Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Maria. A. Nagel, MD
Professor
Neurology
University of Colorado School of Medicine, Aurora
Colorado
United States
Professor
Ophthalmology
University of Colorado School of Medicine, Aurora
Colorado
United States
Varicella zoster virus (VZV) is an exclusively human alphaherpesvirus that produces varicella (chickenpox) on primary infection, establishes latency in ganglionic neurons, then reactivates later in life to produce herpes zoster (HZ; shingles). Historically, varicella and variola (smallpox) were often confused until 1767, when the English physician, Dr. William Heberden, gave the first detailed clinical description that established varicella as a separate disease and noted that those who had previously had chickenpox were not capable of having it again. Numerous studies followed showing that varicella is an infectious disease and could be reproduced in volunteers by inoculation with vesicular fluid or by exposure to patients with either varicella or HZ ; varicella and HZ are caused by the same agent ; and HZ is the result of reactivation of the virus acquired earlier in life. The virus was first isolated in cell culture by Weller and Stoddard in 1952 from vesicular fluid from varicella patients. Later studies indicated that the viruses isolated from subjects with varicella and HZ were morphologically and serologically identical, and the virus was named VZV. Finally, molecular studies demonstrated that the restriction endonuclease patterns of viral genomes from a subject with varicella and subsequent HZ, as well as from live-attenuated varicella vaccine recipients with subsequent HZ, , were be identical, proving that HZ is caused by reactivation of latent VZV.
Varicella is often mild; however, serious complications involving multiple organs can occur in babies, adolescents, adults, pregnant women, and immunocompromised individuals (such as those with human immunodeficiency virus [HIV]/acquired immunodeficiency syndrome [AIDS] or cancer, as well as those on immunosuppressive therapies for autoimmune disease or transplants). For instance, prior to the development of vaccine, varicella could produce pneumonia in both young and adult individuals , ; in acute leukemia patients, cases of fatal pneumonitis were further increased. Before widespread vaccination, illness associated with varicella was responsible for over $333 million in health care costs and more than $1.5 billion in societal costs. Even with effective anti-VZV therapies, including acyclovir and prophylactic passive immunization, the risk of disseminated varicella in immunocompromised children and high risk individuals, as well as the economic burden, warranted development of an effective varicella vaccine.
VZV is highly contagious and predominantly acquired by inhalation of infectious airborne droplets from an individual with varicella or HZ. Nonimmune, immunocompetent children and adults develop varicella 10–21 days after exposure. One to 2 days before rash, fever, malaise, loss of appetite, and headache may occur. The pruritic rash appears in crops—initially as raised pink/red bumps (papules) that become fluid-filled (vesicles); the vesicles then break and leak before crusting over and sometimes scarring. New crops continue to appear for several days with various stages of the rash (bumps, blisters, and scabbed lesions) present throughout the course of disease. The average number of skin vesicles ranges from 250 to 500 in otherwise healthy children. , The rash has a central distribution, with a concentration of lesions on the trunk, scalp, and face, but can spread to the inner mouth, eyelids, and genital area. The height of fever typically parallels the extent of rash, and the subject is usually ill for 5–7 days. Second cases of varicella can occur in immunocompetent persons, but the exact incidence is unknown ; it is hypothesized that the avidity of VZV antibodies is lower in these individuals. Another possibility is that VZV, acquired from infected contacts, subverts and evades the immune system to produce disseminated rash, such as through modulation of immunoinhibitory proteins or natural killer cell dysfunction. Subclinical reinfection with VZV also occurs.
Varicella in otherwise healthy children is usually not severe, but the disease has a wide spectrum of infrequent complications, as well as increased severity, in susceptible, high-risk groups described below. Varicella-associated complications include pneumonia, stroke, encephalitis, cerebellar ataxia, arthritis, myositis, appendicitis, hepatitis, glomerulonephritis, pericarditis, and orchitis. The most common complication is secondary bacterial infection, , predominantly due to Staphylococci or Group A β-hemolytic streptococci. Group A streptococcal infections may be unusually severe and even fatal after varicella. The pathogenesis of group A streptococcal infection following varicella include breakdown of skin providing a portal of entry for bacteria, and possibly transient VZV-mediated immune suppression that facilitates bacterial infection. ,
Acute cerebellar ataxia (ACA) may develop before rash onset or up to weeks afterward. ACA occurs in approximately 1 in 4000 varicella cases among children younger than 15 years old; the prognosis is usually good. A study from the Netherlands showed an incidence rate of ACA after varicella to be 5:100,000 VZV infections for children up to 5 years of age, compared to 0.15:100,000 with doses of varicella vaccine, indicating that vaccination reduces the rate of varicella-associated ACA. Compared to ACA, varicella encephalitis is more serious, yet less common in children (1 in 33,000–50,000 cases); it carries a more guarded prognosis. , Varicella is also associated with an increased risk of arterial ischemic stroke (postvaricella arteriopathy of childhood) that typically involves large vessels and is monophasic ; up to one-third of children with arterial ischemic stroke have varicella in the preceding year compared to 9% in healthy controls.
Compared to children, adults with varicella have significantly higher case morbidity and mortality with primary VZV infection. , In adults, the height and duration of the febrile response are greater and rash is frequently more severe, with a greater number of painful, pruritic lesions and longer time for clearing. , Constitutional signs and symptoms and a prodrome are also of greater intensity in adults with varicella. Varicella-associated extracutaneous complications described above can also occur.
Varicella may be more severe in pregnant women (especially in the last trimester) than in other adults. , Fetal morbidity is increased in pregnant women with varicella, most likely due to transplacental spread of virus. , Varicella during pregnancy may damage the fetal central nervous system and cause permanent scarring of the skin, aplasia of extremities, chorioretinitis, microphthalmia, optic atrophy, cataract, Horner syndrome, blindness, mental retardation, fetal demise, and a high incidence of HZ and death in infancy. This constellation of problems in infants whose mothers had varicella in pregnancy is clinically diagnosed as the congenital varicella syndrome. There is a high correlation between the presence of limb abnormalities and serious central nervous system damage. Various studies have indicated rates of the syndrome after maternal varicella in the range of 0.4–2.0%. Harger and colleagues noted that 0.4% of offspring of 347 women with laboratory-confirmed varicella in pregnancy had the congenital varicella syndrome. A prospective case–control study of 106 pregnant women with varicella, including a meta-analysis combining the results of this study with those of other published prospective studies, estimated a 2% risk of varicella embryopathy if infection occurred during the first trimester of gestation. Enders and associates prospectively studied 1373 women with varicella and 366 with HZ during pregnancy. After maternal varicella, the risk of the fetal syndrome was 0.4% in weeks 1 through 12 and 2.0% in weeks 13 through 20. Although no cases occurred in pregnant women infected after 24 weeks of gestation in this study (346 pregnancies affected between 25 and 36 weeks were followed up), isolated cases with birth defects consistent with congenital varicella syndrome have been described after infections in the third trimester, with the latest occurring at 28 weeks of gestation. , These varying rates may not be significantly different; the syndrome is real, but rare.
The largest prospective study, comprised of 366 women, on the consequences of HZ in pregnancy was conducted by Enders and colleagues. 65 No cases of congenital varicella syndrome were reported. There are rare case reports of fetal abnormalities after maternal HZ, , but whether they represent the congenital varicella syndrome is unclear. If it does occur, the congenital varicella syndrome acquired when a pregnant woman has HZ is exceedingly rare. Infection at any time during fetal life may result in latent infection that is poorly controlled by the host and subsequently reactivates as HZ in infancy or early childhood. , , ,
Maternal varicella that develops within 5 days before or 2 days after delivery is potentially very serious for the newborn infant. Maternal antibodies to VZV may not have formed or crossed the placenta, and VZV may infect the baby before or after delivery. In addition, because of the immaturity of the infant’s cellular immunity, it is at risk for severe varicella especially if there is no maternal antibody to VZV. The infected infant may develop hemorrhagic skin lesions and primary varicella pneumonia. Severe varicella can usually be avoided with prophylactic administration of passive immunization and acyclovir therapy. , Vaccination of women who are seronegative for VZV before pregnancy is the preferred preventive strategy.
Varicella in immunodeficient individuals can present with a more severe and prolonged rash and dissemination. Those receiving chemotherapy, radiation or immunomodulatory therapy, or high doses of corticosteroids for any reason (cancer, transplantation, autoimmune disease, severe asthma), and those with congenital deficits in cell-mediated immunity (CMI), are at greatest risk of developing severe varicella disease. The association between cancer therapy and severe varicella was noted in the early 1950s. By 1975, 30% of children with leukemia who developed varicella had disseminated disease with 7% mortality. In the era of antiviral therapy, the prognosis improved with acyclovir administered early in the course of illness, but deaths continued to occur. Severe varicella became especially common as greater numbers of children were treated successfully for malignant disease, transplantation, and asthma. , Although severe varicella occurred in children with HIV infection, the risk of severe varicella was not as great as for leukemic children.
During the primary infection with VZV, the virus reaches ganglionic neurons by way of viremia and retrograde transport along nerves then becomes latent in neurons within dorsal root, cranial nerve, autonomic, and enteric ganglia ; during latency, no infectious virions are produced. Waning VZV–specific, CMI later in life or during immunosuppression enables VZV to reactivate from one or more ganglia, typically spreading to skin and producing a corresponding, unilateral, dermatomal-distribution rash (HZ). Compared with healthy persons, HZ in immunocompromised persons is more likely to be severe and disseminated. Additional information on HZ is found in Chapter 66 .
VZV is a double-stranded DNA alphaherpesvirus, also known as human herpesvirus-3). Infection is confined to humans and some higher primates; there is one serotype. DNA analysis of >100 VZV isolates indicates that the virus genome does not diverge significantly from the original 124,884 bp sequence obtained in 1986. The linear genome is (80 ± 3) × 10 6 Da with internal inverted terminal sequences resulting in two predominant isomeric DNA molecules. Virions are round or polygonal with a central double-stranded DNA core. The nucleocapsid is approximately 100 nm, formed of 162 hexagonal capsomeres organized into an icosahedron (20 sides) within which the unit length linear virus genome is packaged under ∼293 PSI internal pressure. , The capsid is surrounded by a tegument that contains functional enzymes and an envelope derived in part from cellular membranes. The diameter of the VZV particle is 180–200 nm. Analysis of the first complete VZV DNA sequence predicted 71 open reading frames (ORFs) encoding 67 unique proteins ( Fig. 63.1 ) ; however, recent advances in technology and the recognization of noncoding genes, has more than doubled the number of distinct VZV transcripts that potentially encode newly identified proteins, fusion proteins, as well as long and small noncoding RNA. Current research has matured from identifying new VZV genes to determining if they are present during natural infection or byproducts of tissue culture studies. ,
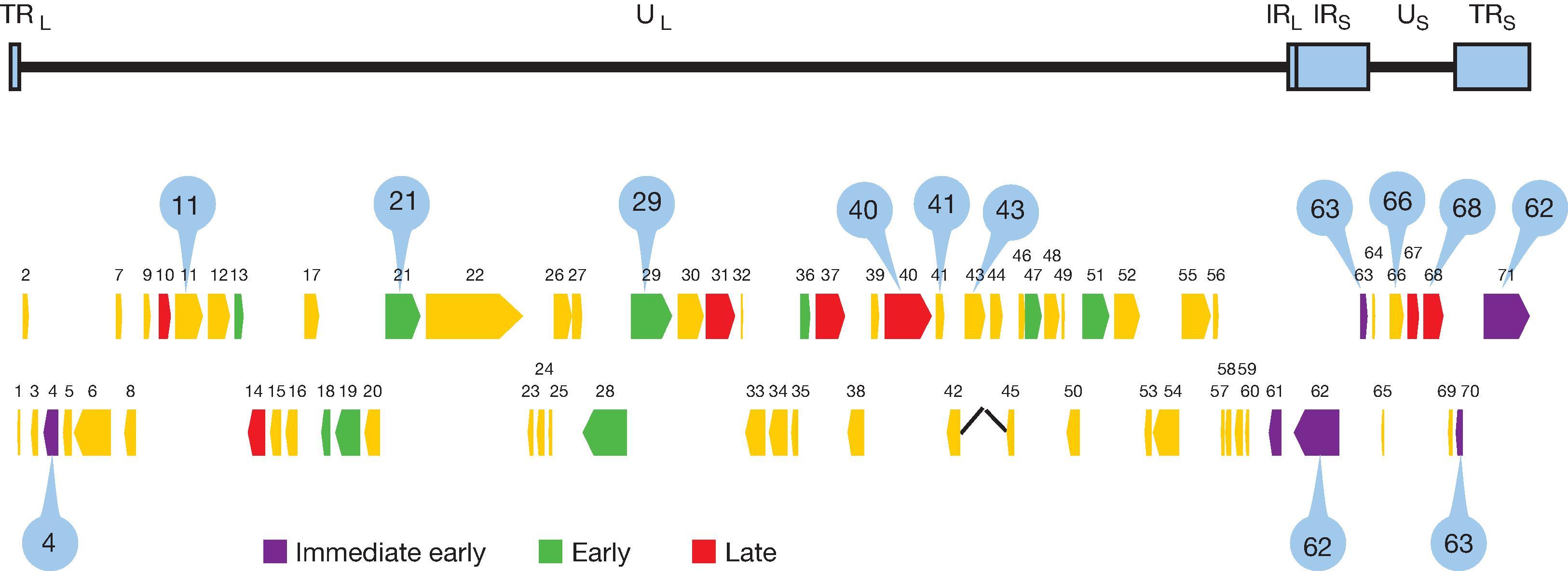
VZV can grow in most human cells and can be adapted to grow in monkey and guinea pig cells. VZV replication follows a coordinated cascade of gene expression that includes immediate early (IE) or α regulatory genes, followed by expression of early (E) or ß genes that encode regulatory and structural proteins, then expression of late (L) or γ genes that encode structural proteins. VZV DNA replication occurs following initiation of early gene expression. Since interruption of the cascade, particularly at the IE or E stages, may result in failure to synthesize infectious virus, these virus proteins are potential targets for chemo- or immuno-therapy. ,
At least 30 polypeptides have been detected in VZV; at least nine are glycosylated. The known glycoproteins (gps) are termed B, C, E, H, I, K, L, M, and N ( Fig. 63.2 ), similar to herpes simplex virus (HSV-1). There is, however, no equivalent of the major gp of HSV-1 gpD, in VZV. Gps are expressed on the virion and on the surface of infected cells; they facilitate infection of other cells and are the target of neutralizing antibodies. , The major gp of VZV is gpE, which is the most abundant gp and highly immunogenic. It is linked to gpI and, with gI, is an Fc receptor on infected cells. It also binds to mannose 6-phosphate receptors, along with other gps in the virus, which are critical to VZV infection. gpE provides trafficking sequences that mediate assembly of viral proteins and envelopment in the trans -Golgi network. , Gp E and gpI are thought to function as navigator gps, directing additional gps to the cell surface and trans -Golgi network, where final envelopment of virions takes place. The current HZ subunit vaccine is based on gpE. , Mannose 6-phosphate receptors play a critical role in VZV entry and egress from cells. Gp H plays a role in spread of VZV, and monoclonal antibodies to gpH prevent spread. , The insulin-degrading enzyme is also a receptor for VZV gpE, and it is important for cell-to-cell spread and infectivity.
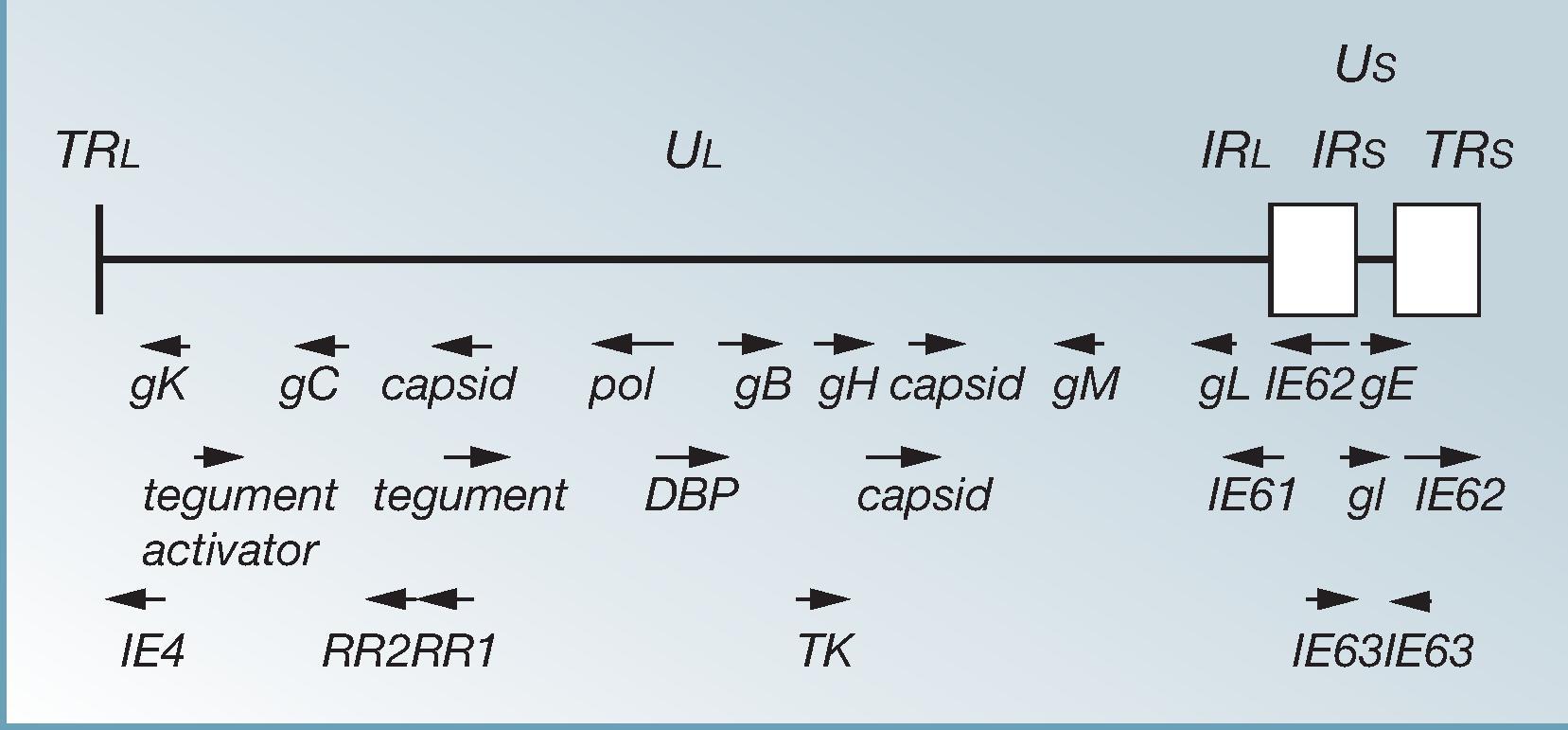
A new VZV variant with a mutated gpE has been isolated from six persons in the United States, Canada, and Europe. These viruses may be escape mutants; their biological significance remains to be defined. An immunocompetent 15-year-old boy with fatal hepatitis caused by a VZV with this mutation was described. Because this is only one patient, and deaths from varicella occur without this mutation, the pathogenic potential of this mutated virus remains unknown. In addition to gps, some IE gene products of VZV are also immunogenic. IE62 protein, which is encoded by VZV ORF62, is closely related to the regulatory HSV-1 ICP4. This protein was at one time thought to be purely nonstructural, as is HSV-1 ICP4. However, IE62 protein has been identified as a major component of the VZV tegument and is highly immunogenic. IE62 protein is the initial transactivating protein of VZV. Other regulatory proteins of VZV are encoded by ORFs 4, 10, 61, and 63.
It was originally reported that during latency, six genes are expressed in sensory ganglia: IE genes (ORFs 4, 62, 63, and 66) and E genes (ORFs 21, 29); L genes are not expressed. , However, further analysis of human trigeminal ganglia obtained at autopsy suggests that these VZV and HSV transcripts may be associated with generalized transcriptional deregulation of virus genes seen during early stages of virus reactivation and that during latency, both viruses transcribe a unique set of latency associated transcripts. , VZV proteins have also been detected in latently infected human ganglia; although, false-positive results are proposed to be the result of cross-reacting antigens in patients with type A blood, and neuromelanin. Interestingly, studies of gastrointestinal neurons from asymptomatic persons undergoing surgery, however, are not subject to all these possibilities, and reveal that the multiple VZV RNA transcripts in human enteric ganglia. , Thus, although VZV gene expression during latency is a highly active field of research, many issues remain unsettled.
Latent VZV occurs only in neurons. Patients with impaired CMI have an increased incidence of HZ, consistent with the hypothesis that suppression of VZV reactivation is under immunologic control. Rodent models of latent VZV infection may help clarify how latent infection with VZV is maintained. , There is an in vitro model of VZV latency in isolated myenteric sensory ganglia from guinea pigs that can be manipulated to reactivate in vitro, , , , as well as models that use the fetal human dorsal root ganglion. Growth of VZV in cell monolayer cultures is slow, with poor infectious yields and an absence of infectious virions in supernatant media since it is highly cell-associated. In the infected person, VZV also spreads almost entirely from cell-to-cell, which is why cellular immunity is crucial in host defense. To obtain cell-free VZV in vitro, infectious virions must be released artificially by disruption of cells by methods such as sonication, but the yield of cell-free infectious virus remains extremely small. , The cell-associated nature of the virus has tended to impede research on VZV and has played a role in slowing vaccine development.
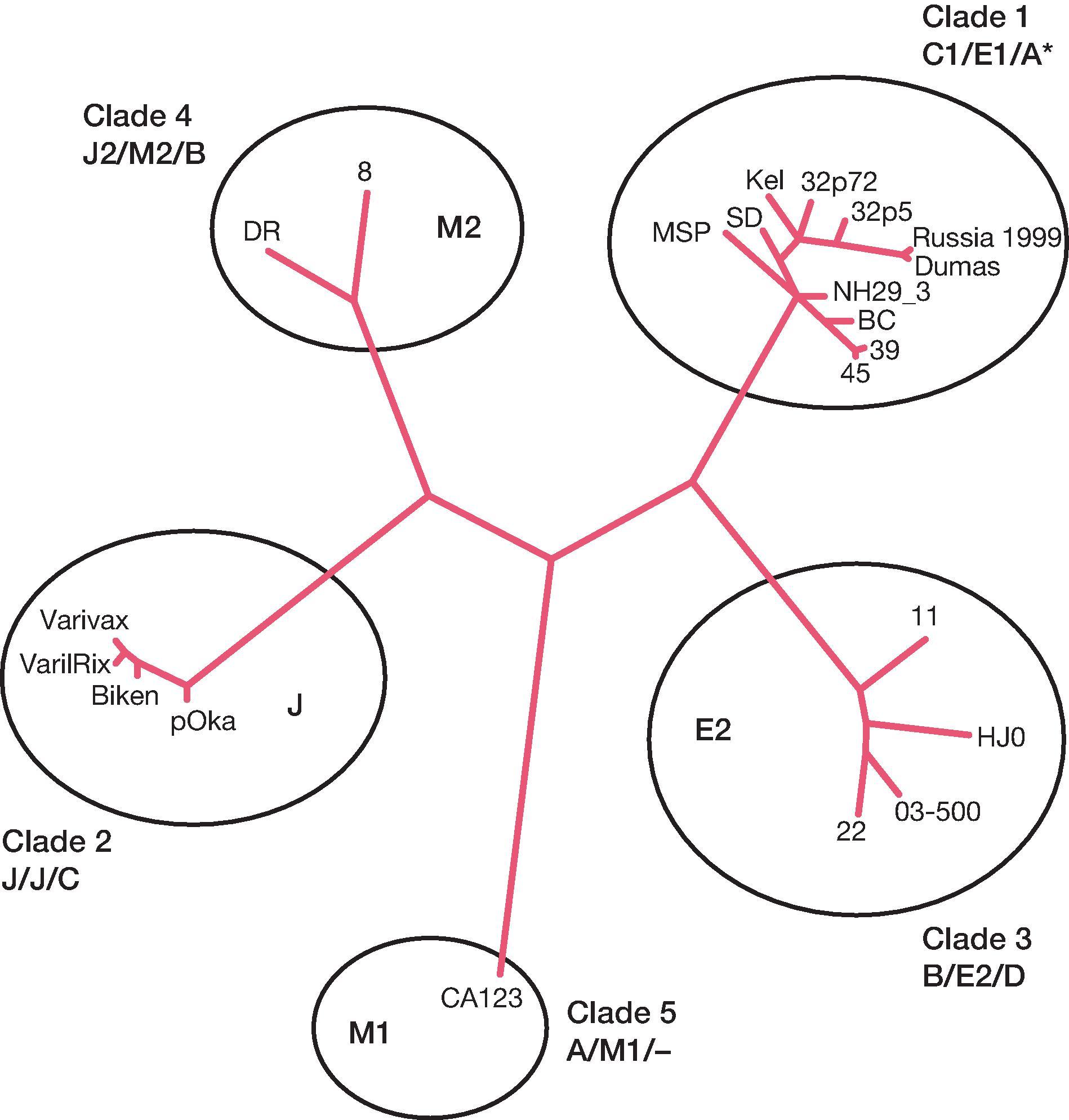
Although there is only one VZV serotype, different genotypes have been identified. According to consensus agreements within the field, five naturally occurring distinct phylogenetic clades have been identified by limited whole genome sequencing and sequence analysis of multiple specific PCR (polymerase chain reaction) amplicon variations within the virus genome ( Fig. 63.3 ). , , , Subsequently, the complete DNA sequence of >1000 virus isolates have been determined, and the number of confirmed clades with at least two complete virus genomic DNA sequences available has risen to eight with one provisional clade (clade VII) yet to be confirmed by a second isolation. The VZV clades show a distinctive geographic distribution, , although all clades circulate at low levels outside the regions where they predominate. For example, occasional clade 2 Asian strains have been identified in the United States and Australia, , and clade 5 African strains have been identified in the United Kingdom. However, only the clade 1 and clade 3 strains most commonly isolated in temperate climates have been observed in most countries in Europe and in New Zealand. , This may reflect the proposed European origin of the different clades, or an ongoing shift in strain distribution related to immigration and travel patterns. All eight confirmed clades have been observed in the United States, although clade 1 and clade 3 isolates predominate (approximately 80% clade 1 and clade 3). Clade 2 strains are the most prevalent genotypes in Japan and China , ; the Oka vaccine is derived from a clade 2 strain bearing a genomic marker present in 10% of wild clade 2 strains (Pst 1 negative in Japan). Rare clade 2 wild-type strains bearing this marker have now been identified in the United States, Australia, and China. , , Sequence analysis of >3000 VZV isolates from throughout the world has not revealed a second member to confirm the provisionary clade VII, and it is generally assumed that this virus may not exist anymore. Similar sequence analysis suggests that clade 6 and the provisional clade VII are within the same supergroup. Recent analyses of whole-genome sequences of VZV revealed the presence of small numbers of strain-specific markers that may prove useful for subgenotyping VZV clades. , , Phylogenetic analysis suggests that circulating genotypes arose through limited mutation and interstrain recombination.
Varicella is a highly contagious disease. Infectivity is postulated to occur predominantly by aerosols from vesicular skin lesions , and to a much lesser extent from respiratory secretions if at all. , Airborne transmission has been described in hospital settings. , Studies of transmission of VZV vaccine in leukemic vaccinees have implicated skin lesions as the source of infectious virus. In patients with varicella, virus can be isolated from skin, but it is difficult to isolate from respiratory secretions. As a result of the loss of mannose 6-phosphate receptor in the superficial epidermis, high concentrations of cell-free VZV develop in skin vesicles; these virions may be aerosolized and transmit the virus to others. PCR analysis of respiratory secretions have yielded contradictory results and therefore have not clarified the issue of whether respiratory spread of VZV is significant. , In one study, VZV DNA was detected in the respiratory tract of 45 patients with varicella, in 28 patients (62%) on day 1, in 50% on day 6, and in 22% after day 6. VZV DNA was detected in the saliva of 100% of 54 HZ patients. VZV DNA was found in throat swab samples from varicella patients for 7 days commencing within 24 hours after the administration of oral acyclovir. Demonstration of VZV DNA by PCR does not necessarily indicate the presence of infectious virus. Prolonged presence of VZV DNA in secretions or dust has not been associated with virus transmission.
The pathogenesis of varicella was conceptualized by Grose in 1981, based on the pathogenesis of mousepox, outlined by Fenner in 1948. Host invasion of VZV is thought to occur in the conjunctivae or the mucosa of the upper respiratory tract (including tonsils). It was hypothesized that, VZV replicates locally in the lymph nodes for several days, causing a low-level primary viremia that delivers the virus to the viscera, where it replicates. A demonstrable secondary viremia of subsequently occurs. Culture of mononuclear cells from 5 days before to 2 days after appearance of rash in patients with natural varicella yielded VZV (confirmed by PCR testing). , Viremia has also been demonstrated in patients with HZ using either virus isolation or PCR. The presence of VZV in CD4 + and CD8 + T lymphocytes also has been demonstrated by in situ hybridization during early varicella. In a model of VZV infection in severe combined immunodeficient mice with human skin grafts (SCID-hu), VZV could also be detected in human T lymphocytes. During acute infection, VZV is transferred to T cells in lymph nodes and the virus reconfigures the T cells to express skin homing markers and reduced immune functions.
VZV-infected human mononuclear cells injected into SCID-hu mice were found to infect the skin implant within 24 hours. Innate immune mechanisms were downregulated in infected keratinocytes, but not in neighboring cells. These observations suggested that the skin may be infected early after VZV enters a host, and that VZV circulates in memory CD4 + T cells to establish focal infection of additional keratinocytes. The long incubation period of varicella is attributed to the time needed for VZV to overcome innate antiviral responses of cutaneous cells. Exactly how this scenario relates to the “Fenner model” with infection of viscera is not yet clear.
The precise roles of humoral immunity and CMI in protection against VZV infection are not entirely understood but are summarized as follows. ,
After natural varicella and HZ, serum VZV immunoglobulin (Ig) M, if it occurs, may be detectable for days to weeks. As with many IgM tests, false-negative and false-positive reactions occur. Fig. 63.4 shows the kinetics of humoral, nasopharyngeal, and cellular immunity in patients with clinical varicella from 10 days before to 20 days after onset of rash.
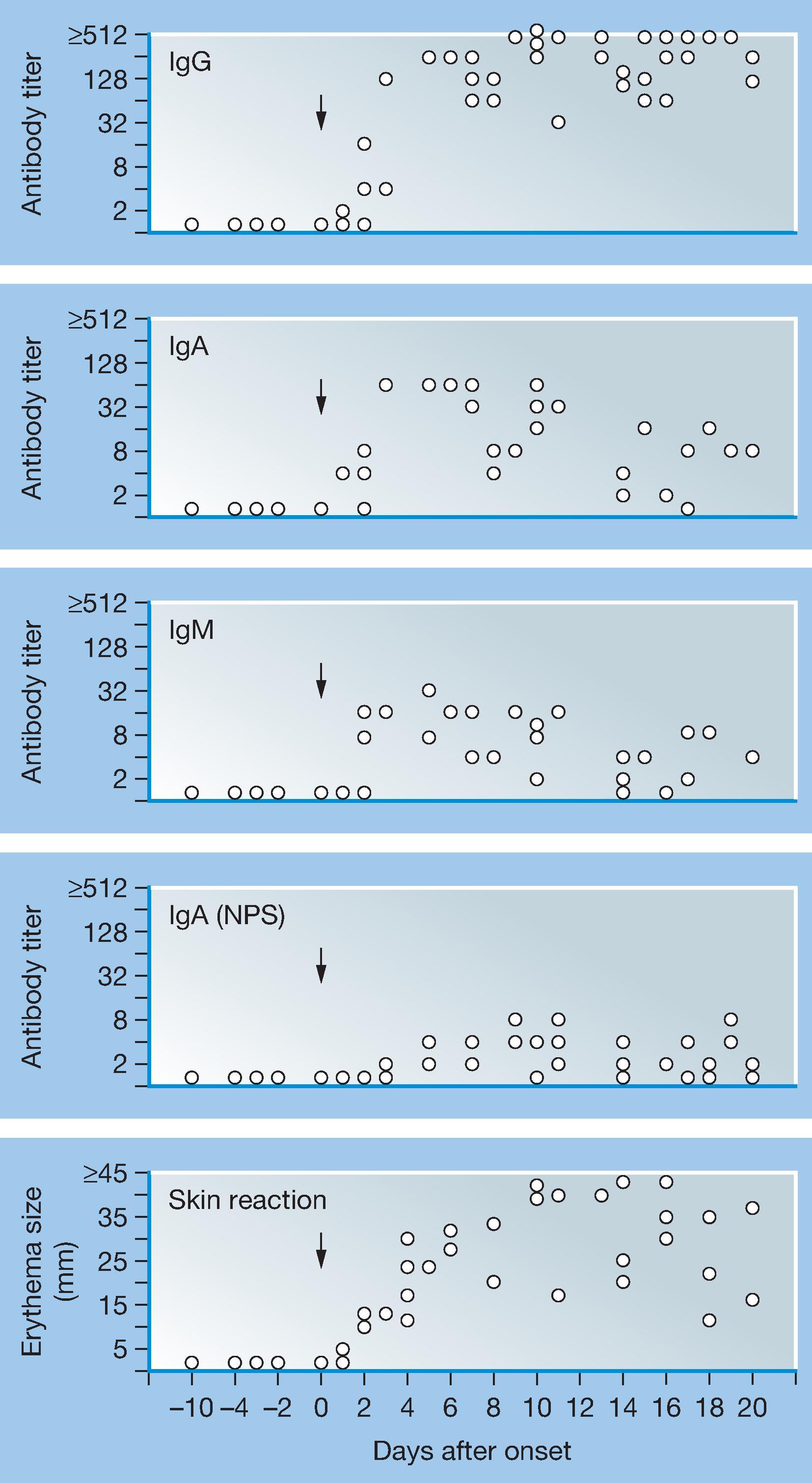
After natural infection, VZV-specific IgG, as measured by the fluorescent antibody to membrane antigen (FAMA) test and which correlates with neutralizing antibodies to VZV, is detected in most patients within the first 4 days after the onset of rash. Peak IgG levels are seen at 4–8 weeks, and levels usually remain high for up to 6–months, after which titers decline twofold to threefold. Positive VZV IgG FAMA titers have been detected decades after clinical varicella. ,
Serum IgA antibody responses to natural infection are detectable early during varicella. Serum IgA antibody was detected in 44% of subjects up to 14 months after varicella.
Nasopharyngeal VZV-specific IgA antibody responses are detectable at the onset of symptoms in patients with natural infections. Maximal titers are attained at the third week of illness.
Cell-mediated immunity appears to be important in control of VZV by the host. Structural and regulatory proteins of VZV are recognized during varicella by T lymphocytes, which induce protection from further VZV infection. Immunity is usually maintained for decades and is mediated by both CD4 + and CD8 + T lymphocytes. Memory T lymphocytes belong to the CD45RO + CCR7 − phenotype. Memory responses may be maintained in part because of periodic exogenous reexposure to others with either varicella or HZ, as well as by possible endogenous reexposure to the virus during subclinical reactivation. T lymphocytes from varicella-immune persons produce cytokines of the T-helper cell type 1, such as interleukin-2 and interferon-γ, result in expansion of virus-specific T cells on exposure to VZV antigens. Natural killer cells, which are part of innate immunity, are another source of interferon-γ and play a role in host defense against VZV. , CD4 + T lymphocytes provide help so that humoral responses to VZV antigens develop and are maintained after varicella. Glycoprotein E-specific CD4 + lymphocytes may play a role in long-term control of multiplication of VZV. ORF4 protein is a target antigen for persistent VZV-specific CD4 + T cells.
Cell-mediated immune responses are promptly detected after natural infection; peak activity occurs within 1–2 weeks which gradually decreases. , CMI to VZV can be detected by stimulation of lymphocytes in vitro with VZV antigens, and by lysis of histocompatible target cells by cytotoxic T cells stimulated with VZV antigens. An enzyme-linked immunospot assay indicates synthesis of interferon-γ by T cells in response to antigenic stimulation by VZV that can be boosted by immunization. Cellular immunity can also be assessed by an intradermal skin test composed of VZV antigens. , , Using tetramer analysis, the importance of the CD4 + T cell response and cellular immunity to gE in recovery from varicella, a role for IE 63 in control of viral latency, and subclinical reactivation of VZV have been demonstrated. , , Declining CMI occurs with advancing age, beginning at age 50. , , The strongest cell-mediated immune responses to VZV appear to be in early adulthood. ,
Clinical observations indicate that patients with isolated agammaglobulinemia have normal courses of varicella and become immune to the disease, whereas those with defects in CMI are at risk to develop disseminated and fatal VZV infections. CMI is probably crucial during VZV disease because spread in the body is by the intracellular route, rather than by release of cell-free virus. That CMI is required to maintain the balance in the interaction between the host and latent VZV is also suggested by the correlation between diminished VZV CMI and an increased risk for HZ. , In contrast, elderly adults usually have high VZV antibody levels and are not particularly subject to second attacks of varicella. Thus, both antibodies and cellular immunity have roles in protection.
Subclinical or clinical reactivation results in both antibody and cellular immune responses. Antibody responses to VZV in healthy subjects with past varicella are characterized by occasional high activity without symptoms, suggesting either exposure to VZV with exogenous boosting of immunity, or subclinical reactivation of VZV. Increases in CMI usually occur after HZ, even in immunocompromised patients. Subclinical reactivation of VZV has been demonstrated by PCR in healthy and immunocompromised persons. ,
As described above, a nonimmune individual acquires varicella predominantly by inhalation of airborne droplets from an individual with varicella. Pregnant women with varicella can transmit virus across the placenta to the fetus, through childbirth, or during the postnatal period. Secondary attack rates in susceptible household contacts of individuals with varicella range from 61% to 100%. , While varicella can develop in susceptible individuals after exposure to HZ, the risk is considerably less than from exposure to varicella. In a household study, 16% of 71 susceptible children younger than 15 years old exposed to HZ developed varicella, a risk approximately five times lower than that from varicella exposure. In a small daycare study, a 3-year-old with HZ transmitted varicella to approximately 30% of susceptible children. Of the 290 HZ cases reported in school and daycare settings in Philadelphia from September 2003-June 2010, 27 (9%) resulted in 84 secondary varicella cases; of 1358 sporadic varicella cases reported, 205 (15%) resulted in 564 secondary varicella cases. VZV transmission was highest from unvaccinated individuals with sporadic varicella.
The diagnosis of VZV infection can usually be made clinically by the characteristic skin rash. In individuals who received the varicella vaccine then developed breakthrough varicella, both clinical and laboratory diagnosis may be difficult because rash may be mild and not as widespread, indicative of immunity conferred by the vaccine. A clinical history that includes age of the patient, the absence of prior varicella, and exposure to persons with varicella or HZ in the preceding 2–3 weeks is essential for diagnosis. Unusual skin manifestations of varicella have been described in areas of chronic dermatitis or after sunburn. Unlike varicella, HZ is characterized by vesicular lesions in a unilateral, dermatomal distribution that are painful and sometimes pruritic. Interestingly, HZ may be confused with recurrent HSV infection. HZ can also occur in the absence of a skin rash and merely present as dermatomal distribution pain (zoster sine herpete), which can make diagnosis extremely difficult.
When it is deemed necessary to confirm varicella or HZ infection, the best test is PCR-detection of VZV DNA in vesicular fluid, swabs from maculopapular lesions, respiratory secretions, saliva or buccal secretions, cerebrospinal fluid (CSF), urine, and tissue specimens. , Because varicella and HZ complications can occur without rash, PCR of saliva is also useful. Detection of VZV DNA by PCR does not necessarily indicate infectivity of the virus. Rapid diagnosis can also be accomplished by direct immunofluorescence. The direct immunofluorescence test is done by swabbing a suspicious skin lesion, making a smear on a glass slide, and staining with a fluorescein-tagged monoclonal antibody to VZV gE. However, PCR is more sensitive than direct immunofluorescence for diagnosis. , Virus isolation of VZV is expensive, insensitive, and time-consuming, so it is not used for diagnostic purposes. For patients with neurological disease due to VZV (with or without associated rash), the best test for diagnosis is detection of anti-VZV IgG antibody in CSF and calculation of intrathecal synthesis; PCR detection of VZV DNA is also useful but much less sensitive than detection of anti-VZV antibodies (30% compared to 93%, respectively). ,
Serologic methods are less useful for the laboratory diagnosis of varicella and have limited value for the diagnosis of HZ. Varicella can be diagnosed by demonstration of IgM antibodies to VZV in serum, but a negative IgM test does not rule out the disease. Further studies of the kinetics of the IgM response are needed for accurate confirmation of varicella infections. Serologic tests are of limited value for the diagnosis of HZ, because heterologous rises in VZV antibody titer may occur when HSV reactivates in a person immune to varicella. A transient IgM response may also follow HZ, as well as varicella.
Serologic tests to detect VZV IgG are used to assess immunity to varicella, in testing of healthcare workers, ideally before employment. Unfortunately, these tests often lack sensitivity and may yield false negative results, especially in vaccinated individuals. Alternatively, a history of at least two doses of live-attenuated varicella vaccine or a history of varicella is indicative of immunity to varicella. The FAMA assay is considered the gold standard for determining VZV antibodies; this test, however, is rarely available. Serologic assay in commercial use is the enzyme-linked immunosorbent assay (ELISA) for measurement of IgG antibodies to VZV. ELISA assays are specific, but as many as 10% false-positive reactions in truly susceptible persons have been recorded. Commercially available ELISA tests are also less sensitive than the FAMA assay, , especially for assessing immunity after immunization. An ELISA that uses VZV gp as the antigen has been reported to be sensitive, but it is not commercially available. Positive antibody titers detected by this method in young unvaccinated children, moreover, indicate that the assay may be overly sensitive. This assay has shown reproducibility when different batches of vaccine were used for immunization, and there was a linear concordance when neutralizing antibody levels and gp-ELISA titers were compared. Specific gp-ELISA units, however, used as end points were not always consistent in published studies.
A latex agglutination assay based on the clumping of latex particles coated with VZV gps, in the presence of VZV antibody, has sensitivity similar to that of FAMA but is no longer commercially available. , , A time-resolved fluorescence immunoassay for measuring antibodies to VZV is being evaluated in the United Kingdom for sensitivity and specificity. While the VZV skin test appears to be sensitive and specific for demonstrating immunity to varicella, , , it is cumbersome to perform on children and is not currently available for clinical use. A serologic assay (termed “line assay”) using five recombinant VZV antigens was described in which vaccinated children had weaker IgG responses to certain antigens (proteins 4, 14, and 49) than children who had experienced natural varicella. If confirmed, this technique might provide a practical means to distinguish between varicella immunes and susceptibles as well as to identify individuals who had been vaccinated. In summary, the most reliable indication of immunity to varicella today is medically diagnosed varicella or a written documentation of two doses of live-attenuated varicella vaccine.
Antiviral therapy for varicella and HZ first became available in the early 1970s. Antiviral therapy is helpful to speed recovery, but it does not terminate virus shedding or prevent latent infection. Vidarabine, the first antiviral drug, was supplanted by acyclovir, a drug that was less toxic. Intravenous acyclovir speeds healing of varicella and HZ in immunocompromised patients, and orally administered acyclovir, at high dosages, may be used to treat patients with HZ. , Orally administered acyclovir may be used to treat otherwise healthy children with varicella, but the antiviral effect on varicella is minimal, in part because of the poor absorption of the drug when given orally and the lower sensitivity of VZV to acyclovir, compared to HSV.
A double-blind placebo-controlled study in which 102 healthy children were given acyclovir (40–80 mg/kg) or placebo daily for 5 days, beginning within 24 hours of rash onset, revealed that the mean number of skin lesions was significantly reduced from over 500–336. On average, there was 1 day less of fever. A multicenter study involving 815 similarly treated children given 80 mg/kg of acyclovir daily yielded similar results. The benefit to secondary household cases (which are typically more severe than the first case in the household) was not different from that of primary cases. The modest benefit conferred by acyclovir therapy is not surprising in view of the typical self-limited nature of chickenpox. Studies in adolescents and adults have not indicated more striking antiviral effects, although acyclovir is recommended for these groups because they are at higher risk of developing severe varicella than healthy children. A study in otherwise healthy children with varicella indicated that 5 days of oral acyclovir is as effective as 7 days.
The oral antiviral prodrug valacyclovir, which is well absorbed from the gastrointestinal tract and is rapidly converted to acyclovir in the body, results in blood levels of acyclovir that are significantly higher than those from oral acyclovir. Although valacyclovir has efficacy for treatment of HZ, because a suspension formulation is not available, clinical experience with this drug in children with varicella is limited. One study, however, addresses dosage of valacyclovir for children. Currently, it is acceptable to use valacyclovir to treat children older than age 2 years who have VZV.
Oral Famciclovir is rapidly converted to penciclovir in the body which has an action like that of acyclovir. At present, either valacyclovir or famciclovir is preferred treatment for HZ in nonimmunocompromised patients. Famciclovir has not been studied for treatment of varicella. , The antiviral drug soruvidine was not approved by the U.S. Food and Drug Administration (FDA) because of the lack of significant benefit over acyclovir and the potential for toxicity in patients being treated for cancer. This drug is available in countries other than the United States.
Postexposure prophylaxis with antiviral therapy for prevention of varicella has been tried and has met with some success in small studies. Acyclovir administered to 25 children after household exposure mostly prevented disease. Immunity appears to persist for several years even with no evidence of clinical disease. Unfortunately, however, less than 85% of patients may develop antibodies to VZV after this prophylaxis, and adequate future protection cannot be ensured. Therefore, two doses of varicella vaccine should be administered subsequently to children who did not develop varicella. Although dosages and timing have varied, administration of acyclovir at a dosage of 10–20 mg/kg four times a day between days 7 and 14 after exposure was most often used. Prophylaxis administered earlier than 1 week after exposure does not seem to provide as good protection. There are no published studies in which immunization and antiviral therapy have been combined. Postexposure antiviral prophylaxis has not been studied in immunocompromised persons, for whom passive immunization with immunoglobulin is preferable. If passive immunization is not available, an alternative would be to treat immunocompromised patients who develop varicella with acyclovir at the very first sign of illness, but this approach may not be successful in all patients. For healthy nonpregnant persons, postexposure vaccination is preferred to preventive antiviral therapy (see “Passive Immunization” Section).
Varicella is a highly contagious disease that occurs worldwide and, in the absence of a vaccination program, affects nearly every person by mid-adulthood in countries with temperate climates. HZ cases represent a method for regular exposure and introduction of VZV into communities that otherwise may not be large enough to sustain VZV endemic transmission. The epidemiology of varicella in temperate climates is different from that in tropical climates; the differences may relate to properties of VZV, climate, population density, and the risk of exposure. In temperate climates, most persons are infected by young adulthood, with the highest incidence of disease occurring among preschool-age children or children in early elementary school. ,
Varicella shows a strong seasonal pattern in temperate and some tropical climates, with a peak incidence during winter and spring. Periodic large outbreaks may occur, with an interepidemic cycle of 2–5 years. , Outbreaks occur in settings where children congregate, such as childcare centers and schools, but have also been described in hospitals, facilities for institutionalized children and adults, refugee camps, and adult settings, including among healthcare workers, military personnel, and correctional facilities.
Prior to the varicella vaccination program in the United States, the annual average varicella incidence measured from national household survey data was 15–16 cases per 1000 population. , Reported incidence from other countries has generally been lower than U.S. survey rates, most likely reflecting less-complete case ascertainment as a result of differences in study methods. , Apart from age, factors associated with differences in varicella incidence or seroprevalence include urban (as opposed to rural) residence, attendance in childcare facilities, attendance at and size of school, and presence of older siblings in the household, all of which are likely to increase the risk of exposure. , In countries where universal varicella vaccination programs are in place, not being vaccinated is a risk factor for varicella. In the United States, where high vaccine coverage has been achieved children of parents who refused varicella immunizations have nine times the risk of requiring a medical visit for varicella than did children whose parents accepted varicella vaccine for their children.
VZV seroprevalence data reflect age-specific disease incidence. National data from the United States before use of varicella vaccine (1988–1994) demonstrated high population VZV seroprevalence: 86% for 6–11-year-olds, 93% for 12–19-year-olds, 95.5% for 20–29-year-olds, and 99% or greater for adults 30 years of age and older. Similarly, more than 90% of adolescents (10–15 years of age) or young adults are VZV seropositive in many countries around the world where seroprevalence has been measured. , A comparative study of prevaccine VZV seroprevalence in 11 European countries using sera from 1995 to 2003 demonstrated that although the vast majority of children acquire VZV during childhood, the rate of transmission of VZV varied considerably between countries; VZV seroprevalence by 5 years of age was 97% in the Netherlands, approximately 70% in Spain and Germany, and 38% in Italy. This study also highlighted that in some European countries, 10% or more of adolescents 10–14 years of age are susceptible to varicella (e.g., 18% in Italy); findings in Australia were similar. Another survey of varicella occurrence among 70,226 school children in Greece showed that 21.4% of sixth-grade children lacked a history of varicella and were probably susceptible as they entered adolescence.
Differences in seroprevalence have been described by race, sex, country of origin, and number of siblings in the household. , , Measuring VZV seroprevalence in countries that have implemented varicella vaccine programs presents new challenges because of the low sensitivity of commercially available VZV IgG tests to measure vaccine-induced immunity. Using the gp-ELISA assay that has good sensitivity for detection of vaccine-induced immunity, the most recent serosurvey in the United States demonstrated that among children 6–11 years of age seroprevalence increased from 86% in the prevaccine era to 98% in 2009–2010, attributed to the childhood vaccination program, while other age-group-specific estimates were similar to prevaccine estimates.
Population-based epidemiology data are less complete for countries in tropical climates but VZV seroprevalence reflects a higher mean age of infection than in temperate climates, , with higher susceptibility among adults. In such countries, seroprevalence among adolescents or young adults has ranged from 10–20% in St. Lucia (an island community) to 80–100% in Taiwan and urban Calcutta, India, with rural-versus-urban and other differences described. , , , , Similarly low VZV seroprevalence has been reported in isolated tribal adults in Eritrea (44%) and in rural Bengali adults 17–25 years of age (42%), compared with much higher VZV seroprevalences among similar-age nonisolated adults in Eritrea (91–96%) and among Bengali adults 17–25 years of age in Calcutta (96%). , Studies in adult subgroups who could be considered for screening and vaccination programs have demonstrated levels of seronegativity among healthcare workers ranging from 11% in Egypt, to 14–19% in Malaysia and Saudi Arabia, to 22% or higher in Thailand, to as high as 51% among first-year medical and engineering students in Sri Lanka. , Studies among women of childbearing age demonstrated that 27% lacked VZV antibodies in Iran and 56% in Sri Lanka, whereas 24% of Singapore military recruits were VZV seronegative.
In island populations that may not have sufficient size to support continuous endemic transmission, seroprevalence can also vary with the timing of the last epidemic year in relation to the serologic study. For a number of countries, the highest incidence was described in the driest, coolest months. , A study examining risk factors for susceptibility among newly arrived migrants in Canada found the highest varicella susceptibility among those originating from climates with the highest temperature and the most months of humidity per year (tropical rainforest). Crowding in densely populated cities or in households may overcome VZV’s diminished ability to spread in tropical climates. In several South American countries (Brazil, Argentina, Mexico, or Bolivia) higher seropositivity rates in children and lower adult susceptibility were reported.
Most nonimmune individuals will develop varicella upon exposure to VZV. Individuals that are at risk for more severe, disseminated disease with organ involvement are detailed in Section IIB above.
More than 90% of the world population harbors latent VZV in neurons of cranial nerve, dorsal root, autonomic, and enteric ganglia with the potential to reactivate from any of these sites (reviewed in Nagel and Bubak ). Kennedy et al. determined that approximately 2–5% of neurons within ganglia could harbor latent virus. Latent VZV has also been detected in 6% of human adrenal glands, most likely within the neural crest-derived chromaffin cells. In an in vitro study of primary human corneal epithelial cells, VZV did not kill the cells but rather induced proliferation, suggesting that corneal epithelial cells may potentially be a reservoir for replicating virus.
Some countries have achieved control or elimination of other vaccine-preventable diseases using vaccines recommended for routine use in childhood vaccine programs. Understanding the varicella and HZ health burden and costs as well as the costs and benefits of vaccination strategies is important when countries consider a varicella vaccination program. In the immediate prevaccine era in the United States, an average of 4 million varicella cases occurred each year, which resulted in an average of 11,000–13,500 hospitalizations (4.1–5.0 hospitalizations per 100,000 population) and 100–150 deaths annually (0.4–0.6 per million population). , , , , , Varicella also contributes significantly to the disease burden from invasive group A streptococcal infections in children. ,
Risk factors for severe varicella include older and younger ages, cellular immune dysfunction caused by illness (e.g., acute leukemia) or medications, and intensity of exposure. , , , In the United States, Australia, and the United Kingdom, overall varicella mortality has ranged from 0.20 to 0.65 per million population. , Population-based case-fatality data have been described only from developed countries with overall case-fatality rates of approximately 2.6 per 100,000 cases as described in the United States in the prevaccine era. The highest case-fatality rate (20 per 100,000 cases; 20–25 times higher than for preschool-age children) occurs among adults. , , Second to mortality in developed countries, hospitalizations caused by varicella provide the next best estimate of severe disease burden. Various studies have described annual rates of varicella hospitalizations in the range of 4.0–4.5 per 100,000 population, , with some studies reporting lower or higher rates (2.6–9.9 per 100,000 population). , Reflecting the high incidence among children, the majority of varicella hospitalizations also occur among children; however, infants and adults are at significantly increased risk of severe disease and hospitalization compared with children 1–9 years of age. , , , , , Varicella also leads to a considerable number of school days missed for children as well as workdays missed for adult patients and for parents looking after sick children. These data are important for describing morbidity and for calculating the economic and societal costs of varicella, which is now vaccine preventable.
There are few data on the health burden of varicella in developing countries, in countries with high HIV seroprevalence, and in countries with tropical climates where a higher proportion of varicella cases may occur among adults. In these situations, varicella morbidity and mortality may be higher than described in developed countries. When comparing results across countries, however, differences in methods that may affect ascertainment of hospitalization, number of patients, and access to care must be considered. A large prospective household study in Guinea-Bissau in 2000–2001 identified 1539 cases of varicella and found almost 80% of cases occurred among children 1–9 years of age and only 4% of cases occurred among those older than 15 years of age. However, 12% of cases occurred in infants, and two patients died, so the case-fatality rate in this small series was 130 in 100,000 cases, approximately 50 times higher than in the United States. Several studies in Sri Lanka have highlighted the high median age of varicella infection—14.5 years—and the associated morbidity. , One study of all patients admitted to the main infectious disease hospital in Sri Lanka from 2000 to 2001 demonstrated that 58% of the total 1690 hospitalizations resulted from varicella. Varicella was the most common disease treated at the infectious disease hospital and a significant cause of morbidity and mortality among adults. The estimated global annual varicella disease burden would include 140 million cases with approximately 6200 deaths.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here