentiated phenotype (see Fig. 3.5 ). Importantly, miR-143/145 knockout mice with increased synthetic VSMCs develop spontaneous neointimal lesions in the femoral artery in the absence of hyperlipidemia and inflammation, supporting a key role for phenotypically altered VSMCs in the pathogenesis of lesion formation.
Whereas miR-143 and miR-145 play keys roles in the contractile phenotype of VSMCs and the response to injury, miR-221 and miR-222 are modulators of VSMC proliferation, although largely by affecting growth-related signaling pathways rather than by controlling VSMC phenotype. miR-221 and miR-222, encoded by a gene cluster on the X chromosome, are upregulated in VSMCs in neointimal lesions and in proliferating cultured VSMCs stimulated by PDGF-BB. Studies show that two CDKIs, p27 KIP1 and p57 KIP2 , have miR-221 and miR-222 binding sites and are gene targets for miR-221 and miR-222 in the rat carotid artery in vivo. Thus miR-221 and miR-222 are proproliferative because they repress two CDKIs, p27 KIP1 and p57 KIP2 . Furthermore, PDGF, via miR-221 induction, inhibits VSMC differentiation via c-kit–induced inhibition of myocardin.
For a more comprehensive list of important miRs in VSMCs, see Table 3.1 and recent reviews.
| miR | Main Targets | Functions | Reference |
|---|---|---|---|
| Procontractile miRNAs (Differentiation) | |||
| miR-10a | HDAC4 | Regulates differentiation of VSMCs from embryonic stem cells | Huang et al. |
| miR-21 | DOCK4, 5, 7 | Promotes contractility | Kang and Hata |
| miR-143/145 | KLF4, KLF5, myocardin, ELK-1, CamkIIδ, ACE, SSH2, SRGAP1/2, ADD3, MRTF-B | Phenotype switching | Boettger et al. Cheng et al. Cordes et al. Xin et al. Wang |
| miR-143/145 | miR-143 (PKC-ε and PDGF-Rα); miR-145 (fascin) |
Regulates podosome formation and migration via downregulation of miR-143/145 | Quintavalle et al. |
| miR-143/145 | KLF4, ELK1 , CamkIIδ |
miR-143/145 in exosomes from ECs transferred to VSMCs and prevents VSMC dedifferentiation (atheroprotective) | Hergenreider et al. |
| miR-143/145 | miR-143/145 mediates EC-VSMC-EC communication via tunneling nanotubes mediated by TGF-β | Climent et al. | |
| miR-143-3p | Modulates migration and apoptosis; miR-143 in PASMC-derived exosomes transferred to PAECs to induce migration | Deng et al. | |
| miR-145 | KLF4 | Restoration of contractile phenotype in metabolic syndrome | Hutcheson et al. |
| miR-145 | KLF4, myocardin | Promotes contractile phenotype | Lovren et al. |
| Prosynthetic miRNAs (Proliferation, Migration) | |||
| miR-1/33 | KLF4, Sp-1 | Proliferation | Chen et al. Xie et al. Torella et al. |
| miR-9 | PDGFR | Inhibits proliferation and migration | Ham et al. |
| miR-15a | CDKN2B | Proliferation, decreases apoptosis | Gao et al. |
| miR-21 | TPM1 , PDCD4, PPARα | Proliferation, migration, apoptosis | Lin et al. Wang et al. Davis et al. Sarkar et al. |
| miR-21 | PTEN, Bcl-2 | Promotes proliferation, decreases apoptosis | Ji et al. Maegdefessel et al. |
| miR-24 | PDGFRB indirectly | Reduces migration and proliferation by myocardin-induced downregulation of PDGFRB | Talasila et al. |
| miR-26 | Contractile gene markers | Regulates VSMC contractile phenotype by exosome transfer of miR-26 from ECs to VSMCs | Lin et al. |
| miR-26a | SMAD1, SMAD2 | Promotes proliferation and migration, inhibits differentiation and apoptosis | Leeper et al. |
| miR-29a | PDGFRB | Reduces migration and proliferation by myocardin-induced downregulation of PDGFRB | Talasila et al. |
| miR-30c | PDGFRB | Inhibits PDGFRB expression and modulates proliferation and apoptosis | Xing et al. |
| miR-31 | CREG | Phenotypic modulation | Wang et al. |
| miR-126 bound to Ago2 from ECs to VSMCs | FOXO3, Bcl2, IRS1 | Modulates VSMC turnover | Zhou et al. |
| miR-129 | Wnt5a | Inhibits proliferation, induces apoptosis | Zhang et al. |
| miR-132 | LRRFIP1 | Proliferation | Choe et al. |
| miR-203 | p63, Abl1 | Inhibits proliferation by downregulating estradiol-induced Abl1 and p63 | Zhao et al. |
| miR-208 | p21 | Proliferation | Zhang et al. |
| miR-221/222 | p27, p57, c-kit | Promotes proliferation and migration, antiapoptosis | Liu et al. Liu et al. Davis et al. |
| let-7d | KRAS | Proliferation | Yu et al. |
| let-7g | LOX-1 | Proliferation and migration | Chen et al. |
| Inflammation | |||
| miR-24 | CHI3L1 | Inhibits vascular inflammation | Maegdefessel et al. |
| miR-200 family | Zeb1 | Upregulates proinflammatory COX-2 and MCP-1 in diabetic mice | Reddy et al. |
| ECM Modifications | |||
| miR-17 cluster | TIMP1, TIMP2 | Downregulates ECM | Wu et al. |
| miR-29 | COL1A1, COL3A1, COL5A1, ELN, MMP2, MMP9 | Downregulates ECM, regulates fibrosis | Boon et al. Maegdefessel et al. |
| miR-29 | ELN | Elastin formation | Latronico et al. Zhang et al. |
| miR-29 | ADAMTS-7 | Promotes calcification by increasing ADAMTS-7 expression | Du et al. |
| miR-29 | COL1A, COL3A | Regulates ECM production | Ulrich et al. |
| miR-29a | MMP2, MMP9 | Downregulates ECM | Jones et al. |
| miR-29b | ELN, MMP2 | Upregulates ECM, promotes apoptosis | Merk et al. |
| miR-133a | RUNX2 | Osteogenic differentiation | Liao et al. |
| miR-143 | Versican mRNA | Attenuates ECM versican expression | Wang et al. |
| miR-181a | OPN | Adhesion and osteopontin formation | Remus et al. |
| miR-181b | TIMP3, ELN | Downregulates ECM | Di Gregoli et al. |
| miR-195 | COL1A1, COL1A2, COL3A1, ELN, MMP2, MMP9 | Regulates ECM | Zampetaki et al. |
| miR-516a | MTHFR, MMP2, TIMP1 | Regulates ECM | Chan et al. |
High-throughput transcriptomics have identified over 100,000 genes for lncRNAs, indicating that lncRNAs outnumber both protein-coding and miR genes combined and are the majority transcript in the human genome. lncRNAs are noncoding RNAs more than 200 nt long transcribed by RNA polymerase. lncRNAs are generally expressed at low levels and are poorly conserved. In contrast to miRs, which usually act to downregulate mRNA and protein expression, lncRNAs have diverse functions and modulate gene expression at all levels: transcriptional, posttranscriptional, translational, and posttranslational via interaction with chromatin modifiers, RNA, RNA-binding proteins, and DNA.
Subclasses of lncRNA are defined on the basis of genomic location relative to other gene loci: sense lncRNAs, antisense lncRNAs, bidirectional lncRNAs, intronic lncRNAs, and intergenic lncRNAs. lncRNAs can be also defined by localization within the cell: nuclear lncRNAs regulate gene transcription either in cis (local) or trans (distal) by acting as guides or scaffolds to localize chromatin remodeling factors on DNA loci; cytoplasmic lncRNAs can act as decoys for miRs or can affect mRNA stability and translation. In addition, lncRNAs in the cytoplasm can act to regulate nuclear translocation of TFs and signaling pathways by RNA-protein interactions. Although few vascular lncRNAs have been identified and described, Table 3.2 contains a list of lncRNAs that have been found in VSMCs.
| lncRNA | Functions | References |
|---|---|---|
| ANRIL | Regulates cyclin dependent kinase inhibitors CDKN2A/B; promotes proliferation, increases adhesion and decreases apoptosis; resides on chromosome 9p21, the strongest genetic factor for coronary artery disease | Congrains et al. Motterle et al. Holdt et al. |
| H19 | Generates miR-675, inhibits tumor suppressor PTEN and promotes proliferation | Lv et al. |
| HAS2_AS1 | Regulates hyaluronan synthesis by altering HAS2 gene chromatin structure and HAS2 transcription; induces migration | Vigetti et al. |
| HIF1A-AS1 | Interacts with BRG1 to regulate proliferation and induce apoptosis | Wang et al. |
| lincRNA-p21 | Represses proliferation and induces apoptosis in atherosclerosis | Wu et al. |
| lnc-Ang362 | Ang II-regulated lnc-Ang362 modulates proliferation by targeting miR-221/222 | Leung et al. |
| lncRNA XR007793 | Regulates cyclic strain-induced proliferation and migration | Yao et al. |
| MYOSLID | Promotes differentiation and inhibits proliferation; first VSMC-selective and SRF/CArG-dependent lncRNA; amplifies differentiation through parallel pathways of TGF-β/SMAD and myocardin-related transcription factor A (MKL1)/SRF | Zhao et al. |
| SENCR | Inhibits migration and stabilizes contractile phenotype | Bell et al. |
| SMILR | Promotes proliferation by regulating proximal HAS2 (hyaluronan synthase 2), an enzyme that synthesizes hyaluronic acid found in restenotic and atherosclerotic lesions | Ballantyne et al. |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here