Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vascular disorders of the hand are relatively uncommon but can pose significant problems for patients suffering from them. Ischemic pain, ulceration, and decreasing hand function are just some of the problems that can occur. Proper management of patients can improve their quality of life dramatically and on occasion prevent loss of tissue and potentially even the limb.
Arterial blood is supplied to the hand through the brachial artery that branches in the proximal forearm primarily into the radial and ulnar arteries. These two major vessels continue down the forearm and supply the major portion of the blood that flows into the hand. The anterior and posterior interosseous vessels can provide flow via collateral circulation in chronic vascular problems; however, these two vessels are much smaller and generally cannot provide adequate oxygenated blood in the case of acute injury to both the radial and ulnar vessels. More recent work has determined that the volar forearm’s median artery can provide significant blood flow to the hand, particularly in the case of injury or harvest of the radial artery. ,
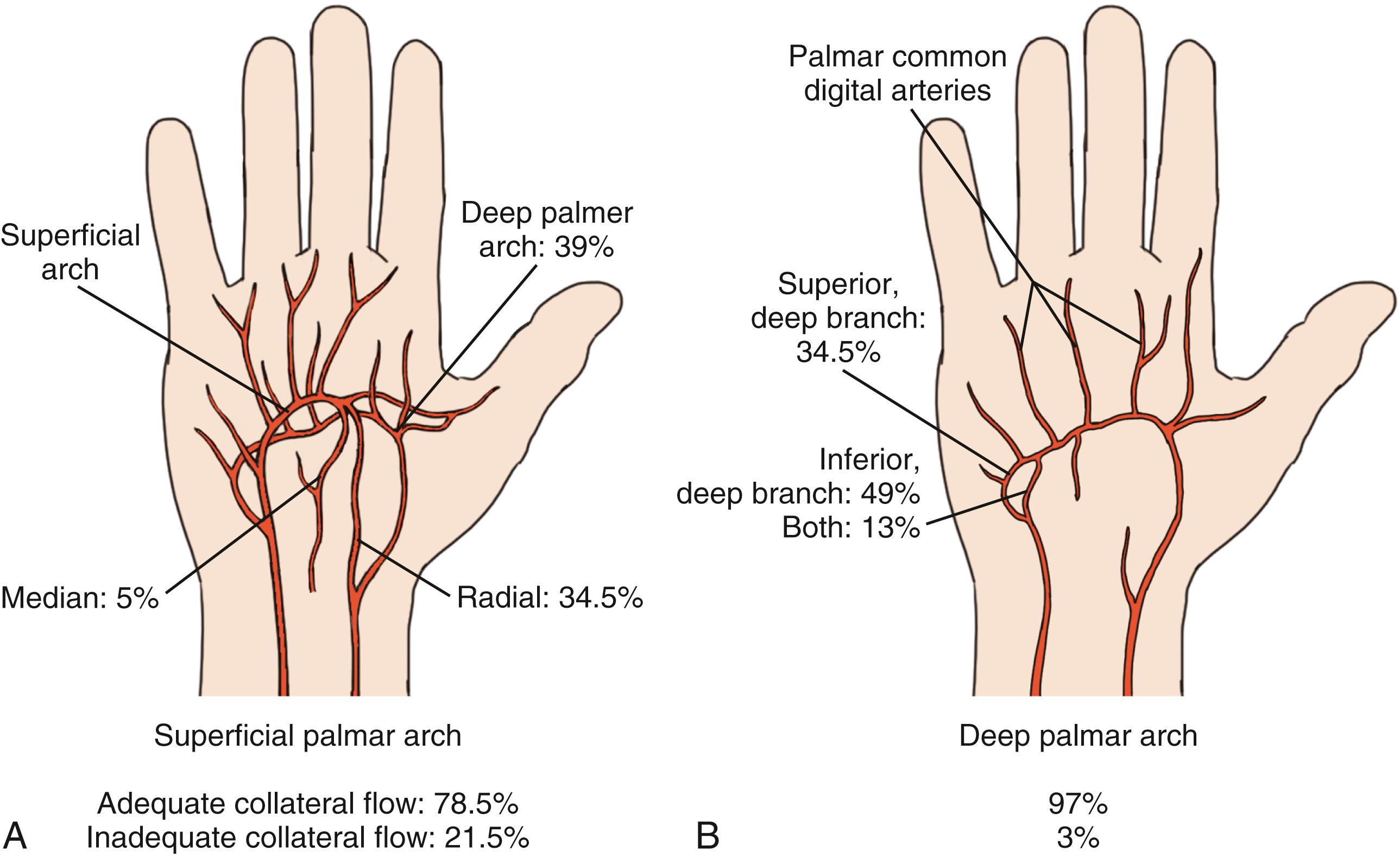
Blood supply to the hand was first clearly elucidated by Coleman and Anson in their classic paper, in which they found that most hands have both a “superficial” and a “deep” arch that supplies the fingers. They found that 78% of the hands studied had a “complete” superficial arch, which theoretically allowed normal flow to all the fingers passing through the arch if flow by means of the radial or ulnar arteries was compromised. The ulnar artery flows into the hand on the palmar surface; its terminal portion becomes the superficial arch, while the radial artery courses around the radial volar wrist into the anatomic snuffbox, then it enters the palm via the first web space. The terminal branches of the radial artery turn into the princeps pollicis and the deep palmar arch ( Fig. 60.1 ). Recent metaanalysis of multiple published papers on the anatomy of the arches has corroborated the findings of Coleman and Anson. In a 2018 paper, Zarzecki et al. reviewed 36 studies which looked at 4481 palmar arches and found that the superficial arch was complete in 81.3% and the deep arch was complete in 95.2% of hands.
Ischemia of the thumb is very uncommon as the blood supply to the thumb is particularly rich. Miletin et al. have recently demonstrated that the thumb has significant arterial flow from both palmar and dorsal circulation, which is probably the reason for ischemia of this digit being rare.
Much has been made of the presence or absence of a complete arch over the years; however, a great deal of clinical experience has shown that acute interruption of flow in either the radial or ulnar artery is extremely unlikely to result in significant ischemia of the hand or fingers. In terms of which vessel provides the dominant circulation to the hand, the results of studies have been variable, with most finding that the ulnar artery is dominant and others finding that it is the radial. I would think that this point is actually moot because, as noted above, injury to one of the two vessels alone rarely causes any ischemia of the hand.
While the arterial supply to the hand enters primarily on the palmar surface, most of the venous drainage begins on the dorsal fingers and continues on the hand’s dorsal surface. The primary arteries of the hand are each followed by two small venae comitantes, the configuration of which actually continues out to the fingers into the digital arteries. The primary drainage of the hand is, however, via the dorsal system, which eventually drains into the cephalic and basilic veins of the forearm and upper arm. Problems with venous drainage of the hand are extremely unusual—with the exception of possible venous thrombosis after replantation—due to the many venous channels available.
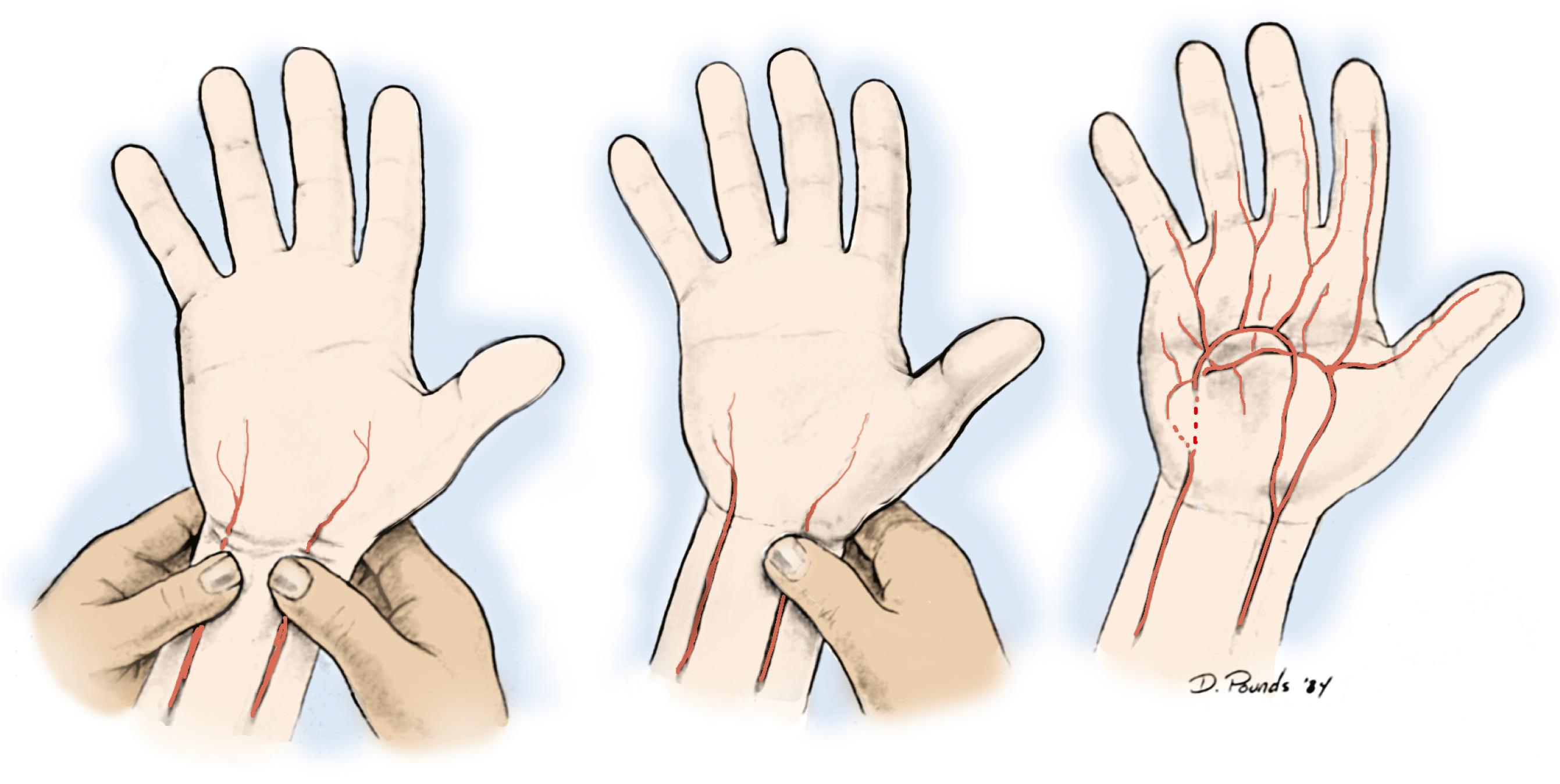
Hand ischemia can result from iatrogenic injury, trauma, or vascular disease. Fortunately, iatrogenic injury is unusual; however, the potential to negatively affect flow to the hand is present in many procedures. An Allen test should be performed routinely prior to any intervention involving the radial artery ( Fig. 60.2 ). Radial artery puncture is a commonly used procedure for harvesting blood to measure gases, and indwelling catheters in the radial artery are commonplace for the monitoring of unstable patients during and after surgery. Although isolated radial artery puncture is exceedingly unlikely to cause ischemia in the hand, indwelling catheters are known to cause permanent ischemic complications in approximately 0.09% of patients.
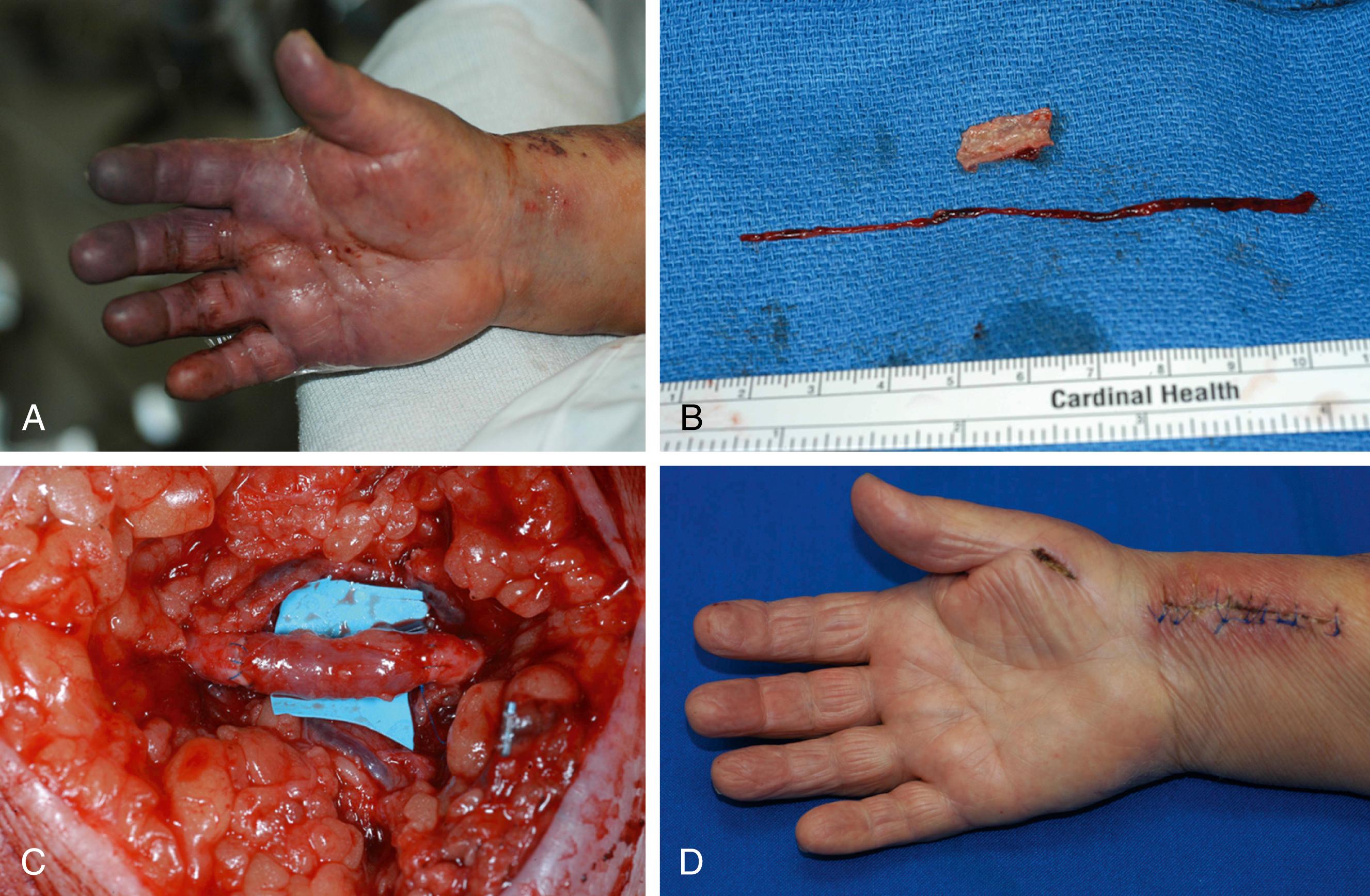
In the group of patients who develop ischemic problems, studies have shown that surgical intervention is not universally successful, probably due to the severity of vascular disease in the radial artery and the effects of distal embolization from the site of injury , ( Fig. 60.3 ). Depending on the patients’ underlying problems, it may be best to manage them with anticoagulation, regional nerve blocks, and possibly intraarterial thrombolytics. The increasing trend toward management of vascular problems with endovascular techniques has led to renewed interest in peripheral artery access, with the radial artery offering a relatively easy way into the arterial system. While early reports minimize the complications of this approach, recent reports have shown some utility in reversing hand ischemia via endovascular techniques. Gandini has reported success using endovascular balloons and stents in the distal arteries of the forearm, but he emphasizes that the endovascular instruments utilized should be those used in cardiac vascular intervention due to the small size of the vessels in the distal forearm.
Currently, the radial artery is often harvested for cardiac revascularization and radial forearm flap reconstruction, which also can lead to hand ischemia. The standard to use to avoid problems is the preoperative Allen test, as noted earlier. Despite the routine use of it, ischemic episodes have been reported after radial artery harvest. This problem can be managed successfully, however, by performing a bypass with the autologous vein to replace the harvested radial artery.
Another iatrogenic cause of ischemia is vascular access in the upper extremity for hemodialysis. This happens in a range of 2% to 5% after a vascular access procedure and can be due to distal embolization or a “steal” phenomenon; in this case, the majority of the arterial flow is diverted across the fistula, leading to ischemia in the distal hand. Often the fistula will have to be banded or even ligated to decrease the shunting of blood from the hand; however, vascular surgeons are usually unwilling to ligate these because of the difficulties of long-term access in dialysis patients. Another option is the so-called distal revascularization interval ligation (DRIL) procedure.
In this operation, a vein graft is sewn into the artery above the site of the fistula, and the bypass is anastomosed to the distal artery at a level beyond it. The native artery is then ligated just distal to the fistula. This theoretically leads to the hand getting the majority of flow rather than the shunt and is effective in many patients. Diabetic patients on dialysis present other difficulties in that they often develop worsening arteriosclerosis of the upper extremity’s vessels and ongoing ischemia; this will be discussed later in this chapter.
The other cause of ischemia of the hand is trauma, which can be either an open or a closed injury to forearm and hand vessels. The classic case of a closed injury to the hand’s arterial supply is in supracondylar fractures in children. In these patients, displacement of the fracture leads to injury and spasm of the brachial artery, which if not recognized can lead to a compartment syndrome and ultimately a Volkmann ischemic contracture of the flexor muscles. Another fairly common type of closed vascular injury unique to the hand is ulnar artery thrombosis, or “hypothenar hammer syndrome.” This injury occurs with closed trauma to the palm of the hand, often when it is used as a hammer or when the hypothenar area suffers a blow. Open injuries, whether by firearms or by lacerations, are particularly common in the forearm and hand. As noted earlier, injury to a single vessel rarely causes acute ischemia but undiagnosed injuries can lead to aneurysm formation and potential distal embolization.
The final cause of ischemia in the hand is intrinsic vascular disease, which can be either arteriosclerotic or vasospastic. They can be seen in combination, which is known as “vasoocclusive” disease. Although Buerger disease has historically been a major cause of arteriosclerotic disease in the upper extremity, in today’s world the most common cause is a combination of diabetes and renal insufficiency. This combination of disease processes leads to accelerated occlusive vascular disease in the upper and lower extremities, and it is not uncommon to see patients with bilateral lower-limb amputations appear with critical ischemia of the hand. Likewise, patients with collagen vascular disease (i.e., scleroderma primarily) suffer from vasculitis and vasospastic disease, which can lead to vasoocclusive disease. The patients can present difficult management issues, and surgical intervention, while helpful, often does not provide a long-term solution.
As with any problem, patients first need to provide a thorough history and undergo a physical examination. Obtaining a medical history, with particular attention to documented diabetes and/or collagen vascular disease, is very important. Obviously, a habit of smoking will have an impact as well. Medical management of the diseases with pharmacologic agents may mitigate some of the symptoms, particularly in those patients with Raynaud disease. The physical examination should pay close attention to the appearance of the hands and the color of the fingers, looking for evidence of ischemia and/or gangrene or ulceration. Likewise, the warmth of the fingers is important, and sensation also plays a role, with the potential for sympathetic overactivity in the presence of nerve compression.
The pulses at the elbow and wrist should be felt, and I feel that Doppler ultrasound plays a very important role in the examination of the patient with a vascular disorder. An Allen test can be done, as described before; however, performing a similar exam with the pencil Doppler may yield much more information. The arch is located with the Doppler, and the radial and ulnar arteries are compressed sequentially at the wrist. The Doppler can be moved over the arch as necessary to follow the signal, listening for a change in character or flow. This technique can almost always diagnose the site of arterial occlusion if performed carefully. Likewise, an arterial signal should be heard with the Doppler placed on the pulp of the finger; if it is not, there is a significant problem with flow to that finger.
It should be noted, however, that the presence of an audible Doppler arterial signal in one of the wrist’s vessels is not an indication of adequate nutritional flow to the hand. By using this simple technique in the office, the surgeon usually can diagnose where the occlusion is; however, this information is not adequate for planning a surgical procedure. For this I prefer using computed tomography angiography (CTA) or arteriography—the latter if fine detail of the hand’s vessels is needed ( Fig. 60.4 ).
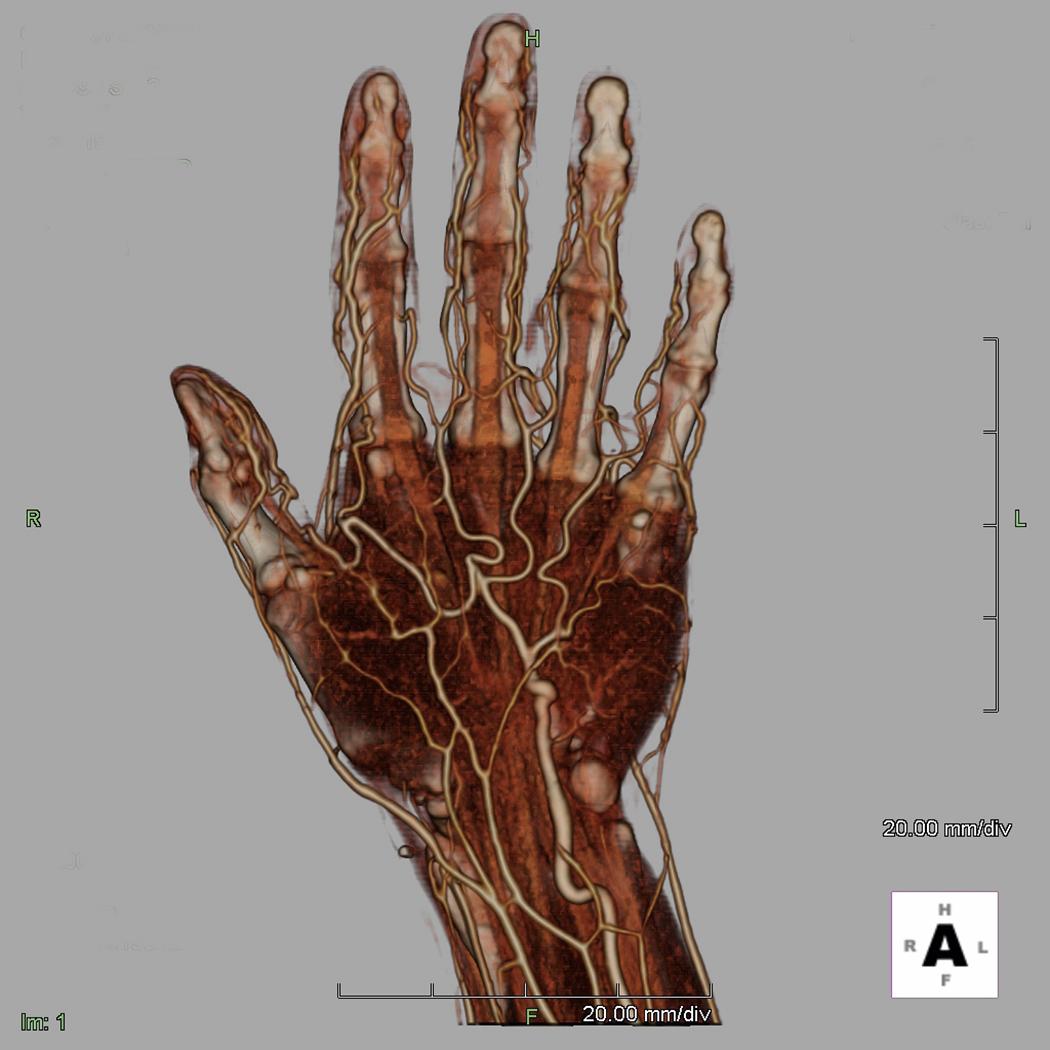
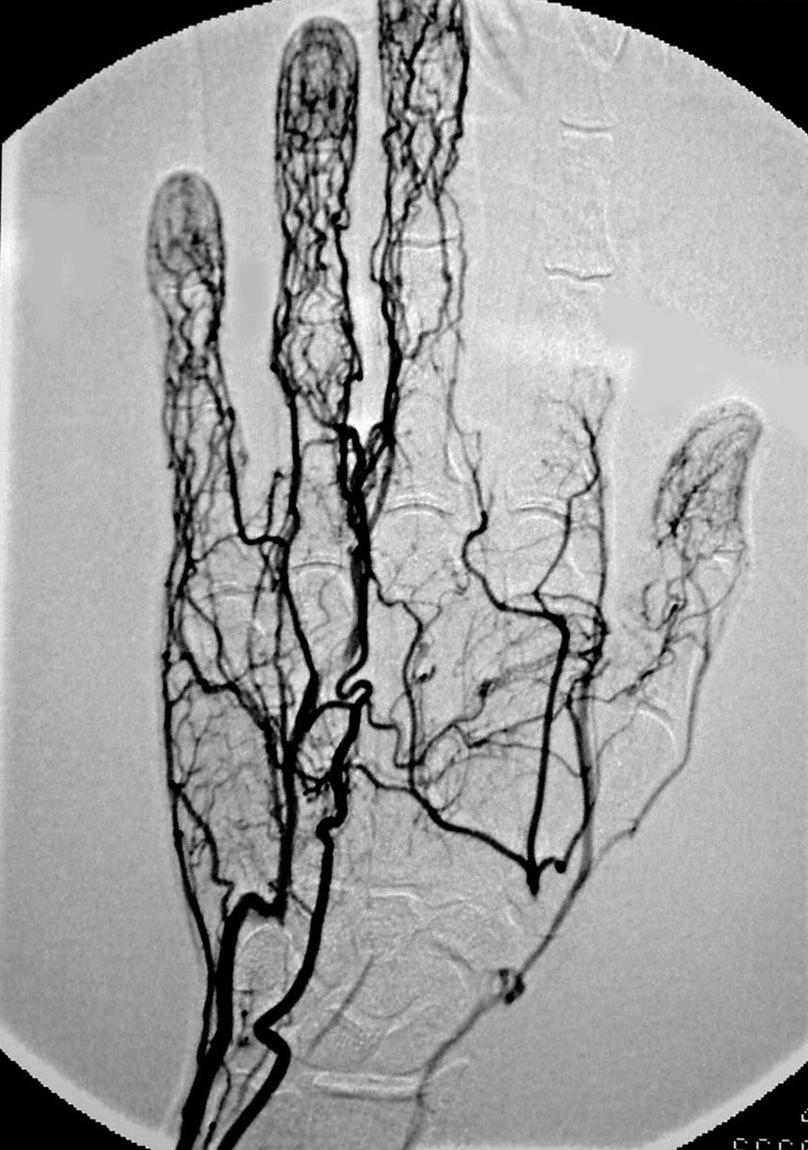
Others prefer a magnetic resonance angiography (MRA), but I have found that the detail in many of these images is inadequate in terms of knowing exactly where and how significant the problem is. The gold standard, however, remains digital subtraction angiography (DSA). Surgeons must view the vascular studies themselves because the radiologist or vascular specialist may or may not see the lesion. A familiarity with the upper extremity’s normal and variant anatomy is essential; however, surgeons who are dealing with vascular problems must evaluate every vascular study, both to review the anatomy and to identify the problem ( Fig. 60.5 ).
There are a number of other types of studies that can be done to aid in the diagnosis of vascular problems in the hand. Color-flow ultrasonography can show real-time flow and be used to evaluate an anastomosis, but it is of primary use in the venous system. A commonly performed test to evaluate vasospasm is cold stress testing, which gives a measure of the vascular system’s ability to recover from exposure to cold. In this test, digital temperature is measured at baseline and the hand is then placed in cold water (5° to 8°C) for 5 minutes. Thermistor probes (or laser Doppler) are used to measure the time required for digital rewarming to baseline. A prolonged rewarming response is often seen in women and can be diagnostic of Raynaud’s. Likewise, smokers often have a delayed rewarming response.
The digital–brachial index, which is the ratio of the blood pressure as measured in the brachial artery and the finger, can be measured with small blood pressure cuffs. This is similar to the ankle–brachial index, and in the upper extremity any value below 0.7 is felt to designate a significant occlusive problem somewhere in the forearm or hand. Blood flow in the capillary bed can be measured by laser Doppler flowmetry, which can give a quantitative measurement of blood flow at skin level. This can be a useful tool to evaluate the results of treatments, particularly for vasospastic problems; however, this equipment is quite expensive and not available to most practitioners.
Similarly, nail fold capillaroscopy shows morphology and flow at the capillary level but is not of practical use for most surgeons. Many of these sophisticated tests are difficult for the practicing surgeon to perform and do not add much, in my opinion, to decision making in terms of patient management. They, however, can be useful in determining the quality of the outcomes of management.
Management of vascular injury or insufficiency in the hand may involve techniques ranging from ligation of the injured vessel to periarterial sympathectomy to complex vein or arterial grafting. Which technique is employed depends on the level of the lesion as well as the cause of the problem. The various techniques used to treat the major causes of ischemia of the hand are discussed in the following subsections, while Box 60.1 offers a historical review.
Hunter (1704): Ligation of the artery proximal to an aneurysm (popliteal); described traumatic aneurysm
Raynaud (1862): Described syndrome of peripheral vasospasm
Mata (1888): Endoaneurysmorrhaphy
Carrell (1902): Described technique of end-to-end anastomosis of blood vessels
Murphy (1905): Resection of cervical ribs causing impingement on the brachial artery
Leriche (1937): Sympathectomy as an effect of excision and ligation of an artery
Korean conflict (1952): Demonstration of successful arterial repair
Allen (1962): Description of Allen test
Kleinert (1965): Revascularization of the superficial palmar arch
Flatt (1980): Periarterial digital artery sympathectomy
Koman (1995): Palmar and wrist sympathectomy for secondary Raynaud phenomenon
Arterial trauma is reasonably common in the upper extremity and can be due to laceration or closed injury, as noted earlier. Reviews have noted that the majority of these injuries are open (i.e., in the 79% range) and that most result from lacerations. The options for management differ somewhat and are discussed here. Open arterial trauma generally requires exploration because there is a wound that needs to be managed, and likewise, injuries to other structures almost always will require repair.
In general, preoperative workup of patients with an open vascular injury is limited to physical examination because a high index of suspicion should be present based on the history and location of the injury. Arterial studies are generally not indicated in open injuries with the exception of gunshot wounds with an otherwise normal examination of motor and sensory systems. It should be noted, however, that clinical studies have demonstrated that up to 50% of vessels with a proximal injury will have a palpable pulse at the wrist. If the limb is well perfused, delay in management of the injury (i.e., beyond 6 hours) if the bleeding is controlled and the patient is hemodynamically stable has not been shown to be detrimental. It also has been shown in the military’s experience during recent conflicts that temporary arterial shunting is a valuable adjunct in the management of arterial injuries. In such patients, shunts were left in as long as 11 hours and in some cases until definitive repair could be performed. The results between patients who had immediate vascular repair and those shunted showed no significant differences in outcomes.
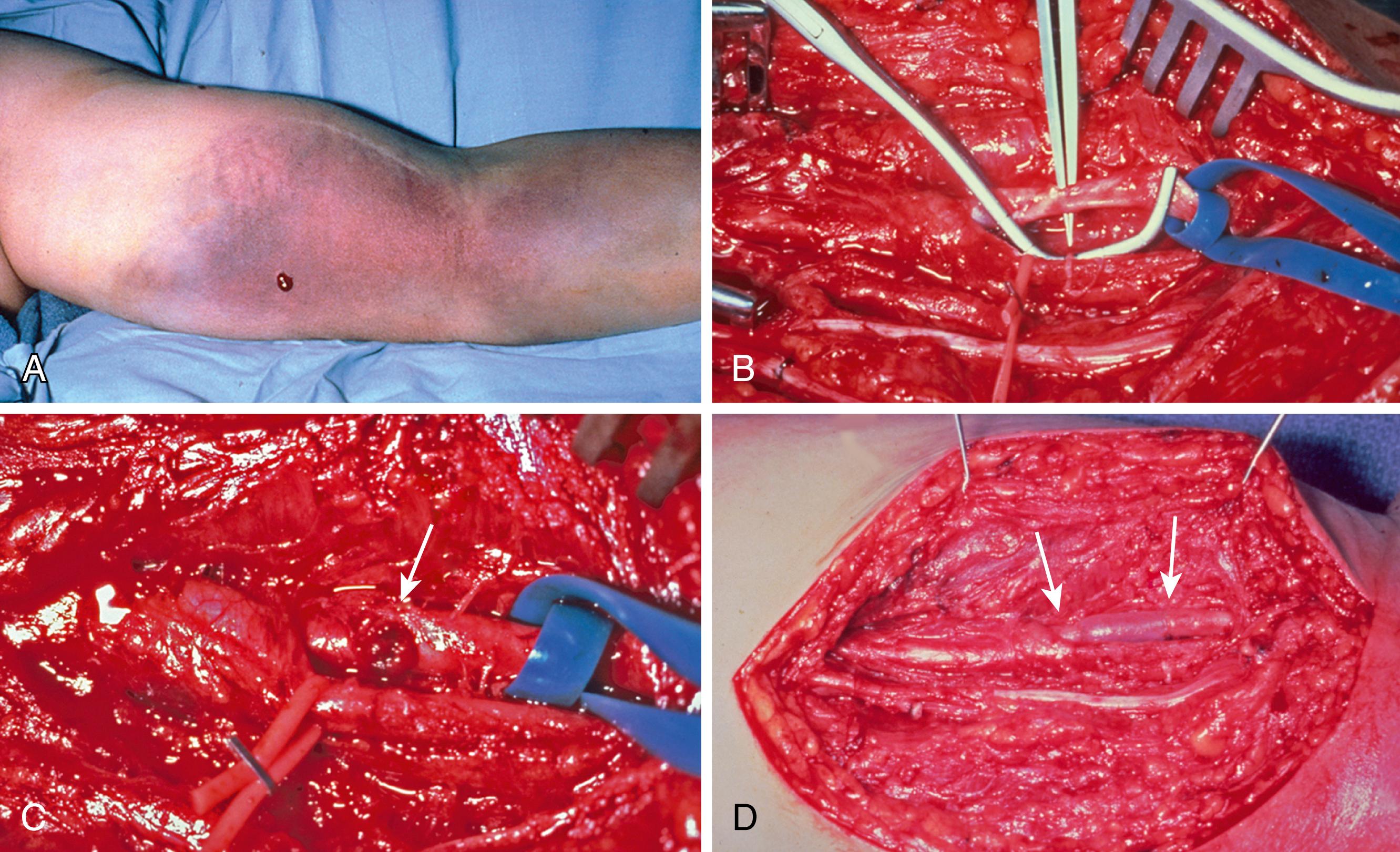
At the time of exploration, the wound will need to be extended in order to allow exploration and repair of the injured structures ( Fig. 60.6 ). If the hand is well perfused, a decision needs to be made as to whether repair of the injured vessel is important to the circulation in the hand. The brachial artery can suffer injury from fractures about the elbow, particularly in children with closed supracondylar humeral fractures. , Such injuries can lead to significant ischemia of forearm muscles and the hand. Studies have demonstrated that single-vessel (i.e., radial or ulnar artery) injuries in the forearm lead to little long-term sequelae even if the injured vessel is simply ligated.
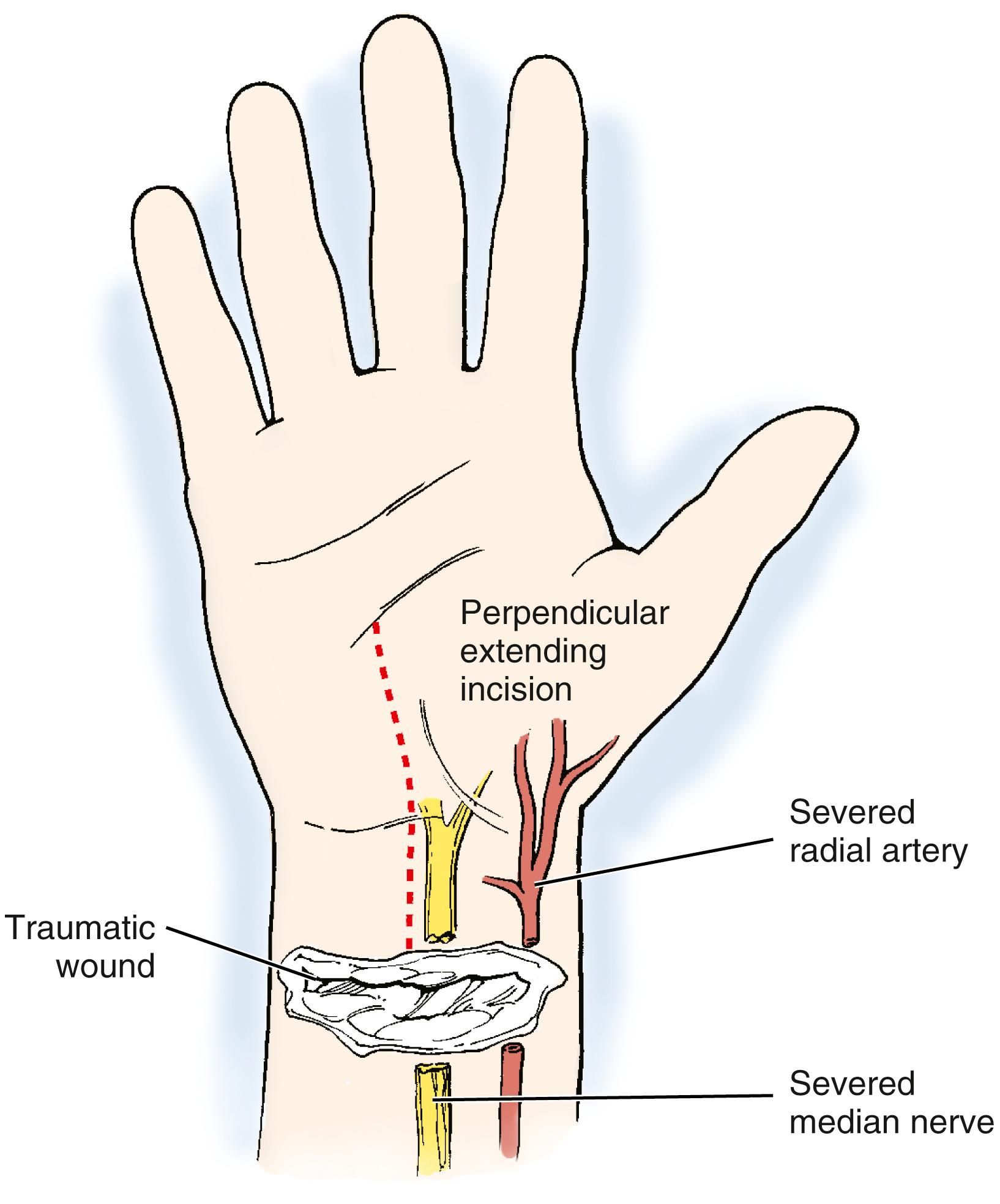
The flow in the remaining vessel has been shown to increase with injury or ligation of the other, and patency rates of repaired single vessels have been shown to be surprisingly low (i.e., in the 50% range), even with proper technique. Arterial injuries combined with median nerve injuries have been shown to lead to increased long-term problems, probably more related to nerve injury than arterial injury. In younger patients and in those with possible vascular compromise from other factors, repair of the radial or ulnar arteries in the forearm almost certainly should be undertaken.
Sharp injuries with complete transection will lead to vessel ends pulling apart, depending on local branches and the surrounding tissue. Cutting injuries usually can be pulled together after careful trimming of the ends of the divided vessel. While forearm vessels can be sewn by most competent hand surgeons with loupes, studies have shown that a better anastomosis can be performed with a microscope. If there is some tension, a large double clamp (e.g., an Acland or Ikuta microvascular) can be used to take the tension off the repair. A repair should be done in standard microsurgical fashion. I prefer 8-0 nylon for forearm vessels, and in some cases use 7-0 polypropylene if the vessel is larger. I always use 6-0 or 7-0 polypropylene for the brachial artery and most proximal vessels. If there is a partial injury, which is sharp, it often can be repaired primarily; however, in some instances damage to the vessel of partial injuries (e.g., gunshot wounds) may well require resection of the injured segment and vein grafting.
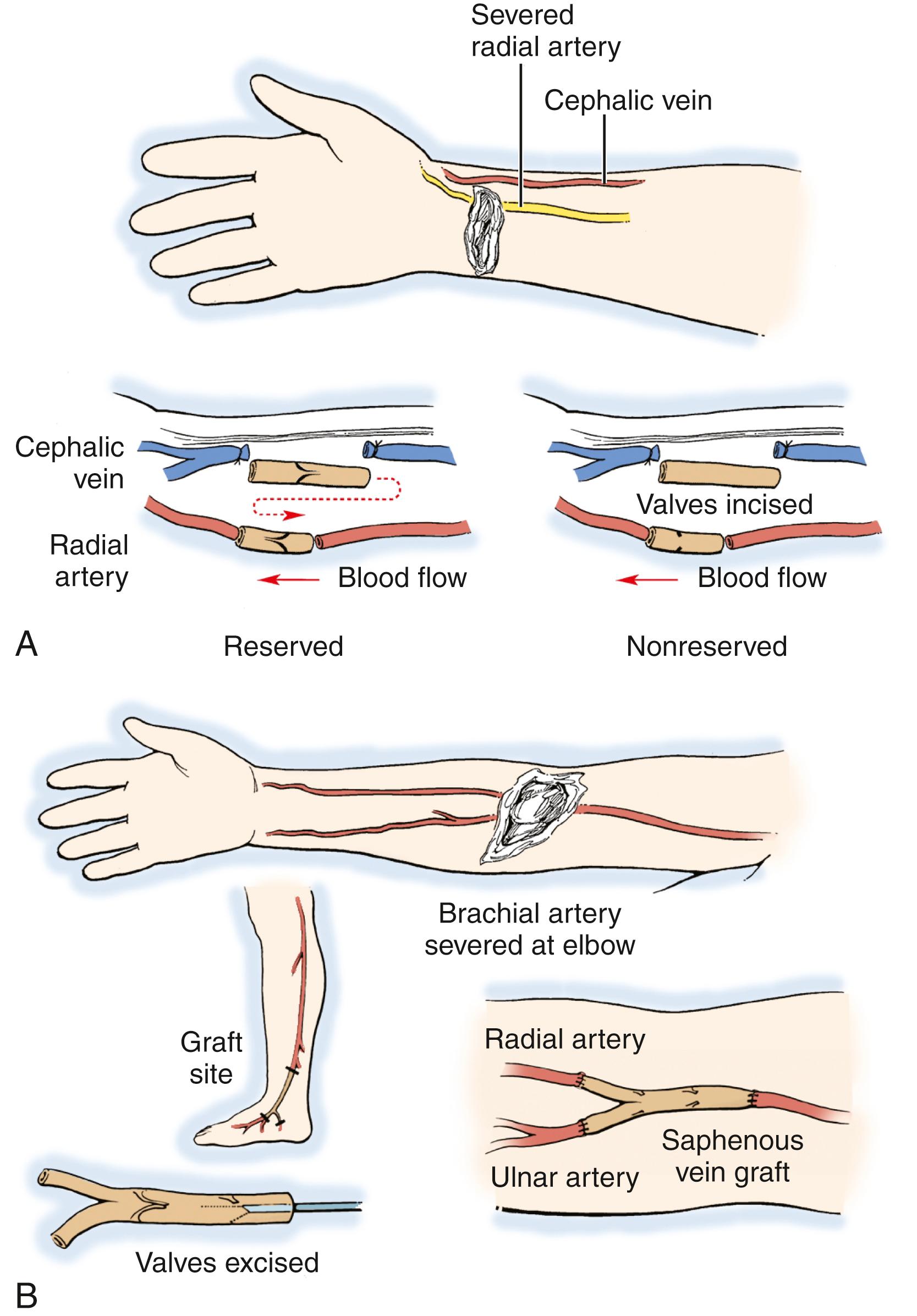
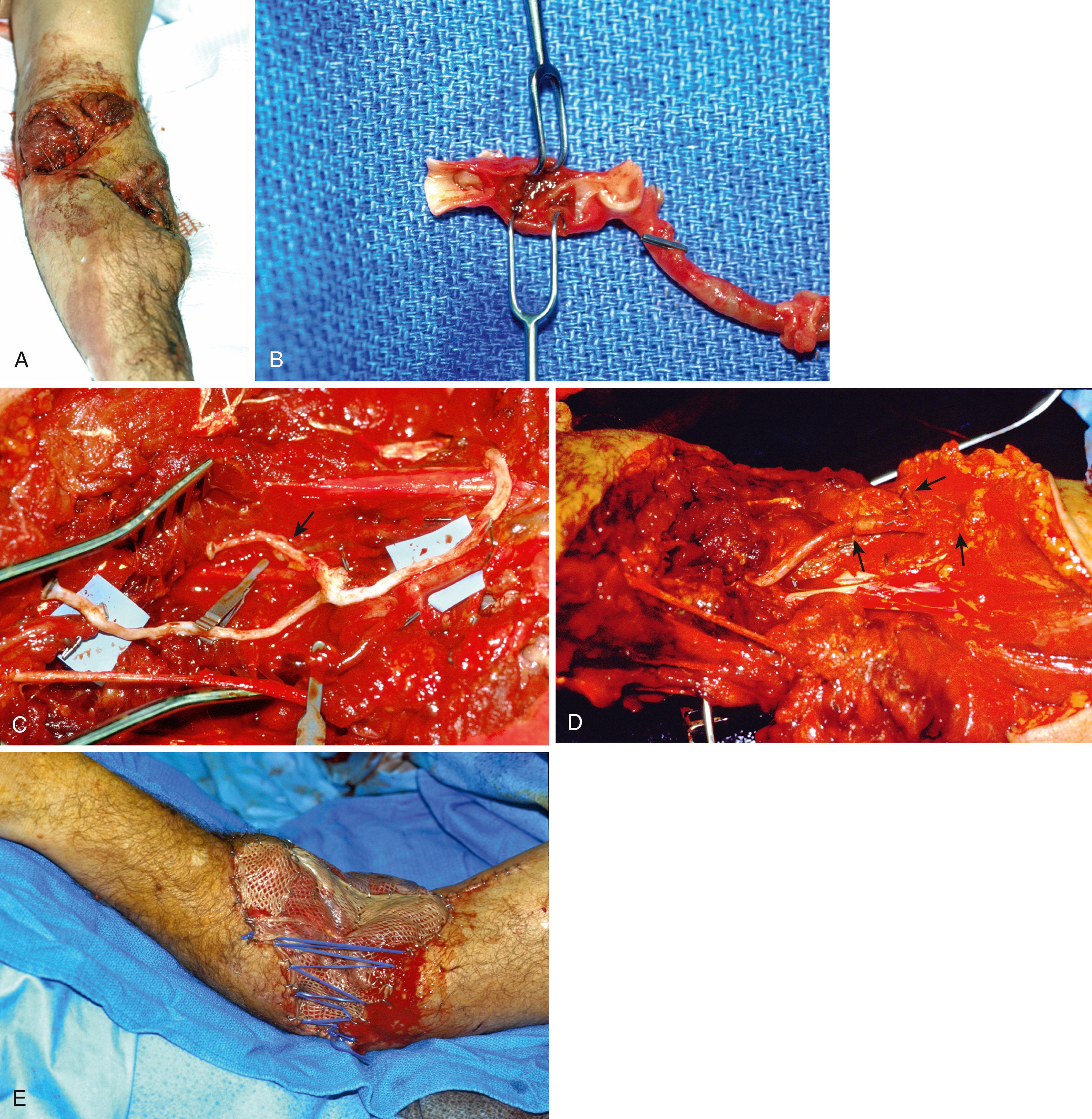
When a vein graft is necessary, it is probably best (and easiest) to harvest it from the injured arm ( Fig. 60.7 ). Although saphenous is an acceptable choice, I have found that it is rather thick-walled and not always the perfect size match for the radial and ulnar arteries in the forearm. There are several maneuvers that can be performed if the vein graft and artery have a circumference mismatch ( Fig. 60.8 ). If a vein graft is going to be done, one must resect an adequate segment of the injured artery to be sure that the anastomosis can be performed out of the zone of injury to decrease the rate of thrombosis. Usually, this is fairly obvious, but any portion of the vessel that has suffered bruising, or has signs of intimal separation when viewed under the microscope, should be resected.
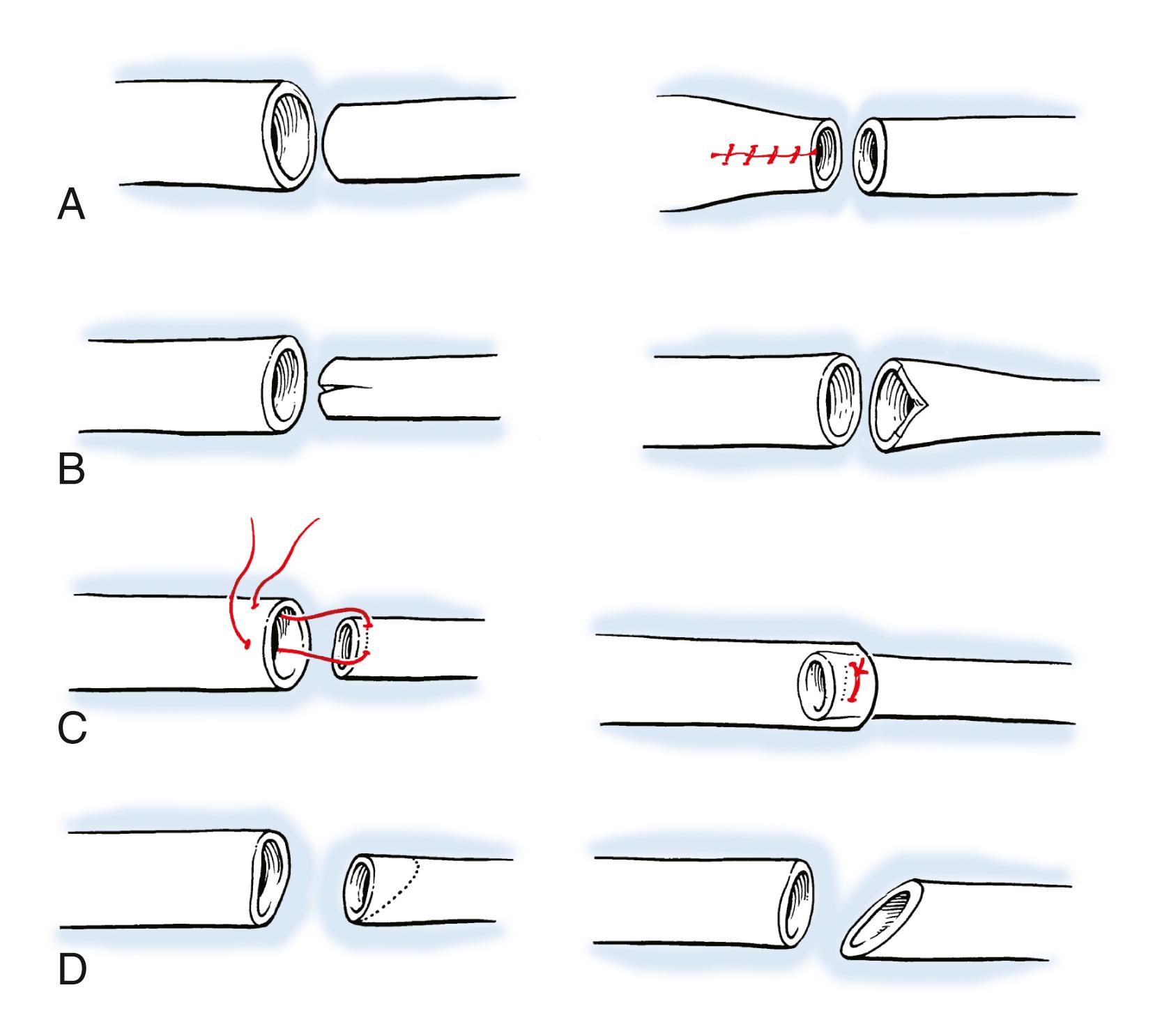
An artery with thrombus in it is not an absolute indication to resect the vessel, as long as the arterial wall does not appear to have suffered damage. After the end is resected, it is probably wise to irrigate the proximal and distal vessels with heparinized saline (generally diluted to 100 units of heparin per milliliter of saline) to decrease the risk of thrombus formation during the time that the clamp is on the artery. Once this is done, I prefer to perform the proximal anastomosis first. When complete, removing the clamp allows the vein graft to fill with blood and both unwind and return to its normal length. After the proximal clamp is removed, the vein is allowed to bleed briefly from the end and is then reclamped. The proper length can then be better estimated than when the vein is empty.
The distal end is trimmed to the proper length if necessary and the distal anastomosis is then performed ( Fig. 60.9 ). Once the anastomosis is completed, distal pulses must be checked. A Doppler can be used, but I feel that a palpable pulse is a much better sign of adequate distal flow. The surgeon should be aware that a clot can propagate distally and embolize into the distal artery or even the hand. Familiarity with the techniques of thrombectomy is essential for those performing vascular repairs (see Fig. 60.3 ). Tension on the repair is checked by ranging the wrist and elbow, as disruption of one of the anastomosis with movement of the hand or arm can be catastrophic.
Arterial repairs and arterial vein grafts cannot be left exposed in a wound nor should they be covered just with skin grafts or an allograft. Viable tissue must be placed over repairs to avoid desiccation and disruption of the repair; at times this may necessitate some type of flap. If the wound is large, as in avulsion-type injuries, selection of a vein for grafting may need to incorporate a branch to allow for placement of a free flap over it with arterial anastomosis to the branch of the graft ( Fig. 60.10 ).
Resect the wounded artery beyond zone of injury (bruising and/or intimal separation).
Select an appropriate length of forearm vein (i.e., basilic or cephalic), alternatively saphenous.
Irrigate proximal and distal arteries with heparinized saline.
Reverse vein graft and repair to proximal artery first, with 7-0 polypropylene or 8-0 nylon.
Allow vein graft to fill with blood and clamp distally; allows vein to unwind and distend.
Trim appropriately and perform distal repair.
I generally splint the elbow and/or wrist after vascular repair or vein grafting in the forearm. If the graft is loose, this is probably not necessary; however, based on the contributing trauma and severity of injury, immobilization is probably prudent. Care must be taken to ensure that the splint does not impinge or put pressure on the graft. Systemic anticoagulation has not been shown to be beneficial and in fact can cause bleeding, so this should be avoided—the patient will have received some systemic heparin from the irrigation of the proximal and distal artery that is probably adequate.
Oral aspirin may have some benefit and should be given in a low dose (i.e., 81 mg) daily for 6 weeks. I allow the patient to begin careful movement after about 3 weeks; however, this depends on which other structures (i.e., bone, tendon, nerve) have been injured and the therapy requirements for them if they have been repaired. Patency rates are generally high in upper extremity vascular repairs done with an autogenous vein. , ,
The primary complication of arterial repair and/or forearm grafting is thrombosis of the repair or graft. This is unlikely to occur unless the surgeon has failed to resect the damaged vessel or has performed a poor vascular repair. Blowout of a repaired artery or vein graft is not unheard of, however, and can be a catastrophic and life-threatening event (see Fig. 60.18 later in the chapter). There must be coverage of the repaired artery or vein graft with well-vascularized soft tissue. If there is wound breakdown, this must be managed aggressively to prevent infection around the repair or graft as well. “Watchful waiting” is not advisable if there are problems with a repaired vessel.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here