Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Therapeutic intervention is optimized when we understand the normal physiological signaling processes that are disrupted by a disease process, the abnormal molecular and cellular mechanisms driving disease pathogenesis, and the pharmacological profile of the intervention. Indeed, the majority of vascular drugs act as replacement therapy to augment endogenous protective signaling processes or as reversal therapy to block or reduce the abnormal activity of pathological mediators or signaling processes. This chapter discusses current and potential future therapies within the context of these processes.
Pharmacology provides a guiding scientific framework to help define optimal approaches to correcting abnormal or perturbed systems. It encompasses the area of pharmacokinetics, which characterizes how our bodies interact with and process drugs, including their absorption, distribution, metabolism, and elimination; and the area of pharmacodynamics, which characterizes the mechanism of action of drugs and how they interact with our bodies to modify cellular and organ function. This chapter focuses mainly on pharmacodynamics and the mechanisms of action of drugs.
The action of most drugs involves their chemical interaction with macromolecular species that regulate cellular activity within important regulatory systems. Indeed, drug receptors generally serve as receptors or signaling intermediates for endogenous mediators. Exogenous drugs and endogenous stimuli that bind to and cause activation of receptor-dependent signaling are termed agonists. Their activity is determined by agonist-dependent and tissue-dependent characteristics. Agonist-dependent activity at receptors is determined by two main drug characteristics: the affinity of the drug for the receptor, which defines the concentration range over which the agonist effectively engages the receptor, and the intrinsic efficacy of the agonist, which defines how well the agonist activates the receptor after it has bound to the site ( Fig. 5.1 ). A key aspect of receptor systems is their remarkable ability, via activation of ion channels and/or serial activation of enzyme systems, to amplify signaling systems. This enables initial discrete agonist-receptor molecular interactions to cause profound alterations in cellular and organ function. High-efficacy agonists are very effective at activating receptors and can generate a maximal response of the system while occupying only a fraction of the receptors. As a result, they are also described as “full” agonists, and the fraction of receptors not required for the maximal response are described as spare receptors or the receptor reserve (see Fig. 5.1 ). In contrast, low-efficacy agonists are less effective at activating the receptors and must engage and activate a greater proportion of receptors to generate a functional response equivalent to that of the higher-efficacy agonists. Low-efficacy agonists will therefore have fewer spare receptors or a smaller receptor reserve as compared with higher-efficacy agonists. When receptor systems are limited (or agonist efficacy is sufficiently low), low-efficacy agonists will not generate the full maximal response in the system; under these conditions, they are also described as “partial” agonists (see Fig. 5.1 ). Receptor systems can become limited as a result of decreased receptor expression or from the reduced efficiency of downstream signaling events, which could reflect reduced expression of signaling mediators or concurrent activation of opposing mechanisms (functional antagonism). Because of their ability to occupy receptors coupled with a reduced ability to activate them, low-efficacy or partial agonists can actually block the activity of higher-efficacy agonists. Indeed, if the receptor system is severely limited or the intrinsic efficacy of the partial agonists is sufficiently low, they may not generate an agonist response and would act like pure antagonists to block the response to higher-efficacy agonists. This variable activity is observed with the low-efficacy α-adrenergic agonist clonidine, which is approved by the US Food and Drug Administration (FDA) for the treatment of hypertension. It activates α2-adrenergic receptors (α2-adrenoceptors, α2-ARs) in the central nervous system to reduce sympathetic outflow, which is thought to be the major mechanism for its hypotensive activity (see the section titled “Therapeutic Intervention and the Autonomic Vascular Innervation”). In the vascular system, clonidine can activate α1- and α2-ARs to cause vasoconstriction, but in the presence of high-efficacy agonists such as the physiological agonists norepinephrine and epinephrine, clonidine has the opposite effect and causes vasodilatation. It is unclear whether this contributes to its antihypertensive activity.
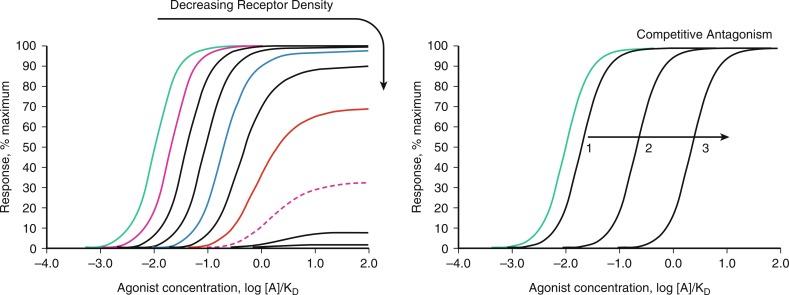
Pure receptor antagonists have significant affinity for receptors but no intrinsic efficacy and are therefore unable to provide activation. Competitive antagonists bind reversibly to the receptor and inhibit the ability of the agonist to bind and cause receptor activation. Increasing the concentration of the agonist will compete away the antagonist and regain functional activity, resulting in a parallel rightward shift in the concentration-response curve with no change in the maximal response (see Fig. 5.1 ). The magnitude of the rightward shift of the curve depends on the concentration of the antagonist and its affinity for the receptor. Indeed, the affinity of the antagonist for the receptors can be determined based on the magnitude of the shift (see Fig. 5.1 ). If the antagonist binds irreversibly or pseudo-irreversibly (i.e., slow dissociation), it will function in a similar manner to a reduced number of receptors, with rightward shifts in the concentration-effect curve (no change in the maximal response) until the receptor reserve is lost, after which it will cause downward shifts in the curve with progressive depression of the maximal response (see Fig. 5.1 ). This type of action is described as noncompetitive antagonism. Phenoxybenzamine, which is FDA-approved for the treatment of pheochromocytoma, is an irreversible noncompetitive antagonist at α-ARs (see section titled “Therapeutic Intervention and the Autonomic Vascular Innervation”).
Some receptors can display spontaneous activity or be activated in an agonist-independent manner. For example, in addition to being activated by angiotensin II (ANGII), AT1 receptors (AT1Rs) are thought to function as mechanoreceptors and to be activated by mechanical stretch independently of ANGII. Because no agonist is bound to the receptor, this type of activity cannot be reversed by simple receptor antagonists. However, some antagonists actually function as inverse agonists: they have negative intrinsic efficacy and cause the receptors to have reduced activity. Inverse agonists therefore have the ability to inhibit receptor activation by agonists and agonist-independent mechanisms. Regarding the example of AT1Rs, both losartan and valsartan are FDA-approved as selective AT1R antagonists to treat hypertension (see section titled “Therapeutic Intervention and the Vascular Media”); however, unlike losartan, valsartan can function as an inverse agonist at AT1Rs and therefore block the ANGII-dependent and independent activity of AT1Rs.
Drug selectivity is a relative, not an absolute quality. In general, as the concentration of a drug is increased, it will interact with additional receptor sites, resulting in distinct concentration-effect curves for its multiple effects. The concentration difference between these concentration-response curves provides a measure of drug selectivity. Although the additional activity could contribute to the therapeutic activity of the drug, it often reflects an unwanted or problematic activity; the selectivity of the compound therefore also provides a measure of its therapeutic index.
Receptors are generally regulated by a negative feedback signaling system whereby sustained activation causes downregulation or desensitization of the receptor system, whereas sustained absence of activation can result in receptor sensitization or increased reactivity to a stimulus. These processes can complicate treatment strategies, causing a diminution in effectiveness of agonist-based therapeutics or rebound activity following cessation or interruption of antagonist-based approaches. For example, treatment of pulmonary arterial hypertension (PAH) with the prostacyclin IP receptor agonist eproprostenil requires continual dose escalation to maintain clinical efficacy (see section titled “Therapeutic Intervention and the Endothelium”). Alterations in the clinical efficacy of drugs can also reflect adaptive changes in pharmacokinetics, including absorption and disposition mechanisms or changes in pharmacodynamics processes resulting, for example, from an ongoing disease process.
The nature of therapeutic intervention is evolving. Emerging therapeutics include biologics such as genetically engineered enzymes, humanized antibodies, and RNA-silencing approaches. Gene therapy approaches using viral vectors to correct genetic mutations are now being approved in the United States and Europe. However, pharmacological concepts can still provide a guiding framework to optimize traditional and novel approaches for correcting abnormal or perturbed systems.
Normal endothelial function is crucial for maintaining cardiovascular and organismal health. Endothelial cells are important regulators of blood vessel constriction, thrombosis, inflammation, permeability, and vascular remodeling. Under physiological conditions, the endothelium exerts a powerful protective influence to inhibit these processes and maintain vascular stability and homeostasis; however, during the development of vascular disease, the endothelium becomes “dysfunctional,” promoting these same processes and contributing to pathological changes in vascular function and structure. Not surprisingly, endothelial-dependent mechanisms are targeted by numerous therapeutics to treat vascular diseases.
Under normal conditions, endothelial cells generate two prominent vasodilator mediators, prostacyclin (prostaglandin I 2 , or PGI 2 ) and nitric oxide (NO) ( Fig. 5.2 ). Although endothelial cells can release another vasodilator, endothelium-derived hyperpolarizing factor (EDHF), it has not yet been implicated or utilized in vascular therapies and is not discussed further here. PGI 2 production is dependent on the enzyme cyclooxygenase (COX), whereas endothelium-derived NO is produced by endothelial NO synthase (eNOS). Basal production of these mediators can be rapidly increased following endothelial activation by numerous stimuli that may be present in the vessel wall and bloodstream, including norepinephrine, thrombin, and bradykinin. Endothelial cells are also mechanosensitive and are regulated by the shear stress exerted by the bloodstream itself, including increased production of PGI 2 and NO (see Fig. 5.2 ). Such flow-mediated dilatation (FMD) has become an important noninvasive mechanism to assess endothelial function and vascular health. Under physiological conditions, endothelial FMD enables dilatation in small arterioles, for example, in response to cellular metabolism, to be conducted upstream to more proximal arterioles and arteries, facilitating targeted increases in blood flow and preventing tissue ischemia.
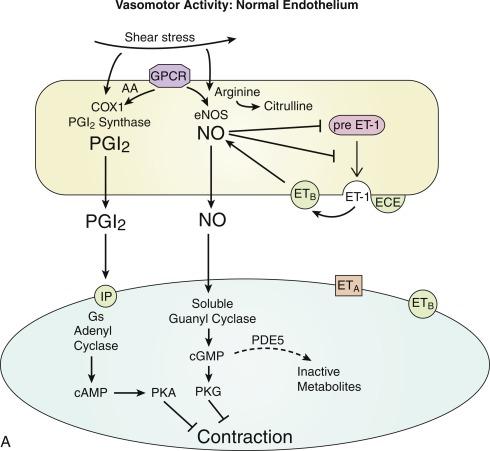
The COX enzyme converts arachidonic acid to an unstable intermediate PGH 2 , which is then converted via specific synthase enzymes to numerous prostanoids, including PGI 2 generated by endothelial PGI 2 synthase (see also the section titled “COX Inhibitors and the Human Vascular System”). A key step in this process is the availability of arachidonic acid, which is released from membrane phospholipids by phospholipase A 2 . eNOS comprises an N-terminal oxygenase domain that binds heme, zinc, tetrahydrobiopterin (BH 4 ), calmodulin and the substrate l -arginine, and a C-terminal reductase domain that binds flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide phosphate (NADPH). Normal enzyme activity requires the formation of homodimers that enables the transfer of electrons from the reductase domain of one eNOS monomer to the oxygenase domain of the other monomer, culminating in the oxidation of l -arginine and NO formation. Owing to their very short half-lives, NO and PGI 2 act locally and are not circulating mediators. PGI 2 activates IP receptors, which are plasma membrane receptors predominantly coupled to the G S -protein, resulting in activation of adenylyl cyclase and increased production of cyclic adenosine monophosphate (AMP). NO diffuses through plasma membranes and activates the cytosolic enzyme soluble guanylyl cyclase and increases production of cyclic guanosine monophosphate (GMP). In vascular smooth muscle cells (VSMCs), cyclic AMP and cyclic GMP activate protein kinase A (PKA) and protein kinase G (PKG), respectively, to initiate vasodilation (see Fig. 5.2 ) (see also the section titled “Therapeutic Intervention and the Vascular Media”). These agents have important additional effects on endothelial, VSMCs, and circulating cells to regulate thrombosis/hemostasis, vascular remodeling, and inflammation.
The activity of NO and PGI 2 is reduced during the development of vascular diseases, resulting in diminished endothelial-mediated dilatation and an increased propensity for vasoconstriction (see Fig. 5.2 ). Indeed, the presence of endothelial dilator dysfunction is predictive of future cardiovascular events, and the assessment of endothelial function may help direct vascular therapy to improve cardiovascular outcomes. Reduced activity of these mediators can reflect alterations in the ability of endothelial cells to be activated or reflect specific changes in enzyme/mediator dynamics such as, for PGI 2 , inactivation of PGI 2 synthase by the pathological oxidant OONO - and/or expression of alternate synthases, and for NO, inactivation by superoxide radical. Indeed, decreased levels of cofactor BH 4 or substrate arginine causes uncoupling of enzyme function resulting in the generation of superoxide rather than NO. eNOS uncoupling is thought to contribute to endothelial dysfunction in numerous vascular disorders including atherosclerosis, diabetes, hypertension and aging. In the setting of endothelial dysfunction, there is not only a decrease in the activity of protective vasodilator mediators but also increased production of the potent vasoconstrictor endothelin-1 (ET-1). ET-1 is formed from precursor peptides by proteolytic processing. PreproET-1 mRNA is translated, stripped of its signal sequence, and further cleaved by a furin-like peptidase to generate BigET-1. Further processing to biologically active ETs is achieved by endothelin-converting enzyme (ECE). BigET-1 and to a lesser extent mature ET-1 are stored in granules within the endothelium. Following stimulation by endothelial secretagogues, for example, thrombin, these peptides are released by exocytosis, resulting in an explosive generation of ET-1 and blood vessel constriction. Endothelium-derived NO is an important functional inhibitor of ET-1, reducing its expression and blocking its exocytotic release and generation. Not surprisingly, there is a minimal role for ET-1 in the normal endothelial regulation of contractility in mature arteries. Indeed, the diminution in NO activity occurring during endothelial dysfunction is likely an important contributor to the increased prominence of ET-1. ET-1 activates two receptor subtypes: the ET A receptor, which is expressed on smooth muscle cells and mediates constriction, and the ET B receptor, which also can be present on VSMCs to mediate constriction but is prominently expressed on endothelial cells and mediates increased production of PGI 2 and NO (see Fig. 5.2 ).
Therapeutic intervention to correct endothelial dilator dysfunction is best exemplified by current approaches to treat PAH (see Fig. 5.2 ). Indeed, first-line therapeutic intervention for PAH is directed toward augmenting or replacing the reduced signaling activity of the endogenous protective mediators NO or PGI 2 and reducing the heightened activity of ET-1. PAH results from a progressive increase in pulmonary vascular resistance and is defined as an elevated mean pulmonary arterial pressure (≥ 25 mm Hg) and increased pulmonary arterial resistance, but with normal pulmonary venous pressure (pulmonary wedge pressure ≤ 15 mm Hg). The disease is predominantly of idiopathic (or unknown) origin but is also associated with other disease processes including scleroderma and ingestion of certain drugs or toxins (e.g., toxic rapeseed oil, fenfluramine). The condition results in right heart failure, which is the major cause of morbidity and mortality. PAH is associated with endothelial dysfunction including reduced activity of NO and PGI 2 and increased expression and circulating levels of ET-1. Before this targeted endothelial mechanistic approach to treatment, the median survival was 2.8 years. This has been dramatically extended to over 7 years, although PAH still represents a devastating disease process.
Intravenous synthetic PGI 2 (epoprostenol) was the first targeted therapy approved for PAH. Epoprostenol improves symptoms, exercise capacity, and hemodynamics and is the only treatment shown to reduce mortality in severe PAH. It is still considered the cornerstone of therapy for PAH, particularly in advanced stages of the disease. As with endogenous PGI 2 , epoprostenol has a very short half-life (~ 3 min) and requires the use of a drug delivery pump system for continuous intravenous infusion. Side effects are consistent with vasodilator therapy and include headaches, flushing, dizziness, and systemic hypotension; potential adverse events are also associated with the delivery system. Two other structural analogues of PGI 2 are FDA-approved for treating PAH. Treprostinil has a longer half-life than epoprostenol and is available for continuous intravenous infusion as well as subcutaneous, inhalation, and oral delivery, whereas iloprost is available in an inhaled formulation. These analogues have favorable effects on exercise capacity, hemodynamics, symptoms, and clinical events, and the vasodilator side effects are similar to those of epoprostenol. Selexipag belongs to a distinct class of PGI 2 -related, FDA-approved treatment options in PAH. It is a selective IP receptor agonist that is structurally distinct from PGI 2 and has shown favorable clinical efficacy in PAH. The active metabolite of selexipag, ACT-333697, has lower intrinsic efficacy at IP receptors compared with iloprost or trepinostil. This reduced intrinsic efficacy results in reduced desensitization and reduced internalization of IP receptors compared with the higher-efficacy agonists. It is unclear how this difference in pharmacodynamics might affect clinical efficacy in PAH.
Cyclic GMP, the predominant signaling mediator of NO activity, is degraded by a family of phosphodiesterase (PDE) enzymes whose expression varies in a cell- and tissue-specific manner. Inhibition of relevant PDE enzymes will augment the basal and stimulated levels of cyclic GMP and therefore amplify the activity of endothelium-derived NO. Pulmonary VSMCs have a high level of expression of the PDE5 subtype. Two orally active selective inhibitors of the PDE5 enzyme, sildenafil and tadalafil, are FDA-approved for the treatment of PAH. Tadalafil differs structurally from sildenafil and has a longer half-life. PDE5 inhibition has shown favorable results in improving exercise capacity, symptoms, and hemodynamics and in decreasing time to clinical worsening. Side effects are mild to moderate and mainly related to vasodilation (headache, flushing, and epistaxis). The therapeutic activity of PDE5 inhibitors will be dependent on the catalytic activity of PDE5 and the basal and NO-stimulated activity of soluble guanylyl cyclase. Endothelial dysfunction is generally associated with reduced bioactivity of endothelium-derived NO. If NO activity is severely limited, it would be expected to negatively affect the clinical efficacy of these agents. In contrast, a new class of agent that directly activates soluble guanylyl cyclase has recently been approved for use in PAH. The first-in-class agent, riociguat, not only directly stimulates soluble guanylyl cyclase independently of NO but also amplifies NO activity by sensitizing soluble guanylyl cyclase to endogenous NO. It has shown favorable effects in improving exercise capacity, symptoms, and hemodynamics and decreasing time to clinical worsening.
Three ET-1 competitive receptor antagonists are currently FDA-approved for treating PAH. Bosentan, macitentan, and ambrisentan are all orally effective. Two of them, bosentan and macitentan, do not discriminate between ET A and ET B receptors and inhibit both receptor subtypes. In contrast, ambrisentan is selective for ET A receptors, displaying an approximate 200-fold increased affinity when compared with ET B receptors. Clinical trials have demonstrated beneficial effects of all three agents in PAH, and data from monotherapy trials do not indicate a difference in clinical efficacy.
Inhaled NO gas is FDA-approved to treat persistent pulmonary hypertension of the newborn, a rare subtype of PAH, and acute hypoxemic respiratory failure. Inhaled NO causes preferential vasodilatation in better-ventilated lung regions rather than poorly inflated areas, resulting in improved ventilation/perfusion matching and increased PaO 2 levels. Because of its mode of delivery and extremely short half-life (2 to 6 seconds), inhaled NO is a selective pulmonary vasodilator that can lower pulmonary artery pressure without altering systemic blood pressure. Although inhaled NO is not approved for use in adult PAH, it is used in this patient group to acutely test sensitivity to vasodilator therapy and to treat acute pulmonary hypertensive crises.
Although it might be assumed that these endothelial-related therapies target vasoconstriction in PAH, they are likely acting through multiple mechanisms. The pathogenesis of PAH involves both functional and structural changes in the pulmonary arterial system. In addition to vasoconstriction, there is hypertrophy and proliferation of VSMCs, with increased muscularization and pulmonary arterial thickening, deposition of extracellular matrix proteins, and increased thrombotic activity, which conspire to increase vascular stiffness and precipitate luminal narrowing or occlusion (see also the section titled “Therapeutic Intervention and the Vascular Media”). Chronic treatment with intravenous epoprostenol has beneficial hemodynamic and clinical effects in PAH even in individuals who lack significant pulmonary arterial vasodilatation to acute administration of the drug. The conclusion is that the long-term beneficial effects of epoprostenol may be only partially related to its vasodilator properties and may reflect alternate actions on disease pathogenesis. Indeed, current guidelines suggest that selected PAH patients should undergo acute vasodilator testing (e.g., inhaled NO or intravenous epoprostenol) to identify the small subgroup of responders (defined as a ≥ 10 mm Hg decrease in pulmonary artery pressure to achieve an absolute level ≤ 40 mm Hg) who might benefit from long-term calcium channel blocker therapy. Only about 10% of idiopathic PAH patients will meet this criterion. In addition to acutely controlling vascular contractility, PGI 2 , NO, cyclic GMP, and ET-1 have important regulatory effects on hemostasis/thrombosis, inflammation, and vascular wall remodeling that likely contribute to the therapeutic effects of drugs targeting these mediators.
Current approaches emphasize combination therapy to treat PAH even as an initial approach to treatment. This is an attractive approach based on the disparate and potentially synergistic mechanisms utilized by these drugs (see Fig. 5.2 ). Indeed, current data suggest that patients may benefit from triple combination therapy comprising intravenous PGI 2 analogues, PDE5 inhibition, and ET-1 receptor blockade.
The endothelium-related therapeutic approaches to treat PAH are also being used in other vascular pathological processes. Intravenous PGI 2 analogues (iloprost, epoprostenol), PDE5 inhibitors (sildenafil, tadalafil, vardenafil), ET receptor antagonists (bosentan) and topical NO-based therapy (topical nitroglycerin) are currently used to treat cutaneous vasospastic episodes and prevent ischemic injury in scleroderma. Scleroderma is associated with a cold-induced cutaneous vasospastic disorder (secondary Raynaud phenomenon) and disruption of endothelial dilator function, which limits FMD, resulting in digital ulceration and necrosis.
Penile erection, which reflects vasodilatation of the venous corpus cavernosum system and results in the organ’s engorgement with blood, is mediated by increased activity of NO and relaxation of cavernosal VSMCs. Cavernosal endothelial cells can produce and liberate NO in response to endothelial agonists and to physical stimuli. However, the predominant source of NO responsible for penile erection is not endothelium-derived but is neuronal NOS (nNOS) located in parasympathetic nonadrenergic noncholinergic nerve fibers innervating the blood vessel wall. In contrast, the sympathetic adrenergic nervous system, acting through norepinephrine and α-ARs, constricts cavernosal smooth muscle and is responsible for maintaining a flaccid penis and the induction of detumescence following erection. Erectile dysfunction can result from psychologic, hormonal, and vascular etiologies, including reduced NO signaling—for example, as a result of increased oxidant activity. Indeed, erectile dysfunction is strongly associated with cardiovascular arterial disease. As with pulmonary smooth muscle cells, cavernosal VSMCs have a high expression of PDE5, responsible for the degradation of cyclic GMP and diminution in NO activity. PDE5 inhibitors—including sildenafil, vardenafil, tadalafil, and avanafil—are approved for use in treating erectile dysfunction and have demonstrated beneficial effects in patients with varying etiologies of sexual dysfunction. As mentioned earlier, their activity is dependent on NO-stimulated activity of soluble guanylyl cyclase, and their use still requires sexual stimulation to create arousal and raise the available levels of NO. Additional vasodilator therapies are being investigated for use in erectile dysfunction, including inhibition of Rho-associated coiled-coil protein kinase or Rho-kinase (ROCK) (see the section titled “Therapeutic Intervention and the Vascular Media”) and direct activation of soluble guanylyl cyclase.
Nitrovasodilators, which generate NO, have been employed for over 150 years to alleviate the chest pain associated with myocardial ischemia. Their activity is reviewed in the section titled “Therapeutic Intervention and the Vascular Media.”
Normally the endothelium actively inhibits hemostasis/thrombosis and promotes fibrinolysis by producing and regulating numerous mediators ( Fig. 5.3 ). It also passively inhibits hemostasis/thrombosis by separating blood from the procoagulant environment of the subendothelium. Indeed, after endothelial injury, hemostasis proceeds in three overlapping phases. —initiation, amplification, and propagation:
Initiation , when subendothelial tissue factor (TF) and activated plasma factor VII (TF/FVIIa) generate trace amounts of thrombin.
Amplification , when platelets are activated by thrombin, by adhesive interactions with the injured vessel, and by platelet-derived mediators (including adenosine diphosphate [ADP], serotonin, and thromboxane A 2 [TXA 2 ]), resulting in their aggregation, release of granule contents, production of procoagulant mediators, increased avidity of adhesion receptors, and reorientation of membrane lipids to expose negatively charged phosphatidylserine. This surface enables positively charged, vitamin K-dependent factors including FIX and FX to assemble the terminal coagulation complexes on the platelet surface.
Propagation , when the tenase (FVIIIa:FIXa) and prothrombinase complexes (FVa:FXa) are assembled on the activated platelet surface. The tenase complex activates FX, whereas the prothrombinase complex generates the burst of thrombin necessary to cleave soluble fibrinogen into insoluble fibrin and formation of the clot.
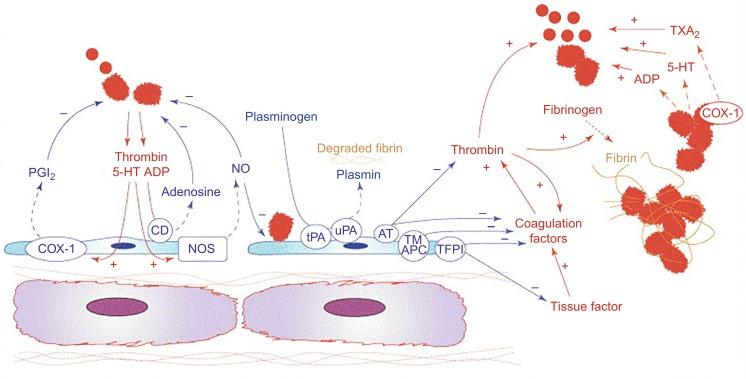
Both endothelium-derived NO and PGI 2 have important antithrombotic activity, acting in a synergistic manner to inhibit platelet activity, inhibit platelet adhesion to the endothelium, and stimulate platelet disaggregation (see Fig. 5.3 ). Importantly, mediators generated during thrombosis—including thrombin, ADP, and serotonin—activate the endothelium to increase the release of NO and PGI 2 . NO is also the most important physiological inhibitor of the exocytosis of Weibel Palade bodies (WPBs), which are endothelial granules containing a highly prothrombotic form of von Willebrand factor (VWF) (ultra-large VWF [ULVWF]) and P-selectin. It is unclear whether antiplatelet and antithrombotic activity contributes to the clinical efficacy of PGI 2 and NO-related therapies (e.g., epoprostenol, riociguat).
There are three major anticoagulant pathways: activated protein C (APC), tissue factor pathway inhibitor (TFPI), and antithrombin. Each pathway is intimately dependent on the endothelium ( Fig. 5.4 ; also see Fig. 5.3 ). Endothelial cells express thrombomodulin, which binds and inhibits the procoagulant activity of thrombin. Moreover, the thrombin-thrombomodulin complex activates protein C, causing an approximately 1000-fold increase in its rate of activation. Endothelial cells also express the endothelial protein C receptor (EPCR), which binds protein C and increases the ability of thrombin-thrombomodulin to activate the protein by another 10-fold. APC inhibits the clotting process by degrading FVa and FVIIIa, key cofactors in the prothrombinase and tenase complexes. Antithrombin is a circulating irreversible inhibitor of several proteinases of the coagulation process, including thrombin, FXa, and FIXa. However, antithrombin circulates in a repressed reactivity state with reduced activity against these mediators. This repression is reversed following the interaction of antithrombin with heparan sulfate, expressed on the endothelial surface. Heparan sulfate is a glycosaminoglycan that forms the bulk of the endothelial glycocalyx, a luminal mesh that covers the endothelial surface. The high-affinity interaction of antithrombin with a small pentasaccharide sequence on heparan sulfate causes a conformational change in the proteinase inhibitor and specifically enhances its activity against FXa and FIXa. Engagement of antithrombin with longer polysaccharide chains of heparan sulfate provides the bridging mechanism that is required for the proteinase inhibitor to block thrombin while also increasing its inhibitory activity against FXa and FIXa. The fourth major anticoagulant mediator, TFPI, is produced predominantly by the endothelium and inhibits FXa and TF/FVIIa activity (see Figs. 5.3 and 5.4 ).
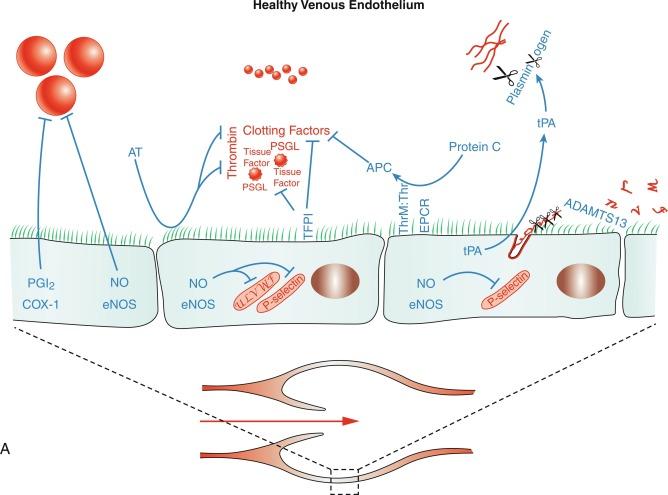
Endothelial cells play a crucial role in fibrinolysis by producing and releasing tissue plasminogen activator (tPA), which converts plasminogen to plasmin, resulting in the degradation of fibrin and dissolution of clot (see Figs. 5.3 and 5.4 ).
Arterial thrombosis occurs predominantly within the context of atherothrombosis and the structural deterioration of atherosclerotic lesions (see the section titled “Atherosclerosis and Intimal Lesion Development”). Atherosclerosis and its clinical sequelae—including coronary heart disease (CHD), ischemic stroke, and peripheral vascular disease—represent the leading cause of death and morbidity in the Western world. The principal mechanism contributing to acute coronary syndromes (ACSs)—such as unstable angina, myocardial infarction, and sudden cardiac death—is rupture of the atherosclerotic plaque, which exposes the bloodstream to large quantities of TF, precipitating arterial thrombosis and blood vessel occlusion. Thrombotic ACS events can also result from superficial erosion of the endothelium, which may reflect endothelial apoptosis.
Non-ST–segment elevation myocardial infarction and unstable angina, which together are defined as NSTE-ACS, represent the leading causes of morbidity and mortality from cardiovascular disease worldwide. The cornerstone of therapy for NSTE-ACS is dual therapy with antiplatelet agents. This approach targets two key platelet-derived mediators involved in platelet activation, TXA 2 and ADP. Low doses of aspirin (ranging from 81 to 325 mg/day) are recommended for all NSTE-ACS patients to inhibit the platelet COX-1 enzyme and platelet generation of TXA 2 , whereas P2Y 12 receptor antagonists (e.g., clopidogrel, ticagrelor) are included to inhibit ADP activity. Because of the rapid inactivation of aspirin in the presystemic circulation and because of its assumed preferential activity at the COX-1 enzyme in platelets, it is often considered that these low doses of aspirin do not influence endothelial COX and therefore do not inhibit the antiplatelet activity of endothelium-derived PGI 2 (see the section titled “COX Inhibitors and the Human Vascular System”). Although the P2Y 12 receptor is expressed on other cell types, including promoting the inflammatory activity of leukocytes and macrophages, distinct purinergic receptors mediate the ADP-induced activation of endothelial cells, suggesting that these agents would preserve the antiplatelet activity resulting from ADP-mediated endothelial production of PGI 2 and NO.
Venous thromboembolism (VTE) is observed clinically as deep-vein thrombosis (DVT) and pulmonary embolism, and is the third most common cause of cardiovascular death after CHD and stroke. Mechanisms contributing to VTE are usually considered within a modern interpretation of the historic Virchow triad, namely endothelial dysfunction, blood flow abnormalities (chiefly stasis), and procoagulant changes in blood constituents. In contrast to atherothrombosis, thrombus formation in human veins is rarely associated with vessel injury and forms on a largely intact endothelium. Furthermore, although platelets make up the core of arterial thrombi and are the closest component to the blood vessel wall, in venous thrombi, fibrin appears to be the substance attaching the thrombus to the vessel wall with platelets attaching to the fibrin downstream. Venous thrombosis is initiated predominantly at venous valves, where blood flow can be sluggish under normal conditions. If blood flow is halted, then this fragile environment can experience a dramatic reduction in oxygen tension. Although venous and valvular endothelial cells express anticoagulant proteins and mediators, hypoxia or inflammation can lead to the downregulation of these protective factors and cause upregulation of procoagulant activity (see Fig. 5.4 ). For example, during inflammatory stimulation, endothelial expression of anticoagulant mediators—including NO, thrombomodulin, EPCR, TFPI, and heparan sulfate—is decreased, whereas endothelial expression of prothrombotic mediators—including PAI-1, TF, and FV—is increased. Moreover, the phosphatidylserine-rich anionic surface necessary for clot formation can result from hypoxia-induced reorientation of endothelial membrane lipids or fusion of the endothelium with circulating microparticles. These are small (< 1 μm) phospholipid vesicles generated by leukocytes, platelets, and endothelial cells. A subpopulation of microparticles, which is increased in individuals at risk for developing VTE, contains high levels of TF and a phosphatidylserine-rich surface. Fusion of these microparticles with venous endothelium can occur subsequent to their interaction with endothelial P-selectin via P-selectin glycoprotein ligand 1 (PSGL-1) expressed by microparticles. Endothelial cells do not normally express P-selectin on their surface, but hypoxia, thrombotic, and inflammatory mediators cause exocytosis of WPBs, which will be amplified by reduced activity of endothelium-derived NO, enabling the translocation of P-selectin to the endothelial cell surface and release of ULVWF onto the luminal surface. P-selectin would then be available to capture PSGL-1–expressing microparticles and focus the crucial components of the coagulation system. ULVWF is normally unfurled by blood flow, enabling it to be degraded by an endothelial surface protease ADAMTS13. However, during stasis, this activity would be diminished preserving the hyperreactive activity of ULVWF on the venous endothelium (see Fig. 5.4 ). Analysis of veins from patients dying of VTE has revealed consistent presence of VWF in venous thrombi, whereas P-selectin is considered a potential biomarker for individuals at risk of VTE, and inhibition of VWF or P-selectin inhibits VTE in preclinical models of the disease.
Unlike atherothrombosis, treatment strategies in venous thrombosis are targeted toward inhibiting prothrombotic mediators. The initial traditional treatment of VTE has been concurrent treatment with heparan/heparin-based anticoagulants and vitamin K antagonists, in particular warfarin, with termination of the heparin-based treatment after a few days once the vitamin K antagonism has become effective. Warfarin inhibits the vitamin K–dependent production of coagulation factors including FIX and FX. Heparin, a product of mast cells, shares considerable structural similarity in its polysaccharide chains to endothelial heparan sulfate and acts in a similar manner to amplify the inhibitory effect of antithrombin against thrombin, FXa, and FIXa (see Fig. 5.4 ). Heparin-based approaches include intravenous unfractionated heparin (UFH), subcutaneous low-molecular-weight heparins (LMWHs), or subcutaneous fondaparinux. UFH is a heterogeneous mixture of heparin polysaccharide chains derived from animal intestines, and only approximately 30% of the molecular species bind to antithrombin. As with endothelial heparan sulfate, the amplification of antithrombin activity against thrombin requires longer polysaccharide chains compared with its activity against FXa and FIXa. The polysaccharide chains in UFH are of sufficient length to support antithrombin-mediated inhibition of thrombin and FXa, resulting in relatively equal inhibitory activity against these mediators. LMWHs are generated by the depolymerization of UFH, resulting in a smaller and variable molecular size, which translates into higher activity against FXa compared with thrombin. Fondaparinux is a synthetic drug based on the small pentasaccharide sequence of heparin/heparan sulfate, which enables it to support antithrombin-mediated inhibition of FXa but not to block thrombin.
Current guidelines recommend the use of direct oral anticoagulants (DOACs), which directly inhibit the enzymatic activity of factor Xa or thrombin, rather than vitamin K antagonists in patients without cancer. Clinical trials have demonstrated that DOACs are noninferior to vitamin K antagonists in preventing recurrent VTE and are associated with less major bleeding. DOACs include the direct thrombin inhibitor dabigatran and the FXa inhibitors rivaroxaban, apixaban, and edoxaban. In patients with cancer, current guidelines recommend long-term use of LMWH, based on clinical trials observing improvement in outcomes compared with vitamin K inhibitors. Aspirin is reported to have beneficial effects in VTE, suggesting that platelets are important contributors to this disease process (see Fig. 5.4 ) ; however, aspirin is inferior to anticoagulants, with DOACs reducing the risk of VTE recurrence by at least 80%, whereas aspirin showed only a 32% risk reduction. Current guidelines recommend aspirin as an available option in individuals with unprovoked VTE who are stopping anticoagulant therapy, but it is not recommended as an alternative to anticoagulant therapy.
Anticoagulants including heparin analogues (e.g., the LMWH enoxaparin) and direct thrombin inhibitors (e.g., bivalirudin) are recommended as additional therapy for NSTE-ACS patients undergoing invasive procedures. Some patients, including those with NSTE-ACS at high risk for VTE, may benefit from long-term triple therapy, combining dual antiplatelet therapy with anticoagulant treatments. In addition to standard warfarin treatment, emerging options for triple therapy anticoagulation include PDE-3 inhibitors, FXa inhibitors, thrombin receptor antagonists, and direct thrombin inhibitors, although this aggressive approach is associated with an increased risk of bleeding.
Thrombolytic therapy (using recombinant tissue-type plasminogen activators, e.g., alteplase) converts endogenous plasminogen to plasmin, which then degrades fibrin in clots (see Fig. 5.4 ). Thrombolytic treatment is indicated for patients with severe pulmonary embolism; it is administered systemically or by catheter infusion directly into the thrombus. Local catheter-directed thrombolysis is occasionally used for the treatment of extensive DVT. Thrombolytic therapy is also utilized to counter arterial thrombotic events, including ischemic stroke and myocardial infarction.
Two COX enzyme subtypes, COX-1 and COX-2, are expressed in human cells. The COX enzyme in mature platelets that is responsible for TXA 2 production is the COX-1 subtype. The nature of the enzyme in endothelial cells that is responsible for PGI 2 production is the surprising subject of debate. The COX-2 subtype is generally an inducible form and is the major source of prostanoids contributing to inflammatory responses. Indeed, inhibition of COX-2 mediates the antipyretic, analgesic, and antiinflammatory actions of nonsteroidal antiinflammatory drugs (NSAIDs) such as ibuprofen or naproxen. The original NSAIDs inhibit both COX-1 and COX-2, although there is variation in their relative selectivity. In contrast, aspirin is a preferential inhibitor of COX-1. This explains why low doses of aspirin (from as low as 75 mg/day) have protective cardiovascular activity by blocking platelet COX-1, but much higher doses are needed for NSAID-like activity mediated by COX-2 inhibition (up to 4 g/day). In each case, the inhibitory effect of aspirin is irreversible. Because COX-1 inhibition in gastric epithelial cells was thought to be responsible for the gastric adverse events associated with NSAID use, a distinct class of NSAIDs with high selectivity for COX-2 was developed. These selective COX-2 inhibitors, described as COXIBs, including rofecoxib and celecoxib, did reduce gastric toxicity while retaining the therapeutic efficacy of NSAIDs. However, there was concern that COXIBs might increase the risk for adverse cardiovascular events, which resulted in a reduction in their use and the withdrawal of rofecoxib from the market. The proposal that endothelial PGI 2 is dependent on COX-2 activity has been promoted to support an increased cardiovascular risk associated with COXIBs compared with traditional NSAIDs. Within that context, arterial thrombosis is considered to be dependent on a balance between the COX-1–dependent generation of TXA 2 in platelets and the COX-2–dependent generation of PGI 2 in the endothelium. Accordingly, low-dose aspirin would be cardioprotective because it selectively inhibits COX-1 in platelets, eliminating prothrombotic TXA 2 while preserving COX-2–mediated endothelial production of antithrombotic PGI 2 . In contrast, COXIBs, by selectively inhibiting COX-2–dependent production of endothelial PGI 2 , would tip the balance in favor of TXA 2 and precipitate vasoconstriction, platelet activation, and atherothrombosis. This “imbalance theory” is based on incorrect assumptions, ignores compelling evidence to the contrary, and is invalid.
Overwhelming evidence confirms that the endothelial COX enzyme responsible for PGI 2 production in the human vascular system is the COX-1 enzyme. However, numerous reports propose that endothelial PGI 2 is derived from COX-2. This proposal is based on the analysis of basal urinary levels of 2,3-dinor-6-keto PGF1α (PGI-M) in healthy volunteers. PGI-M is a PGI 2 metabolite derived from 6-keto PGF1α, the more immediate hydration byproduct of PGI 2 . In healthy volunteers, the basal urinary levels of PGI-M are unaffected or minimally affected by low doses of aspirin (< 160 mg/day), which preferentially inhibit COX-1, but are markedly reduced (up to 80%) by selective COX-2 inhibition with COXIBs. Therefore the basal generation of urinary PGI-M in healthy human volunteers is dependent on COX-2. However, the cellular source of human urinary PGI-M has never been identified. PGI 2 synthase is widely expressed in human nonvascular cells, and COX systems have considerable capacity to generate prostaglandins. Therefore generation of PGI 2 from nonendothelial sources could easily dominate the basal urinary excretion of this PGI 2 metabolite. Although this COX-2–dependent basal urinary excretion of PGI-M was not significantly affected by low-dose aspirin, infusion of the endothelial agonist bradykinin to human volunteers caused a six-fold increase in PGI-M that was abolished by a very low dose of aspirin (75 mg/day). Therefore, in healthy human volunteers, although the basal level of urinary PGI-M is dependent on COX-2 and is derived from an unknown cellular source, the increase in PGI 2 (and urinary PGI-M) resulting from endothelial cell stimulation is entirely dependent on COX-1.
Sensitive molecular, biochemical, and immunochemical analyses of human arteries and veins have consistently demonstrated that native endothelial cells express high levels of COX-1 but do not express COX-2. However, if cells and arteries are placed into the stressful conditions of cell culture, then they begin to express COX-2. One exception is the kidney, where multiple cell types naturally express COX-2, which likely explains why basal levels of urinary 6-keto PGF1α (and perhaps of PGI-M) are thought to be derived from renal COX-2 systems. Unlike urinary 6-keto PGF1α, circulating levels of 6-keto PGF1α were not affected by selective inhibition of COX-2 but were abolished by very low doses of aspirin (35–75 mg/day). Likewise, selective COX-1 inhibition by prior ingestion of low-dose aspirin inhibited the subsequent vascular and endothelial production of PGI 2 from human arteries and veins. Aspirin is rapidly degraded in the bloodstream, and this pharmacokinetic profile was thought to favor inhibition of platelet COX-1 by repetitive administration of low doses of the drug. The ability of low doses of aspirin to inhibit vascular production of PGI 2 is additional compelling evidence that it is derived from COX-1.
Therefore analysis of the human vascular system provides convincing evidence that the endothelial production of PGI 2 is dependent predominantly, if not exclusively, on the COX-1 enzyme. This appears to be valid in normal healthy blood vessels as well as during the development of vascular disease, which can be associated with decreased endothelial production of PGI 2 , likely reflecting inactivation of PGI 2 synthase by reactive oxygen species (ROS).
Atherosclerotic lesions develop preferentially at sites of disturbed blood flow (branches, curvatures) where normal laminar shear stress is distorted by flow separation and directional changes. Although exposure of endothelial cells to sustained normal shear stress is antiinflammatory and atheroprotective, disturbed atheroprone shear stress increases the transcriptional activity of nuclear factor kappa B (NF-κB), a master regulator of the atherosclerotic process, and increases the expression of inflammatory and thrombotic mediators ( Fig. 5.5 ). Regions prone to atherosclerosis also display increased endothelial permeability to macromolecules, including low-density lipoprotein (LDL), and the subendothelial deposition and modification of LDL is an important early amplification step in the atherosclerotic process. These modified lipids further increase inflammatory activity, reducing the activity of protective mediators such as NO and amplifying NF-κB activation. These key changes in endothelial function including increased permeability and inflammatory activity likely reflect the disruptive effects of atheroprone shear stress and modified LDL on endothelial adherens junctions and VE-cadherin–dependent signaling (see Fig. 5.5 and the section titled “Endothelial and Vascular Stabilization”). Expression of inflammatory mediators such as vascular cell adhesion molecule-1 (VCAM-1), monocyte chemoattractant protein-1 (MCP-1), and macrophage colony-stimulating factor (MCSF) increase monocyte recruitment and stimulate their survival and differentiation to macrophages (see Fig. 5.5 ). These cells attempt to clear the modified LDL particles but become engorged with lipids and die, releasing their lipid content into the developing lesion. The evolving lesion reflects interactions between endothelial, immune, and inflammatory cells, with continual remodeling of the atheroma resulting in the formation of a lipid-rich necrotic core, which is capped by a fibrous layer of VSMCs and extracellular matrix that provides stability to the plaque. Although they may remain relatively stable, plaques can develop into chronic active inflammatory lesions with accumulation and activation of macrophages and T cells, which can remodel and weaken the stabilizing fibrous cap, precipitating thrombosis and luminal occlusion (see the section titled “Atherothrombosis and Venous Thromboembolism”).
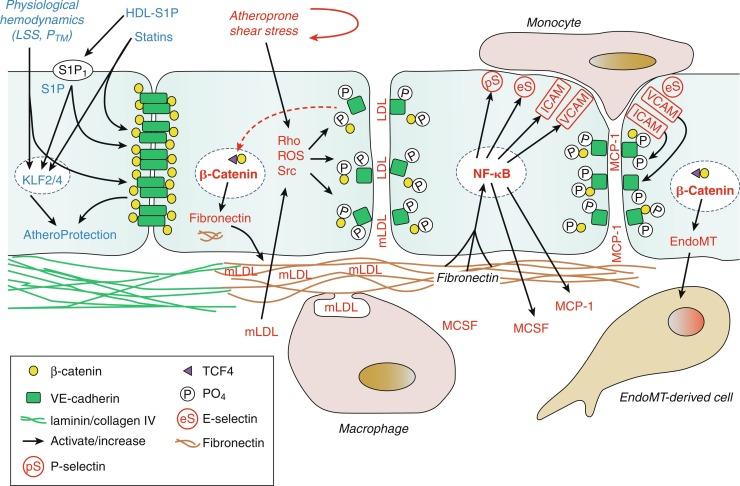
VSMCs expressing endothelial and smooth muscle (mesenchymal) proteins are present in human atherosclerotic lesions, suggesting that they are derived from the endothelium rather than from the arterial media (see Fig. 5.5 ). During endothelial-to-mesenchymal transition (EndoMT), endothelial cells can change their phenotype to that of mesenchymal cells. Indeed, atheroprone shear stress or modified LDL stimulates EndoMT, whereas normal laminar shear stress increases resistance to EndoMT. Because EndoMT cells contribute to vascular and tissue fibrosis, EndoMT-derived mesenchymal cells might be expected to expand and stabilize the protective fibrous cap. However, there is an inverse relationship between the number of EndoMT-derived cells and cap thickness, and an increased number of EndoMT-derived cells are present in ruptured versus nonruptured plaques. The lineage and activity of VSMCs in atherosclerotic plaques is complex: although some VSMC populations contribute to plaque stability, cholesterol accumulation can transform VSMCs into macrophage-like cells and foam cells that contribute to plaque development. EndoMT-derived cells may therefore have an increased propensity to develop a macrophage-like phenotype and contribute to destabilization of the plaque. Preventing the phenotypic switching of VSMCs to macrophage-like cells or inhibiting the EndoMT process may be beneficial in preventing plaque destabilization (see Fig. 5.5 ) (see also the section titled “Endothelial and Vascular Stabilization”).
Owing to their efficacy in reducing morbidity and mortality associated with atherosclerosis, HMG-CoA reductase inhibitors, termed statins, are among the most widely prescribed classes of cardiovascular drugs. Their primary mechanism of action is to inhibit cholesterol biosynthesis, thereby increasing the expression of hepatic LDL clearance receptors and reducing circulating LDL levels. However, the HMG-CoA reductase pathway also plays a fundamental role in cell signaling by generating isoprenoid intermediates involved in the lipid modification and activity of the small GTP-binding proteins (G proteins) Rho, Ras, and Rac. Statins inhibit the activity of these G proteins in numerous cell types, and these pleiotropic effects of statins can produce vascular protective effects that are independent of LDL lowering. Indeed, statins have direct protective effects on endothelial cells that appear to be mediated predominantly by the inhibition of RhoA/ROCK signaling, a key signaling pathway promoting multiple aspects of endothelial dysfunction (see Figs. 5.2 and 5.5 ). Indeed, with regard to reversing endothelial dysfunction, statins increase eNOS expression, eNOS activity, and NO production; they also decrease ET-1 expression, reduce oxidative stress, inhibit apoptosis, increase progenitor cell mobilization, reduce endothelial permeability, reduce prothrombotic activity, enhance fibrinolytic activity, and reduce the production of inflammatory cytokines and mediators. Statins can also inhibit EndoMT. These pleiotropic effects of statins represent a powerful stabilizing influence on the endothelium that would be expected to dramatically inhibit the atherosclerotic process (see also the section titled “Endothelial and Vascular Stabilization”).
Although the idea is appealing, it is currently unclear whether these pleiotropic effects of statins are activated during clinical therapy or contribute to their clinical efficacy. Clinical studies have shown that inflammatory biomarkers predict the risk of initial and recurrent major cardiovascular events (myocardial infarction, stroke, and cardiovascular death). C-reactive protein (CRP), produced by the liver in response to systemic inflammatory mediators, is an independent biomarker of cardiovascular disease. In addition to their effects on lipids, statins have been shown to reduce the level of CRP. Clinical evidence indicates that statins can stabilize vulnerable plaque; this is associated with a decrease in inflammatory biomarkers, suggesting that in addition to LDL lowering, the pleiotropic effects of statins may be involved. Currently available statins are lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, and pitavastatin. They are competitive and reversible inhibitors of HMG-CoA reductase but vary in their elimination half-lives, potency, and lipophilicity.
Although statins are highly effective at lowering LDL cholesterol levels and preventing cardiovascular disease events, there is still a clear need for additional therapies. Indeed, nonstatin approaches are indicated only as adjunctive therapy for patients who are unable to reach their lipid goals despite optimal statin therapy. Ezetimibe, which reduces cholesterol absorption in the small intestine and may increase hepatic LDL clearance, lowers LDL levels by 15% to 25%. A new class of lipid-lowering agents, the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, has the potential to achieve a 50% to 65% decrease in LDL cholesterol levels. PCSK9 promotes degradation of the hepatic LDL clearance receptor. PCSK9 inhibitors therefore increase the LDL receptor–mediated hepatic uptake of LDL, a mechanism shared by statins. However, they do not inhibit the HMG-CoA reductase pathway and therefore do not have similar pleiotropic effects to statins. For example, despite their potent LDL-lowering effects, PCSK9 inhibitors, unlike statins, do not reduce serum markers of inflammation such as CRP, interleukins (ILs), or tumor necrosis factor-α (TNF-α). Two fully human monoclonal antibodies against PCSK9 (evolocumab, alirocumab) are approved for clinical use. Placebo-controlled randomized clinical trials have demonstrated that an aggressive approach to lowering LDL cholesterol levels by combining statin therapy with ezetimibe or a PCSK9 inhibitor can significantly reduce the risk of major adverse cardiovascular events compared to statin use alone. Current clinical guidelines recommend a combined therapeutic approach (statin plus ezetimibe, with or without a PCSK9 inhibitor) in certain patient groups, including individuals at very high risk for atherosclerotic events and whose LDL cholesterol remains ≥70 mg/dL despite maximally tolerated statin use.
Because of the importance of inflammatory mechanisms in the pathogenesis of atherosclerosis, there is increased awareness of the potential for antiinflammatory approaches in treatment for the disease. These approaches include mAbs against IL-6 (e.g., tocilizumab), TNF-α (e.g., infliximab), IL-1β (e.g., canakinumab), and MCP-1 (e.g., MLN-1202). Some of these approaches are already providing promising preliminary results in atherosclerotic disease. These agents would be expected to have protective effects to reverse endothelial dysfunction.
Because cell and tissue function is dependent on adequate vascular perfusion, normal growth and development requires parallel expansion of the vascular system. Healthy tissues can be adequately supplied with oxygen by diffusion only over distances up to 150 μ, requiring cells to maintain a close association with a vascular network and vascular capillaries. Normal cellular expansion beyond this vascular perfusion zone will result in hypoxia, which is the primary stimulus for the necessary expansion of the microvasculature (angiogenesis). Angiogenesis is regulated by a complex interplay between numerous interconnected signaling systems that cause destabilization of the existing microvasculature, enabling invasion and proliferation of “tip” and “stalk” endothelial cells through the extracellular matrix, fusion of neighboring endothelial branches, lumen formation and perfusion of the nascent vessel, followed by stabilization and maturation of the blood vessel. A key regulator of the process is vascular endothelial growth factor (VEGF), which is released by multiple cells types following activation of the hypoxic transcription factor HIF-1α (hypoxia inducible factor). VEGF stimulates angiogenesis following activation of the endothelial VEGFR-2 receptor subtype. Not surprisingly, the angiogenic process can be disrupted by disease processes and is the target of existing and developing therapies to amplify or to inhibit microvascular expansion.
Therapeutic angiogenesis to counter pathological tissue ischemia, such as in peripheral artery disease, has been pursued for decades. However, despite numerous clinical trials targeting a variety of angiogenic mediators and mechanisms including VEGF, the results have been disappointing and no approved therapeutic interventions are currently available.
Folkman originally proposed the groundbreaking hypothesis that the growth of tumors was dependent on angiogenesis and that antiangiogenic therapy would be an effective treatment for human cancers. Angiogenesis is now considered to be an essential component of malignant growth and a “hallmark of cancer.” However, tumor angiogenesis can be a highly abnormal process resulting in heterogeneous, tortuous, and chaotic channels, with an uneven vessel lumen. In addition to abnormal or absent endothelial cells, the stabilizing pericytes are also abnormal or absent, culminating in highly leaky vessels. Such unstable vessels are thought to contribute to metastases and to increased interstitial fluid pressure resulting in heterogeneous blood flow, which can disrupt delivery of therapeutic agents. Although future antiangiogenic therapy will likely take advantage of specific abnormal traits of tumor angiogenesis, current therapy is targeted to mediators involved in normal angiogenesis. FDA-approved antiangiogenic drugs for treating solid tumors are targeted predominantly to VEGF-dependent signaling. Bevacizumab is a recombinant humanized monoclonal antibody that targets circulating VEGF, aflibercept is a recombinant molecule comprising VEGFR-binding domains to sequester VEGF, and ramucirumab is a humanized monoclonal antibody that targets and blocks endothelial VEGFR-2 receptors. A number of protein kinase inhibitors that target the activity of VEGFR receptors have also been approved as antiangiogenic treatment, including sorafenib, sunitinib, axitinib, and cabozantinib. These agents have variable selectivities for the VEGFR-2 receptor, with some agents effectively blocking other mechanisms involved in angiogenesis (including platelet-derived growth factor receptors) and in lymphangiogenesis (e.g., VEGFR-3 receptors). A major problem with current therapies is that the tumors become refractory to VEGF blockade, resulting in treatment failure. A number of mechanisms have been proposed to account for this, including activation of alternate angiogenesis pathways. The inhibition of endothelial VEGFR-2–receptor activity by these different approaches results in a similar spectrum of adverse effects, including hypertension, cardiac toxicity, and thromboembolic events.
Normal endothelial activity is of vital importance to vascular and organismal health, and the destabilization of endothelial structure and function is a common precipitating event in the pathogenesis of vascular disease. Although numerous therapeutic interventions can mimic aspects of endothelial function, no current approaches are specifically targeted toward reversing the diseased endothelial phenotype and reestablishing endothelial stability.
The maintenance of the normal protective endothelial phenotype is an active rather than a passive process. The protective features of the arterial endothelium appear to become fully engaged during the initial postnatal period and to be mediated by increased clustering of VE-cadherin and the formation of adherens junctions. VE-cadherin clustering stimulates the assembly of a macromolecular complex that regulates endothelial cell signaling, function, morphology, and phenotypic identity ( Fig. 5.6 ). This includes increasing eNOS and NO activity, promoting endothelial barrier function (decreased permeability), inhibiting apoptosis, and inhibiting inflammatory activation such as inhibiting leukocyte extravasation and the expression of inflammatory mediators (see Fig. 5.5 ). Inflammatory mediators cause the transient interruption of VE-cadherin clustering, enabling increased endothelial inflammatory activity, increased permeability, and inflammatory cell extravasation. However, chronic disruption of VE-cadherin–dependent signaling precipitates endothelial and vascular destabilization and promotes the development of vascular disease. Indeed, the degradation and loss of VE-cadherin from adherens junctions is responsible for the endothelial dilator dysfunction associated with aging and likely contributes to the prominent endothelial frailty of the aging vasculature, a key aspect of vascular aging. In addition to the loss of protective activity, VE-cadherin degradation and junctional disruption can result in nuclear translocation of its binding partner β-catenin, a transcription factor that stimulates the expression of numerous pathological and inflammatory mediators including the renin angiotensin system (angiotensinogen, renin, angiotensin-converting enzyme [ACE], AT1 receptors), ET-1, TNF-α, IL-6, and fibronectin (see Figs. 5.5 and 5.6 ). Disruption of endothelial adherens junctions and β-catenin transcriptional activity are essential components of EndoMT. Indeed, disruption of VE-cadherin–dependent activity likely contributes to the initiation, progression, and destabilization of atherosclerotic lesions (see Fig. 5.5 ).
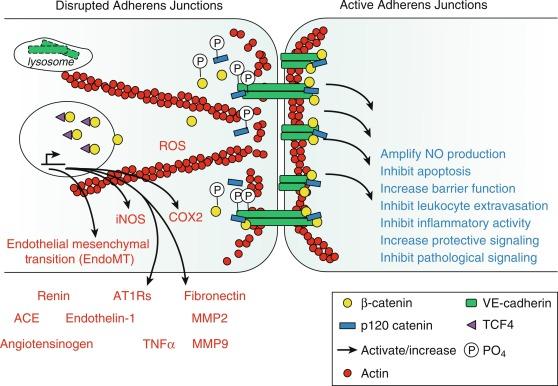
Numerous endogenous protective factors, including sphingosine-1-phosphate, associated with high-density lipoprotein (HDL) and angiopoietin-1, amplify VE-cadherin clustering at adherens junctions (see Fig. 5.5 ). Not surprisingly, vascular disease is associated with alternate signaling mediators that diminish the activity of these protective factors and may be responsible for junctional disruption. Targeted approaches to increasing VE-cadherin clustering at adherens junctions not only decrease inflammatory activity and frailty of endothelial cells but also reduce β-catenin transcriptional activity and stabilize endothelial function and phenotype. This type of therapeutic approach is associated with organ protection and reduced mortality in preclinical inflammatory models. Therapeutic amplification of VE-cadherin–dependent signaling and endothelial adherens junctions would likely be a powerful mechanism to reverse endothelial dysfunction, restore protective endothelial activity and vascular stability, and inhibit the initiation and progression of vascular disease.
The normal regulation of blood flow requires integration of the metabolic requirements of individual organs and tissues with the overall needs of the entire organism. The complex central regulation of the cardiovascular system is achieved in large part though the sympathetic nervous system. The central nervous system receives numerous inputs regarding sensory, emotional, environmental, and hemodynamic challenges and directs acute vascular responses by altering sympathetic activity. Sympathetic nerves innervate and provide a powerful vasoconstrictor influence in most vascular beds, with their activity increasing in arterioles (the vascular faucets) compared with more proximal arteries. Notable exceptions are the cerebral system, where the sympathetic system minimally affects blood flow, and the coronary circulation, where sympathetic activation can initiate vasodilatation. Sympathetic nerves are widely distributed in the venous system, where they play an important role to increase venous return and support an increase in cardiac output and blood pressure. In the arterial system, sympathetic nerves are restricted to the adventitia, requiring sympathetic neurotransmitters to diffuse through the blood vessel wall and regulate vascular cell function. In contrast, in the venous system, the sympathetic nerves penetrate the medial layer to provide a more direct delivery of neurotransmitters to VSMCs, which may contribute to heightened responsiveness of the venous system. The predominant autonomic control of the vasculature is achieved through the sympathetic system, and only a small subset of blood vessels receive a prominent parasympathetic innervation—for example, the pulmonary vasculature. In an upright human, the sympathetic nervous system exerts significant vasoconstrictor activity to numerous tissues and organs, including the splanchnic, renal, skeletal muscle, and cutaneous circulations.
Arterial baroreceptors located in the carotid sinus and aortic arch continually sense blood pressure through the resulting mechanical stretch of the arterial wall. Elevated sensory afferent activity resulting from increased blood pressure is processed in the central nervous system to cause a reflex decrease in efferent sympathetic activity. Likewise, a fall in blood pressure will result in increased sympathetic activity. Although originally considered to be important only for the acute control of blood pressure, it is now believed that the reflex can contribute to long-term blood pressure regulation. Other reflexes contribute to regulation of sympathetic outflow, including peripheral and central chemoreceptors, which respond to changes in the partial pressures of oxygen and carbon dioxide in arterial blood. For example, hypoxia increases sympathetic outflow and is thought to contribute to the chronic sympathoexcitation and hypertension occurring in obstructive sleep apnea.
The primary neurotransmitter released by vascular sympathetic nerves is norepinephrine, which activates α1- and α2-ARs expressed on VSMCs to initiate vasoconstriction ( Fig. 5.7 ). There is generally minimal activation of vasodilator β-ARs by nerve-released norepinephrine except, for example, in the coronary circulation. VSMCs in the peripheral circulation are predominantly of the β2-AR subtype and are activated preferentially by circulating epinephrine, released by the adrenal medulla. Sympathetic nerves also release secondary constrictor neurotransmitters including adenosine triphosphate (ATP) and neuropeptide Y, which can cause vasoconstriction directly or indirectly by amplifying the response to norepinephrine. Following its release from sympathetic nerves, norepinephrine is recaptured by sympathetic nerve varicosities via the norepinephrine transporter (NET) and is then either metabolized by cytoplasmic monoamine oxidase (MAO) or taken into storage vesicles via the vesicular monoamine transporter (VMAT) (see Fig. 5.7 ).
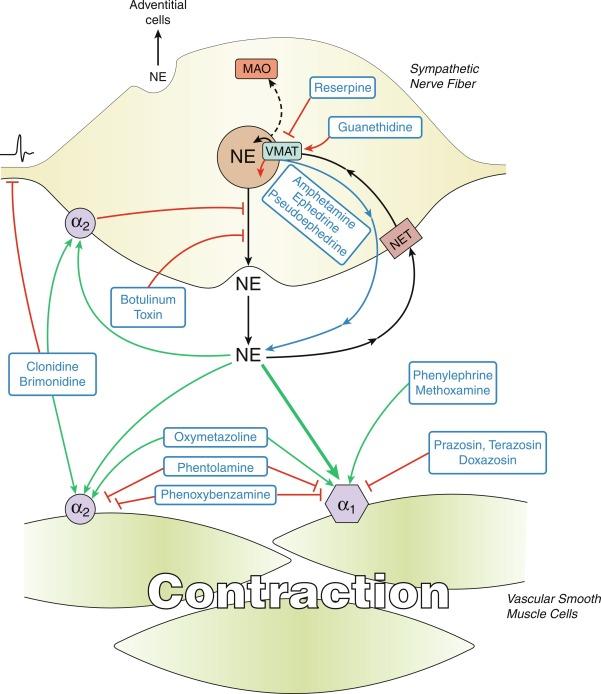
α1-Adrenoceptors are expressed by the VSMCs of most blood vessels regardless of their innervation density. In contrast, functional constrictor α2-ARs have a unique distribution in the human vasculature. VSMC α2-ARs are generally not functional in large proximal arteries, and their activity increases in distal arterioles. This reflects variable expression of VSMC α2-ARs resulting from differential transcriptional activation of α2-AR genes. In most vascular beds, the activity of α2-ARs in distal arteries and arterioles still remains relatively weak compared with α1-ARs, whereas in some systems, notably the cutaneous circulation, α2-AR constrictor activity is greatly increased. This reflects the physiological role of α2-ARs in vascular thermoregulation. In contrast to their selective distribution in the arterial circulation, α2-ARs are widely expressed and functional within the venous system. α2-ARs are also expressed on nerve fibers, including sympathetic nerves, where their activation inhibits the release of neurotransmitters. With vascular sympathetic nerves, this contributes to negative feedback regulation, with prejunctional α2-AR activation causing inhibition of norepinephrine release and vasodilatation (see Fig. 5.7 ). α2-ARs are also expressed by endothelial cells, with their stimulation increasing production of NO and PGI 2 .
In addition to its direct effects to cause arterial and venous constriction in most vascular beds and to stimulate the heart, increasing cardiac output, the sympathetic nervous system has important regulatory effects on the kidneys to increase blood pressure. Increased renal sympathetic nerve activity can elevate blood pressure by three independent mechanisms: (1) increasing renin secretion from the juxtaglomerular apparatus through activation of β1-ARs, (2) increasing sodium reabsorption in the proximal tubules through activation of epithelial α1-ARs, and (3) increasing renal vascular resistance through activation of VSMC α1-ARs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here