Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients may present with symptoms clearly related to the urinary tract, but seemingly unrelated symptoms may also be due to a urologic cause, such as backache from metastatic prostatic carcinoma, fever of unknown origin from renal carcinoma, or lethargy and anaemia from obstructive renal failure.
Afferent innervation of the urinary tract is rudimentary, and pain originating from these organs, though characteristic, may not easily be localised. Renal pain occurs in the angle between the 12th rib and the sacrospinalis muscles. Ureteric pain (or colic) typically radiates forwards and downwards towards the groin, testes or labia, following the dermatomes relating to the nerve roots from which the sympathetic innervation of the ureter originates (i.e., T10–L2). Acute bladder obstruction usually causes central lower abdominal pain. By contrast, chronic bladder obstruction may be virtually asymptomatic. Disease of the bladder and prostate causes ill-defined perineal or penile pains. A prostate that is grossly enlarged can cause rectal symptoms, including tenesmus.
The history aims to distinguish between urinary obstruction (e.g., poor stream), urinary storage issues (e.g., urgency), infection (e.g., frequency, dysuria) and malignancy (e.g., dark, discoloured, brown, pink or red urine). Frequency is recorded numerically: for example, D/N 6/3 (by day, six times; by night, three times). Hesitancy, poor stream and dribbling are characteristic of bladder outflow obstruction (BOO).
Haematuria, or the presence of blood in the urine, is a very specific symptom (visible haematuria) or sign (nonvisible haematuria) of urinary tract disease. Nonvisible haematuria is where the urine appears clear during naked-eye examination but contains red blood cells on microscopic examination or urine dipstick analysis. Visible haematuria is the condition in which the urine is pink, red or brown in colour and occurs when there is substantial bleeding in the urinary tract.
The haematuria may be intermittent in frequency, with periods of clear urine between, or it may be persistent. It may be present either throughout the act of micturition or only during a particular phase of micturition, providing a clue as to the aetiology ( Table 24.1 ).
| Type of haematuria | Site of origin |
|---|---|
| Total/complete | At or above the level of bladder |
| Initial | Prostate, anterior urethra |
| Midstream | At or above the level of bladder |
| Terminal | Posterior urethra, bladder neck, trigone |
Haematuria may be associated with pain, or it may be painless. Conditions that may mimic haematuria are discoloration of urine due to certain drugs like phenazopyridine and rifampicin or certain food items like beetroot. They are of no clinical significance other than that they may cause undue anxiety to the patient.
Dysuria or painful micturition is often described by the patient as a sensation of burning during micturition. It is usually localised to the urethra and is associated with acute inflammatory conditions of the lower urinary tract, such as a urinary tract infection (UTI). The symptom may be associated with urinary frequency and urgency.
These symptoms are interrelated and the result of an inability of the bladder to hold urine. Frequency may be caused by a decrease in the capacity of the bladder (due to diseases causing fibrosis) or by a decrease in the functional capacity of the bladder (due to a large residual volume of urine). Acute inflammatory conditions also decrease the capacity of the bladder, leading to frequency and inability to postpone micturition (urgency).
Nocturia is defined as waking at night to pass urine. Nocturia may have mixed and multiple causes. These include prostate, bladder, cardiac and renal disorders, or excessive intake of fluids, caffeine or alcohol before bedtime.
True incontinence is when the passage of urine occurs without warning and any precipitating factors (exstrophy of bladder, vesicovaginal fistula, ectopic ureteric orifices). Stress incontinence occurs with any increase in the intravesical pressure (straining, coughing, laughing), leading to a loss of urine due to weakness of the sphincter mechanism. Urge incontinence is associated with urgency and is seen in acute inflammatory conditions, patients with upper motor neuron injuries and those with an overactive bladder. Overflow incontinence is seen in patients with chronic urinary retention, when urine dribbles out of the bladder due to an excessive rise in intravesical pressure.
Oliguria denotes decreased urinary output (< 400 mL/day in adults, or < 0.5 mL/kg/h lasting for at least 6 hours), while anuria is a complete absence of urine output. The causes are discussed in the section on acute renal failure.
Rare presenting complaints include pneumaturia (air in urine), faecuria (faecal material in urine), cloudy urine, chyluria (milky urine) and urethral discharge.
Examination should not be confined to the urinary system, as cardiological, neurologic and gynaecologic problems may be associated with urologic symptoms and signs. Many urologic patients are elderly and require an assessment of their fitness for further investigations and operative treatment. Furthermore, the patient’s cardiovascular status may be relevant to subsequent treatment; for example, administration of androgen-deprivation therapy for carcinoma of the prostate.
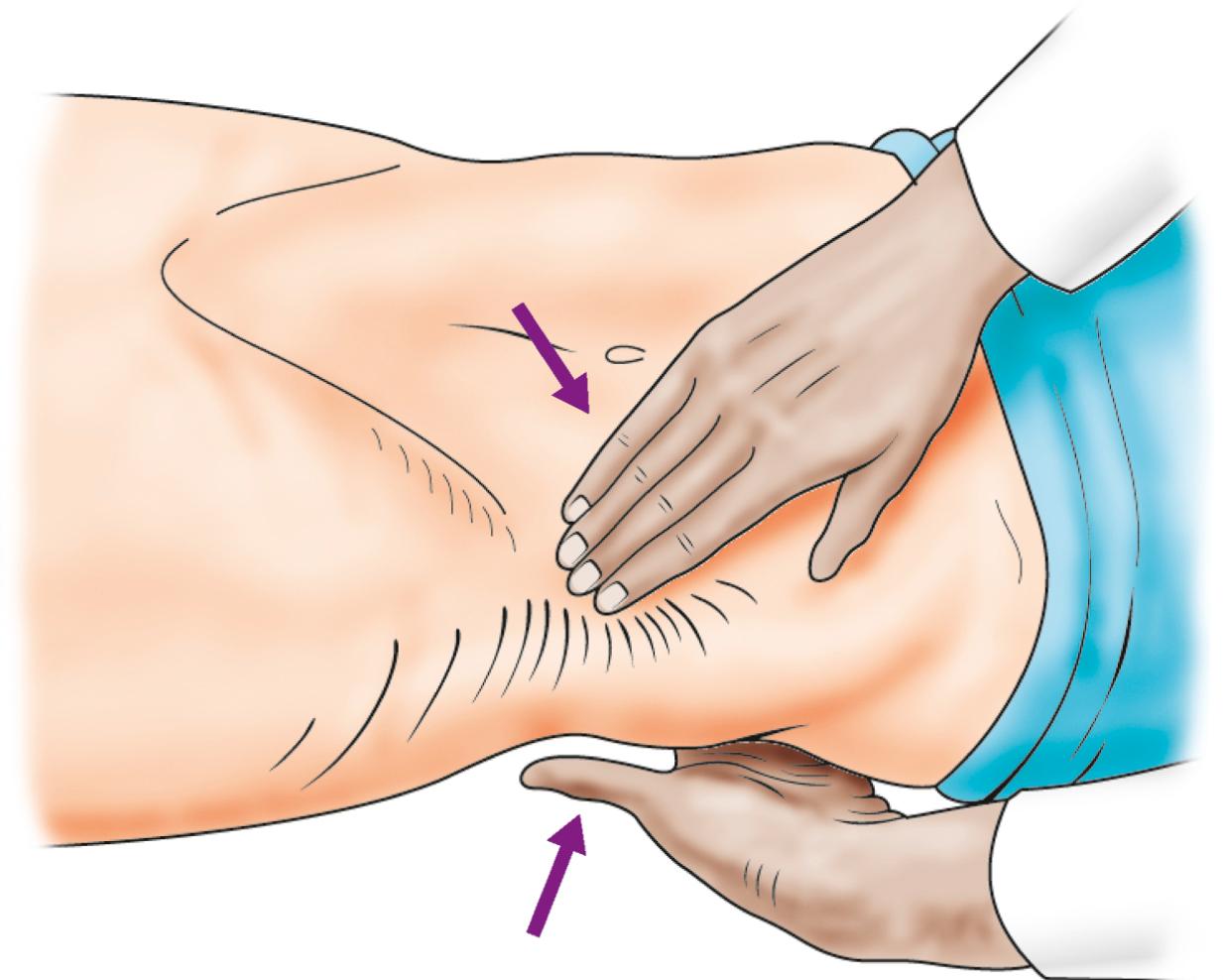
With the patient relaxed, the kidney can be balloted between two hands, one placed posteriorly in the loin and the other one anteriorly; a sufficiently enlarged organ may be bimanually palpable when lifted with one hand placed behind the loin and compressed by the other pressing downwards ( Fig. 24.1 ). The ureter, however, cannot be palpated. An enlarged bladder rises centrally out of the pelvis, is dull to percussion and may be visible in thin patients. In men, the hernial orifices, cords, testes and epididymes are examined with the patient both standing and lying. If the foreskin is uncircumcised, it must be confirmed that it retracts and that the glans and meatus are normal. In women, the vulva, urethra and vagina must also be examined. A speculum examination should be carried out if there is any suspicion of vaginal or cervical abnormality. A full pelvic bimanual examination, whether in males or females, is best carried out under general anaesthesia with a muscle relaxant. A rectal examination is mandatory when evaluating symptoms related to the prostate.
Dipstick testing:
Haematuria. When persistent, nonvisible haematuria indicates a range of urologic or nephrologic conditions.
Proteinuria. In the absence of infection, urine is normally almost protein-free. Proteinuria of more than 150 mg/24 hours mandates further nephrologic investigation.
Glycosuria suggests the presence of diabetes.
Leucocytes and nitrites suggest UTI.
Specific gravity. A loss of concentrating ability of the kidney is seen in conditions affecting the renal medulla, such as chronic renal failure, and presents as urine with a fixed low specific gravity (isosthenuria).
pH. Low urinary pH values predispose to uric acid calculi, while high pH values are often seen in patients with recurrent UTIs.
Microscopy. May detect casts or tubular epithelial cells associated with renal parenchymal disease, crystals in patients with renal calculi, leucocytes in UTIs and inflammatory disease, presence of dysmorphic erythrocytes in glomerulonephritis, or ova in schistosomiasis.
Cytology. This is useful in the diagnosis and follow-up of high-grade bladder cancer and carcinoma in situ (CIS).
Midstream specimen of urine (MSSU). The patient is asked to pass some urine into the toilet. Without interrupting the flow, the next part is directed into a special container and the remainder into the toilet; collected samples are then sent for microbiological assessment.
Creatinine is a breakdown product of skeletal muscle, and serum levels do not begin to rise until the glomerular filtration rate (GFR) is halved. Formulas such as Modification of Diet in Renal Disease (MDRD) or Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) can be used to estimate GFR based on age, gender, race and serum creatinine. Patients with chronic renal disease often have disordered erythropoiesis, leading to normocytic normochromic anaemia in addition to disordered calcium metabolism. The erythrocyte sedimentation rate (ESR) can be markedly raised in idiopathic retroperitoneal fibrosis, a cause of ureteric obstruction. Beta human chorionic gonadotrophin (β-HCG), α-fetoprotein (AFP) and prostate-specific antigen (PSA) are useful tumour markers for testicular cancer and prostate cancer, respectively.
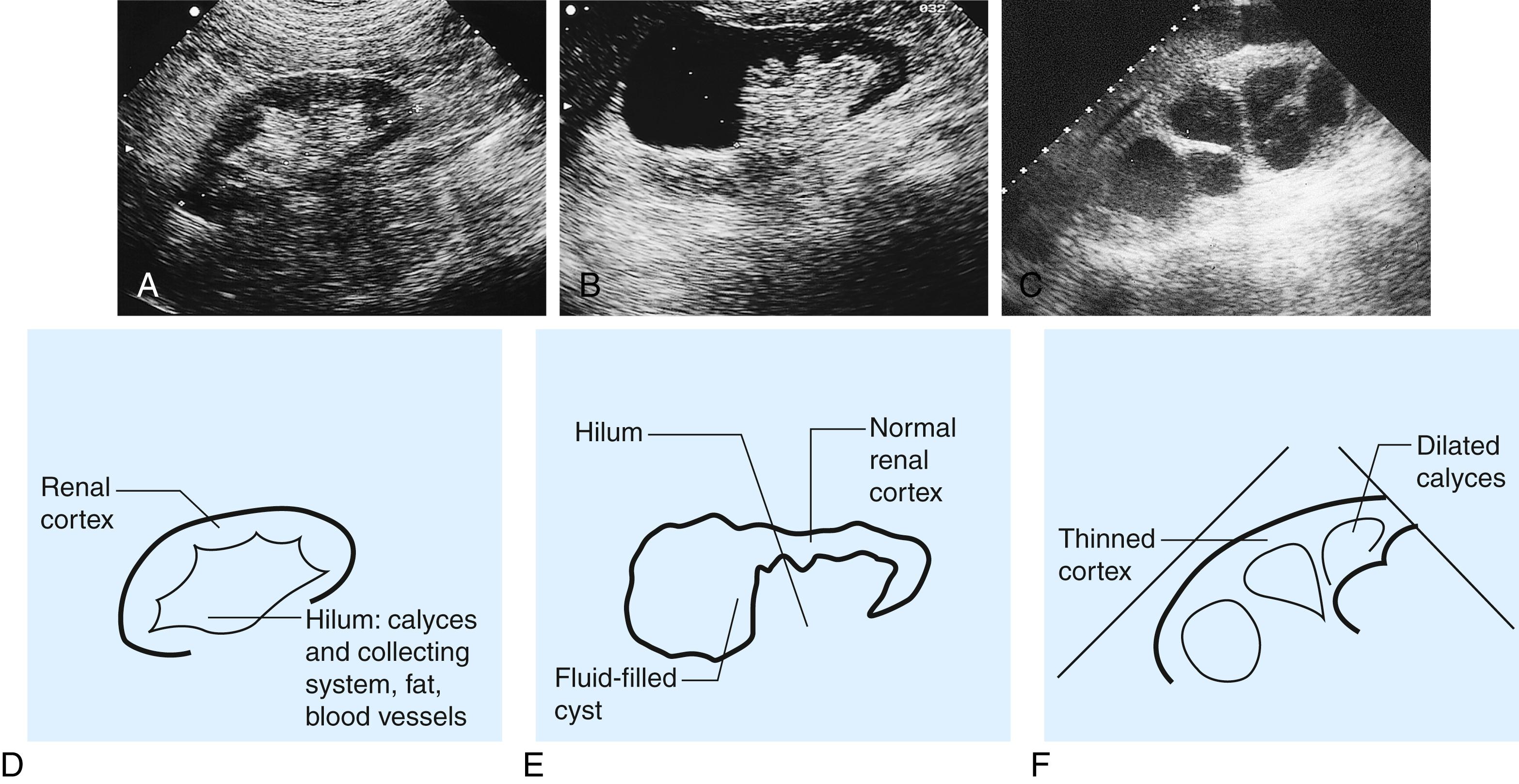
Ultrasound (US) is a common first-line imaging approach ( Fig. 24.2 ), providing information about the renal parenchyma and dilatation of collecting system but less detail about the ureters. It allows visualisation of other related organs, such as the liver, spleen and gynaecologic organs, and is also helpful in the evaluation of bladder, prostate, testis and epididymis.
Transrectal ultrasonography (TRUS) employs a high-frequency transducer via the rectum for evaluation of prostatic disease. The anatomic delineation is much better than with transabdominal US, as the prostate is in direct contact with the anterior wall of the rectum.
A plain film, commonly called a KUB (kidney-ureter-bladder), is a simple imaging investigation for patients with urinary tract symptoms. It provides information about any calcification in the course of the urinary tract.
An intravenous urogram (IVU) involves injecting iodine-containing contrast material intravenously and taking serial x-rays to demonstrate the renal pelvis and calyces, the rate of kidney emptying, the calibre of the ureters and the bladder outline. Once the bladder has filled, a ‘postmicturition’ film will demonstrate bladder emptying and the amount of residual urine. The IVU has largely been replaced by computed tomography (CT) but is still used in some parts of the world, either due to nonavailability of CT or to better appreciate the anatomy if reconstruction is not provided by CT.
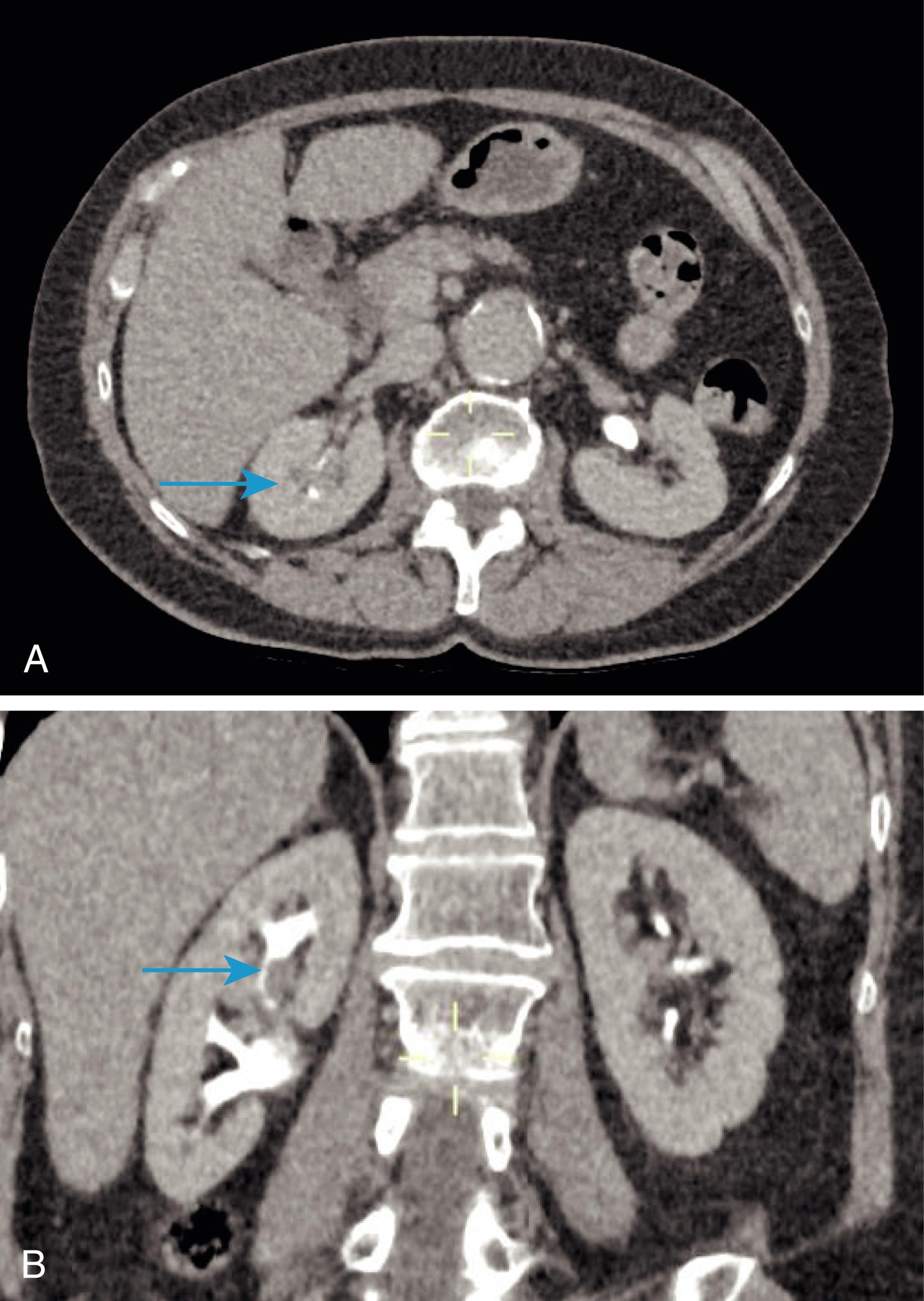
For investigation of suspected urinary tract stone, ‘plain’ (noncontrast) CT KUB is the investigation of choice. CT urography (CTU) allows the ureters to be delineated by contrast ( Fig. 24.3 ) and also allows other structures within the abdomen to be assessed. A CTU may be useful to detect other synchronous pathologies in the abdomen and to stage the extent of local and metastatic spread of any neoplastic lesion. CT has a distinct advantage in evaluation of the retroperitoneum.
Magnetic resonance imaging (MRI) is widely used to stage bladder tumours. It is also increasingly used to evaluate the prostate in patients with raised PSA levels and assess pelvic extent of locally advanced prostate cancer and pelvic lymphadenopathy.
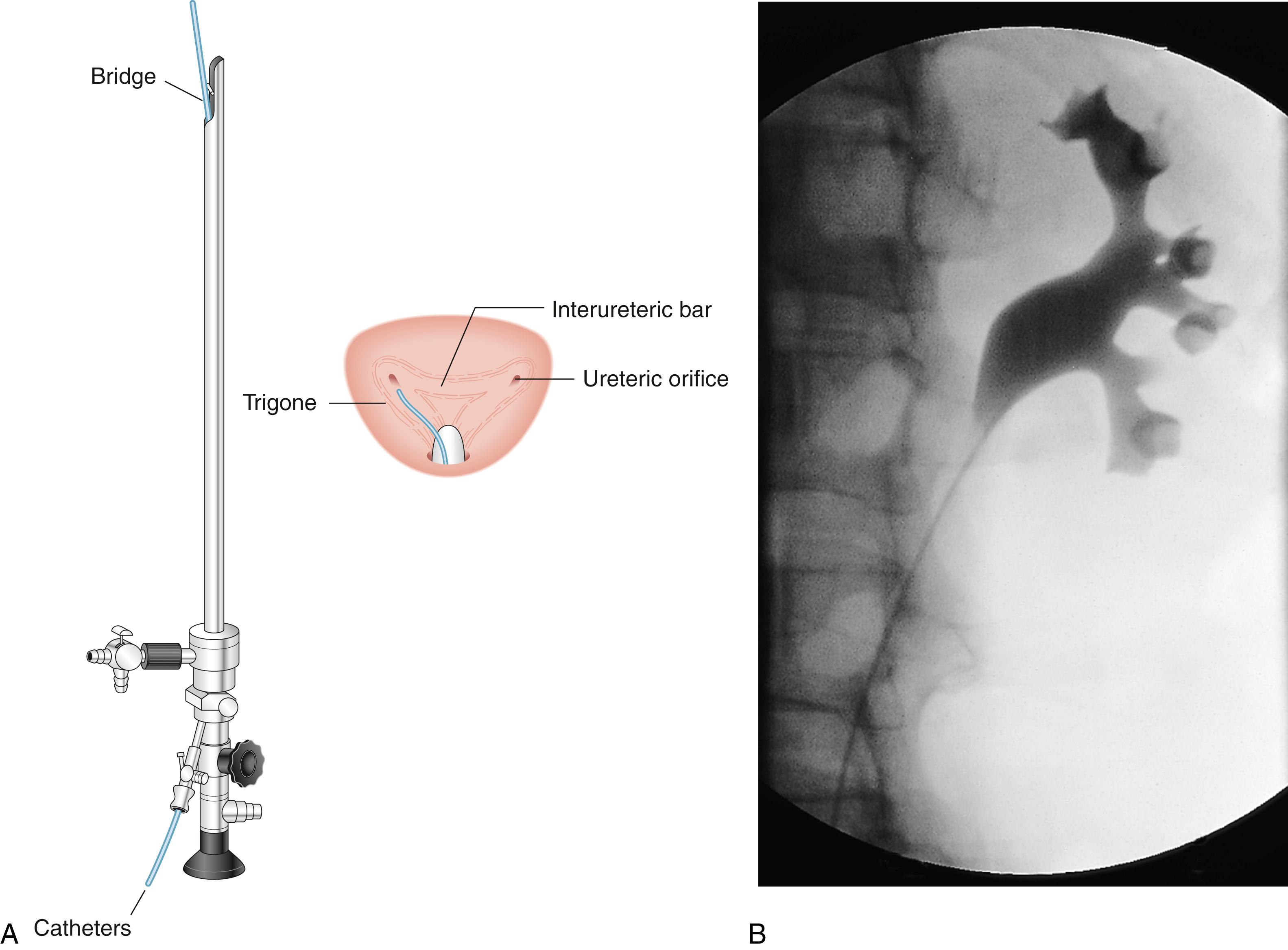
This is an investigation used to outline the collecting system and the ureters when evaluating the upper urinary tract in theatre. Retrograde pyelography may be necessary when ureteric stents are placed or if CTU is inconclusive or contraindicated, for example, in patients with advanced CKD or severe allergy to contrast medium. It is an invasive investigation and carries the risk of introducing infection. The ureter on the side of interest is cannulated under cystoscopic vision in a retrograde fashion using a fine-calibre ureteric access catheter. Radiopaque dye is then injected through the catheter under fluoroscopic screening to outline the collecting system ( Fig. 24.4 ).
Abnormalities of the renal vessels including assessment of active bleeding can be demonstrated by renal angiography or a CT angiogram. A micturating cystourethrogram (MCU) will outline the bladder, detect ureterovesical reflux and examine the bladder neck and urethra ( Fig. 24.5 ). The bladder is filled with contrast material (via a catheter), and emptying is then studied by x-ray screening ( Fig. 24.5 ). An ascending or retrograde urethrogram, in which contrast medium is injected into the urethra, can be used to define strictures. When used in conjunction with an MCU, a descending urethrogram can also be obtained.
Radiolabelled substances are used for two main purposes:
Detecting bony metastases from carcinoma of the prostate (bone scan). 99m Tc-labelled methylene diphosphonate (MDP) is the most reliable method.
Measurement of renal function (scintigraphic renography).
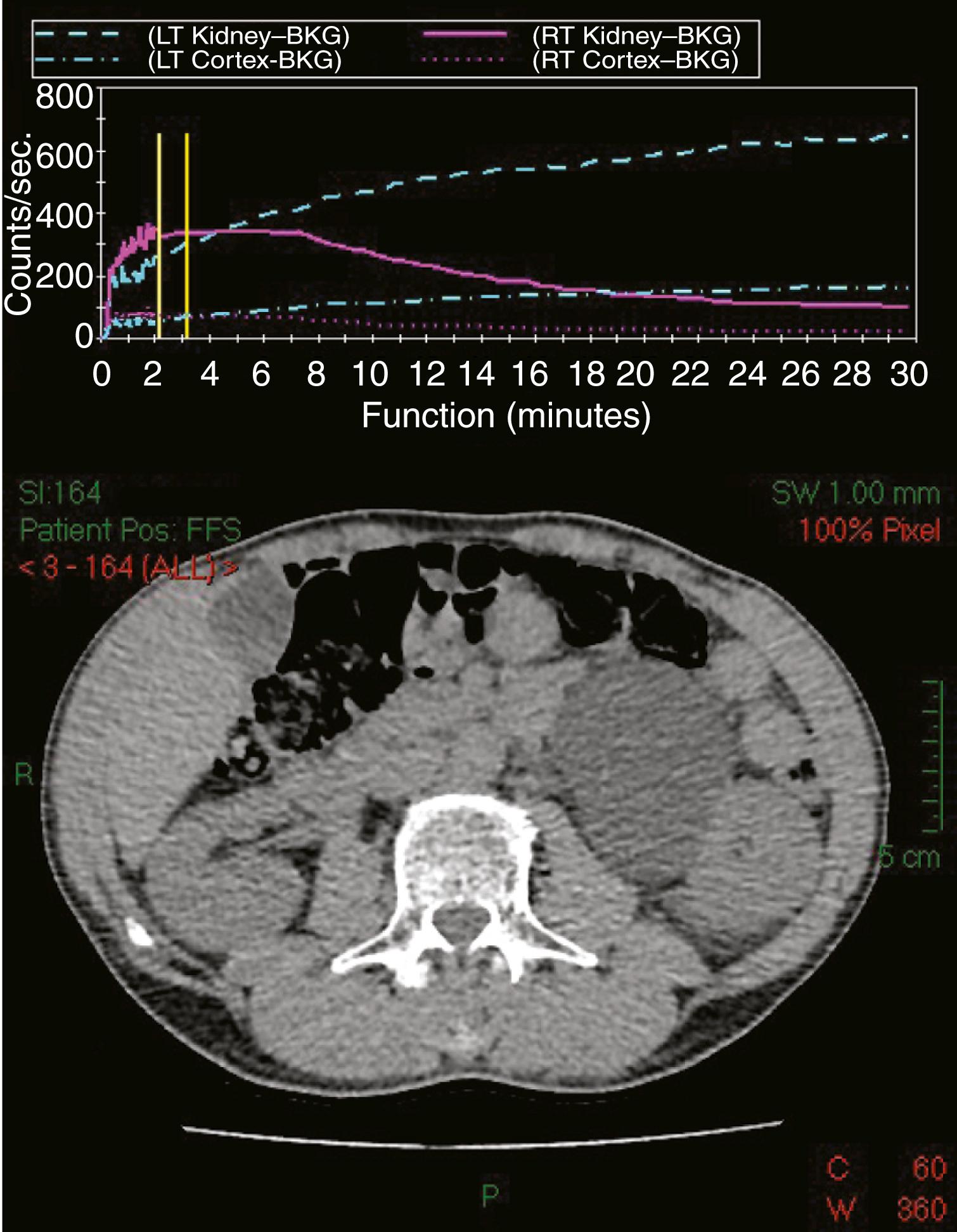
Occasionally, ‘how a kidney looks’ does not correlate with ‘how it behaves’, e.g., hydronephrosis does not always mean the presence of obstruction. Nuclear scintigraphy allows assessment of obstruction of a kidney (e.g., from pelviureteric obstruction), differential kidney function (i.e., how much each kidney is contributing to overall function), assessment for nonfunctioning areas of renal parenchyma (e.g., scarring) and accurate assessment of GFR. Radiolabelled mercaptoacetyltriglycine (MAG-3) has largely superseded technetium-labelled diethylenetriamine pentaacetic acid (Tc-DTPA) for dynamic scanning ( Fig. 24.6 ). MAG-3 is a protein secreted from the renal tubules used in the identification of obstructed kidneys and to assess differential function. During a MAG-3 renogram, furosemide is often given after 20 minutes to differentiate obstruction from dilatation. A MAG-3 scan may also be used to assess vesicoureteral reflux (‘indirect cystogram’). Dimercaptosuccinic acid (DMSA) is concentrated in the renal tubules, and static imaging can be carried out some 2 to 3 hours after injection. Parenchymal defects such as scars, haematomas, lacerations or ischaemia may be demonstrated. Differential renal function can be quantified by measuring the DMSA concentration/density in each kidney. DMSA is generally regarded superior to MAG-3 and DTPA for measuring split renal function of the obstructed kidney and predicting the outcome of surgical intervention, such as nephrectomy.
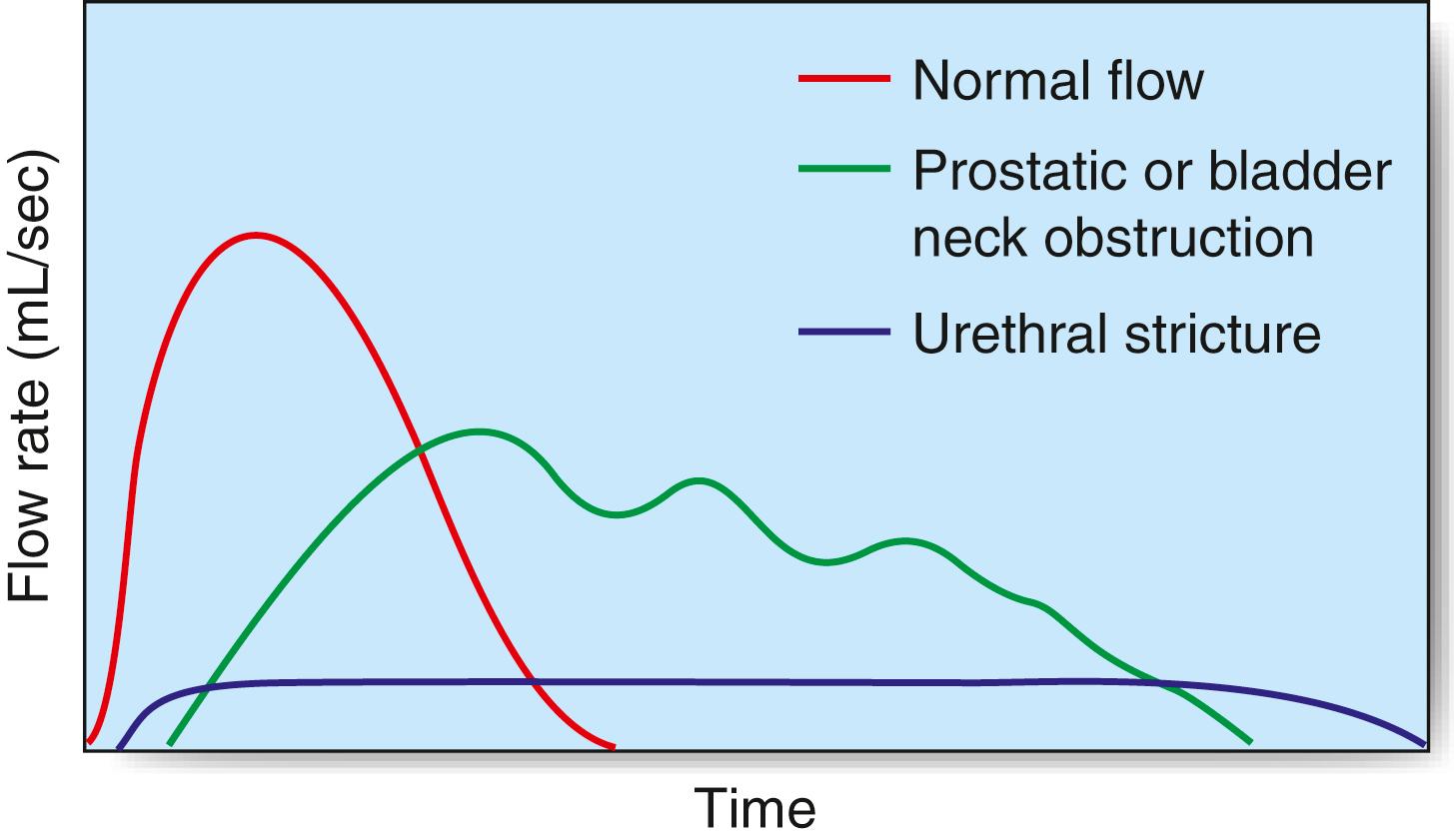
The maximum urinary flow rate during micturition can be measured in the outpatient setting using a flow meter when the voided volume is at least 150 mL or the values may be misleadingly low. The norm in males is 15 to 30 mL/s and in females 20 to 40 mL/s; a flow rate of less than 10 mL/s is abnormal. The flow rate pattern can help determine the cause of obstruction ( Fig. 24.7 ). The residual volume of urine is assessed using US, and more invasive urodynamics (‘pressure-flow studies’) uses rectal and urethral pressure lines to indirectly assess detrusor pressure during filling of the bladder with contrast material or water. The cystometrogram provides a measure of bladder capacity—the capacity at which a desire to void occurs—and the detrusor pressures when the bladder is full and during maximum flow. Spontaneous detrusor contractions during bladder filling may indicate an unstable bladder, a cause of urgency and urge incontinence. The detrusor pressure during voiding and the maximum urinary flow rate are used to define BOO and detrusor contractility through mathematical formulae and nomograms.
Microscopic examination of the semen is a basic investigation in infertile males. The specimen is collected following a period of abstinence of at least 3 days and is examined within 2 hours. Normal semen has a volume of > 1.5 mL and sperm concentration of > 15 million/mL. More than 40% of the sperm should be motile. The morphology, biochemistry and viability of the sperm may also be studied. In selected cases, immunologic tests may help determine the cause of infertility.
Patients should have basic metabolic screening with blood tests (sodium, potassium, calcium, creatinine, urea) and urinary pH. Recurrent urinary tract calculi should raise the suspicion of hyperparathyroidism, idiopathic hypercalciuria, hyperoxaluria, cystinuria, renal tubular acidosis or medullary sponge kidney. Serum creatinine, calcium, phosphate, oxalate and uric acid should be measured, and, if more detailed investigation is required, a 24-hour collection of urine for determination of calcium, phosphate, citrate, oxalate and uric acid excretion can be obtained. The composition of passed or removed stones should be analysed to determine their metabolic type.
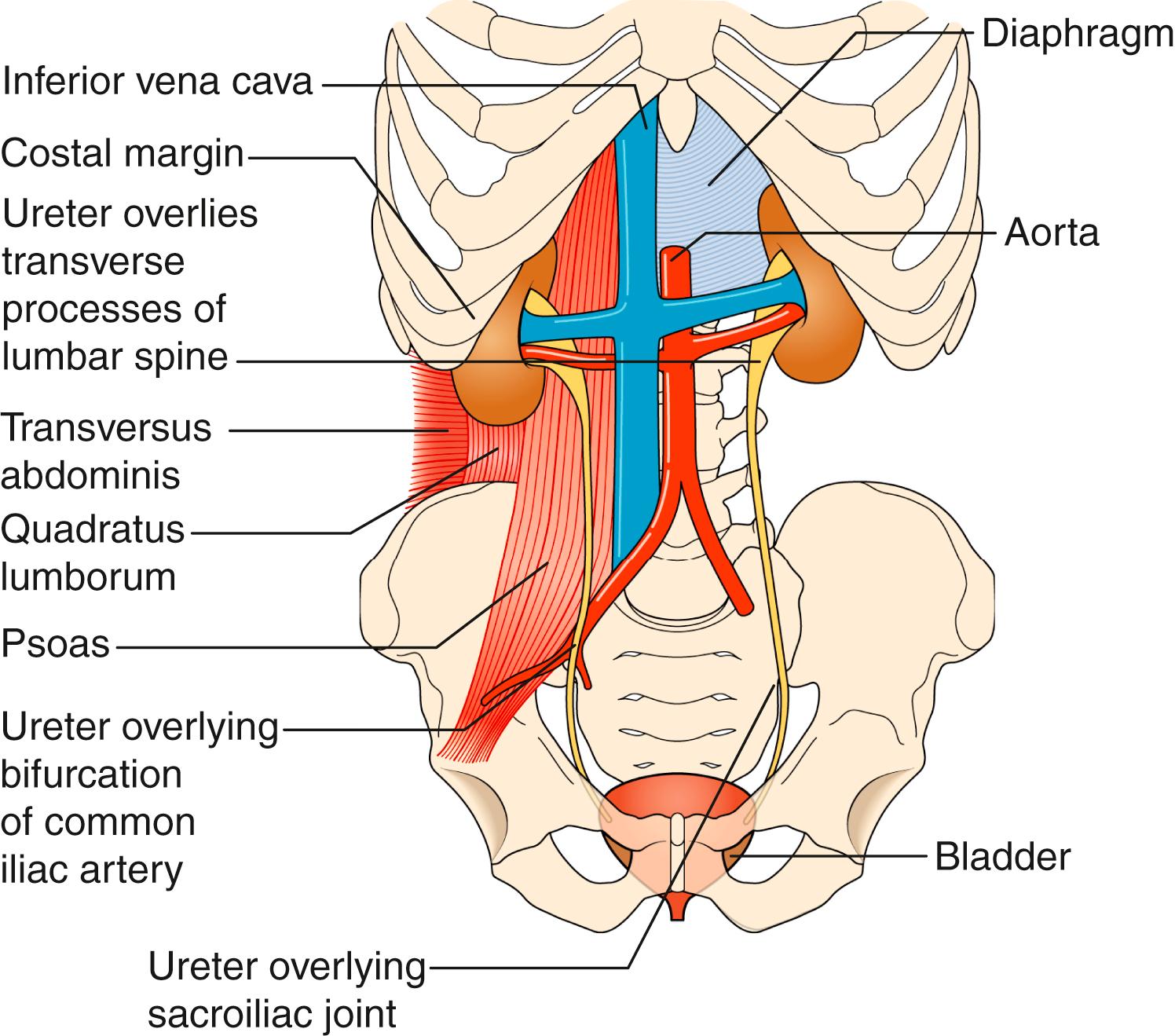
The two kidneys are covered with perinephric fat and lie retroperitoneally on the posterior abdominal wall. Each is approximately 12 cm long, 6 cm wide and 3 cm thick. The upper pole of the kidney lies on the diaphragm, which separates it from the pleura and the 11th and 12th ribs. Below this, it lies on the psoas, quadratus lumborum and transversus abdominis muscles from medial to lateral ( Fig. 24.8 ). Anteriorly, the right kidney is covered by the liver, the second part of the duodenum and the ascending colon. The spleen, stomach, tail of pancreas, left colon and small bowel overlie the left kidney. The renal hilum lies medially and, from front to back, transmits the renal vein, renal artery and renal pelvis. The ureter begins at the renal pelvis and runs for 25 cm to the bladder. The abdominal ureter lies on the medial edge of the psoas muscle, which separates it from the tips of the transverse processes. It then crosses the bifurcation of the common iliac artery, which separates it from the sacroiliac joint, to enter the pelvis. The pelvic ureter runs on the lateral pelvic wall to just in front of the ischial spine, when it then turns medially and forward to enter the bladder. In the male, it is crossed by the vas deferens, and in the female, it lies close to the lateral fornix of the vagina and is crossed by the uterine vessels, where it is vulnerable to damage during hysterectomy. The section of ureter that lies within the bladder wall functions as a flap valve to prevent reflux. Stones tend to impact at the three points where the ureter narrows: namely, the pelviureteric junction (PUJ), the pelvic brim and the ureteric orifice.
The healthy kidney produces between 0.3 and 17 mL of urine per minute, depending on the state of hydration, but on average produces 1 mL of urine per minute. This is transported down the ureter by four or five peristaltic waves per minute to reach the bladder.
These are usually single and almost always asymptomatic. They are often found incidentally during ultrasonography and can usually be differentiated from solid lesions (which may be malignant or benign tumours). Complex cysts containing multiple septa raise the suspicion of malignant change and need further evaluation by CT.
This is an autosomal dominant congenital anomaly affecting both kidneys, often leading to chronic renal failure in middle life. Despite their very large size, the cystic kidneys cause few symptoms. Infection or bleeding may occur in a cyst, causing pain or haematuria.
The incidence is 1 in 400 to 800 live births and is the most common fusion anomaly of the kidney. The fused mass usually has two collecting systems and two ureters. They are almost always malrotated, with the pelves facing anteriorly; they also have an incomplete ascent, lying lower than the normal kidneys. They are usually asymptomatic and are discovered incidentally on imaging. Occasionally they may present with obstruction and resultant pain.
The common developmental anomalies seen are unilateral renal agenesis, renal ectopia, crossed renal ectopia and various degrees of duplication of the collecting system. All of these are compatible with a normal life and are usually detected in adult life as incidental findings on imaging investigations.
Oncocytomas are the commonest benign kidney tumour that can be difficult to differentiate from a kidney cancer on imaging. Angiomyolipomas, either sporadic or associated with tuberous sclerosis complex, are characterised by a typical appearance on CT due to their high fat content. Treatment is observation, embolisation or surgery and is dictated by symptoms and size.
Also known as Wilms tumour, it usually occurs in children under 4 years of age and is the most common childhood urologic malignancy, with an incidence of 7 per million per year. Growth is rapid, and there is early local spread, including invasion of the renal vein. Invasion of the renal pelvis occurs late, thus haematuria is seen in only 15% of cases. Distant metastases most commonly appear in the lungs, liver and bones. Tumours presenting in the first year of life have a better prognosis.
The cardinal sign is a large abdominal mass. Some unusual clinical features associated with a renal carcinoma in adults, such as fever or hypertension, may be present. Clinically it needs to be differentiated from neuroblastoma ( Table 24.2 ).
| Feature | Nephroblastoma | Neuroblastoma |
|---|---|---|
| Frequency | 7 per million children | 1 per 8000–10,000 children |
| Age | Between 3 and 5 years of age | < 2 years of age |
| Origin | Kidney | Adrenal, also extraadrenal |
| Symptoms | Hypertension in 25–60% | Uncommon |
| Abdominal lump | Unilateral, never crosses midline | May cross midline |
| Radiologically | No change in renal axis | Outward and downward displacement of kidney; calcification common |
| Metastases at presentation | Uncommon | Bony metastases common |
| Tumour markers | Serum LDH may be raised | VMA may be raised |
| Treatment | Surgery mainstay, adjuvant chemotherapy for metastases | Chemotherapy, radiotherapy and surgery |
CT of the abdomen and chest is essential for diagnosis and staging. The main differential diagnosis to consider is adrenal neuroblastoma, but other causes of a large kidney, such as hydronephrosis and cystic disease, must also be considered. The tumour is bilateral in 5–10% of cases.
The diagnosis is confirmed by biopsy. Chemotherapy is followed by transabdominal nephrectomy with wide excision of the mass. Further chemotherapy with or without radiotherapy is administered dependent on the histopathologic features; the 5-year survival rate is 70–90%.
Renal cell carcinoma (RCC) arises from the renal tubules and is the most common malignant tumour of the kidney. The prevalence is 2 cases per 1000 and is 1.6-fold more common in males. It is uncommon before the age of 40 years, but there is a sharp increase in incidence from 45 to 49 years and a peak incidence between 80 and 85 years of age. Invasion of the renal vein, often extending into the inferior vena cava and occasionally into the right atrium, can occur. Direct spread into perinephric fat is common. Lymphatic spread occurs to paraaortic nodes; blood-borne metastases (which may be solitary) are most common in the lungs (cannonball metastases) but may develop anywhere.
The triad of pain, haematuria and a palpable mass occurs in only 15% of cases. Historically, 60% presented with haematuria, 40% with loin pain and 25% with a mass, but increasing access to US and CT has increased incidental diagnosis to ∼60%. Patients may present with pyrexia of unknown origin, raised ESR, polycythaemia, disorders of coagulation, abnormalities of liver function tests or with neuromyopathy due to secretion of renin, erythropoietin, parathormone and gonadotrophins, making RCC the ‘great mimic’.
The initial investigation is US or CTU, either to investigate haematuria or as part of imaging for a nonspecific symptom. Subsequently, a CT of chest, abdomen and pelvis ( Fig. 24.9 ) should be performed to complete staging. In young RCC patients (< 45 years), genetic testing for a familial syndrome should be performed.
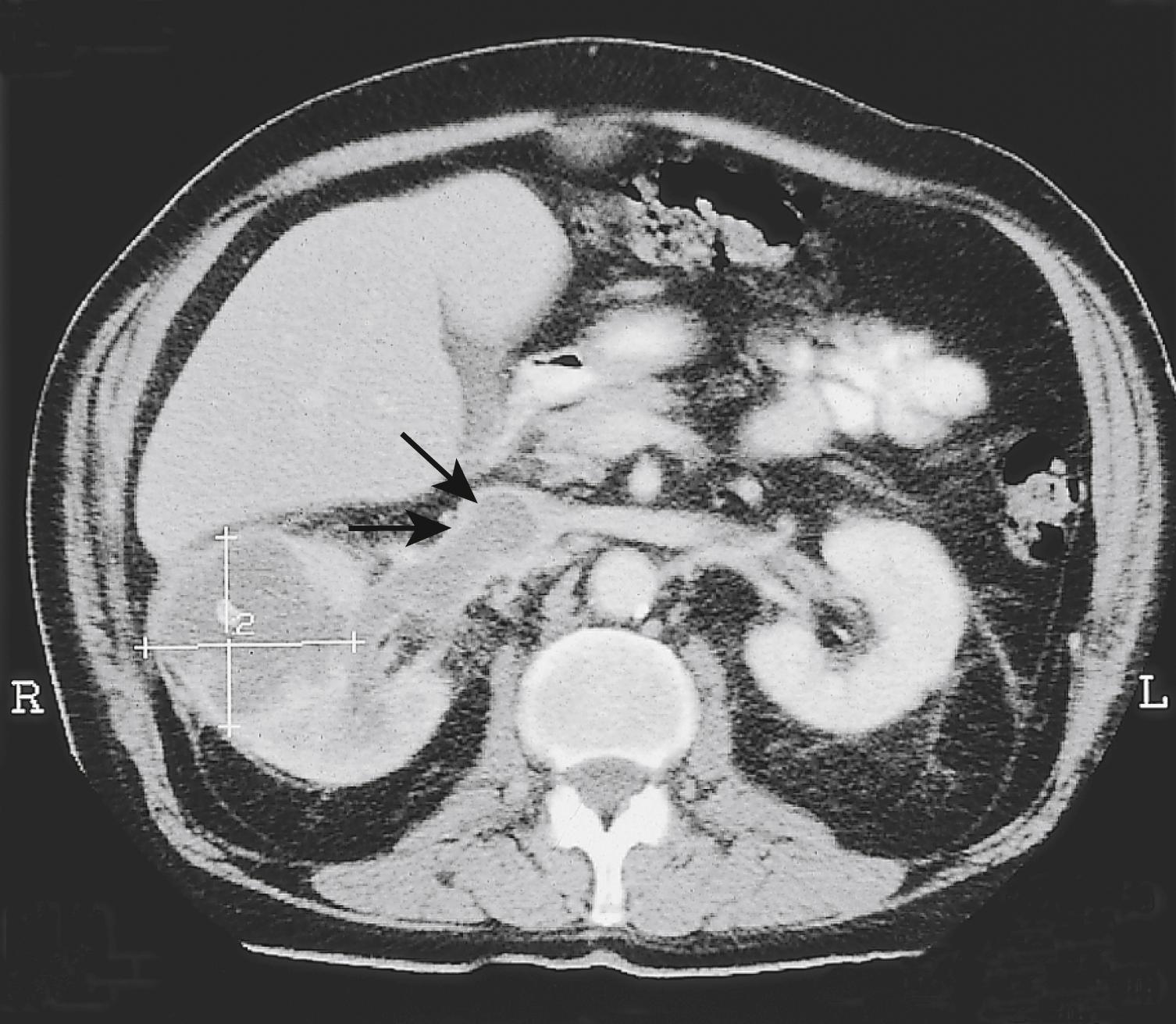
There are numerous histologic subtypes of RCC. The most common is clear cell RCC (85%) followed by papillary RCC (comprised of type 1 and type 2). Chromophobe and collecting duct RCC are less common. RCC may develop in a range of inherited syndromes, i.e., von Hippel–Lindau syndrome and Birt–Hogg–Dubé syndrome.
Tumour, Node, Metastasis (TNM) staging is used ( Table 24.3 ).
| T (Tumour) | |
|
No evidence of primary tumour Primary tumour cannot be assessed Tumour <7 cm in greatest dimension, limited to the kidney Tumour <4 cm in greatest dimension, limited to the kidney Tumour >4 cm but <7 cm in greatest dimension Tumour >7 cm in greatest dimension, limited to the kidney Tumour >7 cm but <10 cm in greatest dimension Tumours >10 cm limited to the kidney Tumour extends into major veins, pelvicalyceal system or perinephric tissues but not into the ipsilateral adrenal gland or beyond Gerota fascia Tumour extends into the renal vein or its segmental branches, or tumour invades the pelvicalyceal system or tumour invades perirenal and/or renal sinus fat (peripelvic fat), but not beyond Gerota fascia Tumour grossly extends into the IVC below the diaphragm Tumour grossly extends into IVC above the diaphragm or invades the wall of the IVC Tumour invades beyond Gerota fascia (including contiguous extension into the ipsilateral adrenal gland) |
| N (Nodes) | |
|
No regional lymph node metastasis Regional lymph nodes cannot be assessed Regional lymph node metastasis |
| M (Metastases) | |
|
No distant metastasis detected Distant metastasis cannot be assessed Distant metastasis |
Organ-confined RCC should be treated with curative intent, either by laparoscopic or open nephrectomy. Tumours (<7 cm) confined to one pole of the kidney can be treated by partial nephrectomy (using an open, robot-assisted or laparoscopic approach) if technically feasible, with oncologic results as good as with radical nephrectomy. Active surveillance (monitoring of symptoms and tumour size with a view to delayed intervention) and thermal ablation (cryoablation, radiofrequency ablation) are well-established management options for comorbid patients with small tumours <4 cm.
Metastatic RCC is relatively radio- and chemoresistant. In the first-line setting, tyrosine-kinase inhibitor monotherapy or combination therapy of a T-cell immune-checkpoint inhibitor with a tyrosine-kinase inhibitor (i.e., pembolizumab + axitinib) or two T-cell immune-checkpoint inhibitors (i.e., ipilimumab + nivolumab) provide a median 12 to 15 months progression-free survival and objective response rates of 40% to 60%. There is little evidence suggesting a survival benefit by performing a cytoreductive nephrectomy prior to starting systemic treatment.
RCC is the most common malignant renal tumour and is 1.6-fold more common in males.
The carcinoma arises in the renal tubules. Renal cancer may involve the renal vein (with bloodstream dissemination), perinephric tissue and lymphatic spread.
The clinical presentation is varied, and the condition often presents incidentally. The triad of pain, haematuria and a mass may be late features, and early systemic effects include fever, polycythaemia, disordered coagulation and pyrexia of unknown origin.
The key investigation is CT of the chest, abdomen and pelvis.
Treatment is usually surgical if the tumour has not spread.
Renal cell cancer is not very radio- or chemotherapy sensitive.
Metastatic renal carcinoma is treated with tyrosine kinase inhibitor and immunotherapy single agent or combinations.
The natural history of renal carcinoma is highly variable, and excision of solitary metastases may be worthwhile.
Upper urinary tract urothelial cell cancer (UUTUCC) of the renal pelvis or ureter are rare tumours, accounting for only 5–10% of urothelial cell carcinomas. They have a similar morphology to bladder carcinomas, and nearly all UUTUCCs are urothelial in origin. UUTUCC is three times more common in males, with a peak incidence in patients aged 70 to 90 years. Patients with a carcinoma of the upper tract have a 30–50% risk of developing bladder carcinoma, whereas conversely, the risk of a patient with bladder carcinoma developing upper tract malignancy is only 1–4%. Smoking, dye and solvent exposure and analgesic abuse have been identified as risk factors.
Visible haematuria is the most common symptom and is seen in 70–90% of patients. Flank pain and dysuria are other presenting symptoms.
UUTUCC is usually identified as an enhancing mass on contrast-enhanced CT or a filling defect on CTU. Suspicious lesions can be evaluated by ureteroscopy and biopsy for pathologic diagnosis. The extent of local and distant spread can be determined by CT of chest, abdomen and pelvis. A cystoscopy is performed to check for concomitant bladder cancer. Urine cytology can be obtained selectively for the affected upper tract.
The standard treatment for UUTUCC is radical (open or laparoscopic) nephroureterectomy along with a bladder cuff, as the disease can be multifocal within the ipsilateral collecting system. Kidney-sparing management (i.e., endoluminal laser treatment) can be offered to select patients but requires close follow-up. In metastatic disease, chemotherapy is used if the patient has an adequate performance status.
The ability of urine to keep compounds in solution and prevent calculus formation is a balance between forces keeping the solute in solution and those that promote crystal formation. Stones form when solute increases (e.g., hypercalciuria) and solvent decreases (e.g., dehydration) or the concentration of inhibitors falls (e.g., decreased citrate excretion). Foreign bodies, anatomic abnormalities and calculi can all act as a nidus for nucleation, promoting further stone formation.
The most common stone types are calcium oxalate (85%), uric acid (10%), mixed calcium phosphate calcium oxalate (10%), magnesium ammonium phosphate (5–15%) and cystine (1%). Calcium oxalate stones are commonly caused by hypercalciuria, hypercalcaemia, hyperoxaluria or hypocitraturia. Uric acid stones form due to increases in uric acid formation, either through gout or myeloproliferative disorders. Approximately 50% of patients with urate stones have gout, but only 20% of patients with gout develop urate stones. Calcium phosphate stones are generally secondary to renal tubular acidosis. Magnesium ammonium phosphate (struvite) stones are usually due to UTI by pathogens that can break urea down into CO 2 and ammonia, thereby alkalinising the urine (e.g., Proteus mirabilis ).
Uric acid, xanthine, indinavir, triamterene and matrix calculi are the common radiolucent varieties and are not visualised on a plain film. All other stones are considered radiopaque; a noncontrast CT KUB is the modality of choice for these.
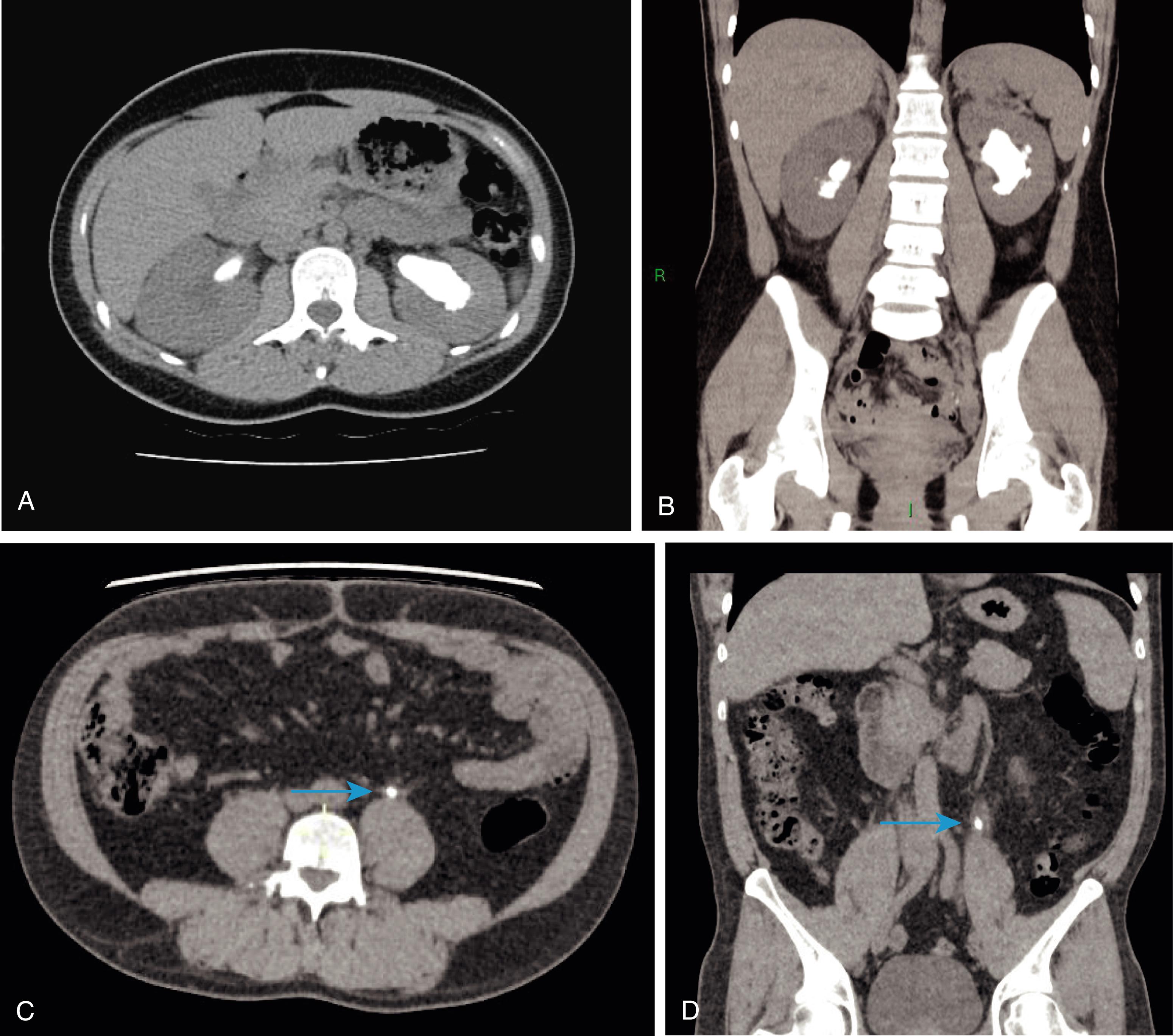
Renal calculi cause flank pain, which may be colicky (arising from the renal pelvis) or a noncolicky dull ache (arising from renal capsule). Ureteric calculi cause colicky pain, and the site of the stone in the ureter determines the site of the pain: upper ureteric calculi cause costovertebral angle or flank pain, midureteric calculi cause pain radiating from ‘loin to groin’ and lower ureteric calculi cause pain radiating to the testicle in males and labia majora in females. Renal pain, renal colic and ureteric colic are characteristically unilateral. A bladder calculus may lead to suprapubic pain, recurrent UTIs, intermittent urinary stream or urgency. Any stone may also cause haematuria. However, a stone in the kidney may remain silent, even one large enough to fill the pelvis and calyces (‘staghorn’ calculus). Urethral calculi may be secondary (passing down from the bladder) or primary (due to a stricture or diverticulum in the urethra) and present with dysuria and pain radiating to the tip of penis.
CT KUB provides all the necessary information on the position and size of the stone ( Fig. 24.10 ). CT also allows measurement of the radiodensity of a stone in Hounsfield Units (HU). In general, uric acid stones show a lower HU (< 500 HU) than calcium stones (> 1000 HU). This information is helpful to identify the most appropriate treatment. Ultrasonography is an easily available noninvasive investigation that provides a lot of information in renal and vesical calculus disease without radiation exposure (preferred in children, women of child-bearing age and pregnant females).
Routine haematologic and biochemical tests are needed to assess renal function and exclude metabolic causes. Urine culture will determine whether there is infection. If obstruction is acute, relief is the prime clinical need; if it is chronic and has caused renal damage, the surgical approach depends on the function of the affected kidney. Function is best determined by radioisotope methods (e.g., DMSA).
Symptomatic treatment should be instituted as soon as the diagnosis is confirmed. Oral or systemic diclofenac, a nonsteroidal antiinflammatory, is the most effective analgesic; pethidine is an alternative. The likelihood of spontaneous passage of a renal or ureteric calculus depends on the size of the stone and its smoothness. A stone < 5 mm in diameter passes in > 90% of cases. The location of the stone in the ureter also determines the ease with which it will pass: small lower ureteric stones have a higher likelihood compared with a larger upper or midureteric calculus. Immediate treatment should be considered in cases of ongoing pain, renal obstruction or, more importantly, when there are signs of sepsis (infected obstructed kidney).
Extracorporeal shockwave lithotripsy (ESWL), the technique of focusing external shockwaves to break up stones, has revolutionised the treatment of renal and ureteric stones. Stones visualised on x-ray or US can be treated by ESWL, especially those that are single and up to 2 cm in size. Other stones can be visualised directly by ureterorenoscopy (URS) and broken up using a holmium laser or removed intact using a Dormia wire basket. Some stones in the kidney that are unlikely to pass, even if broken up, are best treated by direct puncture of the kidney, insertion of a sheath and removal under vision with a nephroscope with or without ultrasonic disaggregation (percutaneous nephrolithotomy [PCNL]). It is now very rare to perform open surgery to remove renal or ureteric stones. Even large staghorn calculi filling the entire renal collecting system can be treated by PCNL with or without ESWL (although multiple sessions may be required). In developing countries in Asia and Africa, where the expertise for the latest noninvasive techniques is not available, large kidney stones continue to be treated by conventional open surgery.
In patients with acute obstruction and sepsis (infected obstructed kidney) or renal impairment, decompression of the kidney either via insertion of a ureteric stent or percutaneous nephrostomy is required. Stones and infection within a kidney can be the cause of renal destruction, and if the kidney contributes less than 15% of total renal function, then nephrectomy is recommended.
Bladder calculi can be treated endoscopically like ureteric calculi, using a stone-crushing device, pneumatic lithotrite or holmium laser. Alternatively, large stones can be dealt with through an open suprapubic cystolithotomy or by suprapubic insertion of a nephroscope and use of ultrasonic shattering.
Primary urethral calculi are treated along with the inciting cause. Secondary calculi impacted in the urethra may be endoscopically removed intact if small or may have to be crushed and removed.
Acute pyelonephritis is a bacterial infection of the renal parenchyma and collecting system. The causative organisms are the same as for UTI (gram-negative enteric organisms), as most infections are ascending infections from the lower tracts. It classically presents as sudden onset of fever with chills and unilateral or bilateral flank pain. It may be associated with lower tract symptoms such as dysuria, frequency and urgency.
The diagnosis is clinical and confirmed by way of urine culture. Blood counts may show the presence of leucocytosis with predominance of neutrophils. Imaging modalities are seldom needed, but US or CT may reveal the presence of an enlarged kidney on the affected side.
Management is aimed at treating the infection. Antibiotics are started empirically with clinical diagnosis and may have to be revised once the culture and sensitivity reports are available. They should be continued for approximately 2 weeks. Patients with sepsis should be hospitalised and administered intravenous antibiotics; absence of response to treatment after 48 to 72 hours should alert the surgeon towards development of complications such as renal abscess or presence of associated urinary tract obstruction, which is an emergency and requires urgent decompression. Imaging (US or CT) is required in these cases.
Chronic pyelonephritis denotes the process of scarring and atrophy of renal parenchyma, ultimately resulting in renal insufficiency. The most common association, especially in children, is with vesicoureteric reflux, hence it is often called reflux nephropathy.
The condition is usually silent and discovered incidentally on investigating abnormal renal functions. Imaging may show a small contracted and scarred kidney that is poorly functioning.
Management is aimed at preventing further damage to the kidneys by recurrent UTIs. The damage that has already resulted is irreversible.
The aetiology and pathogenesis of renal abscesses has changed with the use of antibiotics. Whereas the haematogenous route was the most common route of infection in the past, most infections currently ascend from lower tracts. The haematogenous infections are mostly caused by gram-positive bacteria and are located in the subcapsular and cortical regions of the kidney. Ascending infections, on the other hand, are caused by gram-negative pathogens and are in the corticomedullary region. Perinephric abscesses usually result from extension of renal abscesses in the perinephric space, so the bacteriology is therefore the same.
Patients usually present with fever associated with chills, flank pain and systemic symptoms such as nausea and malaise. Blood counts may reveal neutrophilia, urinalysis pyuria and bacteriuria. Imaging studies are important in making a diagnosis, and CT is the modality of choice.
Management involves hospitalisation, antibiotic therapy and supportive treatment, followed by US- or CT-guided drainage. If percutaneous drainage fails to resolve the abscess, open surgical drainage may be needed.
Emphysematous pyelonephritis (EPN) is a severe necrotising infection of the renal parenchyma; it causes gas formation within the collecting system, renal parenchyma and/or perirenal tissues. It is common in diabetics, and the presentation is similar to that of acute pyelonephritis. However, the clinical course of EPN can be severe and life-threatening if not recognised and treated promptly. Patients are usually unwell and typically present with fever, abdominal or flank pain, nausea and vomiting, and may be in septic shock. Radiologic evaluation with CT diagnoses the problem.
Patients with EPN should be treated with aggressive medical management and, wherever needed, prompt surgical intervention. Initial resuscitation with fluids, control of diabetes and administration of intravenous antibiotics empirically is important in stabilising the patient.
Percutaneous drainage combined with antibiotic therapy is useful in early cases associated with gas in the collecting system alone and when the patient is otherwise stable. It may also be useful in patients with compromised renal function. Nephrectomy is frequently the treatment of choice, especially when patients are sick, with evidence of gas in the renal parenchyma or if there is no access to percutaneous drainage.
The mortality rate associated with EPN was high before the advent of antibiotics; however, advances in imaging technology, control of diabetes, resuscitative management and minimally invasive treatment have improved the outcome.
Genitourinary tuberculosis is usually a secondary infection, the primary focus being somewhere else. Kidneys are the most frequently affected organ, followed by bladder and ureter. Mycobacterium tuberculosis is the causative organism, and the disease is still common in many developing countries and in the presence of HIV infection.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here