Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Multiple mechanisms operate to regulate blood flow to organs – these are also important in the newborn.
Distribution of cardiac output is actively regulated.
Autoregulation may best be described as a degree of capacity rather than an on-off phenomenon.
Cerebral autoregulation, that is, the ability to buffer the effects of blood pressure on cerebral blood flow, is developed even at the limits of viability.
Whereas the brain is relatively large in newborn infants, its blood flow is relatively low.
A “diving” reflex–like response serves to prioritize vital organs during stress.
The cerebral hemispheres may not be privileged, especially in the very preterm neonate.
In most organs the principal role of perfusion is to provide substrates for cellular energy metabolism, with the final purpose of maintaining normal intracellular concentrations of the high-energy phosphate metabolites adenosine triphosphate (ATP) and phosphocreatine. The critical substrate is usually oxygen. Accordingly, organ blood flow is regulated by the energy demand of the given tissue. For instance, during maximal activation by seizures in the brain, cerebral blood flow (CBF) increases threefold, while in the muscle during maximal exercise, blood flow increases up to eightfold. Some organs, such as the brain, heart, and liver, have higher baseline oxygen consumption and thus higher demand for blood flow than others. Finally, in the kidney and skin, perfusion may be considerably above the metabolic needs to serve for glomerular filtration and thermoregulation, respectively. Indeed, during heating, skin blood flow may increase by as much as fourfold without any increase in energy demand.
In the developing organism, metabolic requirements are increased by as much as 40% due to the expenditures of growth. Since growth involves deposition of protein and fat, energy metabolism and oxygen requirements are not increased as much as are the requirements for protein and energy.
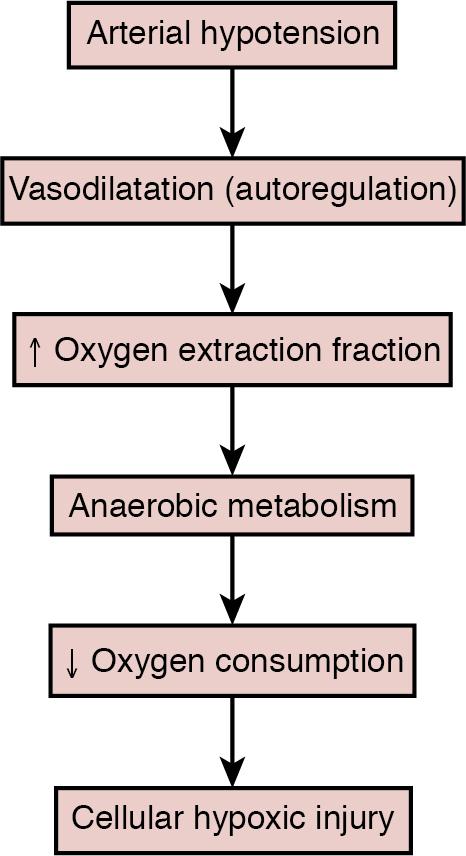
When blood flow is failing, there are several lines of defense mechanisms at the tissue level before the tissue is damaged. First, more oxygen is extracted from the blood. Normal oxygen extraction is about 30%, resulting in a venous oxygen saturation ( S vO 2 ) of 65–70%. Oxygen extraction can increase up to 50% to 60%, resulting in an S v o 2 of 40–50%, which corresponds to a venous (i.e., endcapillary) oxygen tension of 3–4 kPa. This is the critical value for oxygen tension for driving the diffusion of molecular oxygen from the capillary into the cell and to the mitochondria ( Figure 2.1 ). Microvascular anatomy and the pathophysiology of the underlying disease process are both important for these final steps of oxygen delivery to tissue. When the cell senses oxygen insufficiency, its function is affected so that growth stops, organ function fails, and, finally, cellular function; thus, organ survival is threatened ( Figure 2.2 ). Ischemia is the term used for inadequate blood flow to maintain appropriate oxygen delivery and thus cellular function and integrity. Since there are several steps in the cellular reaction to oxygen insufficiency, more than one ischemic threshold may be defined. It is also possible that newborn infants can be, at least, partly protected against hypoxic-ischemic injury by mechanisms akin to hibernation by “hypoxic hypometabolism.”
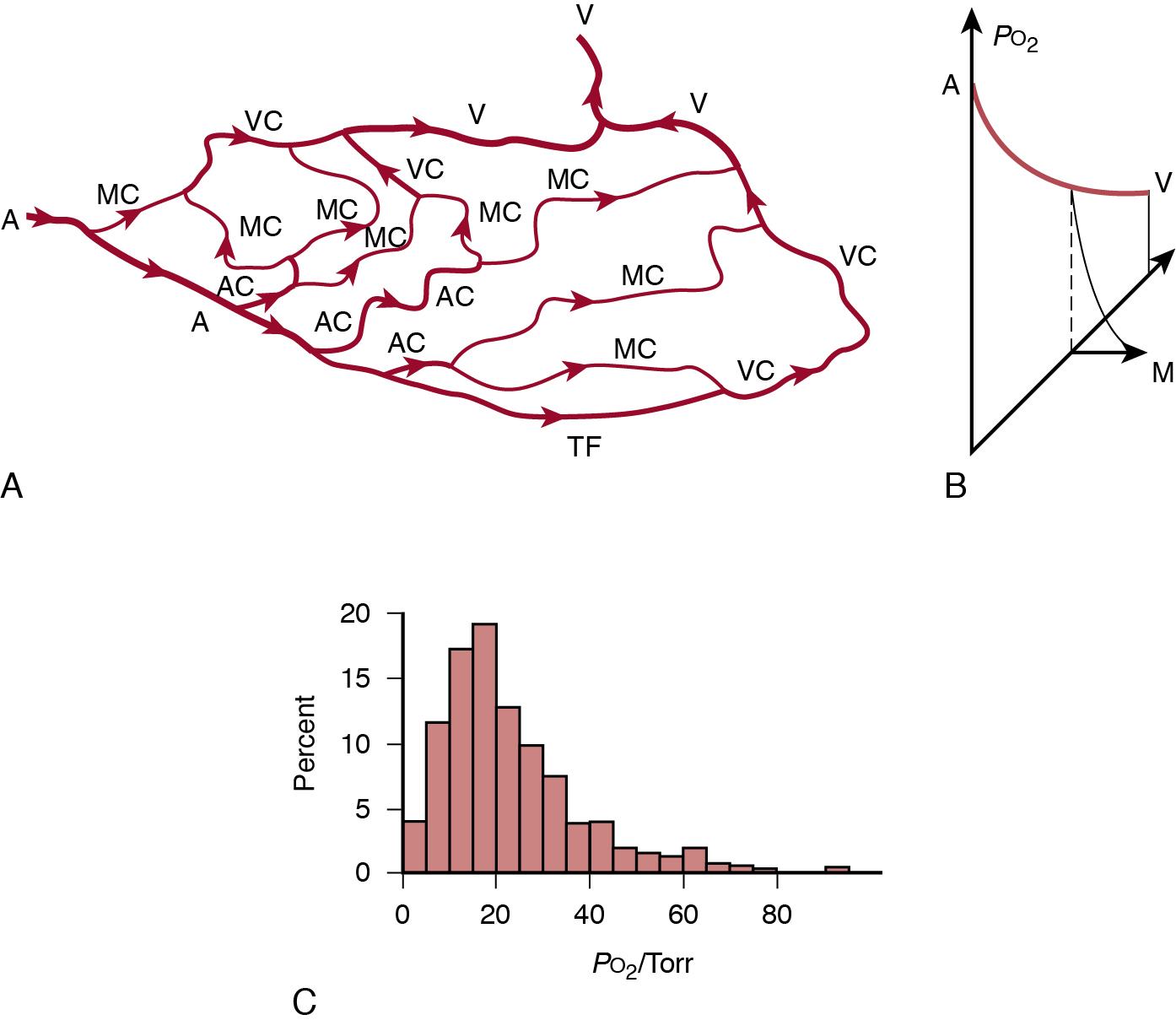
Physically, blood simply flows toward the point of lowest resistance. While flow velocities in the heart are high enough to allow the kinetic energy to thrust the blood forward, flow velocities are minimal in the peripheral circulation. Organs are perfused in parallel and the blood flow through a given tissue is the result of the pressure gradient between the arteries and the veins, the so-called perfusion pressure. Vascular resistance is composed of the limited diameter of blood vessels, particularly the smaller arteries and arterioles, their lengths, and blood viscosity. While veins also hold tone, their contribution to vascular resistance is very limited, so they will be disregarded here. Their importance lies in the regulation of blood return to the heart, which thus influences cardiac output (see Chapter 3 ).
The regulation of organ blood flow takes place by active modification of the arterial diameter, that is, by varying the tone of the smooth muscle cells of the arterial wall. Factors that influence vascular resistance are usually divided into four categories: blood pressure, chemical (P co 2 and P o 2 ), metabolic (functional activation), and neurogenic. Most studies of vascular resistance have been done on cerebral vessels. Therefore the following account refers to cerebral vessels from mature animals, unless stated otherwise.
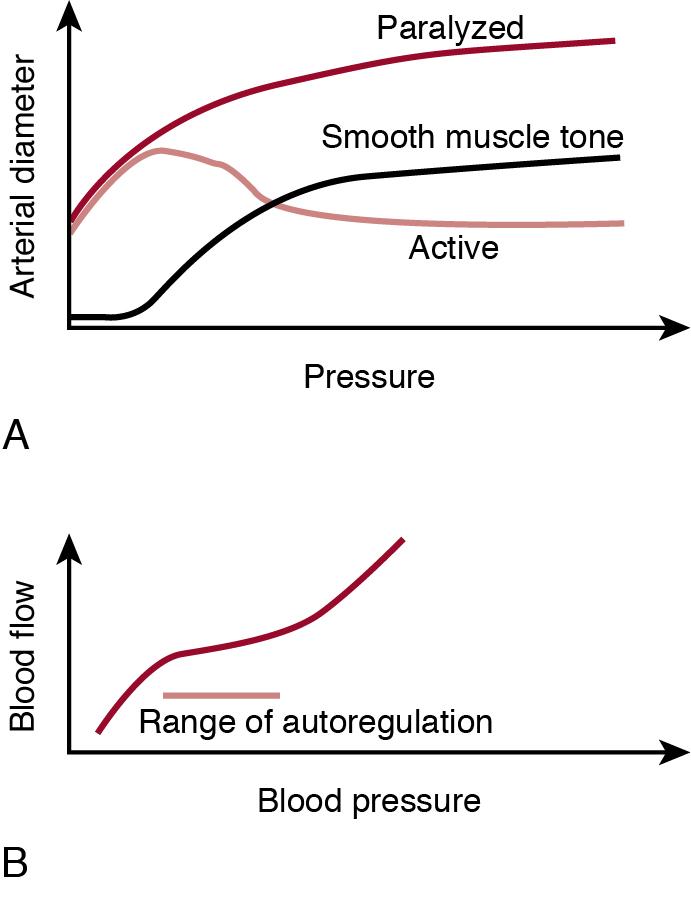
It is often assumed that the arteriole, the precapillary muscular artery with a diameter of 20–50 μm, is the primary determinant of vascular resistance, while the larger arteries are considered passive conduits. However, this is not the case. For instance, in the adult cat the pressure in the small cerebral arteries (150–200 μm) is only 50–60% of the aortic pressure. Thus nearly 50% of the total vascular resistance is “central” and the reactivity of the entire muscular arterial tree is of relevance in regulating organ blood flow and “distributing” cardiac output. The role of the pre-arteriolar arteries is likely to be even more important in the newborn than in the adult. First , the smaller body size translates to smaller conduit arteries (the resistance is proportional to length but is inversely proportional to the diameter to the power of 4). Second , conduit arteries are very reactive in the newborn. The diameter of the carotid artery increases by 75% during acute asphyxia in term lambs, whereas the diameter of the descending aorta decreases by 15% when blood pressure drops. For comparison, flow-induced vasodilatation in the forearm in adults is only in the order of 5%. These findings indicate a roughly 90% reduction of the arterial component of the cerebrovascular resistance coupled with a near doubling of the arterial component of vascular resistance to the lower body. Incidentally, they also suggest that blood flow velocity, as recorded from conduit arteries by Doppler ultrasound, may be potentially misleading and thus likely to be biased as a measure of perfusion in the neonates.
Smooth muscle cells of the arterial wall constrict in response to increased intravascular pressure in the local arterial segment to a degree that more than compensates for the passive stretching of the vessel wall by the increased pressure. The net result is that arteries constrict when pressure increases and dilate when pressure drops. This phenomenon is called the autoregulation of blood flow ( Figure 2.3 ). The response time in isolated, cannulated arterial segments is in the order of 10 seconds. The cellular mechanisms of this process are now better understood. Vessel wall constriction constitutes an intrinsic myogenic reflex and is independent of endothelial function. Rather, pressure induces an increase in the smooth muscle cell membrane potential, which regulates vascular smooth muscle cell activity through the action of voltage-gated calcium channels. Although the precise mechanistic nature of the mechano-chemical coupling is unknown, several plasma membrane–bound receptors are involved, including G protein–coupled receptors and a class of transient potential receptors. Furthermore, the calcium signal is modulated in many ways, but a detailed discussion of this topic is beyond the scope of this chapter. Suffice to mention that phospholipases and activation of protein kinase C are involved, and, at least in the rat middle cerebral artery, the arachidonic acid metabolite 20-HETE has also been implicated. Furthermore, additional modulation of intracellular calcium concentration by alternative sources, such as the calcium-dependent K + channels, also exists. Local variations in the expression of these receptors and channels may, at least in part, explain the difference in responses to blood pressure in different vascular beds and the change in unique blood flow distribution between vital and non-vital organs during the diving reflex (see later).
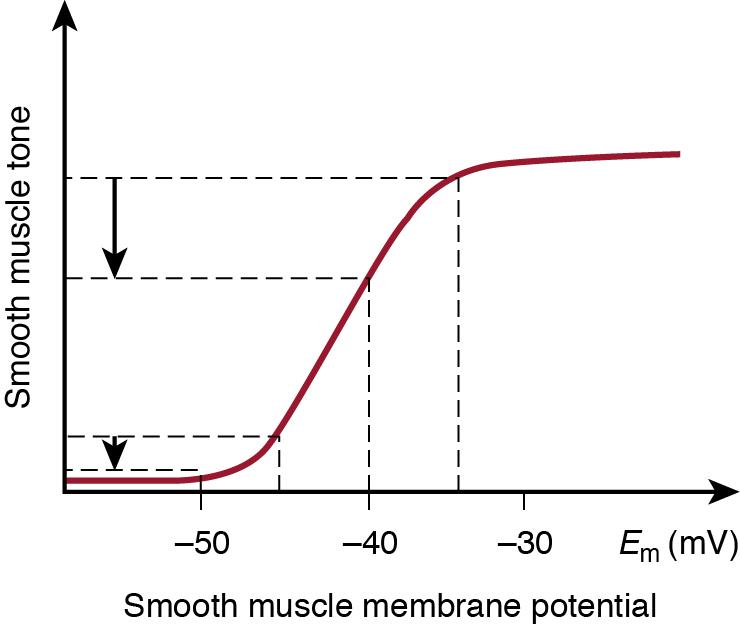
As described earlier, arterial smooth muscle tone is affected by a number of factors, all contributing to determine the level of vascular resistance. Among the vasodilators, hypoxia is one of the most important, more potent, and physiologically relevant factors. Vascular reactivity to O 2 depends, in part, on intact endothelial function ensuring appropriate local nitric oxide (NO) production. Hypoxia also induces tissue lactic acidosis. The decreased pH constitutes a point of interaction between O 2 and CO 2 reactivity (see later). In addition, hypoxia decreases smooth muscle membrane potential by the direct and selective opening of both the calcium-activated and ATP-sensitive K + channels in the cell membrane. In the immature brain adenosine is also an important regulator of the vascular response to hypoxia. The membrane potential response to hypoxia is independent of the existing intravascular pressure. However, at lower pressures, the decrease in membrane potential only leads to minimal further arterial dilation because, since at low vascular tone, the membrane potential-muscular tone relationship is already outside the steep part of the slope ( Figure 2.4 ). In other words at low perfusion pressures, the dilator pathway has already been near maximally activated. Therefore in a hypotensive neonate, a superimposed hypoxic event cannot be appropriately compensated due to the low perfusion pressure. The end result is tissue hypoxia-ischemia with the potential of causing irreversible damage to organs, especially to the brain. This is a clinically highly relevant point when providing care for the hypotensive and hypoxic neonate.
Arteries and arterioles in the brain constrict with hypocapnia and dilate with hypercapnia. The principal part of this reaction is mediated through changes in pH, that is, H + concentration. Perivascular pH has a direct effect on the membrane potential of arterial smooth muscle cells since, mostly via activation of both the ATP-sensitive and calcium-activated K + channels, extracellular H + concentration is one of the main determinants of the potassium conductance of the plasma membrane in arterial smooth muscle cells regulating the outward K + current. Therefore when the pH decreases, the K + outflow from the vascular smooth muscle cell increases, resulting in hyperpolarization of the cell membrane and, via the closure of voltage-gated Ca ++ channels, it causes vasodilatation. Furthermore, increased extracellular and, to a lesser degree, intracellular H + concentrations reduce the conductance of the voltage-dependent calcium channels, further enhancing vascular smooth muscle cell relaxation.
Hypercapnic vasodilatation is reduced by up to 50% when NO synthase (NOS) activity is blocked in the brain of the adult rat. The hypercapnic response is restituted by the addition of an NO donor. This finding suggests that unhindered local NO production is necessary for the pH to exert its vasoregulator effects. Indeed, it has been suggested that, although the calcium-activated and ATP-sensitive K + channels play the primary role in the vascular response to changes in P co 2 , the function of these channels is regulated by local NO production.
The role of prostanoids in mediating the vascular response to P co 2 is less clear. , The fact that indomethacin abolishes the normal CBF-CO 2 response in preterm infants is likely a direct effect of the drug independent of its inhibitory action on prostanoid synthesis. This notion is supported by the finding that ibuprofen is devoid of such effects on the brain blood flow–CO 2 response.
Several mechanisms operate to match local blood flow to metabolic requirements, including changes in pH, local production of adenosine, ATP and NO, and local neural mechanisms. It appears that, in the muscle, there is not a single factor dominating, since the robust and very fast coupling of activity and blood flow is almost unaffected by blocking any of these mechanisms one by one. In the brain astrocytes may be the central sites of regulation of this response in the neurovascular unit via their perivascular end-feet and by using many of the aforementioned cellular mechanisms, such as changes in K + ion flux and the local production of prostanoids, ATP, and adenosine. Among these cellular regulators, adenosine has been proposed to play a principal role. Adenosine also works by regulating the activity of the calcium-activated and ATP-sensitive K + channels.
Endothelial cells sense flow by shear stress and produce NO in reaction to high shear stress at high flow velocities. NO diffuses freely and reaches the smooth muscle cell underneath the endothelium. NO acts on smooth muscle K + channels using cyclic guanosine monophosphate (GMP) as the secondary messenger and then a series of intermediate steps. Since NO is a vasodilator, the basic arterial reflex to high flow is vasodilation. Thus when a tissue is activated (e.g., a muscle contracts), the local vessels first dilate, as directed by the mechanisms of the metabolic flow control described earlier, and blood flow increases. This initial increase in blood flow is then sensed in the conduit arteries through the shear stress-induced increase in local NO production, and vascular resistance is further reduced, allowing flow to increase yet again. The action remains local as the generated NO diffusing into the bloodstream is largely inactivated by hemoglobin.
Epinephrine in the blood originates from the adrenal glands, whereas norepinephrine is produced by sympathetic nerve endings and extra-adrenal chromaffin tissue. Sympathetic nerves are present in nearly all vessels, located in the adventitia and mixed in with the smooth muscle cells. Adrenoreceptors are widely distributed in the cardiovascular system located in the smooth muscle and endothelial cell membranes. Several different adrenoreceptors exist; alpha-1 receptors with at least three subtypes are present primarily in the arteries and the myocardium, while alpha-2, beta-1, and beta-2 receptors are expressed in all types of vessels and the myocardium. In the arteries and veins alpha-receptor stimulation causes vasoconstriction, and beta-receptor stimulation results in vasodilatation. Both alpha- and beta-adrenoreceptors are frequently expressed in the membrane of the same cell. Therefore the response of the given cell to epinephrine or norepinephrine depends on the relative abundance of the receptor types expressed. Points of clinical importance are (1) the regulation of the expression of the cardiovascular adrenergic receptors are regulated by corticosteroids, (2) the high incidence of relative adrenal insufficiency in preterm neonates and critically ill term infants (see Chapter 23 ), and (3) the downregulation of the cardiovascular adrenergic receptors in response to the increased release of endogenous catecholamines or the administration of exogenous catecholamines given in critical illness. Typically, arteries and arterioles of the skin, gut, and muscle constrict in response to increases in endogenous catecholamine production, whereas those of the heart and brain neither constrict nor even dilate (see later). The response also depends on the resting tone of the given vessel. Furthermore, circulating norepinephrine may be more effective than norepinephrine produced by sympathetic nerve activity, since alpha-1 receptors may be particularly abundant in the membrane regions close to where the nerve secretes the transmitter. Furthermore, the signaling pathways of the adrenoreceptors are complex and dependent on the receptor subtype. Activation of alpha-adrenoreceptors generally results in vasoconstriction mediated by increased release of calcium from intracellular stores as a first step, while beta-receptor-induced vasodilation is mediated by increased cyclic adenosine monophosphate (AMP) generation. However, the system is more complex and, among other mechanisms, receptor activation-associated changes in K + conductance and local NOS are also involved. Finally, the sympathetic nervous system is activated during hypoxia, hypotension, or hypovolemia via the stimulation of different chemoreceptors and baroreceptors in the vessel walls and the vasomotor centers in the medulla. Activation of the sympathetic nervous system plays a central role in the cardiovascular response to stress, and it is the mainstay of the diving reflex response during hypoxia-ischemia.
Several endogenous vasoactive factors, other than those mentioned earlier, play a role in the processes of organ blood flow regulation, such as angiotensin II, arginine-vasopressin, vasointestinal peptide, neuropeptide gamma, and endothelin-1. However, none of these vasoactive factors has been shown to have any significant importance in themselves under normal conditions, except for the role of angiotensin II in regulating renal microhemodynamics.
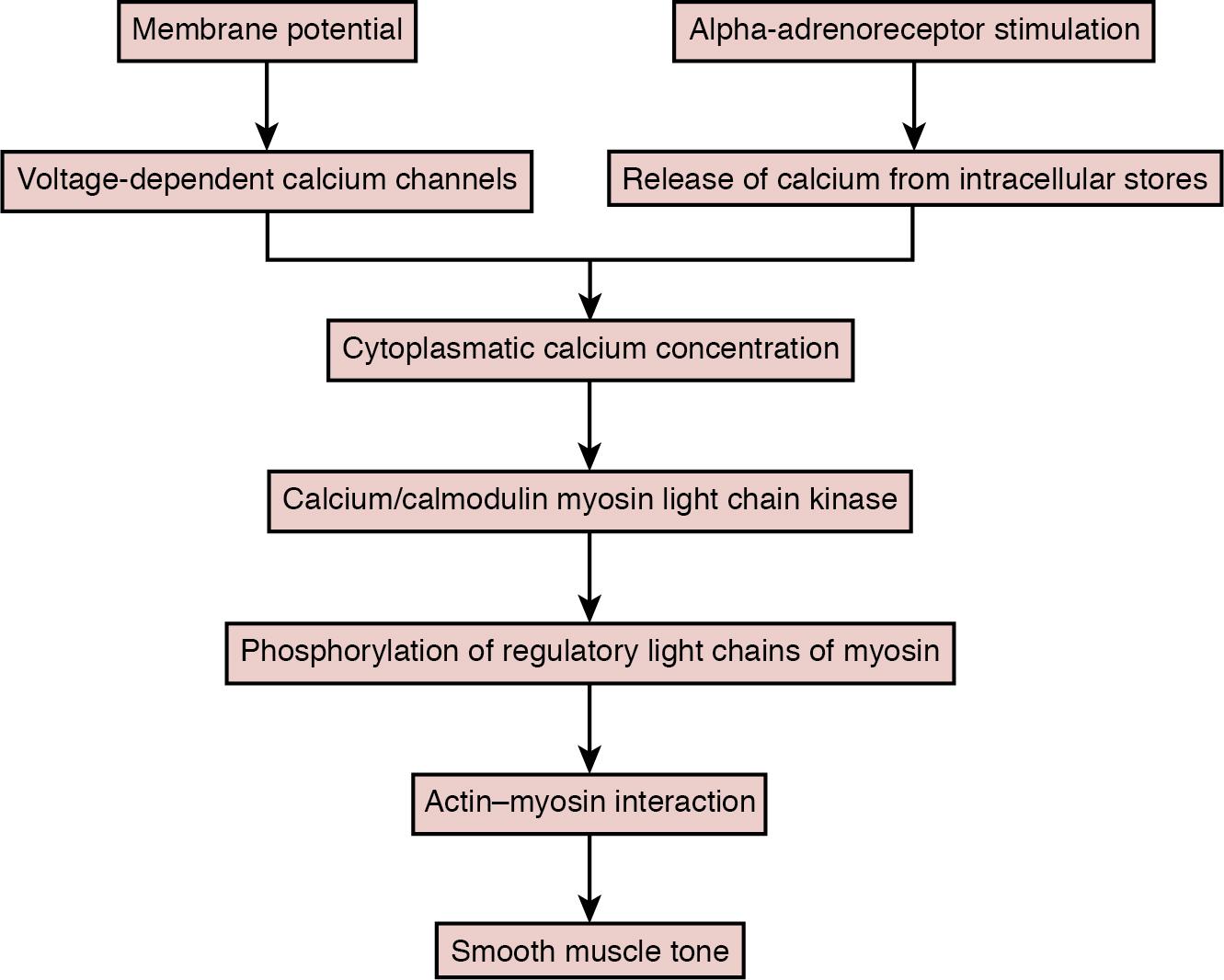
In conclusion, many factors have an input and interact to define the degree of contraction of the local vascular smooth muscle cell to regulate local arterial and arteriolar tone (see Figure 2.4 ). Although many details are unknown, especially in the developing immature animal or human, the final common pathway appears to involve the smooth-cell membrane potential, cytoplasmic calcium concentration, and the calcium/calmodulin myosin light chain kinase–mediated phosphorylation of the regulatory light chains of myosin, resulting in the interaction of actin and myosin ( Figure 2.5 ). The complexity of the known factors and their interplay, as well as the differences in the response among the different organs, are overwhelming, and no simple or unifying principle of vascular tone regulation has gained a foothold. Indeed, the complexity predicts that vascular tone and reactivity in a particular arterial segment in a particular tissue may differ markedly from that in other segments or other tissues. Practically, this means that the insights that are summarized above are as yet insufficient to allow any quantitative predictions for different organs or segments of the vascular tree.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here