Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lung vascular development occurs as highly choreographed sequence, regulated by hypoxia inducible factors, vascular endothelial growth factor, nitric oxide, and other transcription factors and mediators.
In addition to arterial vessels, pulmonary veins are highly reactive vessels that contribute to the overall regulation of pulmonary vascular resistance in the fetus and newborn.
Antenatal pulmonary vascular development can be disrupted by events such as placental insufficiency, genetic abnormalities such as Down syndrome, prolonged oligohydramnios, and congenital diaphragmatic hernia.
Postnatal development of the lung circulation can be disrupted by numerous stresses such as preterm birth, asphyxia, and hypoxia or hyperoxia.
Therapeutic controversies include optimal oxygenation targets, the role of adjunctive therapies such as cGMP and cAMP phosphodiesterase inhibitors, endothelin receptor antagonists, and prostanoids, the role of cardiac dysfunction (particularly in congenital diaphragmatic hernia), and treatment of acute and chronic pulmonary hypertension in the premature infant.
The development of the pulmonary vasculature during fetal and neonatal life is highly coordinated with airway growth and plays a key role in normal lung development. In comparison with adult pulmonary vascular disease, disruption of lung vascular development plays a central role in the pathobiology of pulmonary vascular disease and airway development in the neonate and young infant.
Lung development is classically divided into five overlapping stages in humans and rodents based on gross histological features, termed the embryonic (weeks 4–7 of gestation), pseudoglandular (weeks 5–17), canalicular (weeks 16–26), saccular (24–38), and alveolar stages (week 36 to infancy). The development of the pulmonary vasculature is closely correlated with and interacts with airway growth. Lung vascularization initially originates in the mesenchyme, distal to the epithelium. In response to epithelial-derived vascular endothelial growth factor (VEGF), the endothelial cells move toward the epithelium, where they form the epithelium-capillary interface needed for gas exchange. Growth of the lung vasculature continues after birth and into adulthood.
Pulmonary hypertension (PH) is a normal physiologic state during fetal life and permits survival on placental support. Fetal pulmonary vascular resistance (PVR) is high in part due to hypoxic pulmonary vasoconstriction ( Fig. 3.1 ). In the fetal lamb, pulmonary arterial blood has a PO 2 of approximately 18 mm Hg and oxygen saturation of 50%. Because of high PVR, only about 16% of the combined ventricular output is directed to the lungs; the majority of right ventricular output passes through the ductus arteriosus to the descending aorta. The blood is then oxygenated by the placenta and returns to the body through the umbilical vein, with a PO 2 of ∼32 to 35 mm Hg in lambs. Based on the oxygen saturation difference between the umbilical vein (85%) and umbilical artery (52%), the fetus can achieve sufficient oxygen delivery at the low PO 2 levels needed for normal lung development.
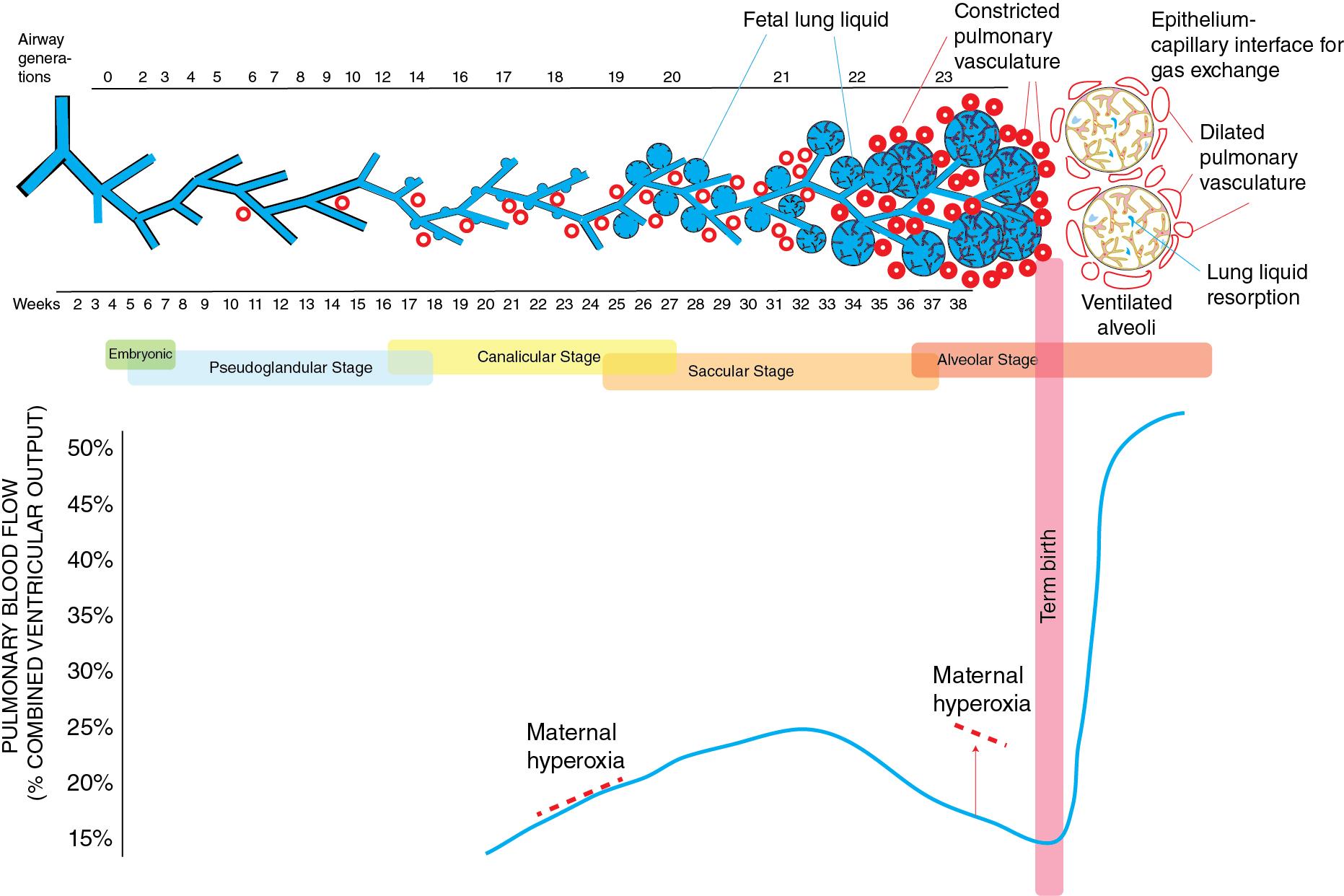
In human fetuses, Doppler studies demonstrate that pulmonary blood flow is only 13% of combined ventricular output at 20 weeks of gestation (canalicular stage), representing a nadir during lung development ( Fig. 3.1 ). This finding is largely secondary to the lower cross-sectional area of the very immature pulmonary vascular bed. Furthermore, in fetal lambs at an equivalent point in gestation (∼65% gestation), pulmonary blood flow does not increase in response to hyperoxia and PVR does not increase in response to hypoxia. , Similarly, in human pregnancies, maternal hyperoxygenation with face mask oxygen at 20 to 26 weeks of gestation does not result in pulmonary vasodilation. Birth at this gestational age (23–26 weeks) is associated with a 2% risk of acute PH, , which perhaps explains the high rates of inhaled nitric oxide (iNO) therapy (6–8%) in extremely premature infants.
As the lung develops through the early saccular stage, a rapid proliferation of pulmonary vessels and a marked increase in cross-sectional area of the pulmonary vascular bed occur, which decreases fetal PVR. At the same time, pulmonary vessels become more reactive to vasoconstrictors, such as hypoxia and endothelin, and vasodilators, such as oxygen. The net result is higher PVR and increased reactivity of the pulmonary vasculature. For instance, maternal hyperoxygenation testing (60% oxygen by face mask) is used to measure the reactivity of the fetal pulmonary vasculature in response to oxygen in late gestation and to predict postnatal survival in congenital diaphragmatic hernia. Later in fetal life, studies in nonhuman primate and sheep models have shown that PVR is very sensitive to maternal hypoxemia (by administration of 12% oxygen) and hyperoxemia (by administration of 100% oxygen), independent of changes in umbilical flow. , These changes in PVR in turn determine the distribution of fetal cardiac output and oxygen delivery to the brain and heart. For instance, in pathologic conditions such as congenital diaphragmatic hernia, reduced pulmonary venous return and left ventricular filling may contribute to left ventricular hypoplasia. ,
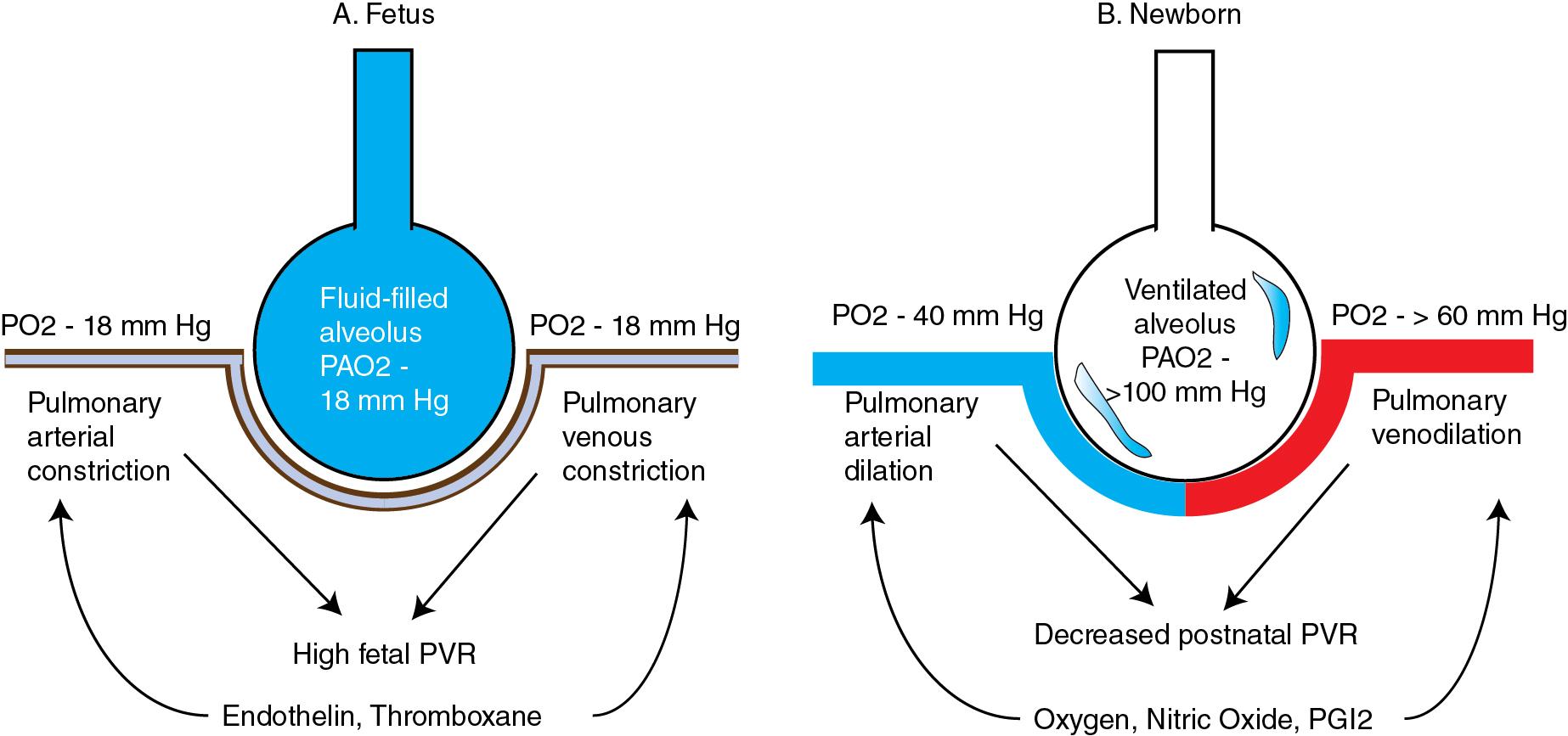
Pulmonary veins were originally regarded as passive conduit vessels, but they are now recognized to be reactive vessels that contribute to the overall regulation of PVR. In the fetus, pulmonary veins contribute a significant fraction to total PVR and may play a more important role in regulating the fetal and newborn pulmonary circulation than in adults ( Fig. 3.2 ). In perinatal sheep, NO stimulated endogenously by acetylcholine or given exogenously causes greater relaxation and accumulation of cGMP in pulmonary veins than in arteries. At birth, the veins, as well as the arteries, relax in response to NO and dilator prostaglandins, thereby assisting in the fall in PVR ( Fig. 3.3 ). These effects are oxygen-dependent and modulated by protein kinase G. In a number of species, including the human, pulmonary veins are also the primary sites of action of certain vasoconstrictors, such as endothelin and thromboxane ( Figs. 3.2 and 3.3 ).
![Fig. 3.3, Overview of endothelium-derived vasodilator (prostacyclin [PGI 2 ] and nitric oxide [NO] ) and vasoconstrictor (endothelin [ET-1] ) pathways. Fig. 3.3, Overview of endothelium-derived vasodilator (prostacyclin [PGI 2 ] and nitric oxide [NO] ) and vasoconstrictor (endothelin [ET-1] ) pathways.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Vasculardevelopmentandpulmonaryhypertension/2_3s20B9780323878746000126.jpg)
The hypoxic conditions of fetal life support the tremendous lung vascular growth that occurs before birth. Hypoxia inducible factors (HIFs) are regarded as the “master regulators” of the transcriptional response to hypoxia and are involved in angiogenesis, survival, and metabolic pathways ( Fig. 3.4 ). HIFs are heterodimers consisting of oxygen-sensitive α-subunits (HIF-1α, HIF-2α) and constitutively expressed β-subunits. Hypoxia stabilizes the α-subunit leading to nuclear accumulation and activation of multiple target genes. HIF-1 regulates genes involved in angiogenesis (e.g., VEGF), oxygen transport (e.g., erythropoietin), and energy metabolism (e.g., glycolytic enzymes), among others. The importance of HIFs in fetal lung development has been demonstrated by studies revealing that deletion of HIF-1 causes embryonic lethality, and deletion of HIF-2 reduces VEGF levels and leads to early death due to respiratory failure. In the adult lung, hypoxia induces abnormal vascular remodeling, potentially by inducing HIF activity. However, hypoxia is the normal fetal condition and is a required environmental stimulus to sustain normal fetal lung and vascular development.
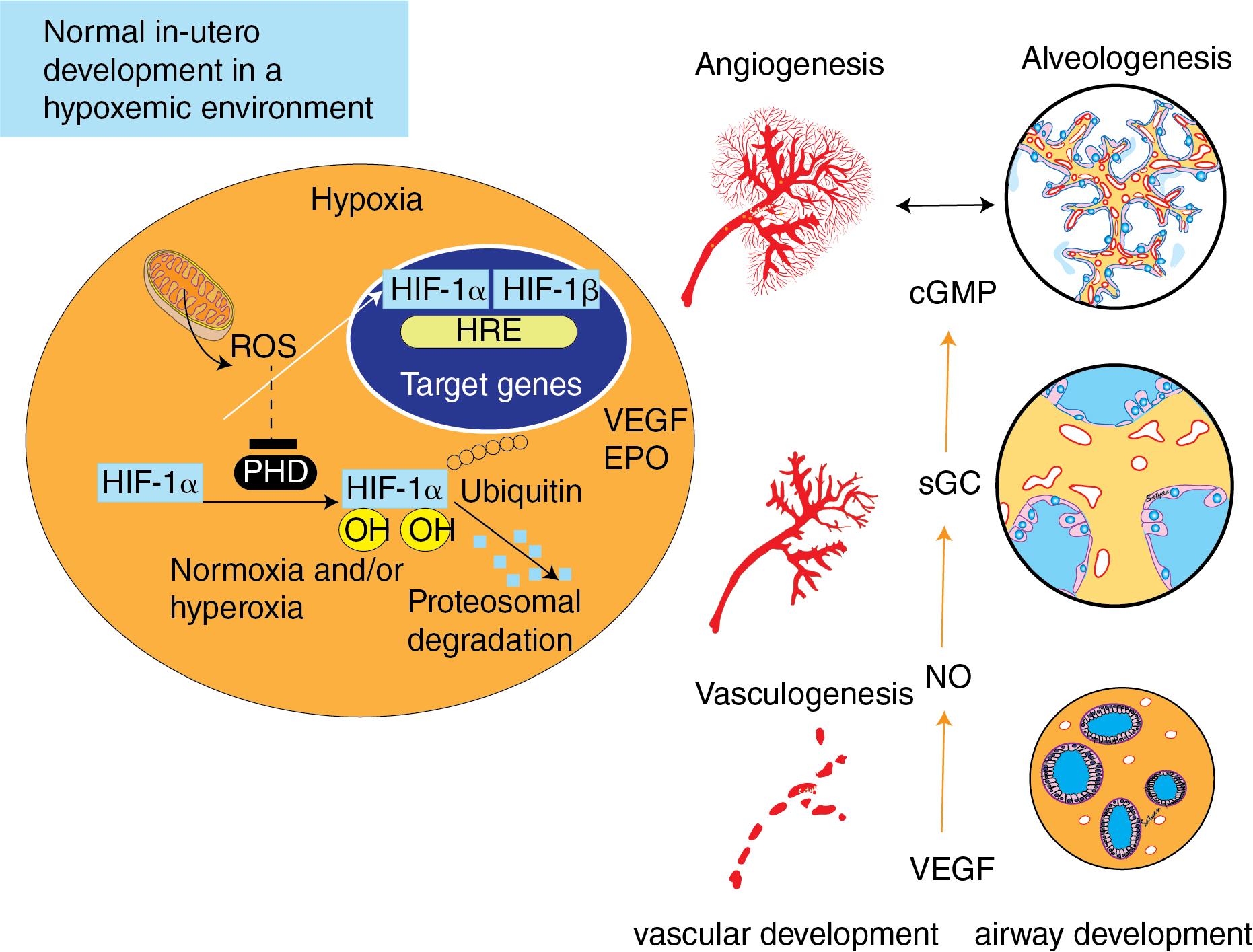
VEGF is a key regulator of lung vascular growth and development during fetal and postnatal life. VEGF transcription is regulated by HIF, and its signaling is transduced via two transmembrane tyrosine kinase receptors: VEGFR-2 and VEGFR-1. VEGF RNA and protein are localized to distal airway epithelial cells, while VEGFR-1 and VEGFR-2 mRNA expression is localized to the pulmonary endothelial cells closely approximated to the developing epithelium. The importance of VEGF for vascular development has been demonstrated by a number of studies that inactivate or knock out VEGF or its receptors. Each of these will produce a lethal phenotype that is characterized by deficient organization of endothelial cells. Furthermore, VEGFR-1 and VEGFR-2 inhibitors (e.g., SU5416) impair alveolar development in fetal and newborn rodent models, producing pathologic findings similar to those seen in clinical bronchopulmonary dysplasia (BPD). Even in adult rats, chronic treatment with SU5416 causes PH and enlarges the air spaces, suggesting that normal VEGF function is required not only for the formation but also for the maintenance of the pulmonary vasculature and alveolar structures well after lung development is completed.
VEGF-induced lung angiogenesis is in part mediated by NO. While NO is best known for its vasoactive properties, it also plays an important role in the structural development of the pulmonary vasculature. Lung endothelial NO synthase (eNOS) mRNA and protein are present in early fetal life in rats and sheep, and increase with advancing gestation in utero. , Lungs of fetal and neonatal mice deficient in eNOS have reduced alveolarization and vascularization and are more susceptible to the effects of hypoxia on vascular and alveolar growth. , VEGF inhibition is associated with decreased lung eNOS protein expression and NO production, and treatment with inhaled NO improves vascular and alveolar growth after VEGF inhibition. However, in neonatal mice that are eNOS deficient, recombinant human VEGF protein treatment restores lung structure after exposure to mild hyperoxia, suggesting that VEGF operates in part through mechanisms independent of eNOS.
Numerous transcription factors important to lung vascular development have been identified. The Forkhead box (Fox) family of transcription factors regulate expression of genes involved in cellular proliferation and differentiation. Newborn mice with low Foxf1 levels die with defects in lung vascularization and alveolarization, and endothelial-specific deletion of Foxf1 produces embryonic lethality, growth retardation, and vascular abnormalities in the lung, placenta, and retina. These findings are directly relevant to human lung development, as, Foxf1 haploinsufficiency is found in 40% of infants with alveolar capillary dysplasia (ACD), a lethal disorder of lung vascular development. NF-κB is a transcription factor traditionally associated with inflammation, but recent data suggest that it may play a very different, protective role in the neonatal lung. Blocking NF-κB activity during the alveolar stage of lung development in neonatal mice induced alveolar simplification and reduced pulmonary capillary density like that observed in BPD, effects that appear to be regulated by VEGFR-2. Anecdotal reports of exacerbation of PH following intravitreal injection of VEGF inhibitors for retinopathy of prematurity suggest an ongoing role of VEGF in postnatal pulmonary vascular development in preterm infants.
Lung endothelial progenitor cells (EPCs) have been recently identified as mediators of lung development, although their mechanistic role is not yet well understood. In rats, microvascular pulmonary endothelial cells proliferate twice as fast as endothelial cells isolated from large pulmonary arteries. These highly proliferative cells express endothelial cell markers (CD31, CD144, eNOS, and von Willebrand factor) and progenitor cell antigens (CD34 and CD309). Thus, the pulmonary microcirculation seems to be enriched with EPCs that support vasculogenesis while maintaining endothelial microvascular functionality.
Resident microvascular EPCs also share features of human cord blood–derived endothelial colony-forming cells (ECFCs). Developing human fetal and neonatal rat lungs contain ECFCs with robust proliferative and vasculogenic potential. The functionality of these cells can be disrupted during or after birth, potentially contributing to arrested alveolar growth after extremely preterm birth: for instance, human fetal lung ECFCs exposed to hyperoxia in vitro proliferate less and form fewer capillary-like networks. These findings also suggest a role for ECFCs in lung repair: in rodents, exogenous administration of human cord blood–derived ECFCs restored alveolar and lung vascular growth in hyperoxic rodents. Lung engraftment was low, suggesting that the ECFCs may support lung growth and repair through paracrine effects. Recent studies in a mouse model of ACD found that administration of EPCs stimulated neonatal lung angiogenesis through Foxf1-mediated signaling. These findings suggest that pulmonary EPCs from patient-derived induced pluripotent stem cells could be a therapeutic avenue to restore vascular growth and function in the lungs of patients with pediatric pulmonary disorders such as ACD.
As gestation progresses, NO and cGMP become central to the emergence of pulmonary vascular reactivity. Inhibition of eNOS increases basal PVR as early as 0.75 gestation (112 days) in the fetal lamb, indicating that endogenous NOS activity contributes to vasoregulation during late gestation. Pulmonary vasodilation in response to NO (an endothelium-independent mediator) precedes the response to endothelium-dependent mediators such as acetylcholine and oxygen. The response to NO is dependent on expression and activity of soluble guanylate cyclase (sGC) in the smooth muscle cell ( Fig. 3.3 ), which remains low through early preterm (126 days) gestation in fetal sheep and markedly increases toward the end of third trimester. Intracellular cGMP levels are also tightly regulated by cGMP-specific phosphodiesterase (PDE5) activity, which increases during late gestation.
Pulmonary endothelial cells produce the prostaglandin molecules PGI 2 and PGE 2 , which are both potent vasodilators. Prostacyclin (PGI 2 ) acts on its receptor in the smooth muscle cell to produce cyclic adenosine monophosphate (cAMP), which also mediates smooth muscle cell vasodilation (similar to cGMP, Fig. 3.3 ) and is inactivated by cAMP-specific phosphodiesterase 3A (PDE3A). While stimuli such as shear stress induce release of PGI 2 , overall, prostaglandin release appears to play a less important role than NO in regulating fetal and transitional pulmonary vascular tone.
Constrictors also play a role in regulating the pulmonary vascular tone of the fetus. Endothelin-1 (ET-1) is produced by vascular endothelium and acts on the ET-A receptors in the smooth muscle cell to induce vasoconstriction by increasing ionic calcium concentrations. A second endothelial receptor, ET-B, on the endothelial cell stimulates NO release and vasodilation ( Fig. 3.3 ). Although capable of both vasodilator and constrictor responses, ET-1 appears to primarily act as a pulmonary vasoconstrictor in the fetal pulmonary circulation and is elevated in infants with severe persistent pulmonary hypertension of the newborn (PPHN). Lipid mediators, such as thromboxane A 2 , leukotrienes C 4 and D 4 , and platelet-activating factor are potent pulmonary vasoconstrictors, but there is scant evidence that these agents influence PVR during fetal life and transition.
Endogenous serotonin (5-HT) production is another contributor to the high PVR of the fetus. Infusions of 5-HT increase PVR, , and infusions of ketanserin, a 5-HT 2A receptor antagonist, decrease fetal PVR in a dose-related fashion. Conversely, brief infusions of selective serotonin reuptake inhibitors (SSRIs), such as sertraline and fluoxetine, cause potent and sustained elevations of PVR. Together, these findings suggest that 5-HT causes pulmonary vasoconstriction and contributes to maintenance of high PVR in the normal fetus through stimulation of 5-HT 2A receptors and Rho kinase activation. These findings have important implications for SSRI treatment for maternal depression, as described below.
At birth, a rapid and dramatic series of circulatory events occur as the fetus transitions to extrauterine life. After birth and initiation of air breathing, several mechanisms operate simultaneously to rapidly reduce pulmonary arterial resistance and increase pulmonary blood flow. Of these, the most important stimuli are ventilation of the lungs and an increase in oxygen tension ( Figs. 3.1 and 3.2 ). Pulmonary blood flow increases by eightfold, resolving fetal PH. Clamping of the umbilical cord removes the low-resistance placental circulation, increasing systemic arterial pressure as pulmonary arterial pressure falls. In some infants with in utero adverse events or with abnormalities of pulmonary transition at birth, PH persists into the newborn period, resulting in PPHN.
Pulmonary endothelial NO production increases markedly at the time of birth. NOS inhibitors (e.g., nitro-l-arginine) attenuate the decline in PVR after delivery of fetal lambs, , suggesting that the release of NO may be responsible for 50% of the rise in pulmonary blood flow at birth. Oxygen is an important catalyst for this increased NO production. In near-term fetal lambs, maternal hyperoxia induced by hyperbaric oxygenation increased pulmonary arterial PO 2 from 19 ± 1.5 to 48 ± 9 mm Hg, and pulmonary blood flow from 34 ± 3.3 to 298 ± 35 mL/kg/min, a 10-fold rise that nearly replicates the normal transition and is blocked by pretreatment with NOS inhibitors. However, mice deficient in eNOS can successfully make the transition at birth without evidence of PPHN, suggesting the presence of alternate or compensatory vasodilator mechanisms, such as upregulation of other NOS isoforms or dilator prostaglandins. Interestingly, eNOS-deficient mouse pups develop PH after relatively mild decreases in PaO 2 and have higher neonatal mortality when exposed to hypoxia after birth. It is possible that eNOS deficiency alone may not be sufficient for the failure of postnatal adaptation, but that a decreased ability to produce NO during perinatal stressors such as hypoxia or inflammation may contribute to the development of postnatal PH. The similar timing of the peak in activity of pulmonary sGC and cGMP phosphodiesterase (PDE5) allows for fine regulation of vascular cGMP concentrations in the transitional and early neonatal period ( Fig. 3.4 ).
The arachidonic acid-prostacyclin pathway also plays an important role in the transition at birth. Rhythmic lung distension and shear stress stimulate both PGI 2 and NO production in the late gestation fetus, although the effect of O 2 tension is predominantly on NO activity. PDE3A catalyzes the breakdown of cAMP ( Fig. 3.3 ) and appears to create important cross-talk with the cGMP pathway.
Less is known about the pulmonary vascular transition after preterm birth, although similar mechanisms appear to be in effect. In premature lambs at ∼70% of gestation (112-115 days), the pulmonary vasodilator responses to rhythmic distension of the lung or increased PaO 2 are partly due to stimulation of NO release. In human preterm infants, the decrease in PAP after birth is significantly slower compared to term infants, particularly if respiratory distress syndrome also exists. Pulmonary arterial pressure elevations may persist for several days in extremely preterm infants. , Skimming et al. have questioned as to whether a “natural” increase in PVR affords benefit to the preterm infant by reducing the ductal steal and stabilizing systemic circulation. However, recent prospective studies clearly indicate that early PH in extremely preterm babies is associated with BPD and late PH, so it more likely indicates abnormal vascular development or function.
After birth, structural development of the lung and its vasculature continues. More than 90% of lung alveolarization occurs postnatally, with a prominent surge between birth and 6 months of age. Similarly, there is marked growth and development of the microvascular network during the alveolarization phase. A double capillary network is characteristic of the fetal and neonatal lung, but as alveolarization progresses, the interalveolar septae thin and the double capillary layer fuses into the single layer characteristic of the mature vasculature. The capillary network continues to expand its surface area through childhood by nearly 20-fold.
The histological features of early neonatal PPHN have been described in animal models and in fatal cases of PPHN in term infants. , In two autopsy series of infants with PPHN, vascular remodeling resulted in muscularization of the smallest arteries (< 30μM external diameter) at the level of the alveolar duct and wall, , and a doubling of the medial wall thickness of the intra-acinar arteries ( Fig. 3.5 ). These findings suggest that structural maldevelopment of the peripheral pulmonary arterial bed begins in utero and does not merely represent a failure of the fetal pattern to regress. Very similar patterns of remodeling are observed in animal models of PPHN, including the lamb model of antenatal ductal ligation.
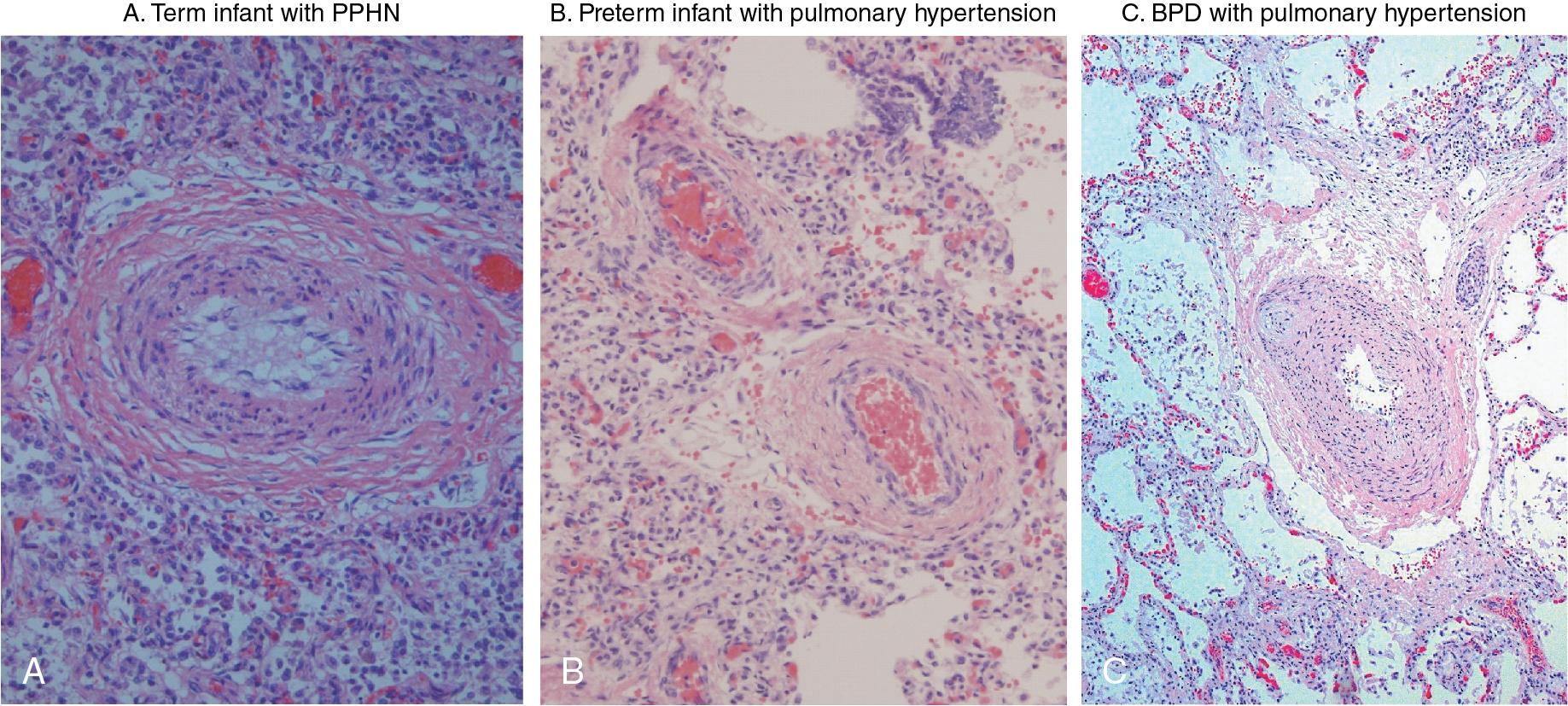
Thickening of the adventitia is observed in remodeled pulmonary vessels ( Fig. 3.5 ) and likely contributes to pulmonary artery stiffness. Adventitial cells (including fibroblasts, pericytes, progenitor cells, etc.) also appear to be regulators of vascular wall function from the “outside in.” , For example, NO is less potent when administered to the adventitial side of vessels. This may be partly due to the presence of constitutively active NADPH oxidase in adventitial cells, which generate superoxide anions that actively scavenge NO.
In contrast to PPHN, after very preterm birth, the developing lung is exposed to an extrauterine environment that disrupts the normal fetal vascular developmental patterns. On histology, the lungs of very preterm infants with BPD display evidence of arrested development, with reduced numbers of both alveoli and intra-acinar arteries. The pulmonary circulation in animal models and infants with BPD is characterized by vascular pruning, decreased vascular branching, and altered patterns of vascular distribution within the lung interstitium. Similar to early PPHN, smooth muscle proliferation also extends abnormally into the smaller peripheral arteries. In addition, intrapulmonary bronchopulmonary anastomoses have recently been identified that act as arteriovenous shunts that contribute to hypoxemia. These anastomotic vessels may represent a compensatory mechanism to overcome the reduction in vascular surface area, or as a protective “pop-off” mechanism to reduce the severity of PH and protect the right ventricle.
Signaling abnormalities in the remodeled vasculature include decreased expression of eNOS and reduced urinary levels of NO metabolites. , sGC expression and activity is also diminished in animal models of neonatal PH and congenital diaphragmatic hernia (CDH), , which is partly secondary to oxidation of sGC that renders it NO-insensitive. , Because NO and cGMP also inhibit vascular smooth muscle growth, it is likely that a combination of diminished eNOS expression, inactivation of sGC, and reduced cGMP levels also contribute to excessive muscularization of pulmonary vessels in PPHN and BPD. Ventilation with high concentrations of inspired oxygen and exposure to reactive oxygen species (ROS) also decrease cGMP levels through increasing PDE5 activity, effects that appear to be mediated by ROS produced from the mitochondria ( Fig. 3.3 ). In fetal lambs with PPHN, pulmonary prostacyclin synthase (PGIS) and PGI 2 receptor (IP) protein levels in the lung are decreased, but levels of adenylate cyclase and PDE3A are not altered.
Circulating levels of endothelin (ET-1), a potent vasoconstrictor and smooth muscle mitogen, are increased in human infants with PPHN, and lung and vascular ET-1 levels are increased in fetal lambs with PPHN. , ET-1 appears also to be a marker for chronic PH, in that infants with congenital diaphragmatic hernia and poor outcomes have higher plasma ET-1 levels at 2 weeks of age and severity of PH than infants discharged on room air. The constrictor effects of endothelin are mediated in part through activation of the RhoA-Rho kinase (ROCK) pathway. Increased Rho kinase activity leads to phosphorylation of myosin light-chain kinase, which in turn increases intracellular calcium and causes vascular contraction. The ROCK pathway plays an important role in hypoxic pulmonary vasoconstriction, and as a mediator of the impaired angiogenesis and increased contractility associated with chronic fetal PH ( Fig. 3.3 ).
A number of gene mutations including mutations in the gene coding bone morphogenic protein receptor type 2 (BMPR2) and other genes (e.g., CAV1, KCNK3, EIF2AK4) have been identified in adults with PH. In contrast, PH is rarely familial in newborn infants and relatively few genetic mutations have been identified. Candidate gene analyses have not identified polymorphisms of the eNOS, VEGF, or other NO pathway genes in infants with PPHN. However, polymorphisms have been identified in carbamoyl-phosphate synthetase 1, which catalyzes the first, rate-determining step of the urea cycle that leads to L-arginine synthesis, a key substrate required for NO generation. , In addition, higher rates of genetic variants for cortisol signaling (corticotropin releasing hormone receptor-1 [ CRHR1 ] and CRH-binding protein) were observed in neonates with PPHN, as well as evidence for functional adrenal insufficiency. More recently, variants in the T-box transcription factor 4 gene (TBX4) have been reported in infants with prolonged PH. While the contribution of these novel variants to PPHN remains poorly characterized, whole genome sequencing should be considered in infants with atypical or prolonged PPHN.
Children with Down syndrome (trisomy 21) commonly develop PH in association with structural heart defects, but also have a 10-fold increased risk for idiopathic PPHN. In a Dutch cohort, PPHN was documented in 5.2% of Down syndrome infants without cardiac disease. In addition, Down syndrome infants have worse pulmonary arterial hypertension in conjunction with anatomic cardiac disease than genetically normal infants with similar lesions and they are more likely to require ECMO support for PPHN. One recent study showed that 85% of autopsy specimens from Down syndrome children displayed pulmonary vascular remodeling, suggesting that PH may occur even more commonly than clinically recognized.
Dysregulation of angiogenic factors likely contributes to the pathogenesis of PH associated with Down syndrome. Chromosome 21 includes at least three genes with potent antiangiogenic properties which could affect fetal vascular development. One likely candidate, endostatin, is a known antiangiogenic factor that downregulates signaling of VEGF, which would be expected to impair angiogenesis and adversely affect lung structure. A threefold increase of endostatin mRNA expression has been reported in prenatal Down syndrome lungs, along with reduced microvascular density, thickened large and small pulmonary artery walls, and a persistent double capillary network. More recent biomarker analysis of older children (ages 1–11 years) found elevated endostatin levels in the Down syndrome group relative to age-matched controls; levels were highest in those Down syndrome children with active or resolved PH. On the other hand, Bush et al. reported elevated endostatin levels in Down syndrome children regardless of the presence of PH, raising questions about whether circulating endostatin levels are predictive of PH in this population.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here